Relationship between Preoperative Nutritional Status and Clinical Outcomes in Patients with Head and Neck Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Populations
2.2. Definition of Prognostic Nutritional Index and other Confounders
2.3. Endpoint
2.4. Statistical Analysis
3. Results
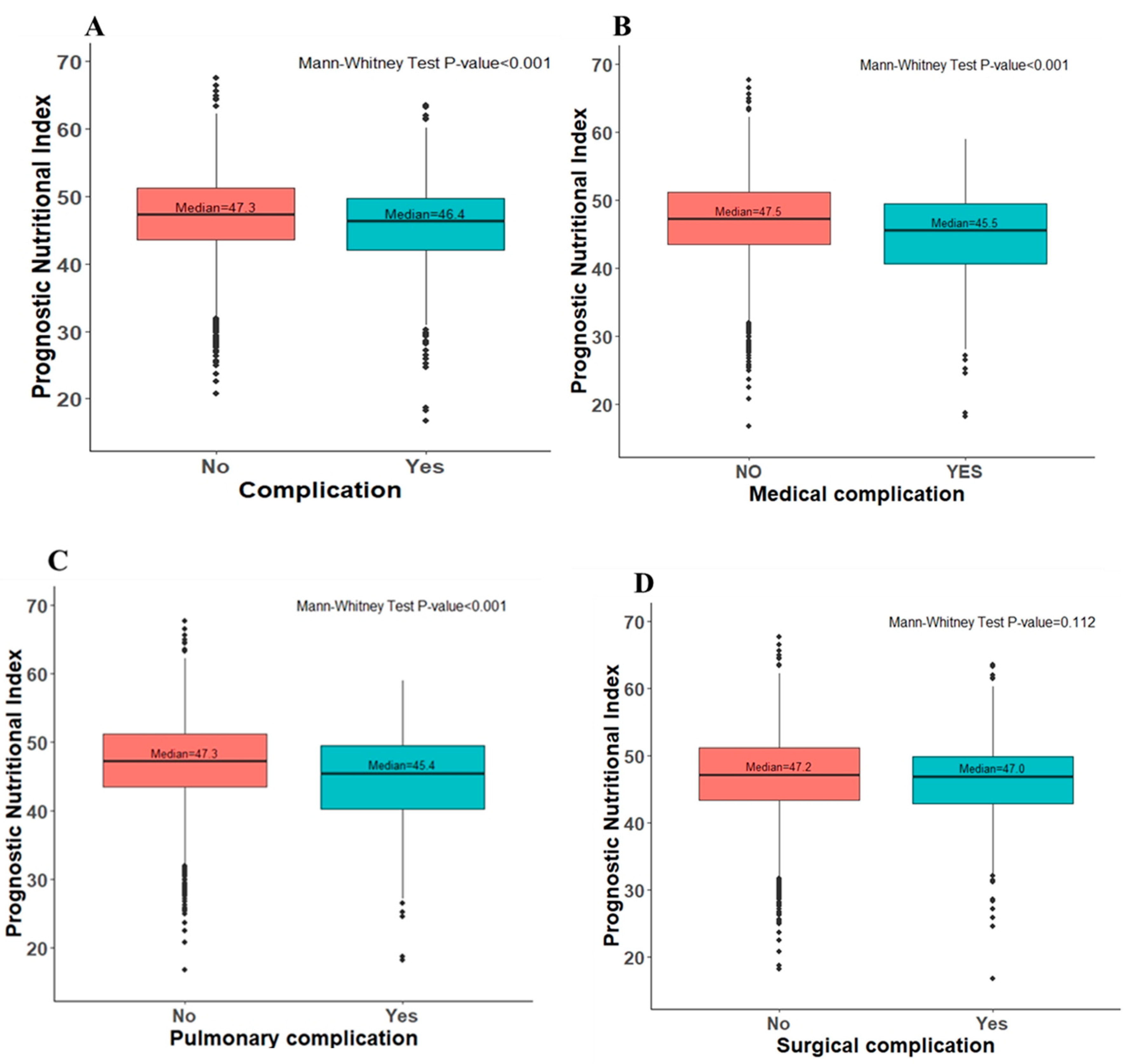
3.1. Post-Operative Complications
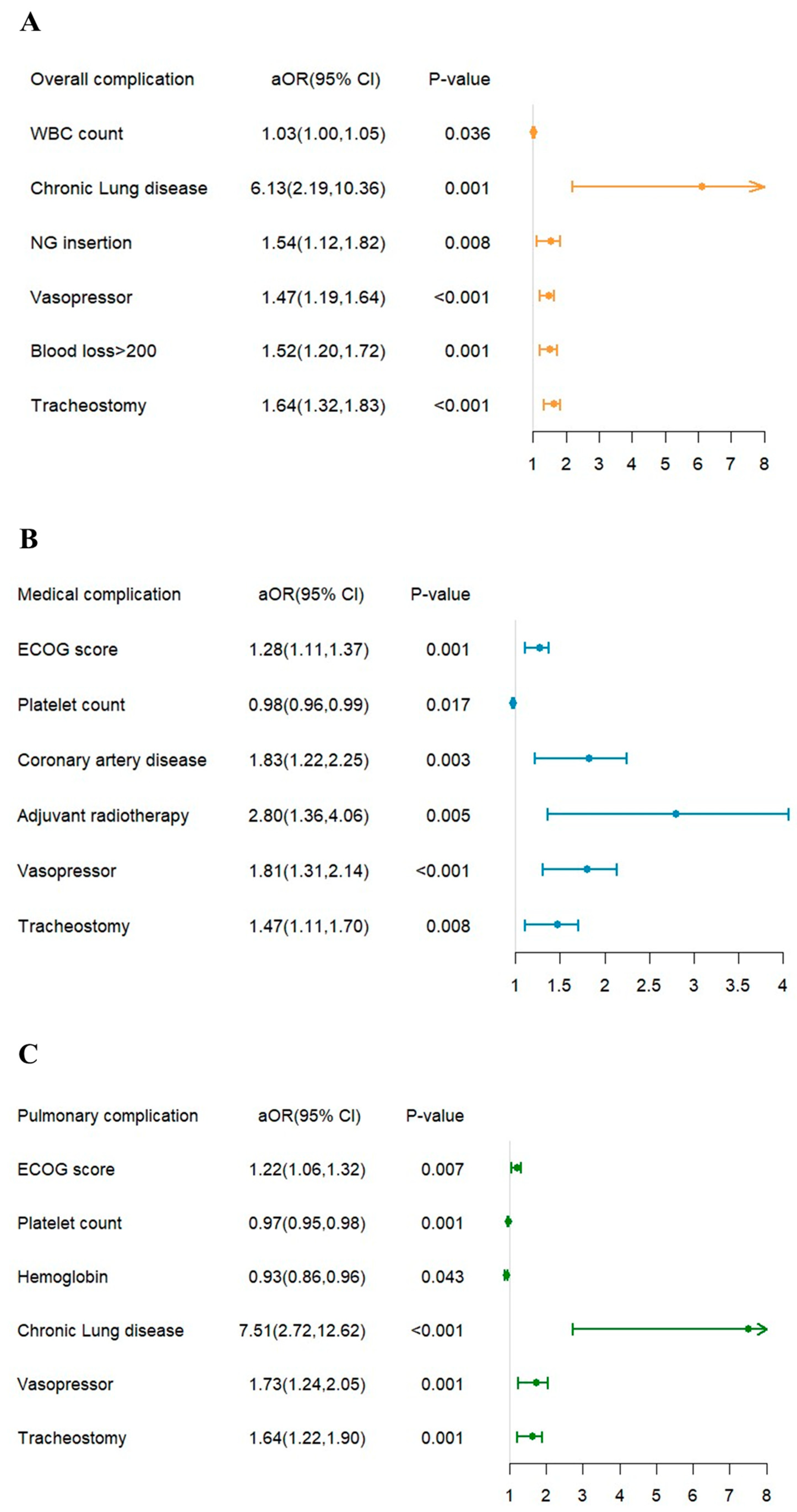
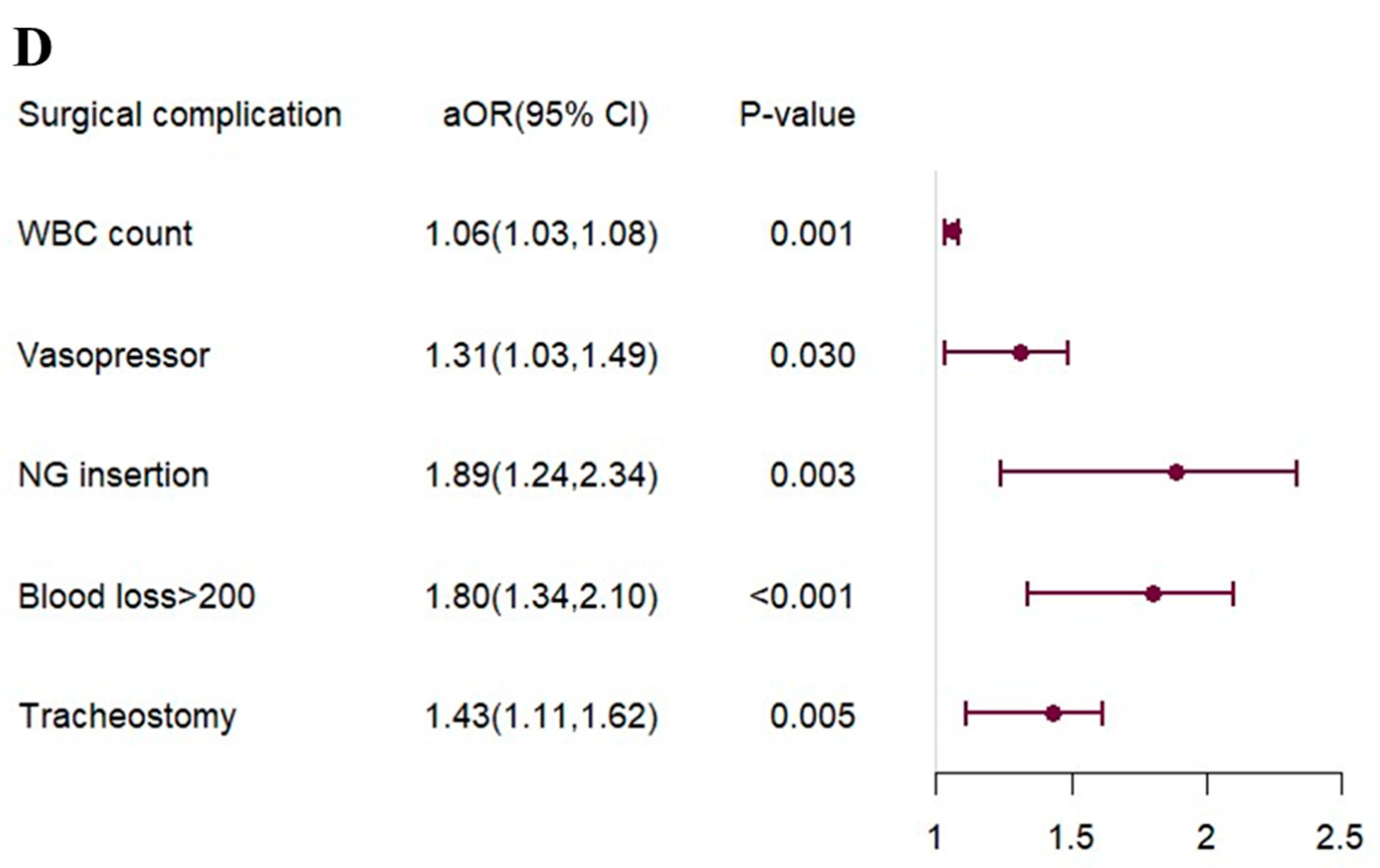
3.2. Risk Factor for Postoperative Complications
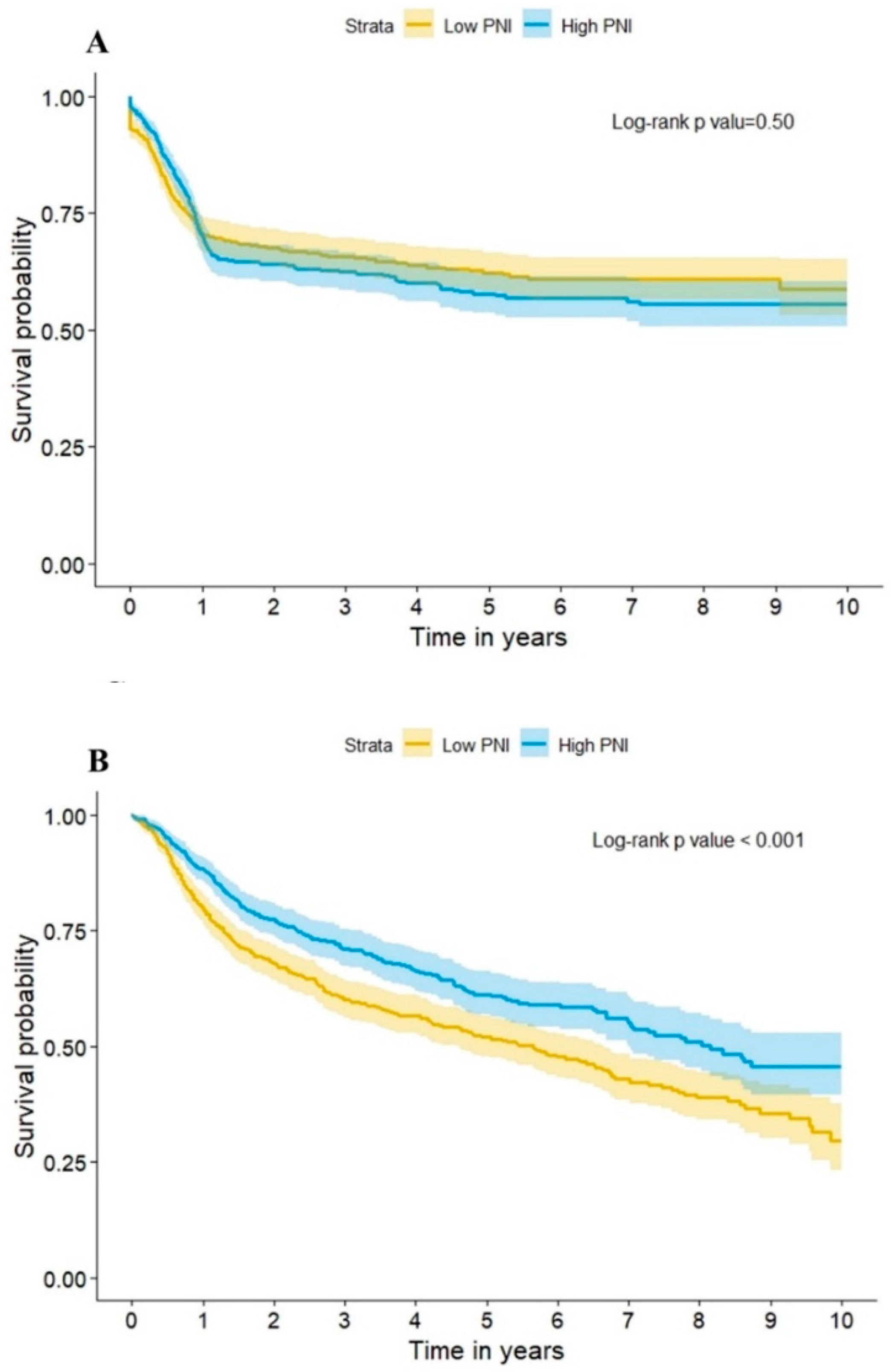
3.3. Long-Term Outcome
4. Discussion
Limitations and Future Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abe, A.; Hayashi, H.; Ishihama, T.; Furuta, H. Prognostic impact of the prognostic nutritional index in cases of resected oral squamous cell carcinoma: A retrospective study. BMC Oral. Health 2021, 21, 40. [Google Scholar] [CrossRef] [PubMed]
- Bruixola, G.; Caballero, J.; Papaccio, F.; Petrillo, A.; Iranzo, A.; Civera, M.; Moriana, M.; Bosch, N.; Maroñas, M.; González, I.; et al. Prognostic Nutritional Index as an independent prognostic factor in locoregionally advanced squamous cell head and neck cancer. ESMO Open 2018, 3, e000425. [Google Scholar] [CrossRef] [PubMed]
- Caburet, C.; Farigon, N.; Mulliez, A.; Mom, T.; Boirie, Y.; Gilain, L.; Saroul, N. Impact of nutritional status at the outset of assessment on postoperative complications in head and neck cancer. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2020, 137, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Cerantola, Y.; Grass, F.; Cristaudi, A.; Demartines, N.; Schäfer, M.; Hübner, M. Perioperative nutrition in abdominal surgery: Recommendations and reality. Gastroenterol. Res. Pract. 2011, 2011, 739347. [Google Scholar] [CrossRef]
- Chang, P.H.; Hsieh, J.C.; Yeh, K.Y.; Chen, E.Y.; Yang, S.W.; Huang, J.S.; Lai, C.H.; Wu, T.H.; Huang, Y.M.; Chang, Y.S.; et al. Prognostic nutritional index relevance in chemoradiotherapy for advanced oral cavity, oropharyngeal and hypopharyngeal cancer. Asia Pac. J. Clin. Nutr. 2018, 27, 996–1001. [Google Scholar] [CrossRef]
- Chen, Q.J.; Qu, H.J.; Li, D.Z.; Li, X.M.; Zhu, J.J.; Xiang, Y.; Li, L.; Ma, Y.T.; Yang, Y.N. Prognostic nutritional index predicts clinical outcome in patients with acute ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Sci. Rep. 2017, 7, 3285. [Google Scholar] [CrossRef]
- Correa-Rodríguez, M.; Pocovi-Gerardino, G.; Callejas-Rubio, J.L.; Fernández, R.R.; Martín-Amada, M.; Cruz-Caparros, M.G.; Ortego-Centeno, N.; Rueda-Medina, B. The Prognostic Nutritional Index and Nutritional Risk Index Are Associated with Disease Activity in Patients with Systemic Lupus Erythematosus. Nutrients 2019, 11, 638. [Google Scholar] [CrossRef]
