Protection of Vitamin C on Oxidative Damage Caused by Long-Term Excess Iodine Exposure in Wistar Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Dose Calculation
2.3. Animal and Treatments
2.4. Samples Collection
2.5. Preparation of Tissue Homogenate
2.6. Determination of Antioxidant Enzymes Activity and MDA Content
2.7. Determination of Urinary Iodine
2.8. Statistical Analysis
3. Results
3.1. Water and Food Consumption and Iodine Intake
3.2. Urinary Iodine
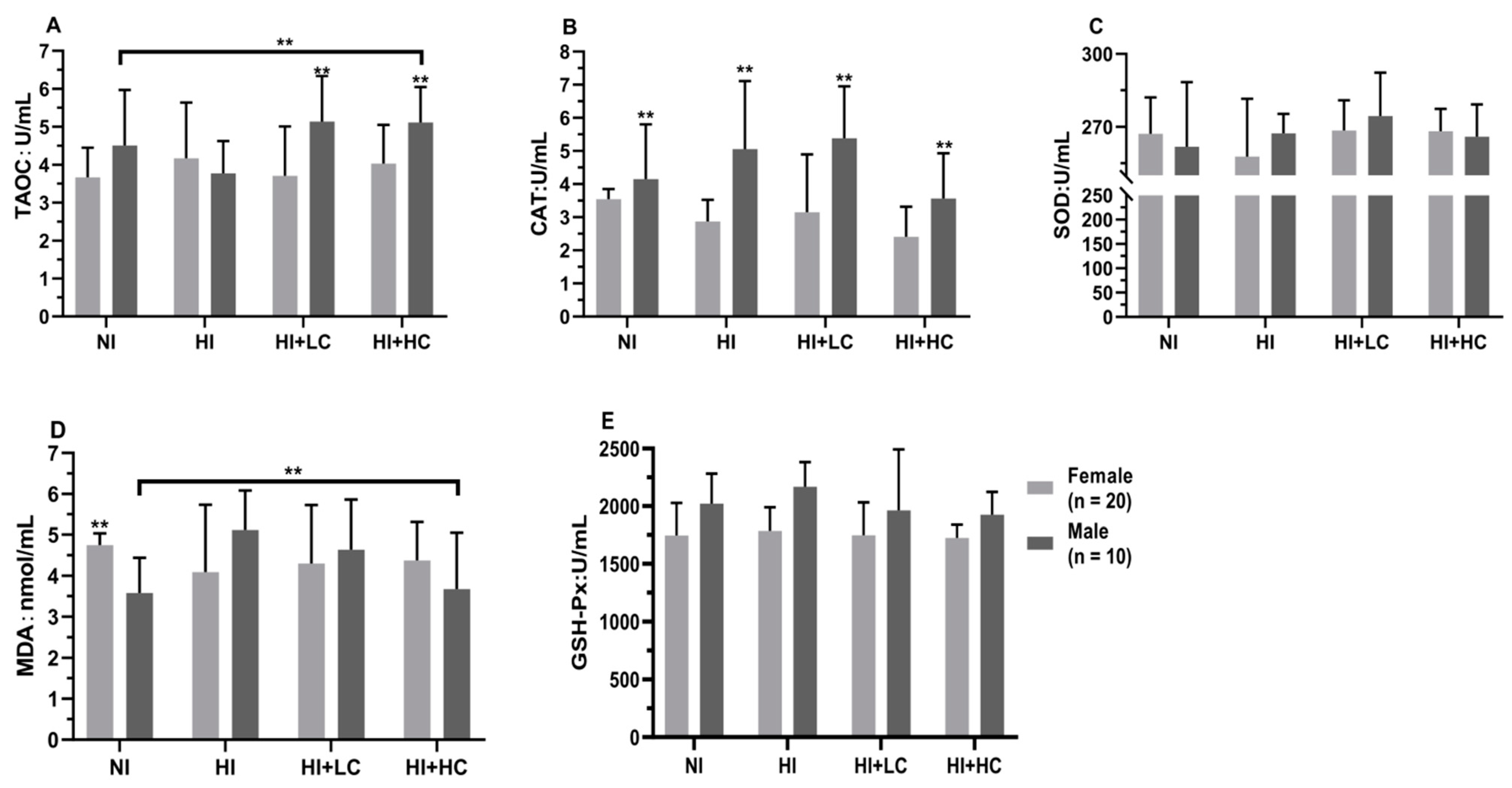
3.3. Protection of Vitamin C from Oxidative Damage in Serum Caused by Excess Iodine
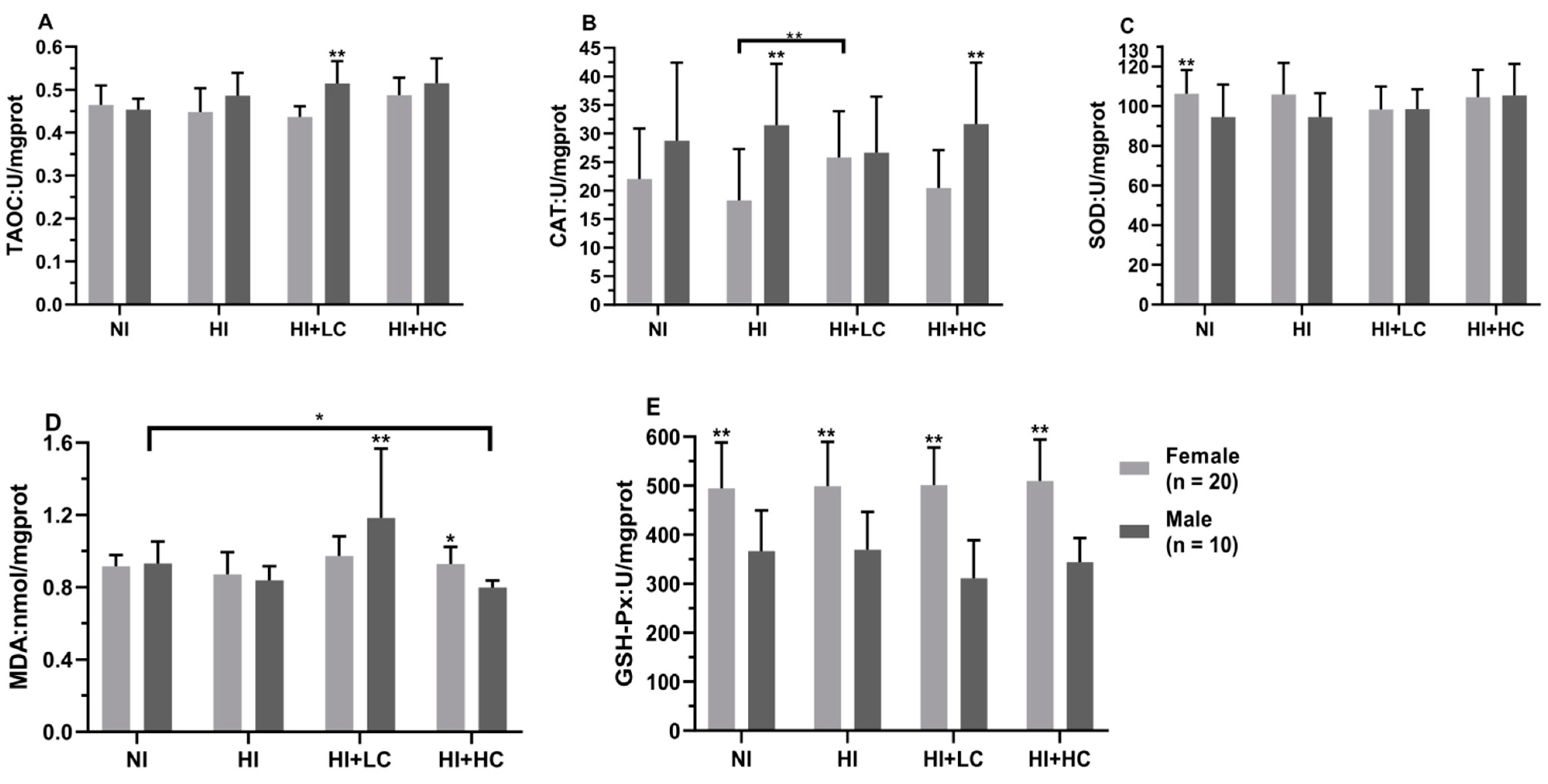
3.4. Protection of Vitamin C from Oxidative Damage in Liver Caused by Excess Iodine
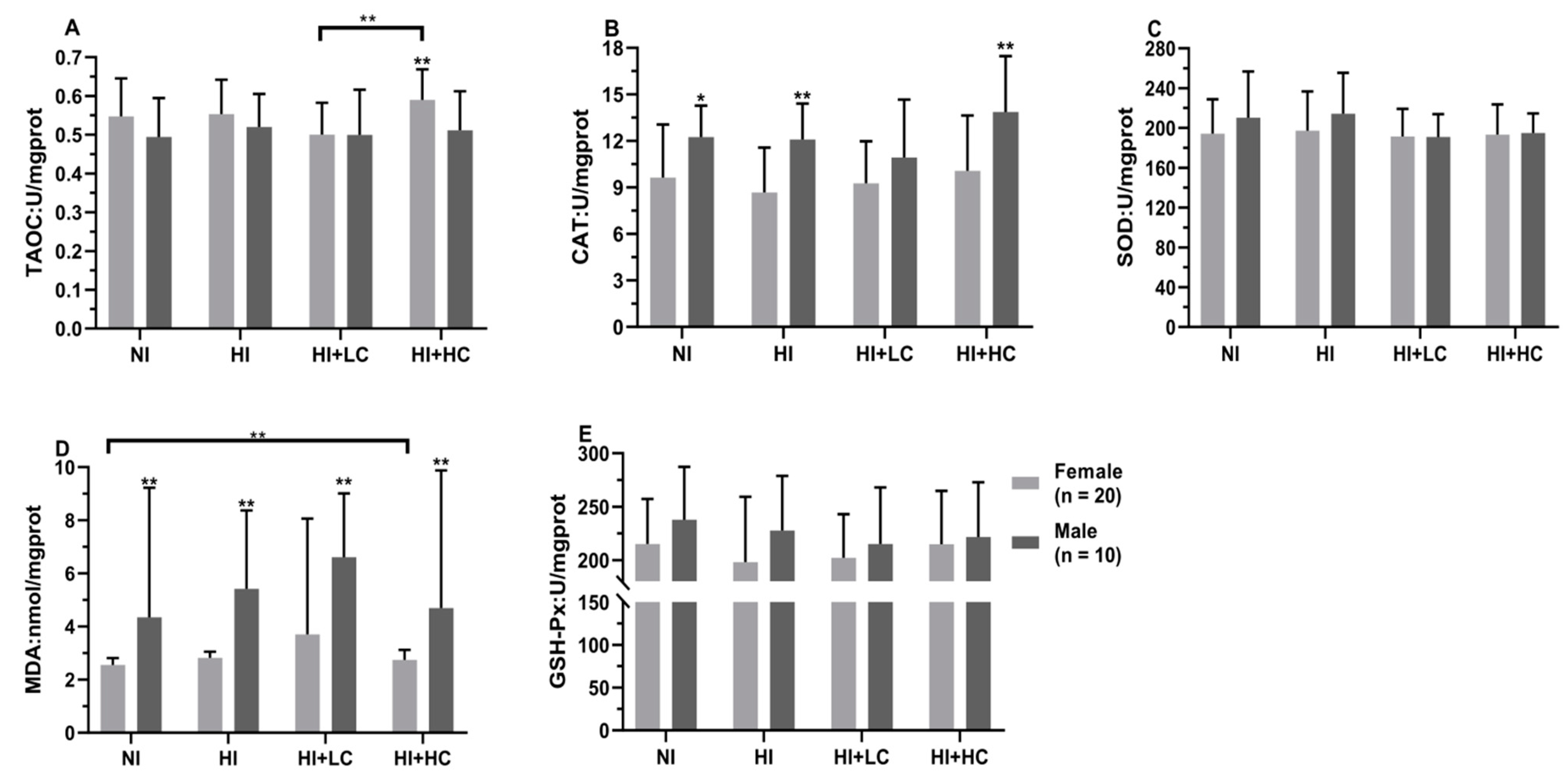
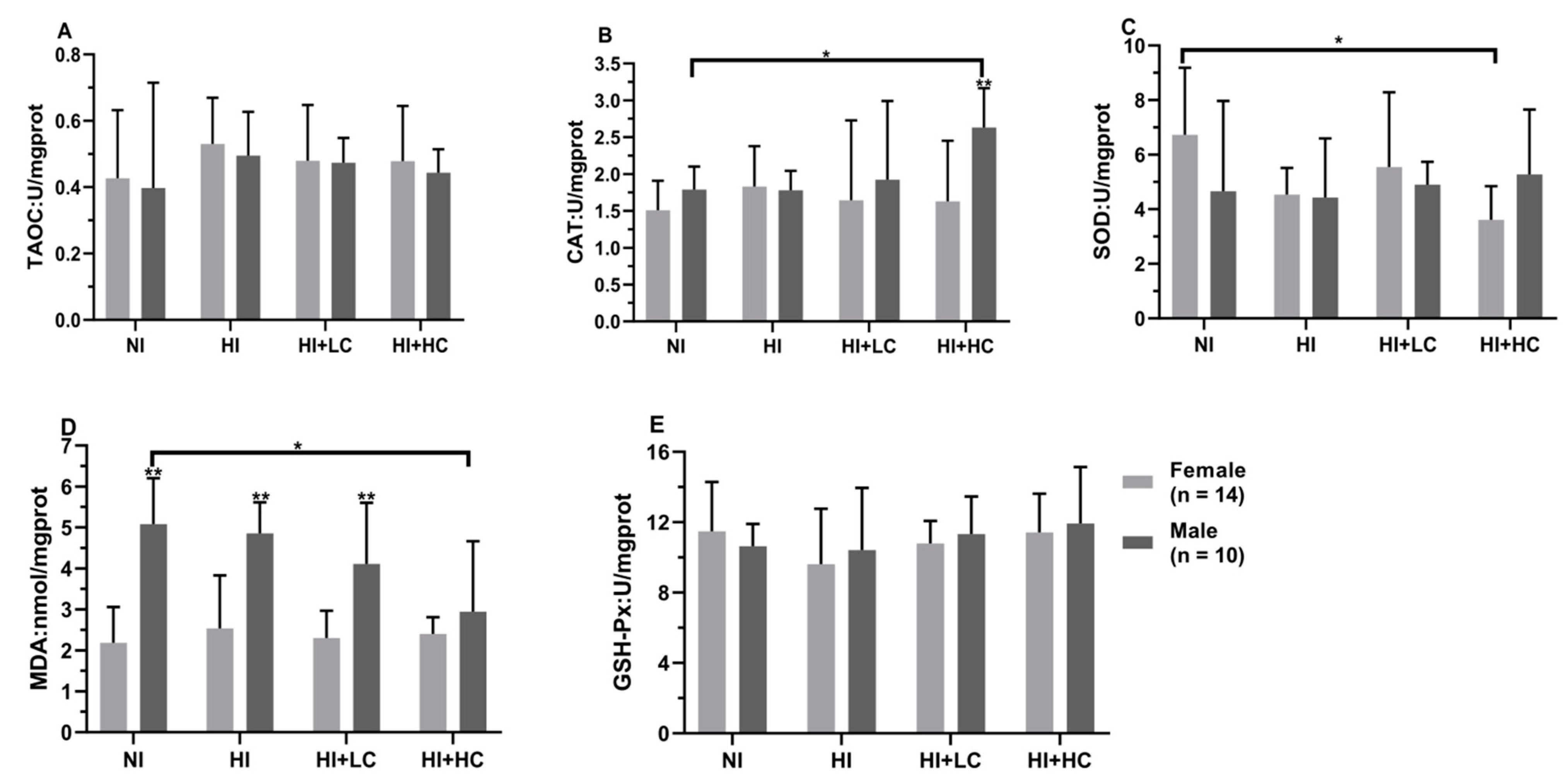
3.5. Protection of Vitamin C from Oxidative Damage in Kidney Caused by Excess Iodine
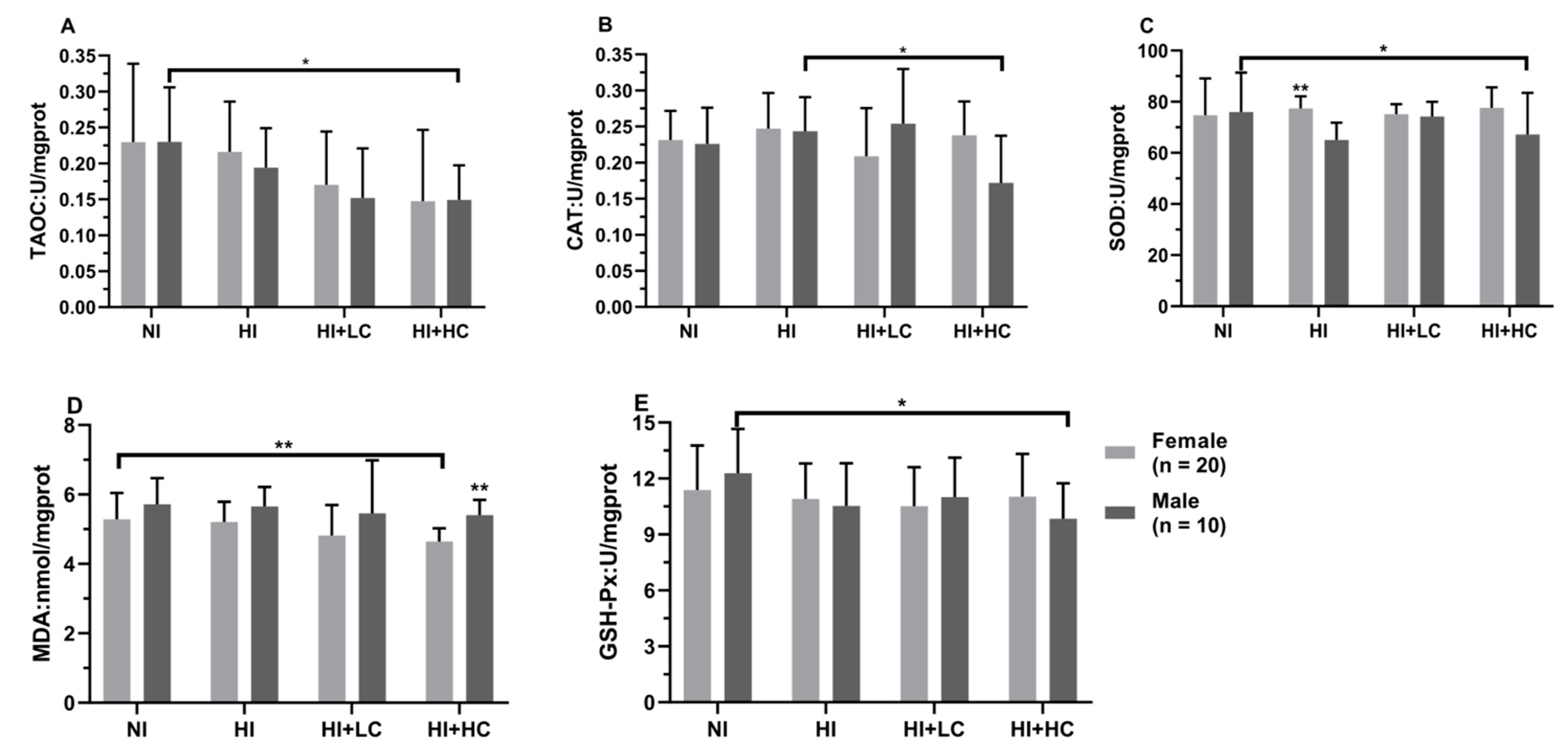
3.6. Protection of Vitamin C from Oxidative Damage in the Brain Caused by Excess Iodine
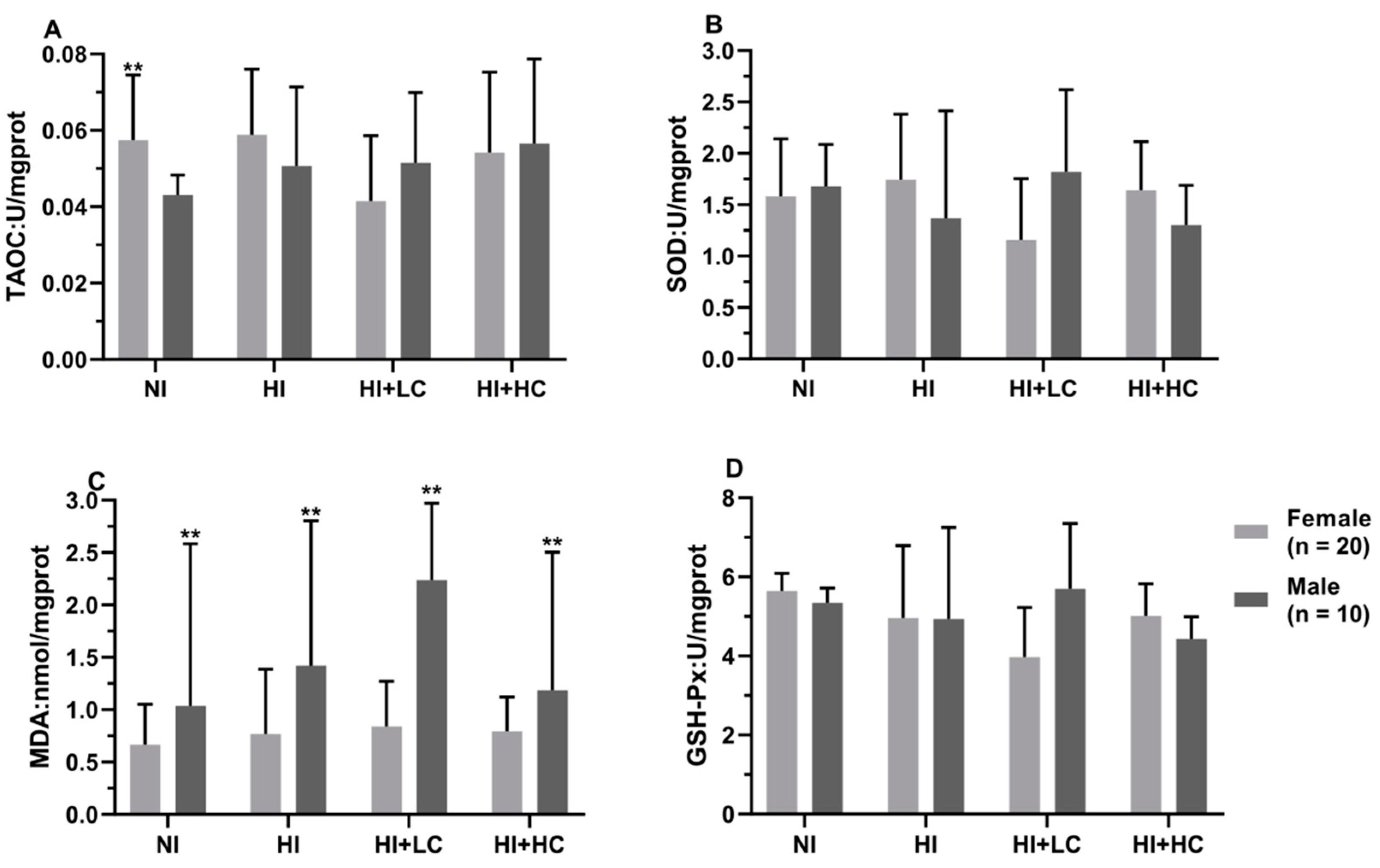
3.7. Protection of Vitamin C on Oxidative Damage in the Thyroid Caused by Excess Iodine
3.8. Protection of Vitamin C from Oxidative Damage in the Lens Caused by Excess Iodine
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, R.; Fan, L.; Du, Y.; Liu, L.; Qian, T.; Zhao, M.; Che, W.; Liu, P.; Sun, D. The relationship between different iodine sources and nutrition in pregnant women and adults. Front. Endocrinol. 2022, 13, 924990. [Google Scholar] [CrossRef] [PubMed]
- Brent, G.A. Mechanisms of thyroid hormone action. J. Clin. Investig. 2012, 122, 3035–3043. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, D.P.; Dupuy, C. Thyroid hormone biosynthesis and release. Mol. Cell Endocrinol. 2017, 458, 6–15. [Google Scholar] [CrossRef]
- Sun, R.; Qian, T.T.; Liu, L.C.; Zhao, M.; Che, W.J.; Zhang, L.; Li, W.D.; Jia, Q.Z.; Wang, J.H.; Li, J.S.; et al. Effect of Iodine Supplementation on Iodine Nutrition and Thyroid Function in Pregnant Women: A Cross-Sectional Study. Biomed. Environ. Sci. 2022, 35, 641–647. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Codling, K.; Chang, S.; Zhang, S.; Shen, H.; Su, X.; Chen, Z.; Scherpbier, R.W.; Yan, J. Eliminating Iodine Deficiency in China: Achievements, Challenges and Global Implications. Nutrients 2017, 9, 361. [Google Scholar] [CrossRef]
