Effect of Oral Iron Supplementation on Cognitive Function among Children and Adolescents in Low- and Middle-Income Countries: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. The Study Protocol and Search Strategy
2.2. Selection Criteria
- (1)
- Randomized controlled trials (RCTs);
- (2)
- Children and adolescents within the age range of 5–19 years;
- (3)
- From LMICs classified by the World Bank according to the year of the study;
- (4)
- Oral iron supplementation where iron was the only micronutrient provided;
- (5)
- The control group could be a placebo or no intervention.
- (1)
- Interventional studies without appropriate control groups;
- (2)
- Observational studies;
- (3)
- Editorials, reviews, opinions, or review articles were ineligible; however, these articles were reviewed to determine eligible research;
- (4)
- Participants with pregnancy or HIV/AIDS, or lactating.
2.3. Data Extraction
2.4. Outcome
2.5. Quality Assessment
2.6. Statistical Analysis
3. Results
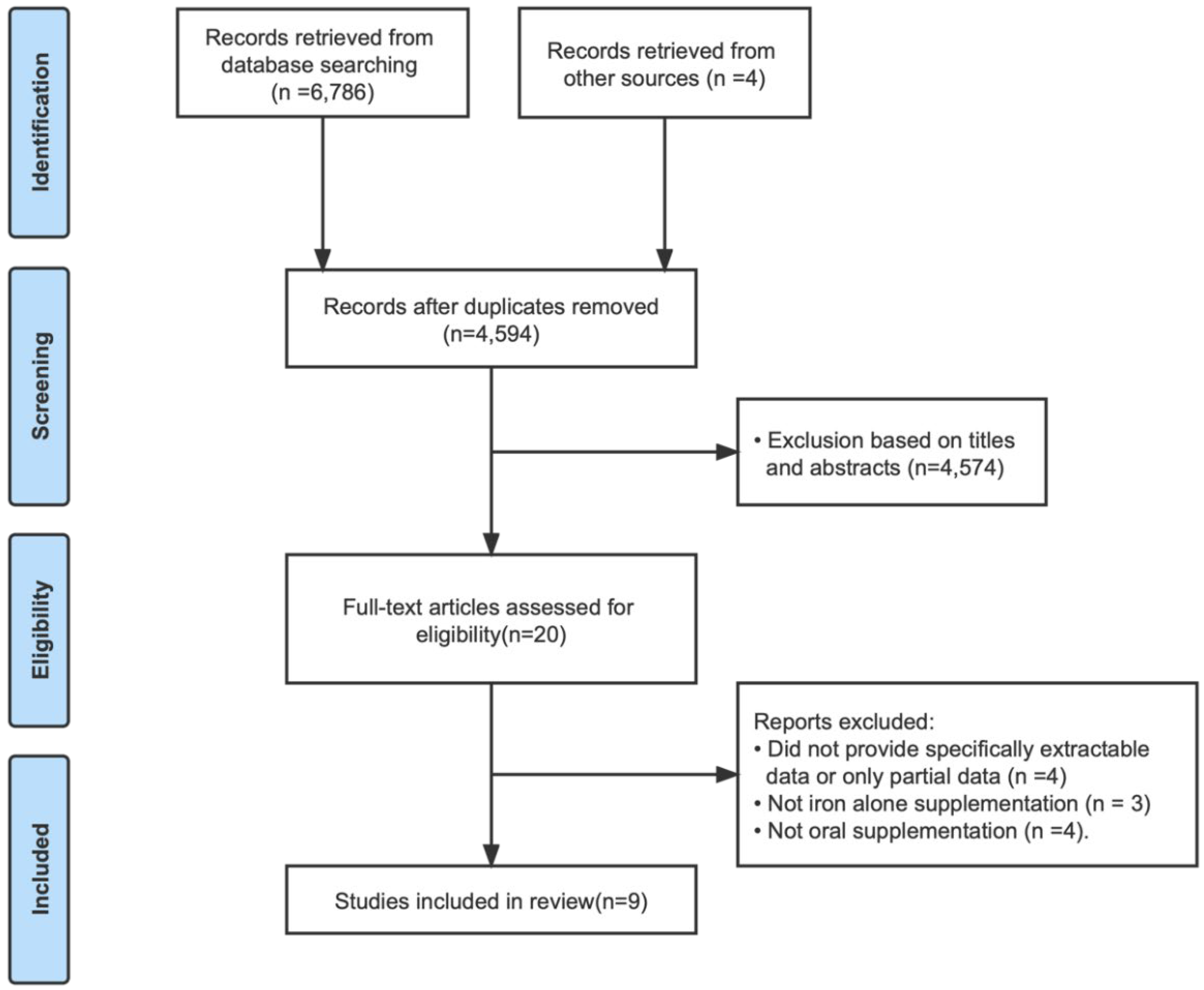
3.1. Document Retrieval Results
3.2. Basic Features Included in The Study
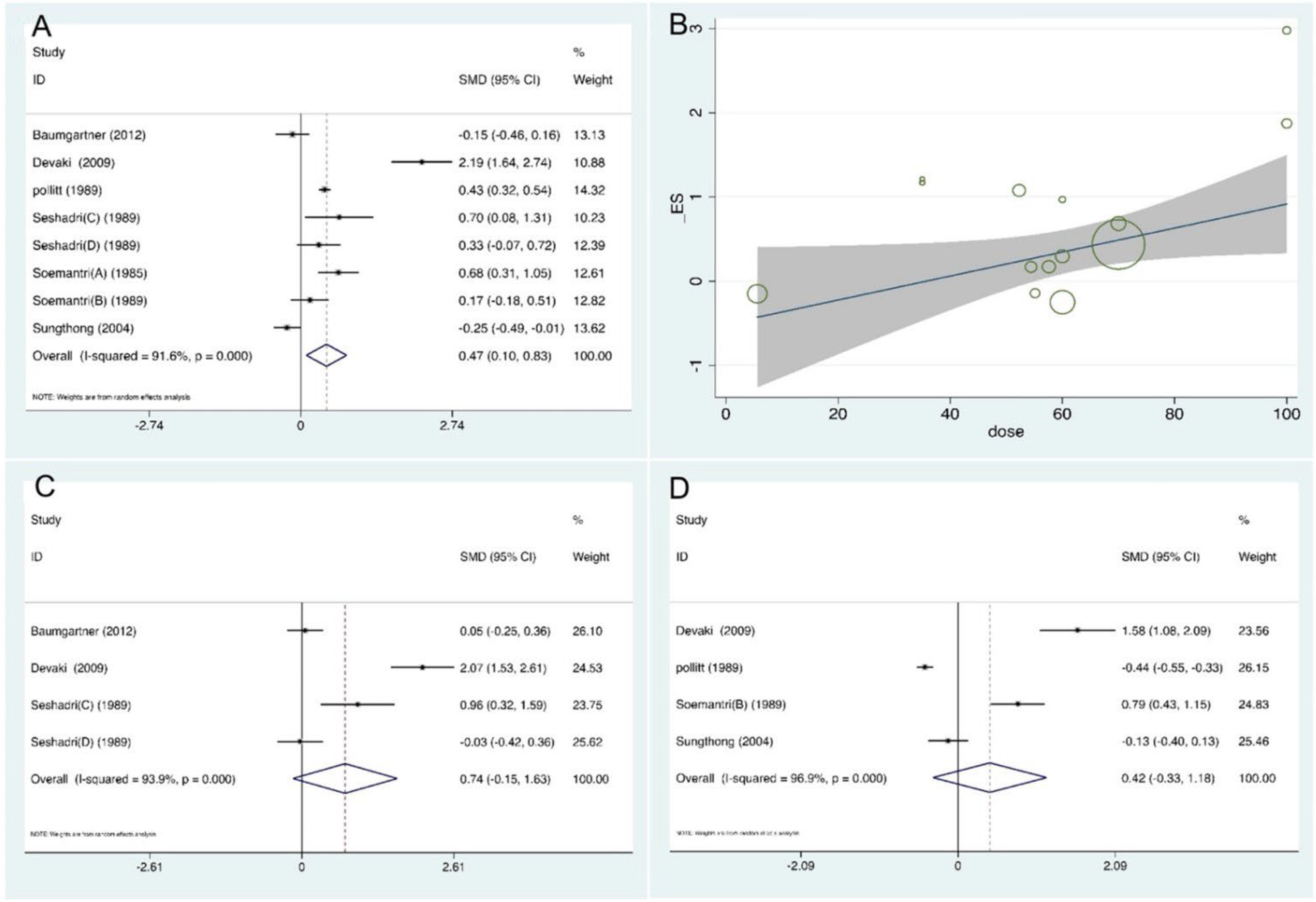
3.3. Outcomes
3.4. Subgroup Comparisons
3.5. Methodological Quality, Publication Bias, and Sensitivity Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Beard, J.L. Iron requirements in adolescent females. J. Nutr. 2000, 130, 440s–442s. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Mohammed, S.; Saxena, A.; Rahi, S.; Mohan, A. Operational Framework: Weekly Iron and Folic Acid Supplementation Programme for Adolescents. Available online: http://www.tripuranrhm.gov.in/Guidlines/WIFS.pdf (accessed on 20 November 2022).