- Eu, C.W.; Ajit Singh, V.; Yasin, N.F. Effective nutritional status screening in orthopaedic oncology patients and post-operative complications. J. Orthop Surg. (Hong Kong) 2019, 27, 2309499019847232. [Google Scholar] [CrossRef]
- Fanetti, G.; Polesel, J.; Fratta, E.; Muraro, E.; Lupato, V.; Alfieri, S.; Gobitti, C.; Minatel, E.; Matrone, F.; Caroli, A.; et al. Prognostic Nutritional Index Predicts Toxicity in Head and Neck Cancer Patients Treated with Definitive Radiotherapy in Association with Chemotherapy. Nutrients 2021, 13, 1277. [Google Scholar] [CrossRef]
- Fowler, A.J.; Ahmad, T.; Phull, M.K.; Allard, S.; Gillies, M.A.; Pearse, R.M. Meta-analysis of the association between preoperative anaemia and mortality after surgery. Br. J. Surg. 2015, 102, 1314–1324. [Google Scholar] [CrossRef]
- Fujiwara, D.; Tsubaki, M.; Takeda, T.; Miura, M.; Nishida, S.; Sakaguchi, K. Objective evaluation of nutritional status using the prognostic nutritional index during and after chemoradiotherapy in Japanese patients with head and neck cancer: A retrospective study. Eur. J. Hosp. Pharm. 2021, 28, 266–270. [Google Scholar] [CrossRef] [PubMed]
- Ghiam, M.K.; Langerman, A.; Sargi, Z.; Rohde, S. Head and Neck Cancer Patients: Rates, Reasons, and Risk Factors for 30-Day Unplanned Readmission. Otolaryngol. Head Neck. Surg. 2018, 159, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Guller, M.; Herberg, M.; Amin, N.; Alkhatib, H.; Maroun, C.; Wu, E.; Allen, H.; Zheng, Y.; Gourin, C.; Vosler, P.; et al. Nutritional Status as a Predictive Biomarker for Immunotherapy Outcomes in Advanced Head and Neck Cancer. Cancers 2021, 13, 5772. [Google Scholar] [CrossRef]
- Imai, T.; Asada, Y.; Morita, S.; Saijo, S.; Fujii, K.; Kishimoto, K.; Yamazaki, T.; Goto, T.; Matsuura, K. Preoperative prognostic nutritional index as a method to predict postoperative complications after major head and neck surgery with free tissue transfer reconstruction. Jpn J. Clin. Oncol. 2020, 50, 29–35. [Google Scholar] [CrossRef]
- Johannet, P.; Sawyers, A.; Qian, Y.; Kozloff, S.; Gulati, N.; Donnelly, D.; Zhong, J.; Osman, I. Baseline prognostic nutritional index and changes in pretreatment body mass index associate with immunotherapy response in patients with advanced cancer. J. Immunother. Cancer 2020, 8, e001674. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, T.; Wasano, K.; Yamamoto, S.; Tomisato, S.; Ogawa, K. Utility of clinico-biological data for long-term prognosis of head and neck terminal cancer. Acta Otolaryngol. 2017, 137, 895–898. [Google Scholar] [CrossRef] [PubMed]