- Leung, A.M.; Lewis, E.B. Consequences of excess iodine. Nat. Rev. Endocrinol. 2014, 10, 136–142. [Google Scholar] [CrossRef]
- Teti, C.; Panciroli, M.; Nazzari, E.; Pesce, G.; Mariotti, S.; Olivieri, A.; Bagnasco, M. Iodoprophylaxis and thyroid autoimmunity: An update. Immunol. Res. 2021, 69, 129–138. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, N.K.; Park, H.K.; Byun, D.W.; Suh, K.; Yoo, M.H.; Min, Y.-K.; Kim, S.W.; Chung, J.H. Strong association of relatively low and extremely excessive iodine intakes with thyroid cancer in an iodine-replete area. Eur. J. Nutr. 2017, 56, 965–971. [Google Scholar] [CrossRef]
- Bürgi, H.; Schaffner, T.H.; Seiler, J.P. The toxicology of iodate: A review of the literature. Thyroid 2001, 11, 449–456. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Zhou, J.Z.; Jia, X.D. Research on the safety of potassium iodate. J. Occup. Environ. Med. 2017, 34, 175–178. [Google Scholar] [CrossRef]
- Li, Q.; Mair, C.; Schedle, K.; Hellmayr, I.; Windisch, W. Effects of varying dietary iodine supplementation levels as iodide or iodate on thyroid status as well as mRNA expression and enzyme activity of antioxidative enzymes in tissues of grower/finisher pigs. Eur. J. Nutr. 2013, 52, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Zhang, M.; Wang, X.; Wang, M.; Zhang, B.; Jiang, W.; Bian, J.; Liu, X. Effects of long-term excessive iodine intake on blood lipids in Chinese adults: A cross-sectional study. Eur. J. Clin. Nutr. 2021, 75, 708–714. [Google Scholar] [CrossRef] [PubMed]
- Esra, B.C.; Yasin, S.G. Bilateral retinal toxicity as a result of poisoning with pure iodine. Rom. J. Ophthalmol. 2021, 65, 73–75. [Google Scholar] [CrossRef]
- United Nations Children’s Fund. Guidance on the Monitoring of Salt Iodization Programmes and Determination of Population Iodine Status; United Nations Children’s Fund: New York, NY, USA, 2018. [Google Scholar]
