Citri Reticulatae Pericarpium (Chenpi) Protects against Endothelial Dysfunction and Vascular Inflammation in Diabetic Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of CRP Extract
2.2. Animal Protocols
2.3. Oral Glucose Tolerance Test (OGTT)
2.4. Blood Pressure Measurement
2.5. Determination of Plasma Lipid Profile
2.6. Determination of Liver Function Biomarkers in Plasma
2.7. Ex vivo Culture of Rat and Mouse Aortas
2.8. Isometric Force Measurement in Wire Myograph
2.9. Western Blotting
2.10. Statistical Analysis
3. Results
3.1. CRP Extract Mitigates High Blood Pressure in Diabetic Rats
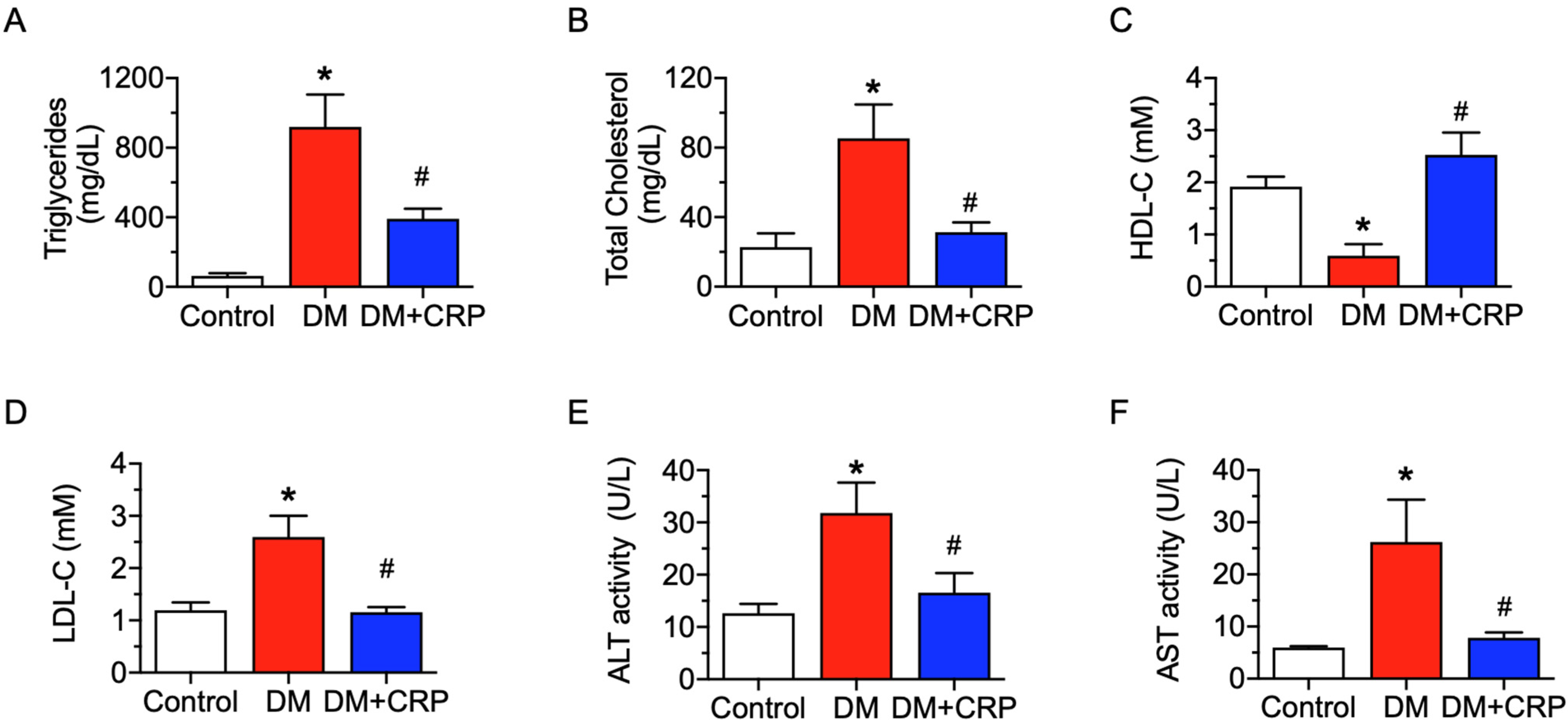
