No Effect of Vitamin C Administration on Neutrophil Recovery in Autologous Stem Cell Transplantation for Myeloma or Lymphoma: A Blinded, Randomized Placebo-Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Observational Study
2.2. Run-In Phase
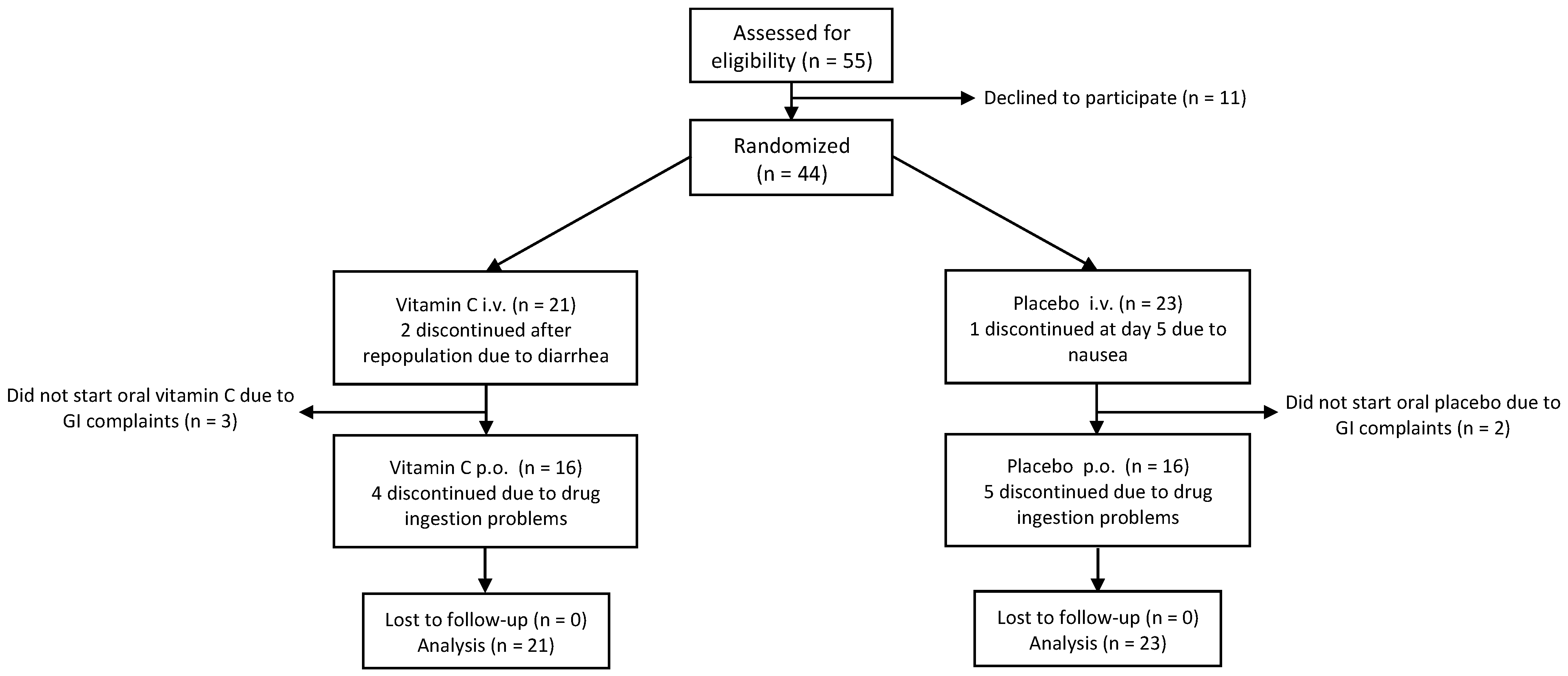
2.3. Patient Enrolment of Randomized, Placebo-Controlled Phase
2.4. Administration of Intervention
2.5. Collection of Clinical Data
2.6. Collection and Analysis of Blood Samples
2.7. Collection of Quality of Life Data
2.8. Statistical Analyses
3. Results
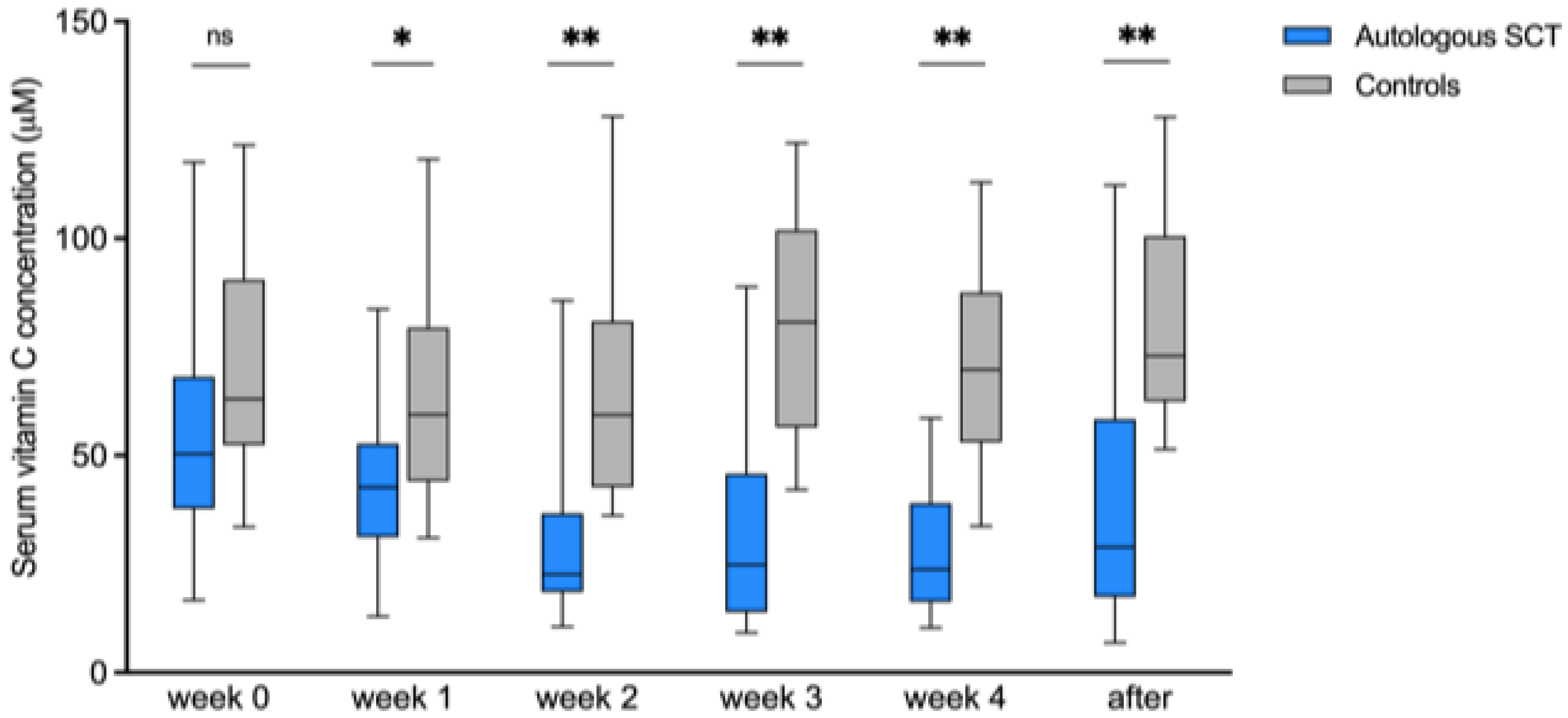
3.1. Observational Study
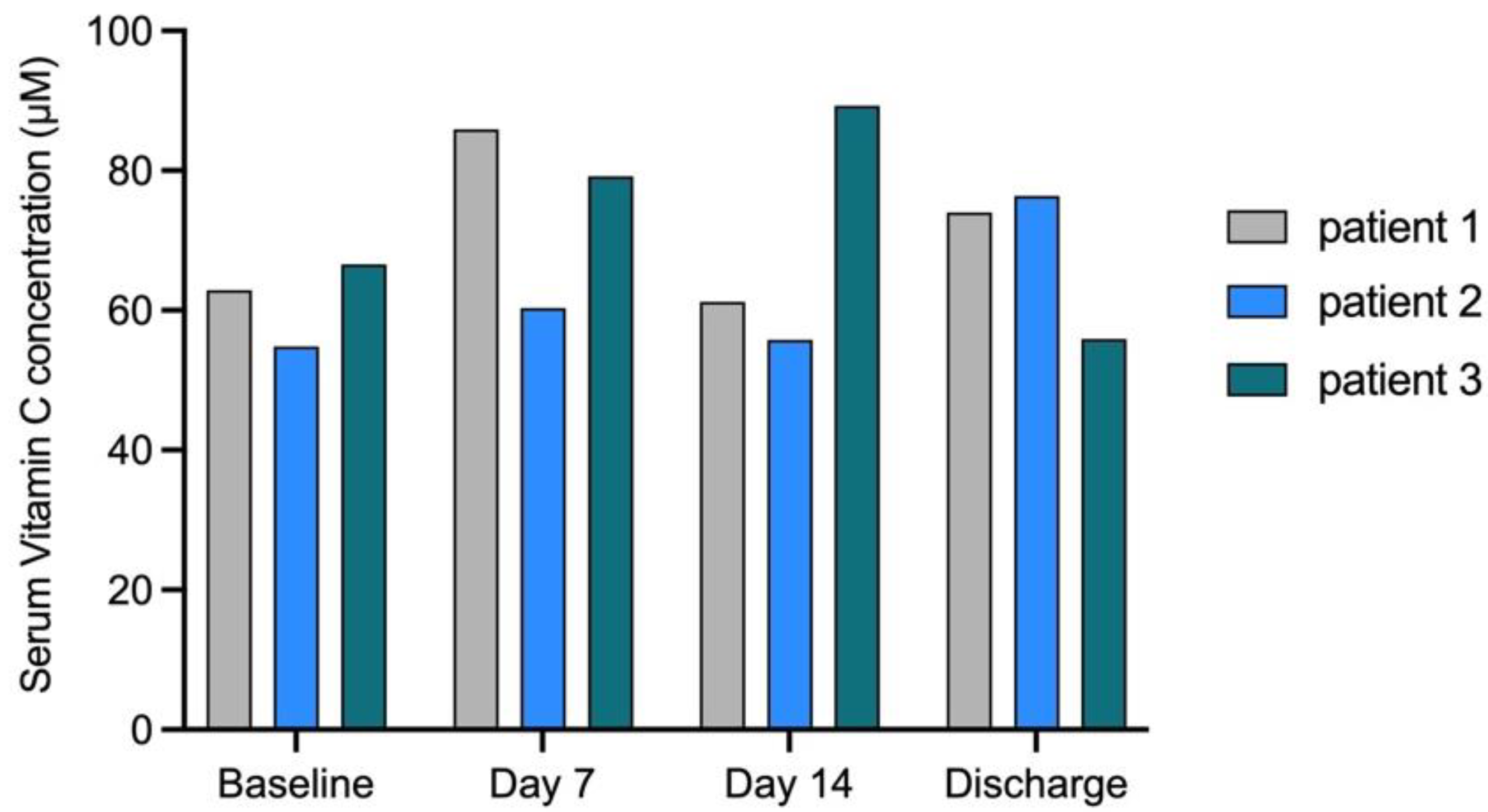
3.2. Run-In Phase
3.3. Randomized Placebo-Controlled Phase
3.3.1. Patient Characteristics
3.3.2. Vitamin C Kinetics
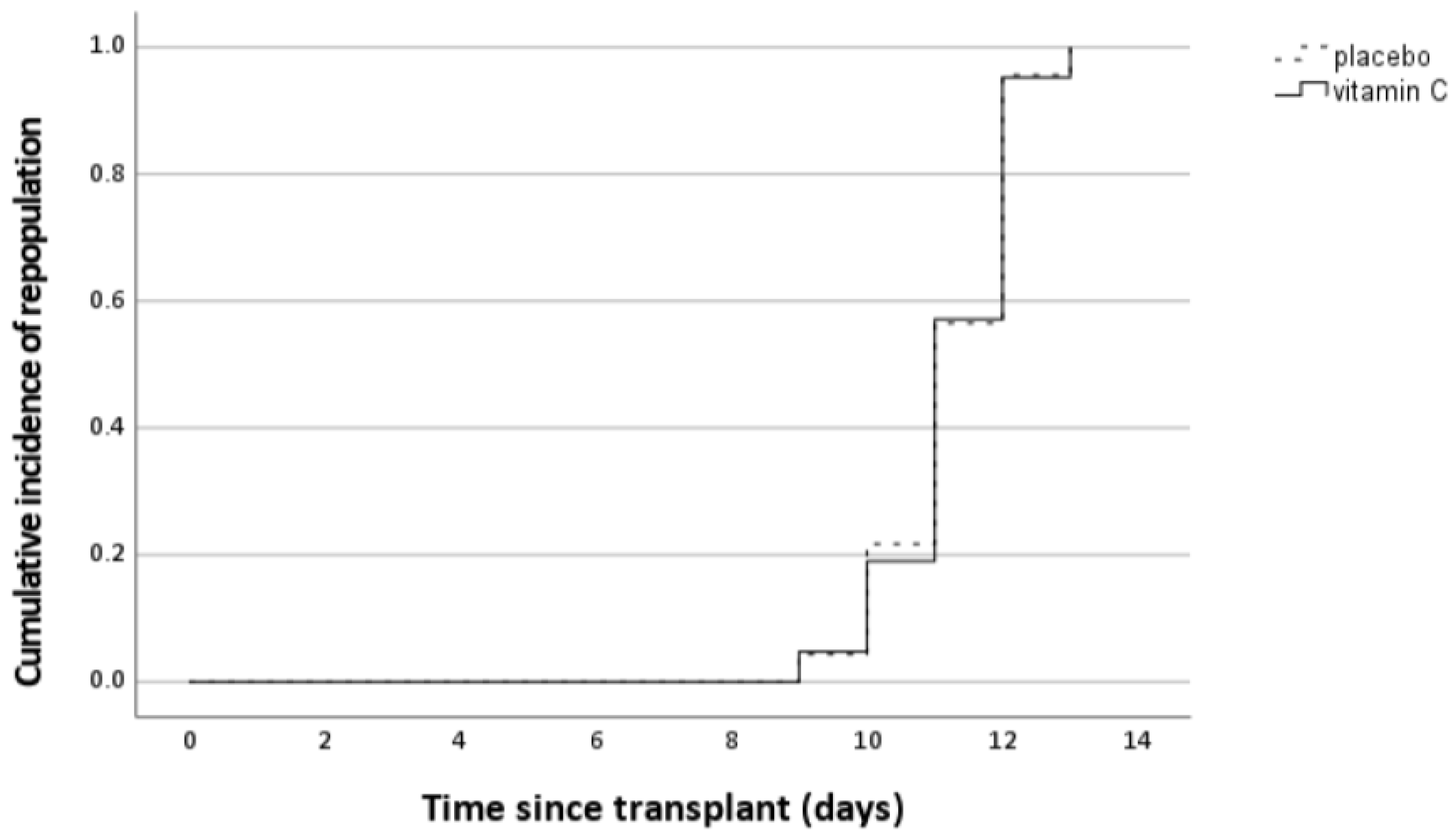
3.3.3. Effect of Intervention on Neutrophil Engraftment
3.3.4. Effect of Intervention on Other Clinical Parameters
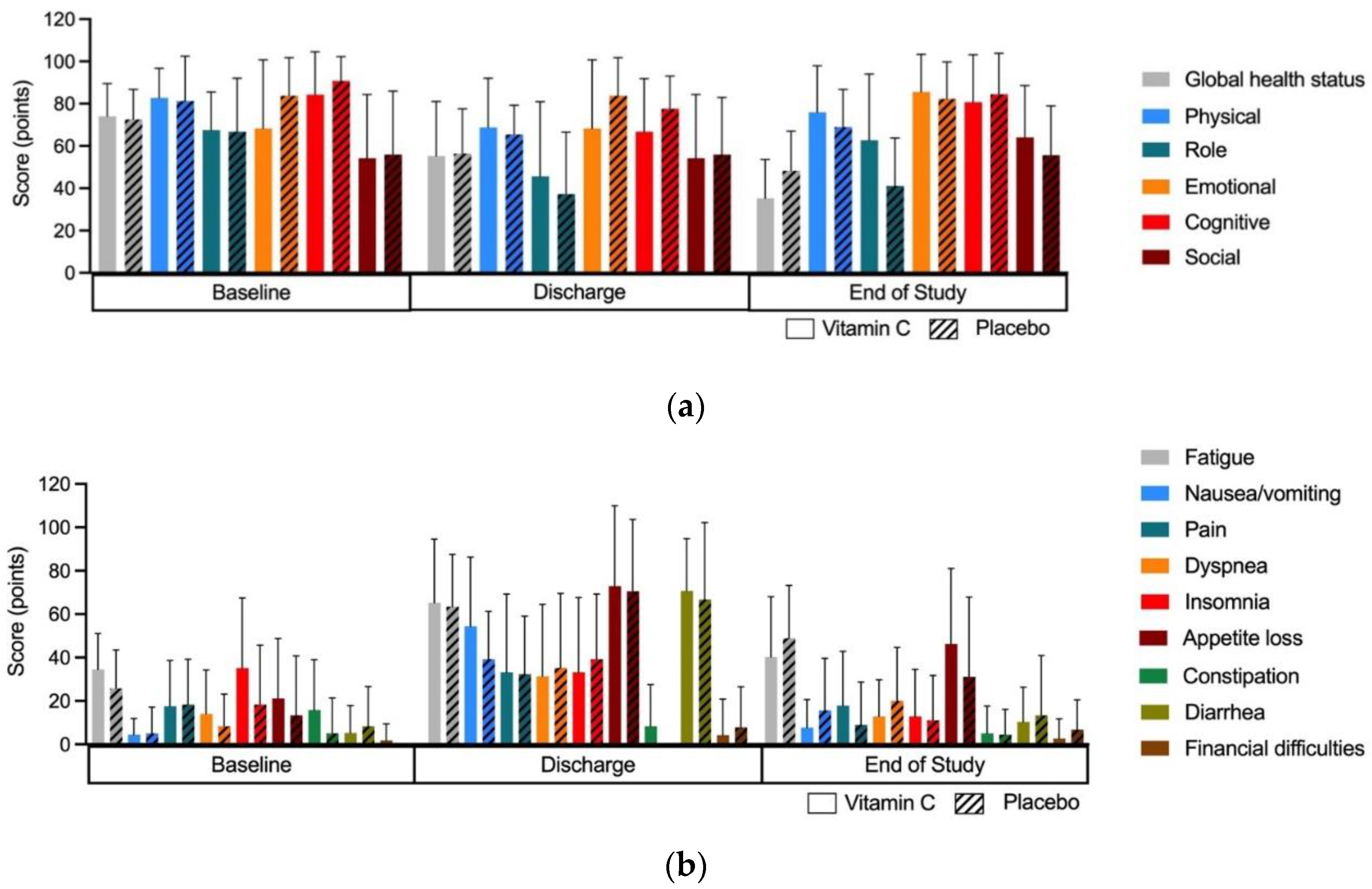
3.3.5. Effect of Intervention on Quality of Life