- Keskin, M.; Hayıroğlu, M.I.; Keskin, T.; Kaya, A.; Tatlısu, M.A.; Altay, S.; Uzun, A.O.; Börklü, E.B.; Güvenç, T.S.; Avcı, I.I.; et al. A novel and useful predictive indicator of prognosis in ST-segment elevation myocardial infarction, the prognostic nutritional index. Nutr. Metab. Cardiovasc. Dis. 2017, 27, 438–446. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.Y.; Kim, S.Y.; Song, J.H.; Kim, Y.S.; Jeong, S.J.; Lee, J.G.; Paik, H.C.; Park, M.S. Usefulness of the preoperative prognostic nutritional index score as a predictor of the outcomes of lung transplantation: A single-institution experience. Clin. Nutr. 2019, 38, 2423–2429. [Google Scholar] [CrossRef]
- Ling, H.H.; Yeh, K.Y.; Ng, S.H.; Wang, C.H.; Lai, C.H.; Wu, T.H.; Chang, P.H.; Chou, W.C.; Chen, F.P.; Lin, Y.C. Determining Malnutrition Assessment Criteria to Predict One-Year Mortality for Locally Advanced Head and Neck Cancer Patients Undergoing Concurrent Chemoradiotherapy. Nutrients 2020, 12, 836. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, X.; Kadasah, S.; Peng, C. Clinical Value of the Prognostic Nutrition Index in the Assessment of Prognosis in Critically Ill Patients with Stroke: A Retrospective Analysis. Comput. Math Methods Med. 2022, 2022, 4889920. [Google Scholar] [CrossRef]
- Luan, C.W.; Tsai, Y.T.; Yang, H.Y.; Chen, K.Y.; Chen, P.H.; Chou, H.H. Pretreatment prognostic nutritional index as a prognostic marker in head and neck cancer: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 17117. [Google Scholar] [CrossRef] [PubMed]
- Lyell, N.J.; Kitano, M.; Smith, B.; Gleisner, A.L.; Backes, F.J.; Cheng, G.; McCarter, M.D.; Abdel-Misih, S.; Jones, E.L. The effect of preoperative nutritional status on postoperative complications and overall survival in patients undergoing pelvic exenteration: A multi-disciplinary, multi-institutional cohort study. Am. J. Surg. 2019, 218, 275–280. [Google Scholar] [CrossRef]
- Nozoe, T.; Kimura, Y.; Ishida, M.; Saeki, H.; Korenaga, D.; Sugimachi, K. Correlation of pre-operative nutritional condition with post-operative complications in surgical treatment for oesophageal carcinoma. Eur. J. Surg. Oncol. 2002, 28, 396–400. [Google Scholar] [CrossRef] [PubMed]
- Nozoe, T.; Kohno, M.; Iguchi, T.; Mori, E.; Maeda, T.; Matsukuma, A.; Ezaki, T. The prognostic nutritional index can be a prognostic indicator in colorectal carcinoma. Surg. Today 2012, 42, 532–535. [Google Scholar] [CrossRef] [PubMed]