- Zimmermann, M.B. Iodine deficiency and excess in children: Worldwide status in 2013. Endocr. Pract. 2013, 19, 839–846. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.F.; Xu, J.; Hou, X.H.; Guo, H.L.; Hao, L.P.; Yao, P.; Liu, L.G.; Sun, X.F. Developmental toxic effects of chronic exposure to high doses of iodine in the mouse. Reprod. Toxicol. 2006, 22, 725–730. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Liu, S.; Sun, D.; Zhang, S.; Su, X.; Shen, Y.; Han, H. Geographical distribution of drinking-water with high iodine level and association between high iodine level in drinking-water and goitre: A Chinese national investigation. Br. J. Nutr. 2011, 106, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Sui, H.X.; Li, J.W.; Mao, W.F.; Zhu, J.H.; Na He, Y.; Song, X.Y.; Ma, N.; Zhang, L.; Na Liu, S.; Liu, Z.P.; et al. Dietary iodine intake in the Chinese population. Biomed. Environ. Sci. 2011, 24, 617–623. [Google Scholar] [CrossRef]
- Sang, Z.; Chen, W.; Shen, J.; Tan, L.; Zhao, N.; Liu, H.; Wen, S.; Wei, W.; Zhang, G.; Zhang, W. Long-term exposure to excessive iodine from water is associated with thyroid dysfunction in children. J. Nutr. 2013, 143, 2038–2043. [Google Scholar] [CrossRef]
- Iwan, P.; Stepniak, J.; Karbownik-Lewinska, M. Melatonin reduces high levels of lipid peroxidation induced by potassium iodate in porcine thyroid. Int. J. Vitam. Nutr. Res. 2021, 91, 271–277. [Google Scholar] [CrossRef]
- Parhizkar, E.; Rashedinia, M.; Karimi, M.; Alipour, S. Design and development of vitamin C-encapsulated proliposome with improved in-vitro and ex-vivo antioxidant efficacy. J. Microencapsul. 2018, 35, 301–311. [Google Scholar] [CrossRef]
- Caritá, A.C.; Fonseca-Santos, B.; Shultz, J.D.; Michniak-Kohn, B.; Chorilli, M.; Leonardi, G.R. Vitamin C: One compound, several uses. Advances for delivery, efficiency and stability. Nanomedicine 2020, 24, 102–117. [Google Scholar] [CrossRef] [PubMed]
- Sheraz, M.A.; Khan, M.F.; Ahmed, S.; Kazi, S.H.; Ahmad, I. Factors affecting formulation characteristics and stability of ascorbic acid in water-in-oil creams. Int. J. Cosmet. Sci. 2014, 36, 494–504. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.S.; Deng, Z.Y.; Li, Y.X.; Yang, R.; Wang, W.T.; Wang, N.; Chen, Y.J.; Wang, B.Z. Protective effect and mechanism of proanthocyanidins on thyroid oxidative damage induced by potassium iodate. Chin. J. Gerontol. 2017, 37, 1838–1840. [Google Scholar] [CrossRef]
- Iwan, P.; Stepniak, J.; Karbownik-Lewinska, M. Pro-Oxidative Effect of KIO3 and Protective Effect of Melatonin in the Thyroid-Comparison to Other Tissues. Life 2021, 11, 592. [Google Scholar] [CrossRef]