3.3.6. Effect of Intervention on Lymphocyte and NK Cell Immune Reconstitution
3.3.7. Adverse Events
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

References
- Cameron, E.; Pauling, L. Supplemental ascorbate in the supportive treatment of cancer: Reevaluation of prolongation of survival times in terminal human cancer. Proc. Natl. Acad. Sci. USA 1978, 75, 4538–4542. [Google Scholar] [CrossRef]
- Cameron, E.; Pauling, L. Supplemental ascorbate in the supportive treatment of cancer: Prolongation of survival times in terminal human cancer. Proc. Natl. Acad. Sci. USA 1976, 73, 3685–3689. [Google Scholar] [CrossRef] [PubMed]
- Creagan, E.T.; Moertel, C.G.; O’Fallon, J.R.; Schutt, A.J.; O’Connell, M.J.; Rubin, J.; Frytak, S. Failure of high-dose vitamin C (ascorbic acid) therapy to benefit patients with advanced cancer. A controlled trial. N. Engl. J. Med. 1979, 301, 687–690. [Google Scholar] [CrossRef] [PubMed]
- Moertel, C.G.; Fleming, T.R.; Creagan, E.T.; Rubin, J.; O’Connell, M.J.; Ames, M.M. High-dose vitamin C versus placebo in the treatment of patients with advanced cancer who have had no prior chemotherapy. A randomized double-blind comparison. N. Engl. J. Med. 1985, 312, 137–141. [Google Scholar] [CrossRef]
- Murata, A.; Morishige, F.; Yamaguchi, H. Prolongation of survival times of terminal cancer patients by administration of large doses of ascorbate. Int. J. Vitam. Nutr. Res. Suppl. 1982, 23, 103–113. [Google Scholar] [PubMed]
- Carr, A.C.; Maggini, S. Vitamin C and Immune Function. Nutrients 2017, 9, 1211. [Google Scholar] [CrossRef] [PubMed]
- Liugan, M.; Carr, A.C. Vitamin C and Neutrophil Function: Findings from Randomized Controlled Trials. Nutrients 2019, 11, 2102. [Google Scholar] [CrossRef]
- Bozonet, S.M.; Carr, A.C. The Role of Physiological Vitamin C Concentrations on Key Functions of Neutrophils Isolated from Healthy Individuals. Nutrients 2019, 11, 1363. [Google Scholar] [CrossRef]