- Odor, P.M.; Bampoe, S.; Gilhooly, D.; Creagh-Brown, B.; Moonesinghe, S.R. Perioperative interventions for prevention of postoperative pulmonary complications: Systematic review and meta-analysis. BMJ 2020, 368, m540. [Google Scholar] [CrossRef]
- Oh, C.A.; Kim, D.H.; Oh, S.J.; Choi, M.G.; Noh, J.H.; Sohn, T.S.; Bae, J.M.; Kim, S. Nutritional risk index as a predictor of postoperative wound complications after gastrectomy. World J. Gastroenterol. 2012, 18, 673–678. [Google Scholar] [CrossRef]
- Okada, S.; Shimada, J.; Kato, D.; Tsunezuka, H.; Teramukai, S.; Inoue, M. Clinical Significance of Prognostic Nutritional Index After Surgical Treatment in Lung Cancer. Ann. Thorac. Surg. 2017, 104, 296–302. [Google Scholar] [CrossRef]
- Okada, S.; Shimada, J.; Teramukai, S.; Kato, D.; Tsunezuka, H.; Miyata, N.; Ishihara, S.; Furuya, T.; Nakazono, C.; Ishikawa, N.; et al. Risk Stratification According to the Prognostic Nutritional Index for Predicting Postoperative Complications After Lung Cancer Surgery. Ann. Surg. Oncol. 2018, 25, 1254–1261. [Google Scholar] [CrossRef]
- Onodera, T.; Goseki, N.; Kosaki, G. Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients. Nihon Geka Gakkai Zasshi 1984, 85, 1001–1005. [Google Scholar]
- Peng, J.C.; Nie, F.; Li, Y.J.; Xu, Q.Y.; Xing, S.P.; Gao, Y. Prognostic Nutritional Index as a Predictor of 30-Day Mortality Among Patients Admitted to Intensive Care Unit with Acute Exacerbation of Chronic Obstructive Pulmonary Disease: A Single-Center Retrospective Cohort Study. Med. Sci. Monit. 2022, 28, e934687. [Google Scholar] [CrossRef]
- Peng, L.; Wang, Y.; Liu, F.; Qiu, X.; Zhang, X.; Fang, C.; Qian, X.; Li, Y. Peripheral blood markers predictive of outcome and immune-related adverse events in advanced non-small cell lung cancer treated with PD-1 inhibitors. Cancer Immunol. Immunother 2020, 69, 1813–1822. [Google Scholar] [CrossRef]
- Pinato, D.J.; North, B.V.; Sharma, R. A novel, externally validated inflammation-based prognostic algorithm in hepatocellular carcinoma: The prognostic nutritional index (PNI). Br. J. Cancer 2012, 106, 1439–1445. [Google Scholar] [CrossRef] [PubMed]
- Saito, H.; Kono, Y.; Murakami, Y.; Kuroda, H.; Matsunaga, T.; Fukumoto, Y.; Osaki, T. Influence of prognostic nutritional index and tumor markers on survival in gastric cancer surgery patients. Langenbecks Arch. Surg. 2017, 402, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Shiihara, M.; Higuchi, R.; Izumo, W.; Yazawa, T.; Uemura, S.; Furukawa, T.; Yamamoto, M. Impact of the controlling nutritional status score on severe postoperative complications of pancreaticoduodenectomy for pancreatic cancer. Langenbecks Arch. Surg. 2021, 406, 1491–1498. [Google Scholar] [CrossRef]