- Zhang, Y.P.; Zhang, S.Q.; Li, N. Experiment on scavenging iodate ion by ascorbic acid in simulated gastric juice. Chin. J. Endemiol. 2017, 36, 176–181. [Google Scholar] [CrossRef]
- Chinese Nutrition Society. Reference intake of nutrients for Chinese residents. Acta Nutr. Sin. 2001, 2, 190. [Google Scholar] [CrossRef]
- OECD. Guidance Notes for Analysis and Evaluation Chronic Toxicity and Carcinogenicity Studies. Ser. Test. Assess. 2002, 35, 64. [Google Scholar]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1974, 239, 70–76. [Google Scholar] [CrossRef]
- Winterbourn, C.C.; Hawkins, R.E.; Brian, M.; Carrell, R.W. The estimation of red cell superoxide dismutase activity. J. Lab. Clin. Med. 1975, 85, 337–341. [Google Scholar]
- Siddique, Y.H.; Ara, G.; Afzal, M. Estimation of lipid peroxidation induced by hydrogen peroxide in cultured human lymphocytes. Dose-Response A Publ. Int. Hormesis Soc. 2012, 10, 1–10. [Google Scholar] [CrossRef]
- Hafeman, D.G.; Sunde, R.A.; Hoekstra, W.G. Effect of dietary selenium on erythrocyte and liver glutathione peroxidase in the rat. J. Nutr. 1974, 104, 580–587. [Google Scholar] [CrossRef] [PubMed]
- Fortunato, R.S.; Ferreira, A.C.; Hecht, F.; Dupuy, C.; Carvalho, D.P. Sexual dimorphism and thyroid dysfunction: A matter of oxidative stress? J. Endocrinol. 2014, 221, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.X. Effects of Different Iodine Agents on Antioxidant Capacity of Iodine Deficiency Rats. Master’s Thesis, Tianjin Medical University, Tianjin, China, 2002. [Google Scholar]
- Zhang, Y.; Gu, Y.Y. Potassium iodate and potassium iodide and peroxidation damage. Chin. J. Control Endemic. Dis. 2012, 27, 3. [Google Scholar]
- Leoni, S.G.; Kimura, E.T.; Santisteban, P.; De, V.A. Regulation of thyroid oxidative state by thioredoxin reductase has a crucial role in thyroid responses to iodide excess. Mol. Endocrinol. 2011, 25, 1924–1935. [Google Scholar] [CrossRef] [PubMed]