- Tauler, P.; Aguiló, A.; Gimeno, I.; Noguera, A.; Agustí, A.; Tur, J.A.; Pons, A. Differential response of lymphocytes and neutrophils to high intensity physical activity and to vitamin C diet supplementation. Free Radic. Res. 2003, 37, 931–938. [Google Scholar] [CrossRef]
- Kizhina, A.; Pechorina, E.; Mikheeva, V. Effect of vitamin C supplementation on some leukocyte parameters in American mink (Neovison vison) with abnormal granulogenesis. Tissue Cell 2022, 77, 101870. [Google Scholar] [CrossRef]
- Huijskens, M.J.A.J.; Walczak, M.; Koller, N.; Briedé, J.J.; Senden-Gijsbers, B.L.M.G.; Schnijderberg, M.C.; Bos, G.M.J.; Germeraad, W.T.V. Technical advance: Ascorbic acid induces development of double-positive T cells from human hematopoietic stem cells in the absence of stromal cells. J. Leukoc. Biol. 2014, 96, 1165–1175. [Google Scholar] [CrossRef] [PubMed]
- Huijskens, M.J.; Walczak, M.; Sarkar, S.; Atrafi, F.; Senden-Gijsbers, B.L.; Tilanus, M.G.; Bos, G.M.; Wieten, L.; Germeraad, W.T. Ascorbic acid promotes proliferation of natural killer cell