- Sun, K.; Chen, S.; Xu, J.; Li, G.; He, Y. The prognostic significance of the prognostic nutritional index in cancer: A systematic review and meta-analysis. J. Cancer Res. Clin. Oncol. 2014, 140, 1537–1549. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- van Bokhorst-de van der Schueren, M.A.; van Leeuwen, P.A.; Sauerwein, H.P.; Kuik, D.J.; Snow, G.B.; Quak, J.J. Assessment of malnutrition parameters in head and neck cancer and their relation to postoperative complications. Head Neck. 1997, 19, 419–425. [Google Scholar] [CrossRef]
- Wu, C.Y.; Lin, Y.H.; Lo, W.C.; Cheng, P.C.; Hsu, W.L.; Chen, Y.C.; Shueng, P.W.; Hsieh, C.H.; Liao, L.J. Nutritional status at diagnosis is prognostic for pharyngeal cancer patients: A retrospective study. Eur. Arch. Otorhinolaryngol. 2022, 279, 3671–3678. [Google Scholar] [CrossRef]
- Yagi, T.; Oshita, Y.; Okano, I.; Kuroda, T.; Ishikawa, K.; Nagai, T.; Inagaki, K. Controlling nutritional status score predicts postoperative complications after hip fracture surgery. BMC Geriatr. 2020, 20, 243. [Google Scholar] [CrossRef]
- Yamamoto, H.; Sugimoto, S.; Soh, J.; Shiotani, T.; Miyoshi, K.; Otani, S.; Okazaki, M.; Yamane, M.; Toyooka, S. The prognostic nutritional index is correlated negatively with the lung allocation score and predicts survival after both cadaveric and living-donor lobar lung transplantation. Surg. Today 2021, 51, 1610–1618. [Google Scholar] [CrossRef]
- Yeap, E.; Teoh, W.M.K.; Nguyen, T.C.; Suhardja, T.S. Preoperative anaemia and thrombocytopenia are associated with venous thromboembolism complications after colorectal resection. ANZ J. Surg. 2021, 91, e32–e37. [Google Scholar] [CrossRef] [PubMed]
- Cancer Registry Annual Report of Taiwan. Available online: https://www.hpa.gov.tw/Pages/List.aspx?nodeid=269 (accessed on 9 November 2020).
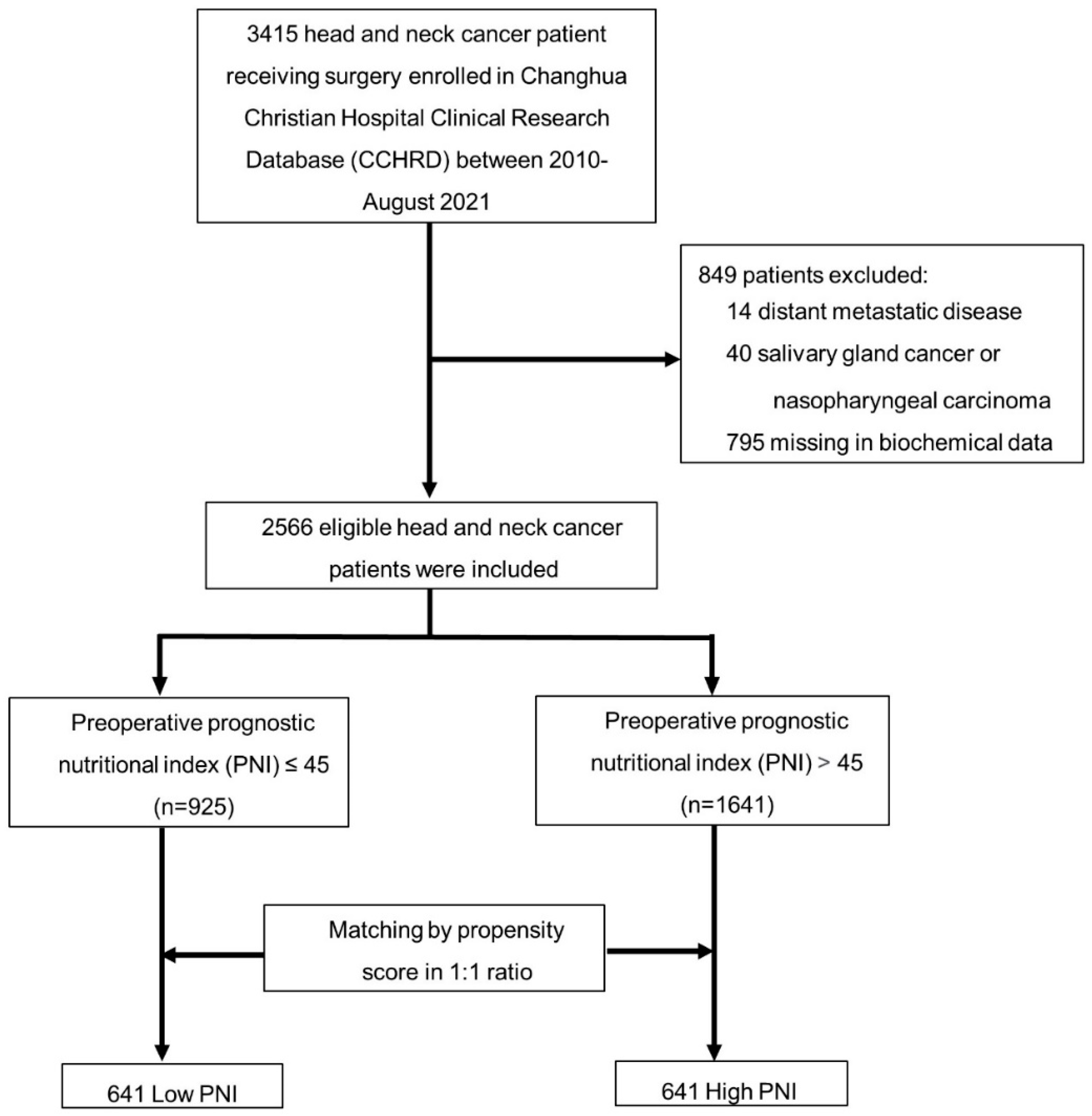
| Before Propensity Score Matching | After Propensity Score Matching | |||||
|---|---|---|---|---|---|---|
| Low PNI (n = 925) | High PNI (n = 1641) | p-Value | Low PNI (n = 641) | High PNI (n = 641) | p-Value | |
| BMI (kg/m2) | 22.7 (20.3, 25.6) | 24.6 (22.2, 27.2) | <0.001 | 23.3 (20.8, 26.6) | 23.6 (21.3, 26.1) | 0.303 |
| Gender, Male | 886 (95.8%) | 1557 (94.9%) | 0.304 | 612 (95.5%) | 611 (95.3%) | 0.894 |
| Age (years) | 60 (53, 67) | 56 (49, 64) | <0.001 | 59 (52, 67) | 59 (52, 68) | 0.603 |
| Education | <0.001 | 0.755 | ||||
| Primary school or below | 420 (45.4%) | 554 (33.8%) | 273 (42.6%) | 272 (42.4%) | ||