- Murray, C.J.; Aravkin, A.Y.; Zheng, P.; Abbafati, C.; Abbas, K.M.; Abbasi-Kangevari, M.; Abd-Allah, F.; Abdelalim, A.; Abdollahi, M.; Abdollahpour, I. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1223–1249. [Google Scholar] [CrossRef] [PubMed]
- Stevens, G.A.; Finucane, M.M.; De-Regil, L.M.; Paciorek, C.J.; Flaxman, S.R.; Branca, F.; Peña-Rosas, J.P.; Bhutta, Z.A.; Ezzati, M. Global, regional, and national trends in haemoglobin concentration and prevalence of total and severe anaemia in children and pregnant and non-pregnant women for 1995-2011: A systematic analysis of population-representative data. Lancet Glob. Health 2013, 1, e16–e25. [Google Scholar] [CrossRef] [PubMed]
- Podder, R.; DellaValle, M.D.; Tyler, R.T.; Glahn, R.P.; Tako, E.; Vandenberg, A. Relative Bioavailability of Iron in Bangladeshi Traditional Meals Prepared with Iron-Fortified Lentil Dal. Nutrients 2018, 10, 354. [Google Scholar] [CrossRef]
- Yusoff, H.; Wan Daud, W.N.; Ahmad, Z. Effectiveness of Nutrition Education vs. Non-Nutrition Education Intervention in Improving Awareness Pertaining Iron Deficiency among Anemic Adolescents. Iran J. Public Health 2013, 42, 467–471. [Google Scholar]
- Kapil, U.; Kapil, R.; Gupta, A. National Iron Plus Initiative: Current status & future strategy. Indian J. Med. Res. 2019, 150, 239–247. [Google Scholar] [CrossRef]
- Rashid, M.; Flora, M.; Moni, M.; Akhter, A.; Mahmud, Z. Reviewing Anemia and iron folic acid supplementation program in Bangladesh-a special article. Bangladesh Med. J. 2010, 39. [Google Scholar] [CrossRef]
- World Health Organization. Guideline Daily Iron Supplementation in Infants and Children; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Guideline, W. Daily Iron Supplementation in Adult Women and Adolescent Girls; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Lozoff, B.; Beard, J.; Connor, J.; Barbara, F.; Georgieff, M.; Schallert, T. Long-lasting neural and behavioral effects of iron deficiency in infancy. Nutr. Rev. 2006, 64, S34–S43, discussion S72–S91. [Google Scholar] [CrossRef]
- Radlowski, E.C.; Johnson, R.W. Perinatal iron deficiency and neurocognitive development. Front. Hum. Neurosci. 2013, 7, 585. [Google Scholar] [CrossRef]
- Valerio, L.G. Mammalian iron metabolism. Toxicol. Mech. Methods 2007, 17, 497–517. [Google Scholar] [CrossRef]
- Ortiz, E.; Pasquini, J.M.; Thompson, K.; Felt, B.; Butkus, G.; Beard, J.; Connor, J.R. Effect of manipulation of iron storage, transport, or availability on myelin composition and brain iron content in three different animal models. J. Neurosci. Res. 2004, 77, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Berglund, S.K.; Westrup, B.; Hägglöf, B.; Hernell, O.; Domellöf, M. Effects of iron supplementation of LBW infants on cognition and behavior at 3 years. Pediatrics 2013, 131, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Bandhu, R.; Shankar, N.; Tandon, O. Effect of iron on growth in iron deficient anemic school going children. Indian J. Physiol. Pharmacol. 2003, 47, 59–66. [Google Scholar] [PubMed]
- Scott, S.P.; Murray-Kolb, L.E. Iron Status Is Associated with Performance on Executive Functioning Tasks in Nonanemic Young Women. J. Nutr. 2016, 146, 30–37. [Google Scholar] [CrossRef]