populations in culture systems applicable for natural killer cell therapy. Cytotherapy 2015, 17, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Storek, J. Immunological reconstitution after hematopoietic cell transplantation—Its relation to the contents of the graft. Expert Opin. Biol. Ther. 2008, 8, 583–597. [Google Scholar] [CrossRef] [PubMed]
- Huijskens, M.J.; Wodzig, W.K.; Walczak, M.; Germeraad, W.T.; Bos, G.M. Ascorbic acid serum levels are reduced in patients with hematological malignancies. Results Immunol. 2016, 6, 8–10. [Google Scholar] [CrossRef]
- Levine, M.; Conry-Cantilena, C.; Wang, Y.; Welch, R.W.; Washko, P.W.; Dhariwal, K.R.; Park, J.B.; Lazarev, A.; Graumlich, J.F.; King, J.; et al. Vitamin C pharmacokinetics in healthy volunteers: Evidence for a recommended dietary allowance. Proc. Natl. Acad. Sci. USA 1996, 93, 3704–3709. [Google Scholar] [CrossRef] [PubMed]
- van Gorkom, G.N.Y.; Lookermans, E.L.; Van Elssen, C.; Bos, G.M.J. The Effect of Vitamin C (Ascorbic Acid) in the Treatment of Patients with Cancer: A Systematic Review. Nutrients 2019, 11, 977. [Google Scholar] [CrossRef]
- European Food Safety Authority. Scientific opinion on dietary reference values for vitamin C. EFSA J. 2013, 11, 3418. [Google Scholar]
- van Gorkom, G.; Gijsbers, B.; Ververs, E.-J.; El Molla, A.; Sarodnik, C.; Riess, C.; Wodzig, W.; Bos, G.; Van Elssen, C. Easy-to-Use HPLC Method to Measure Intracellular Ascorbic Acid Levels in Human Peripheral Blood Mononuclear Cells and in Plasma. Antioxidants 2022, 11, 134. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.; Ferrara, J.L. Immunobiology of acute graft-versus-host disease. Blood Rev. 2003, 17, 187–194. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Bethesda (MD): National Library of Medicine (US). Identifier NCT03613727, Therapeutic Use of Intravenous Vitamin C in Allogeneic Stem Cell Transplant Recipients. 3 August 2018. Available online: https://clinicaltrials.gov/ct2/show/NCT03613727 (accessed on 13 July 2022).