| Secondary school | 270 (29.2%) | 548 (33.4%) | 193 (30.1%) | 189 (29.5%) | ||
| High school | 198 (21.4%) | 438 (26.7%) | 149 (23.2%) | 152 (23.7%) | ||
| University or above | 37 (4.0%) | 101 (6.1%) | 26 (4.1%) | 28 (4.3%) | ||
| Stage | <0.001 | 0.316 | ||||
| I | 183 (19.8%) | 529 (32.3%) | 155 (24.2%) | 142 (22.2%) | ||
| II | 145 (15.7%) | 366 (22.3%) | 108 (16.8%) | 123 (19.2%) | ||
| III | 122 (13.2%) | 215 (13.1%) | 79 (12.3%) | 96 (15%) | ||
| IV | 475 (51.4%) | 531 (32.4%) | 299 (46.6%) | 280 (43.7%) | ||
| Lymphatic invasion | 293 (31.7%) | 431 (26.3%) | 0.003 | 190 (29.6%) | 215 (33.5%) | 0.133 |
| Tumor Grade | 0.744 | 0.916 | ||||
| Well-differentiated | 88 (9.5%) | 178 (10.8%) | 64 (10%) | 63 (9.8%) | ||
| Moderately differentiated | 671 (72.5%) | 1181 (72%) | 462 (72.1%) | 456 (71.1%) | ||
| Poorly differentiated | 66 (7.1%) | 112 (6.8%) | 49 (7.6%) | 48 (7.5%) | ||
| Undifferentiated/unknown | 100 (10.8%) | 170 (10.4%) | 66 (10.3%) | 74 (11.5%) | ||
| Tumor site | <0.001 | 0.233 | ||||
| Oral cavity | 656 (70.9%) | 1346 (82%) | 477 (74.4%) | 500 (78%) | ||
| Oropharyngeal | 120 (13%) | 154 (9.4%) | 73 (11.4%) | 74 (11.5%) | ||
| Hypopharyngeal | 95 (10.3%) | 84 (5.1%) | 52 (8.1%) | 44 (6.9%) | ||
| Laryngeal cancer | 47 (5.1%) | 54 (3.3%) | 34 (5.3%) | 21 (3.3%) | ||
| Nasal cavity and sinus | 7 (0.8%) | 3 (0.2%) | 5 (0.8%) | 2 (0.3%) | ||
| Smoking habits | 0.008 | 0.310 | ||||
| Never | 64 (6.9%) | 116 (7.1%) | 52 (8.1%) | 44 (6.9%) | ||
| Quit | 563 (60.9%) | 1090 (66.4%) | 383 (59.8%) | 409 (63.8%) | ||
| Current | 298 (32.2%) | 435 (26.5%) | 206 (32.1%) | 188 (29.3%) | ||
| Betel nut habits | 0.071 | 0.976 | ||||
| Never | 60 (6.5%) | 117 (7.1%) | 47 (7.3%) | 49 (7.6%) | ||
| Quit | 756 (81.7%) | 1281 (78.1%) | 516 (80.5%) | 515 (80.3%) | ||
| Current | 109 (11.8%) | 243 (14.8%) | 78 (12.2%) | 77 (12%) | ||
| Alcohol habits | 0.732 | 0.972 | ||||
| Never | 471 (50.9%) | 831 (50.6%) | 325 (50.7%) | 329 (51.3%) | ||
| Quit | 124 (13.4%) | 205 (12.5%) | 83 (12.9%) | 81 (12.6%) | ||
| Current | 330 (35.7%) | 605 (36.9%) | 233 (36.3%) | 231 (36%) | ||
| ECOG | 0.001 | 0.796 | ||||
| 0 | 822 (88.9%) | 1529 (93.2%) | 582 (90.8%) | 582 (90.8%) | ||
| 1 | 50 (5.4%) | 67 (4.1%) | 31 (4.8%) | 37 (5.8%) | ||
| 2 | 10 (1.1%) | 17 (1%) | 8 (1.2%) | 8 (1.2%) | ||
| 3 | 3 (0.3%) | 6 (0.4%) | 2 (0.3%) | 1 (0.2%) | ||
| 4 | 40 (4.3%) | 22 (1.3%) | 18 (2.8%) | 13 (2%) | ||
| Operating time, min | 424 (285, 565) | 375 (261, 525) | <0.001 | 421 (281, 560) | 400 (269, 540) | 0.298 |
| Blood loss, cc | 300 (150, 400) | 200 (100, 300) | <0.001 | 250 (150, 400) | 200 (150, 350) | 0.129 |