- Milczarek, M.; Stępniak, J.; Lewiński, A.; Karbownik-Lewińska, M. Potassium iodide, but not potassium iodate, as a potential protective agent against oxidative damage to membrane lipids in porcine thyroid. Thyroid Res. 2013, 30, 10. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Qu, W.; Zhao, L.-N.; Han, H.; Yang, X.-F.; Sun, X.-F.; Hao, L.-P.; Xu, J. Iodine excess induces hepatic steatosis through disturbance of thyroid hormone metabolism involving oxidative stress in BALB/c mice. Biol. Trace Elem. Res. 2013, 154, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Corvilain, B.; Collyn, L.; Sande, J.; Dumont, J.E. Stimulation by iodide of H2O2 generation in thyroid slices from several species. Am. J. Physiol. Endocrinol. Metab. 2000, 278, 692–699. [Google Scholar] [CrossRef]
- Joanta, A.E.; Filip, A.; Clichici, S.; Andrei, S.; Daicoviciu, D. Iodide excess exerts oxidative stress in some target tissues of the thyroid hormones. Acta Physiol. Hung. 2006, 93, 347–359. [Google Scholar] [CrossRef]
- Hussein, A.A.; Abbas, A.M.; Wakil, G.A.; Elsamanoudy, A.Z.; Aziz, A.A. Effect of chronic excess iodine intake on thyroid function and oxidative stress in hypothyroid rats. Can. J. Physiol. Pharmacol. 2012, 90, 617–625. [Google Scholar] [CrossRef]
- Karbownik-Lewinska, M.; Stepniak, J.; Milczarek, M.; Lewinski, A. Protective effect of KI in mtDNA in porcine thyroid: Comparison with KIO3 and nDNA. Eur. J. Nutr. 2015, 54, 319–323. [Google Scholar] [CrossRef]
- Duran, B.O.S.; de Góes, G.A.; Zanella, B.T.T.; Freire, P.P.; Valente, J.S.; Salomão, R.A.S.; Fernandes, A.; Mareco, E.A.; Carvalho, R.F.; Dal-Pai-Silva, M. Ascorbic acid stimulates the in vitro myoblast proliferation and migration of pacu (Piaractus mesopotamicus). Sci. Rep. 2019, 9, 2229. [Google Scholar] [CrossRef] [PubMed]
- Deicher, R.; Hörl, W.H. Vitamin C in chronic kidney disease and hemodialysis patients. Kidney Blood Press. Res. 2003, 26, 100–106. [Google Scholar] [CrossRef]
- Gueguen, S.; Pirollet, P.; Leroy, P.; Guilland, J.-C.; Arnaud, J.; Paille, F.; Siest, G.; Visvikis, S.; Hercberg, S.; Herbeth, B. Changes in serum retinol, alpha-tocopherol, vitamin C, carotenoids, xinc and selenium after micronutrient supplementation during alcohol rehabilitation. J. Am. Coll. Nutr. 2003, 22, 303–310. [Google Scholar] [CrossRef]