- Carr, A.C.; Vissers, M.C.M.; Cook, J.S. The effect of intravenous vitamin C on cancer- and chemotherapy-related fatigue and quality of life. Front. Oncol. 2014, 4, 283. [Google Scholar] [CrossRef]
- Yeom, C.H.; Jung, G.C.; Song, K.J. Changes of terminal cancer patients’ health-related quality of life after high dose vitamin C administration. J. Korean Med. Sci. 2007, 22, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Mizuno, H.; Yanagisawa, A. High-dose intravenous vitamin C improves quality of life in cancer patients. Pers. Med. Universe 2012, 1, 49–53. [Google Scholar] [CrossRef]
- Xia, J.; Xu, H.; Zhang, X.; Allamargot, C.; Coleman, K.L.; Nessler, R.; Frech, I.; Tricot, G.; Zhan, F. Multiple Myeloma Tumor Cells are Selectively Killed by Pharmacologically-dosed Ascorbic Acid. EBioMedicine 2017, 18, 41–49. [Google Scholar] [CrossRef] [PubMed]

| Vitamin C, (n = 21) | Placebo, (n = 23) | p Value | |
|---|---|---|---|
| Age (years), mean (range) | 61.7 (31–71) | 61.4 (34–72) | 0.92 |
| Gender | 1.0 | ||
| Male, n, (%) | 9 (43) | 10 (43) | |
| Female, n, (%) | 12 (57) | 13 (57) | |
| Disease | 0.75 | ||
| MM, n, (%) | 15 (71) | 15 (65) | |
| Lymphoma, n, (%) | 6 (29) | 8 (35) | |
| Weight (kg), mean (range) | 72.7 (47–125) | 75.7 (46–105) | 0.60 |
| Length (cm), mean (range) | 169.1 (153–197) | 169.3 (150–191) | 0.96 |
| BMI, mean (range) | 25.1 (16.5–36.1) | 26.2 (16.9–36) | 0.43 |
| Comorbidities, n, (%) | 1.0 | ||
| Yes: | 12 (57) | 13 (57) | |
| Cardiac | 4 | 3 | |
| Pulmonary | 0 | 2 | |
| Renal | 1 | 1 | |
| Hepatic | 1 | 0 | |
| Other | 6 | 7 | |
| No: | 9 (43) | 10 (43) | |
| WHO, n, (%) | 0.57 | ||
| 0 | 13 (62) | 15 (65) | |
| 1 | 8 (38) | 7 (30) | |
| 2 | 0 (0) | 1 (4) | |
| Number of previous treatment lines, n, (%) | 0.62 | ||
| 1 | 14 (67) | 15 (65) | |
| 2 | 7 (33) | 7 (30) | |
| >2 | 0 (0) | 1 (4) | |
| Previous autologous transplantation, n, (%) | 1.0 | ||
| Yes | 3 (14) | 4 (17) | |
| No | 18 (86) | 19 (83) | |
| Time diagnosis to study (months), mean (range) | 12.3 (4.0–77.7) | 21.2 (4.1–137.1) | 0.28 |
| Vitamin C | Placebo | p Value | |
|---|---|---|---|
| Duration of hospitalization (days), mean (range) | 19.7 (13–47) | 19.1 (14–31) | 0.80 |
| Neutropenic fever, n (%) | 0.20 | ||
| Yes: | 12 (57) | 18 (78) | |
| No: | 9 (43) | 5 (22) | |
| Duration of neutropenic fever (days), mean (range) | 2.3 (1–4) | 2.8 (1–7) | 0.48 |
| Duration of antibiotic treatment (days), mean (range) | 3.7 (0–10) | 4.8 (0–9) | 0.28 |
| Presence of bacteremia, n (%) | 0.07 | ||
| Yes: | 2 (10) | 8 (35) | |
| No: | 19 (90) | 15 (65) | |
| Readmission, n (%) | 0.67 | ||
| Yes: | 2 (10) | 4 (17) | |
| No: | 19 (90) | 19 (83) | |
| PFS 3 months | 90.5% (SE 6.4) | 95.7% (SE 4.3) | 0.49 |
| OS 3 months | 90.5% (SE 6.4) | 100% | 0.13 |
| Baseline | Repopulation | End of Study | |||||||
| Vitamin C | Placebo | p | Vitamin C | Placebo | p | Vitamin C | Placebo | p | |