| Tracheostomy | 635 (62.0%) | 953 (53.1%) | <0.001 | 435 (60.5%) | 442 (60.63%) | 0.960 |
| Preoperative laboratory tests | ||||||
| Hemoglobin (g/dL) | 12.6 (11.1, 14) | 14.4 (13.3, 15.3) | <0.001 | 13.2 (11.8, 14.3) | 14 (12.9, 15) | <0.001 |
| Platelet count (1000/μL) | 220 (168, 283) | 222 (183, 267) | 0.313 | 224 (173, 281) | 222 (183, 272) | 0.855 |
| RDW (%) | 14.1 (13.3, 15.4) | 13.5 (13, 14.3) | <0.001 | 13.8 (13.2, 14.8) | 13.8 (13.2, 14.8) | 0.707 |
| WBC count (1000/μL) | 6.5 (5.1, 8.5) | 7.3 (6.1, 8.7) | <0.001 | 6.6 (5.2, 8.8) | 7 (5.9, 8.5) | 0.008 |
| Absolution lymphocyte count (1000/μL) | 1.2 (0.8, 1.5) | 1.8 (1.5, 2.2) | <0.001 | 1.3 (1, 1.6) | 1.6 (1.3, 1.9) | <0.001 |
| Neutrophil (%) | 69.6 (62.4, 75.9) | 63.3 (57.2, 69.1) | <0.001 | 67 (60.5, 73.8) | 66.8 (61.5, 71.7) | 0.366 |
| Albumin (g/dL) | 3.5 (3.2, 3.7) | 4.1 (3.9, 4.3) | <0.001 | 3.5 (3.3, 3.7) | 4.1 (3.9, 4.3) | <0.001 |
| Creatinine (mg/dL) | 0.9 (0.7, 1.1) | 0.9 (0.8, 1) | 0.811 | 0.9 (0.8, 1.1) | 0.9 (0.8, 1.1) | 0.625 |
| ALT (U/L) | 19 (15, 29) | 24 (17, 34) | <0.001 | 20 (15, 29) | 20 (16, 31) | 0.067 |
| PNI score | 41.7 (38.1, 43.6) | 49.9 (47.4, 52.8) | <0.001 | 42.2 (39.7, 43.9) | 48.7 (46.7, 51) | <0.001 |
| Comorbidity disease | ||||||
| Hypertension | 330 (35.7%) | 565 (34.4%) | 0.525 | 229 (35.7%) | 223 (34.8%) | 0.726 |
| Diabetes mellitus | 208 (22.5%) | 314 (19.1%) | 0.043 | 139 (21.7%) | 138 (21.5%) | 0.946 |
| Coronary artery disease | 85 (9.2%) | 125 (7.6%) | 0.163 | 60 (9.4%) | 56 (8.7%) | 0.697 |
| Chronic Kidney Disease | 31 (3.4%) | 17 (1%) | <0.001 | 13 (2%) | 15 (2.3%) | 0.702 |
| Chronic Lung disease | 10 (1.1%) | 6 (0.4%) | 0.027 | 5 (0.8%) | 6 (0.9%) | 0.762 |
| Treatment | ||||||
| Adjuvant chemotherapy | 248 (26.8%) | 218 (13.3%) | <0.001 | 114 (17.8%) | 122 (19%) | 0.564 |
| Adjuvant radiotherapy | 34 (3.7%) | 4 (0.2%) | <0.001 | 4 (0.6%) | 4 (0.6%) | 1.000 |
| Vasopressor | 641 (69.3%) | 1091 (66.5%) | 0.144 | 434 (67.7%) | 421 (65.7%) | 0.441 |
| Nutritional supplement | 234 (25.3%) | 171 (10.4%) | <0.001 | 114 (17.8%) | 113 (17.6%) | 0.942 |
| Glutamine supplement | 9 (1%) | 3 (0.2%) | 0.005 | 4 (0.6%) | 3 (0.5%) | 0.705 |
| Amino acid supplement | 9 (1%) | 9 (0.5%) | 0.216 | 5 (0.8%) | 6 (0.9%) | 0.762 |
| Albumin supplement | 223 (24.1%) | 163 (9.9%) | <0.001 | 109 (17%) | 108 (16.8%) | 0.941 |
| Feeding method | ||||||
| Jejunostomy | 13 (1.4%) | 10 (0.6%) | 0.040 | 5 (0.8%) | 6 (0.9%) | 0.762 |
| NG insertion | 757 (81.8%) | 1423 (86.7%) | 0.001 | 546 (85.2%) | 549 (85.6%) | 0.812 |