- Chakraborthy, A.; Ramani, P.; Sherlin, H.J.; Premkumar, P.; Natesan, A. Antioxidant and pro-oxidant activity of Vitamin C in oral environment. Indian J. Dent. Res. 2014, 25, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Karbownik, M.; Lewiński, A. Melatonin reduces Fenton reaction-induced lipid peroxidation in porcine thyroid tissue. J. Cell Biochem. 2003, 90, 806–811. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.L.; Li, P.J.; Chen, T.D.; Yang, L.; Zhang, L. Changes of serum malondialdehyde, superoxide dismutase and glutathione peroxidase in patients with acute carbon monoxide poisoning and the intervention effect of vitamin C. Chin. J. Emerg. Med. 2003, 12, 2. [Google Scholar] [CrossRef]
- Ristow, M.; Zarse, K.; Oberbach, A.; Klöting, N.; Birringer, M.; Kiehntopf, M.; Stumvoll, M.; Kahn, C.R.; Blüher, M. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc. Natl. Acad. Sci. USA 2009, 106, 8665–8670. [Google Scholar] [CrossRef]
| Iodine Type | Classification | Different Sex (μg/L) M, (P25–P75) | Urinary Iodine M, (P25–P75) (n = 15, μg/L) | p (HI, HI + LC, and HI + HC) | |
|---|---|---|---|---|---|
| Female (n = 10) | Male (n = 5) | ||||
| KI | NI | 74.98 (52.07–88.42) | 70.14 (55.83–96.37) | 73.53 (52.62–89.36) | |
| HI | 3371.40 ** (3163.89–5007.75) | 3188.63 ** (2628.76–4145.06) | 3334.50 ** (2388.63–4904.93) | 0.183 | |
| HI + LC | 4363.17 ** (3132.34–5136.92) | 4935.32 ** (3427.49–5914.56) | 4540.89 ** (3263.55–4981.27) | ||
| HI + HC | 3951.08 ** (2830.47–5167.89) | 3424.45 ** (2460.64–4278.64) | 3581.56 ** (2007.12–4663.44) | ||
| KIO3 | NI | 68.50 (55.28–90.44) | 64.20 (49.01–90.93) | 67.18 (52.50–96.42) | |
| HI | 3592.30 ** (2710.815803.82) | 4755.90 ** (3562.59–7044.47) | 3662.14 ** (2984.43–5859.09) | 0.840 | |
| HI + LC | 3910.33 ** (2668.45–5807.05) | 3513.58 ** (2857.31–4001.91) | 3656.47 ** (2776.23–4512.95) | ||
| HI + HC | 4256.03 ** (2642.97–4991.33) | 4035.67 ** (3264.24–6639.86) | 3957.14 ** (3012.70–4904.93) | ||
| Indicators | Classification | KI (n = 15) | KIO3 (n = 15) | p |
|---|---|---|---|---|
| TAOC U/mgprot | NI | 0.45 ± 0.04 | 0.47 ± 0.06 | 0.486 |
| HI | 0.48 ± 0.08 | 0.45 ± 0.07 | 0.217 | |
| HI + LC | 0.46 ± 0.06 | 0.47 ± 0.09 | 0.902 | |
| HI + HC | 0.49 ± 0.05 | 0.51 ± 0.07 | 0.838 | |
| p | 0.242 | 0.113 | ||
| CAT U/mgprot | NI | 26.93 ± 11.12 | 21.62 ± 10.43 | 0.189 |
| HI | 22.60 ± 10.85 | 22.73 ± 12.24 | 0.976 | |
| HI + LC | 28.65 ± 9.20 | 23.52 ± 7.25 | 0.101 | |
| HI + HC | 25.08 ± 10.93 | 23.33 ± 8.52 | 0.628 | |
| p | 0.404 | 0.951 | ||