- Borgna-Pignatti, C.; Marsella, M. Iron deficiency in infancy and childhood. Pediatr. Ann. 2008, 37, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Lozoff, B.; Smith, J.B.; Kaciroti, N.; Clark, K.M.; Guevara, S.; Jimenez, E. Functional significance of early-life iron deficiency: Outcomes at 25 years. J. Pediatr. 2013, 163, 1260–1266. [Google Scholar] [CrossRef]
- Devaki, P.B.; Chandra, R.K.; Geisser, P. Effects of oral iron(III) hydroxide polymaltose complex supplementation on hemoglobin increase, cognitive function, affective behavior and scholastic performance of adolescents with varying iron status: A single centre prospective placebo controlled study. Arzneimittelforschung 2009, 59, 303–310. [Google Scholar] [CrossRef]
- Muthayya, S.; Thankachan, P.; Hirve, S.; Amalrajan, V.; Thomas, T.; Lubree, H.; Agarwal, D.; Srinivasan, K.; Hurrell, R.F.; Yajnik, C.S.; et al. Iron fortification of whole wheat flour reduces iron deficiency and iron deficiency anemia and increases body iron stores in Indian school-aged children. J. Nutr. 2012, 142, 1997–2003. [Google Scholar] [CrossRef]
- Wang, B.; Zhan, S.; Gong, T.; Lee, L. Iron therapy for improving psychomotor development and cognitive function in children under the age of three with iron deficiency anaemia. Cochrane Database Syst. Rev. 2013, 2013, Cd001444. [Google Scholar] [CrossRef]
- Shamseer, L.; Moher, D.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: Elaboration and explanation. BMJ 2015, 349, g7647. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions; John Wiley & Sons: Hoboken, NJ, USA, 2019. [Google Scholar]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, J.; Smuts, C.M.; Malan, L.; Kvalsvig, J.; van Stuijvenberg, M.E.; Hurrell, R.F.; Zimmermann, M.B. Effects of iron and n-3 fatty acid supplementation, alone and in combination, on cognition in school children: A randomized, double-blind, placebo-controlled intervention in South Africa. Am. J. Clin. Nutr. 2012, 96, 1327–1338. [Google Scholar] [CrossRef] [PubMed]
- Pollitt, E.; Hathiral, P.; Kotchabhakdi, N.J.; Missell, L.; Valyasevi, A. Iron deficiency and educational achievement in Thailand. Am. J. Clin. Nutr. 1989, 50, 687–697. [Google Scholar] [CrossRef] [PubMed]
- Rezaeian, A.; Ghayour-Mobarhan, M.; Mazloum, S.R.; Yavari, M.; Jafari, S.-A. Effects of iron supplementation twice a week on attention score and haematologic measures in female high school students. Singapore Med. J. 2014, 55, 587. [Google Scholar] [CrossRef] [PubMed]
- Seshadri, S.; Gopaldas, T. Impact of iron supplementation on cognitive functions in preschool and school-aged children: The Indian experience. Am. J. Clin. Nutr. 1989, 50, 675–686. [Google Scholar] [CrossRef] [PubMed]
- Soemantri, A.; Pollitt, E.; Kim, I. Iron deficiency anemia and educational achievement. Am. J. Clin. Nutr. 1985, 42, 1221–1228. [Google Scholar] [CrossRef]
- Soemantri, A. Preliminary findings on iron supplementation and learning achievement of rural Indonesian children. Am. J. Clin. Nutr. 1989, 50, 698–702. [Google Scholar] [CrossRef]