| Propensity Score | 0.5 ± 0.2 | 0.7 ± 0.2 | <0.001 | 0.6 ± 0.2 | 0.6 ± 0.2 | 0.981 |
| 30 days short-term outcome | ||||||
| Complication | 208 (22.5%) | 272 (16.6%) | <0.001 | 139 (21.7%) | 108 (16.8%) | 0.028 |
| Medical complication: | 108 (11.7%) | 118 (7.2%) | <0.001 | 68 (10.6%) | 39 (6.1%) | 0.003 |
| Pulmonary complication | 100 (10.8%) | 106 (6.5%) | <0.001 | 62 (9.7%) | 35 (5.5%) | 0.004 |
| Surgical complication: | 115 (12.4%) | 171 (10.4%) | 0.120 | 81 (12.6%) | 75 (11.7%) | 0.608 |
| Long-term outcome | ||||||
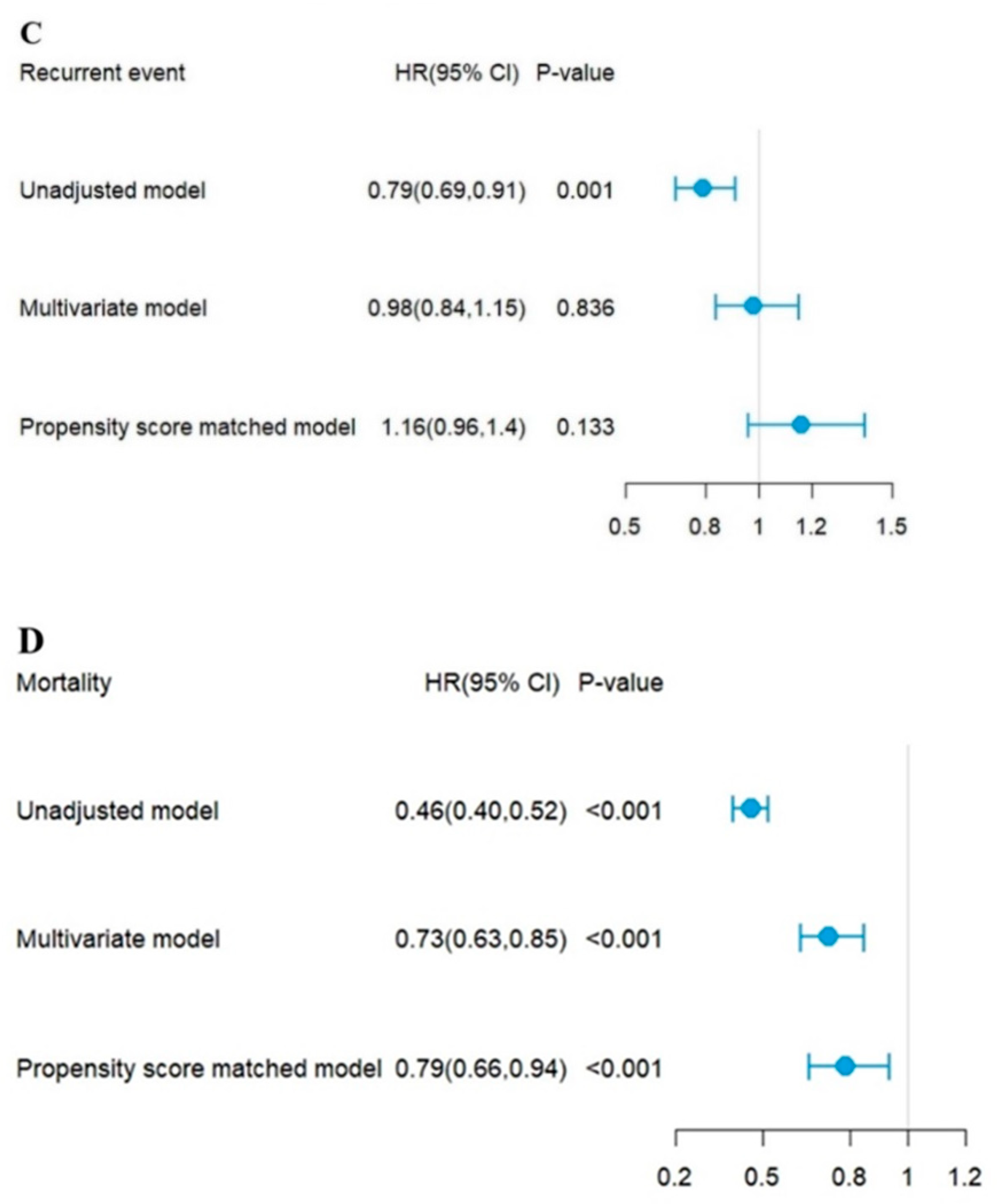
| Recurrent | 325 (36.3%) | 521 (33.4%) | 0.155 | 214 (34.6%) | 235 (38.7%) | 0.138 |
| Death | 494 (55.1%) | 490 (31.5%) | <0.001 | 307 (49.6%) | 212 (34.9%) | <0.001 |
| Univariate Analysis | Multivariate Analysis | Propensity Score Match Analysis | ||||
|---|---|---|---|---|---|---|
| cOR (95% CI) | p-Value | aOR (95% CI) | p-Value | PSM (95% CI) | p-Value | |
| Overall Complication | ||||||
| Low PNI | 1 | 1 | 1 | |||
| High PNI | 0.68 (0.56, 0.84) | <0.001 | 0.71 (0.59, 0.78) | <0.001 | 0.72 (0.54, 0.96) | 0.027 |
| Medical complication | ||||||
| Low PNI | 1 | 1 | 1 | |||
| High PNI | 0.59 (0.45, 0.77) | <0.001 | 0.70 (0.54, 0.81) | 0.011 | 0.56 (0.37, 0.85) | 0.007 |
| Pulmonary complication | ||||||
| Low PNI | 1 | 1 | 1 | |||
| High PNI | 0.57 (0.43, 0.76) | <0.001 | 0.71 (0.53, 0.82) | 0.017 | 0.54 (0.35, 0.84) | 0.007 |
| Surgical complication | ||||||
| Low PNI | 1 | 1 | 1 | |||
| High PNI | 0.82 (0.64, 1.05) | 0.120 | 0.77 (0.62, 0.86) | 0.022 | 0.92 (0.66, 1.28) | 0.610 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, E.-Y.; Chen, M.-K.; Hsieh, M.-Y.; Kor, C.-T.; Liu, Y.-T. Relationship between Preoperative Nutritional Status and Clinical Outcomes in Patients with Head and Neck Cancer. Nutrients 2022, 14, 5331. https://doi.org/10.3390/nu14245331
Wang E-Y, Chen M-K, Hsieh M-Y, Kor C-T, Liu Y-T. Relationship between Preoperative Nutritional Status and Clinical Outcomes in Patients with Head and Neck Cancer. Nutrients. 2022; 14(24):5331. https://doi.org/10.3390/nu14245331
Chicago/Turabian StyleWang, En-Ying, Mu-Kuan Chen, Ming-Yu Hsieh, Chew-Teng Kor, and Yen-Tze Liu. 2022. "Relationship between Preoperative Nutritional Status and Clinical Outcomes in Patients with Head and Neck Cancer" Nutrients 14, no. 24: 5331. https://doi.org/10.3390/nu14245331
APA StyleWang, E.-Y., Chen, M.-K., Hsieh, M.-Y., Kor, C.-T., & Liu, Y.-T. (2022). Relationship between Preoperative Nutritional Status and Clinical Outcomes in Patients with Head and Neck Cancer. Nutrients, 14(24), 5331. https://doi.org/10.3390/nu14245331