| SOD U/mgprot | NI | 101.88 ± 17.58 | 106.65 ± 19.36 | 0.486 |
| HI | 100.20 ± 15.77 | 103.96 ± 15.61 | 0.517 | |
| HI + LC | 97.59 ± 10.82 | 99.18 ± 11.35 | 0.698 | |
| HI + HC | 104.65 ± 12.57 | 104.96 ± 16.26 | 0.954 | |
| p | 0.542 | 0.575 | ||
| MDA nmol/mgprot | NI | 0.86 ± 0.16 | 0.92 ± 0.16 | 0.512 |
| HI | 0.89 ± 0.13 | 0.86 ± 0.10 | 0.217 | |
| HI + LC | 1.08 ± 0.24 | 1.09 ± 0.36 | 0.713 | |
| HI + HC | 0.96 ± 0.25 | 0.86 ± 0.09 | 0.174 | |
| p | 0.023 * HI + LC > NI, HI, HI + HC | 0.058 | ||
| GSH-Px U/mgprot | NI | 459.81 ± 133.67 | 444.52 ± 78.29 | 0.706 |
| HI | 439.16 ± 89.81 | 472.77 ± 119.85 | 0.392 | |
| HI + LC | 430.91 ± 131.21 | 444.88 ± 107.96 | 0.753 | |
| HI + HC | 454.28 ± 105.77 | 454.78 ± 114.50 | 0.990 | |
| p | 0.905 | 0.886 |
| Indicators | Classification | KI (n = 15) | KIO3 (n = 15) | p |
|---|---|---|---|---|
| TAOC U/mgprot | NI | 0.50 ± 0.09 | 0.57 ± 0.11 | 0.061 |
| HI | 0.55 ± 0.10 | 0.53 ± 0.08 | 0.653 | |
| HI + LC | 0.50 ± 0.09 | 0.50 ± 0.10 | 0.976 | |
| HI + HC | 0.55 ± 0.10 | 0.58 ± 0.09 | 0.482 | |
| p | 0.350 | 0.141 | ||
| CAT U/mgprot | NI | 10.90 ± 3.17 | 10.15 ± 3.37 | 0.542 |
| HI | 9.88 ± 3.64 | 9.75 ± 2.68 | 0.914 | |
| HI + LC | 9.78 ± 4.05 | 9.87 ± 1.99 | 0.922 | |
| HI + HC | 10.99 ± 3.64 | 11.69 ± 4.35 | 0.638 | |
| p | 0.790 | 0.367 | ||
| SOD U/mgprot | NI | 200.75 ± 36.75 | 201.10 ± 42.52 | 0.981 |
| HI | 202.37 ± 43.74 | 203.60 ± 37.72 | 0.935 | |
| HI + LC | 192.73 ± 27.71 | 189.76 ± 24.78 | 0.759 | |
| HI + HC | 192.18 ± 19.63 | 195.69 ± 33.05 | 0.726 | |
| p | 0.835 | 0.707 | ||
| MDA nmol/mgprot | NI | 4.01 ± 2.71 | 3.76 ± 2.68 | 0.983 |
| HI | 4.68 ± 2.94 | 3.30 ± 1.61 | 0.161 | |
| HI + LC | 5.94 ± 3.51 | 6.04 ± 3.13 | 0.653 | |
| HI + HC | 4.32 ± 2.82 | 3.85 ± 2.43 | 0.624 | |
| p | 0.015 * HI + LC > NI, HI, HI + HC | 0.005 ** HI + LC > NI, HI, HI + HC | ||
| GSH-Px U/mgprot | NI | 214.62 ± 46.49 | 231.71 ± 44.08 | 0.319 |
| HI | 216.04 ± 59.11 | 199.77 ± 59.77 | 0.450 | |
| HI + LC | 201.57 ± 47.57 | 211.36 ± 42.79 | 0.558 | |
| HI + HC | 210.20 ± 56.12 | 224.12 ± 42.96 | 0.452 | |
| p | 0.875 | 0.281 |
| Indicators | Classification | KI (n = 12) | KIO3 (n = 12) | p |
|---|---|---|---|---|
| TAOC U/mgprot | NI | 0.47 ± 0.20 | 0.52 ± 0.22 | 0.486 |