- Sungthong, R.; Mo-Suwan, L.; Chongsuvivatwong, V.; Geater, A.F. Once-weekly and 5-days a week iron supplementation differentially affect cognitive function but not school performance in Thai children. J. Nutr. 2004, 134, 2349–2354. [Google Scholar] [CrossRef][Green Version]
- Falkingham, M.; Abdelhamid, A.; Curtis, P.; Fairweather-Tait, S.; Dye, L.; Hooper, L. The effects of oral iron supplementation on cognition in older children and adults: A systematic review and meta-analysis. Nutr. J. 2010, 9, 4. [Google Scholar] [CrossRef]
- Sachdev, H.; Gera, T.; Nestel, P. Effect of iron supplementation on mental and motor development in children: Systematic review of randomised controlled trials. Public Health Nutr. 2005, 8, 117–132. [Google Scholar] [CrossRef]
- Felt, B.T.; Beard, J.L.; Schallert, T.; Shao, J.; Aldridge, J.W.; Connor, J.R.; Georgieff, M.K.; Lozoff, B. Persistent neurochemical and behavioral abnormalities in adulthood despite early iron supplementation for perinatal iron deficiency anemia in rats. Behav. Brain Res. 2006, 171, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Ward, K.L.; Tkac, I.; Jing, Y.; Felt, B.; Beard, J.; Connor, J.; Schallert, T.; Georgieff, M.K.; Rao, R. Gestational and lactational iron deficiency alters the developing striatal metabolome and associated behaviors in young rats. J. Nutr. 2007, 137, 1043–1049. [Google Scholar] [CrossRef] [PubMed]
- Lozoff, B. Iron deficiency and child development. Food Nutr. Bull. 2007, 28, S560–S571. [Google Scholar] [CrossRef] [PubMed]
- Lam, L.F.; Lawlis, T.R. Feeding the brain—The effects of micronutrient interventions on cognitive performance among school-aged children: A systematic review of randomized controlled trials. Clin. Nutr. 2017, 36, 1007–1014. [Google Scholar] [CrossRef]
- Wildman, R.E.; Medeiros, D.M. Advanced Human Nutrition; CRC Press: Boca Raton, FL, USA, 2000. [Google Scholar]
- Youdim, M.B.; Ben-Shachar, D.; Yehuda, S. Putative biological mechanisms of the effect of iron deficiency on brain biochemistry and behavior. Am. J. Clin. Nutr. 1989, 50, 607–615, discussion 615–607. [Google Scholar] [CrossRef]
- Higgins, J.; Deeks, J.; Altman, D.G. Chapter 16: Special topics in statistics. In Cochrane Handbook for Systematic Reviews of Interventions; SAGE Publications Ltd.: Thousand Oaks, CA, USA, 2011. [Google Scholar] [CrossRef]
| Author | Country | Age at Intervention, Years | Intervention Group | Control Group | Duration of Intervention | Outcomes |
|---|---|---|---|---|---|---|
| Baumgartner [26], 2012 | South Africa | 6–11 | 50 mg*4/wk FeSO4 (n = 81) | Placebo (n = 80) | 8.5 months | KABC sequential processing: Triangles, Hand movement, Atlantis Delayed |
| Devaki [20], 2009 | India | 15–18 | 100 mg*6/wk EFe (n = 30) | Control placebo (n = 30) | 8 months | WAIS, Short term memory, long term memory, Scholastic performance test |
| Pollitt [27], 1989 | Thailand | 9–11 | FeSO4 50 mg/d for 2 weeks and FeSO4 100 mg/d for 14 weeks (n = 5) | Placebo (n = 4) | 4 months | IQ, Mathematics |
| Rezaeian [28], 2014 | Iran | 14–18 | 50 mg*2/wk FeSO4 (n = 100) | Control group (n = 100) | 16 weeks | Attention score |