| Lymphocytes | 1040.7 (497–2098) | 1099.9 (352–221) | 0.68 | 473.1 (10–1800) | 289.5 (58–840) | 0.10 | 1702.8 (415–3895) | 1395.8 (469–3071) | 0.35 |
| NK cells | 192.6 (4–863) | 186.2 (76–373) | 0.88 | 91.8 (3–715) | 59.0 (1–220) | 0.41 | 412.3 (130–1314) | 293.2 (31–1005) | 0.34 |
| B cells | 54.2 (0–148) | 73.1 (0–288) | 0.40 | 0.5 (0–4) | 3.1 (0–47) | 0.32 | 20.3 (0–130) | 15.1 (0–126) | 0.68 |
| Total T cells | 812.2 (309–1809) | 833.4 (141–1872) | 0.87 | 370.4 (6–1106) | 216.8 (36–579) | 0.06 | 1260.3 (162–3720) | 1067.7 (350–2894) | 0.57 |
| Th cells | 435.6 (76–952) | 454.8 (28–745) | 0.78 | 199.2 (5–618) | 156.8 (31–431) | 0.31 | 378.6 (98–1048) | 318.0 (124–714) | 0.47 |
| Cytotoxic T cells | 366.6 (47–909) | 357.8 (73–1445) | 0.92 | 173.4 (1–825) | 60.8 (3–207) | 0.03 | 842.9 (66–2638) | 733.9 (46–2159) | 0.69 |
| Vitamin C (n = 21) | Placebo (n = 23) | p-Value | |
|---|---|---|---|
| Patients with AE (grade 3) | 9 | 8 | 0.76 |
| AE grade 3 | |||
| Hypokalemia | 8 | 7 | 0.75 |
| Mucositis | 4 | 1 | 0.18 |
| Gastric hemorrhage | 0 | 1 | 1.0 |
| Pneumonia | 1 | 1 | 1.0 |
| Hypertension | 1 | 0 | 0.48 |
| Toxicodermia | 1 | 0 | 0.48 |
| Malnutrition | 1 | 0 | 0.48 |
| Hypomagnesaemia | 1 | 0 | 0.48 |
| TOTAL | 17 | 11 | |
| Death | 2 (COVID-19) | 0 | 0.22 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van Gorkom, G.N.Y.; Boerenkamp, L.S.; Gijsbers, B.L.M.G.; van Ojik, H.H.; Wodzig, W.K.W.H.; Wieten, L.; Van Elssen, C.H.M.J.; Bos, G.M.J. No Effect of Vitamin C Administration on Neutrophil Recovery in Autologous Stem Cell Transplantation for Myeloma or Lymphoma: A Blinded, Randomized Placebo-Controlled Trial. Nutrients 2022, 14, 4784. https://doi.org/10.3390/nu14224784
van Gorkom GNY, Boerenkamp LS, Gijsbers BLMG, van Ojik HH, Wodzig WKWH, Wieten L, Van Elssen CHMJ, Bos GMJ. No Effect of Vitamin C Administration on Neutrophil Recovery in Autologous Stem Cell Transplantation for Myeloma or Lymphoma: A Blinded, Randomized Placebo-Controlled Trial. Nutrients. 2022; 14(22):4784. https://doi.org/10.3390/nu14224784
Chicago/Turabian Stylevan Gorkom, Gwendolyn N. Y., Lara S. Boerenkamp, Birgit L. M. G. Gijsbers, Heidi H. van Ojik, Will K. W. H. Wodzig, Lotte Wieten, Catharina H. M. J. Van Elssen, and Gerard M. J. Bos. 2022. "No Effect of Vitamin C Administration on Neutrophil Recovery in Autologous Stem Cell Transplantation for Myeloma or Lymphoma: A Blinded, Randomized Placebo-Controlled Trial" Nutrients 14, no. 22: 4784. https://doi.org/10.3390/nu14224784
APA Stylevan Gorkom, G. N. Y., Boerenkamp, L. S., Gijsbers, B. L. M. G., van Ojik, H. H., Wodzig, W. K. W. H., Wieten, L., Van Elssen, C. H. M. J., & Bos, G. M. J. (2022). No Effect of Vitamin C Administration on Neutrophil Recovery in Autologous Stem Cell Transplantation for Myeloma or Lymphoma: A Blinded, Randomized Placebo-Controlled Trial. Nutrients, 14(22), 4784. https://doi.org/10.3390/nu14224784