| HI | 0.58 ± 0.33 | 0.50 ± 0.13 | 0.838 | |
| HI + LC | 0.43 ± 0.14 | 0.53 ± 0.19 | 0.161 | |
| HI + HC | 0.46 ± 0.15 | 0.51 ± 0.14 | 0.345 | |
| p | 0.714 | 0.960 | ||
| CAT U/mgprot | NI | 1.38 ± 0.31 | 1.96 ± 0.71 | 0.008 ** |
| HI | 1.58 ± 0.68 | 2.08 ± 1.15 | 0.265 | |
| HI + LC | 1.91 ± 0.78 | 2.06 ± 0.81 | 0.806 | |
| HI + HC | 2.10 ± 0.74 | 2.16 ± 0.92 | 0.998 | |
| p | 0.036 * NI, HI < HI + LC, HI + HC | 0.972 | ||
| SOD U/mgprot | NI | 5.51 ± 2.92 | 6.75 ± 2.29 | 0.246 |
| HI | 4.71 ± 2.10 | 4.39 ± 2.08 | 0.583 | |
| HI + LC | 5.14 ± 2.89 | 5.53 ± 2.23 | 0.533 | |
| HI + HC | 4.84 ± 2.36 | 5.03 ± 2.53 | 0.653 | |
| p | 0.967 | 0.039 * NI, HI + LC > HI, HI + HC | ||
| MDA nmol/mgprot | NI | 3.51 ± 2.27 | 2.85 ± 1.44 | 0.775 |
| HI | 4.09 ± 1.85 | 3.08 ± 1.30 | 0.137 | |
| HI + LC | 2.77 ± 1.25 | 2.93 ± 1.59 | 0.806 | |
| HI + HC | 2.51 ± 1.16 | 3.04 ± 1.28 | 0.250 | |
| p | 0.105 | 0.958 | ||
| GSH-Px U/mgprot | NI | 10.54 ± 2.66 | 11.72 ± 1.79 | 0.215 |
| HI | 9.28 ± 3.20 | 10.62 ± 3.31 | 0.326 | |
| HI + LC | 11.37 ± 1.93 | 10.67 ± 9.91 | 0.309 | |
| HI + HC | 11.92 ± 2.41 | 11.34 ± 2.88 | 0.596 | |
| p | 0.074 | 0.696 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, R.; Liu, L.; Qian, T.; Zhao, M.; Che, W.; Hou, X.; Xie, H.; Su, Y.; Pan, H.; Li, J.; et al. Protection of Vitamin C on Oxidative Damage Caused by Long-Term Excess Iodine Exposure in Wistar Rats. Nutrients 2022, 14, 5245. https://doi.org/10.3390/nu14245245
Sun R, Liu L, Qian T, Zhao M, Che W, Hou X, Xie H, Su Y, Pan H, Li J, et al. Protection of Vitamin C on Oxidative Damage Caused by Long-Term Excess Iodine Exposure in Wistar Rats. Nutrients. 2022; 14(24):5245. https://doi.org/10.3390/nu14245245
Chicago/Turabian StyleSun, Rong, Lanchun Liu, Tingting Qian, Meng Zhao, Wenjing Che, Xin Hou, Honglei Xie, Yue Su, Haowen Pan, Jia Li, and et al. 2022. "Protection of Vitamin C on Oxidative Damage Caused by Long-Term Excess Iodine Exposure in Wistar Rats" Nutrients 14, no. 24: 5245. https://doi.org/10.3390/nu14245245
APA StyleSun, R., Liu, L., Qian, T., Zhao, M., Che, W., Hou, X., Xie, H., Su, Y., Pan, H., Li, J., & Liu, P. (2022). Protection of Vitamin C on Oxidative Damage Caused by Long-Term Excess Iodine Exposure in Wistar Rats. Nutrients, 14(24), 5245. https://doi.org/10.3390/nu14245245