| Seshadri (C) [29], 1989 | India | 8–15 | (1) 40 mg/d EFe (n = 16) (2) 30 mg/d EFe (n = 16) | Placebo (n = 16) | 4 months | Mazes, Digit span, Visual recall |
| Seshadri (D) [29], 1989 | India | 8–15 | 60 mg/d EFe (n = 65) | Placebo (n = 65) | 8 months | Mazes, Digit span, Visual recall |
| Soemantri (A) [30], 1985 | Indonesia | Average 9.5 | (1) anemia: 10 mg/kg/d FeSO4 (n = 43) (2) Non-anemia: 10 mg/kg/d FeSO4 (n = 16) | (1) anemia placebo (n = 35) (2) non-anemia placebo (n = 25) | 3 mouths | IQ |
| Soemantri (B) [31], 1989 | India | 8.1–11.6 | 2 mg/kg/d EFe (n = 37) | Placebo (n = 35) | 3 months | IQ, math scores |
| Sungthong [32], 2004 | Thailand | 6–13 | (1) 300 mg/d FeSO4 (n = 140); (2) 300 mg/wk. FeSO4 (n = 134) | Placebo (n = 123) | 16 weeks | IQ, mathematics |
| Subgroup Analyses | Heterogeneity Test | Pooled SMD Values (95% CI) | Pooled SMD Values’ Tests Statistic | |||
|---|---|---|---|---|---|---|
| q | d.f. | I2 | Z | p | ||
| Sex | ||||||
| Whole population | 116.61 | 8 | 93.1% | 0.67 (0.12, 1.22) | 2.38 | 0.017 |
| Male | 2.83 | 2 | 29.3% | 0.64 (0.14, 1.13) | 2.53 | 0.011 |
| Female | 1.64 | 1 | 39.1% | 0.50 (−0.11, 1.11) | 1.62 | 0.106 |
| Age, year | ||||||
| ≤11 | 47.32 | 6 | 87.3% | 0.29 (−0.03, 0.61) | 1.75 | 0.08 |
| >11 | 56.39 | 6 | 89.4% | 1.18 (0.33, 2.04) | 2.7 | 0.007 |
| Dose, mg/day | ||||||
| <60 | 23.98 | 6 | 75.0% | 0.38 (−0.06, 0.81) | 1.71 | 0.088 |
| ≥60 | 97.44 | 6 | 93.8% | 0.91 (0.38, 1.45) | 3.37 | 0.001 |
| Duration, month | ||||||
| <4 | 12.24 | 3 | 75.5% | 0.34 (−0.19, 0.86) | 1.26 | 0.208 |
| ≥4 | 112.71 | 9 | 92.0% | 0.81 (0.38, 1.25) | 3.66 | <0.001 |
| Iron status | ||||||
| Anemia | 43.98 | 5 | 88.6% | 1.01 (0.34, 1.68) | 2.97 | 0.003 |
| Non-anemia | 28.26 | 5 | 82.3% | 0.68 (0.17, 1.18) | 2.63 | 0.009 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Z.; Yang, H.; Wang, D.; Sudfeld, C.R.; Zhao, A.; Xin, Y.; Chen, J.C.; Fawzi, W.W.; Xing, Y.; Li, Z. Effect of Oral Iron Supplementation on Cognitive Function among Children and Adolescents in Low- and Middle-Income Countries: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 5332. https://doi.org/10.3390/nu14245332
Chen Z, Yang H, Wang D, Sudfeld CR, Zhao A, Xin Y, Chen JC, Fawzi WW, Xing Y, Li Z. Effect of Oral Iron Supplementation on Cognitive Function among Children and Adolescents in Low- and Middle-Income Countries: A Systematic Review and Meta-Analysis. Nutrients. 2022; 14(24):5332. https://doi.org/10.3390/nu14245332
Chicago/Turabian StyleChen, Zekun, Huanhuan Yang, Dongqing Wang, Christopher R. Sudfeld, Ai Zhao, Yiqian Xin, Jiawen Carmen Chen, Wafaie W. Fawzi, Yan Xing, and Zhihui Li. 2022. "Effect of Oral Iron Supplementation on Cognitive Function among Children and Adolescents in Low- and Middle-Income Countries: A Systematic Review and Meta-Analysis" Nutrients 14, no. 24: 5332. https://doi.org/10.3390/nu14245332
APA StyleChen, Z., Yang, H., Wang, D., Sudfeld, C. R., Zhao, A., Xin, Y., Chen, J. C., Fawzi, W. W., Xing, Y., & Li, Z. (2022). Effect of Oral Iron Supplementation on Cognitive Function among Children and Adolescents in Low- and Middle-Income Countries: A Systematic Review and Meta-Analysis. Nutrients, 14(24), 5332. https://doi.org/10.3390/nu14245332