3.2. CRP Extract Ameliorates Lipid Metabolism Dysregulation and Liver Function Injury in Diabetic Rats
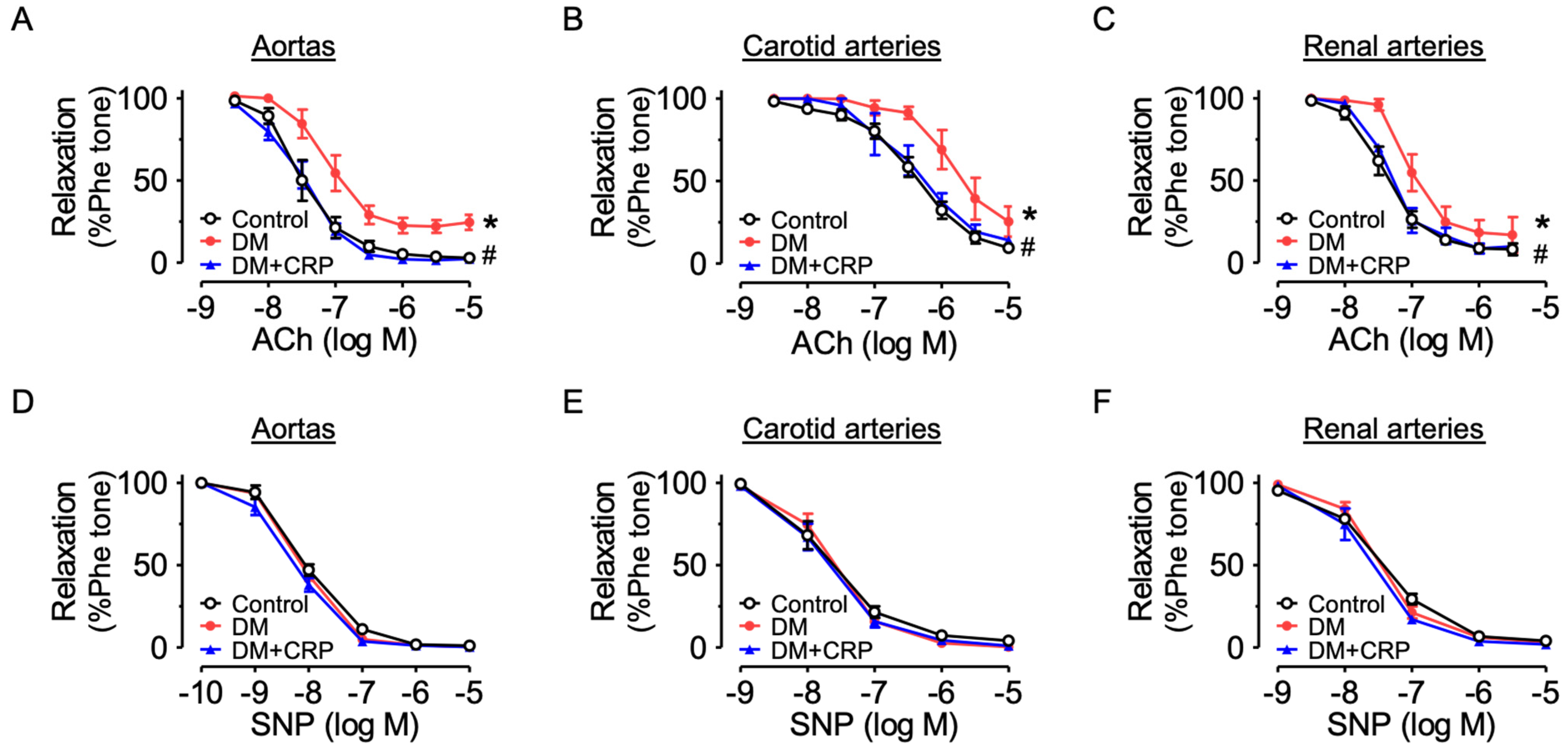
3.3. CRP Extract Alleviates Endothelial Dysfunction Associated with Diabetes
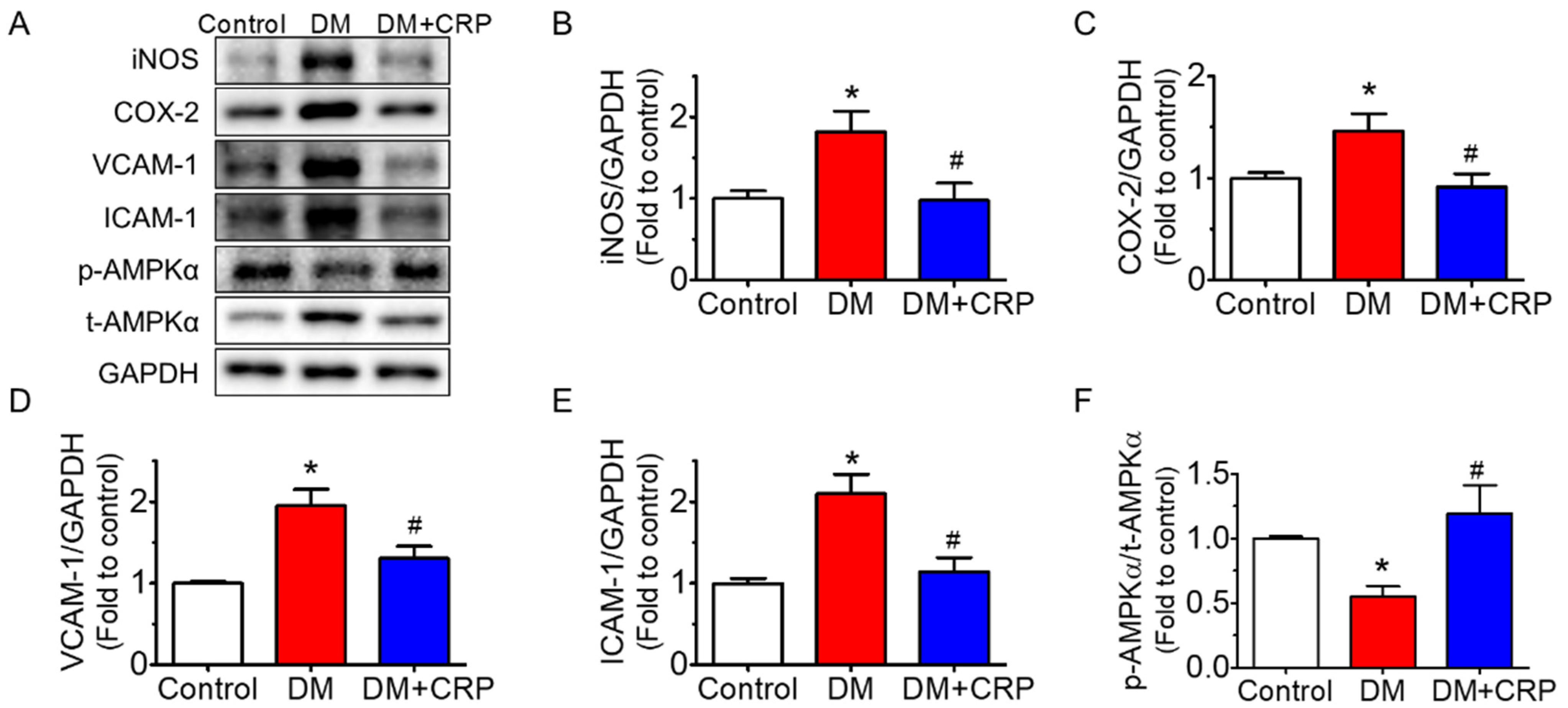
3.4. CRP Extract Inhibited Inflammation in the Aortas of Diabetic Rats
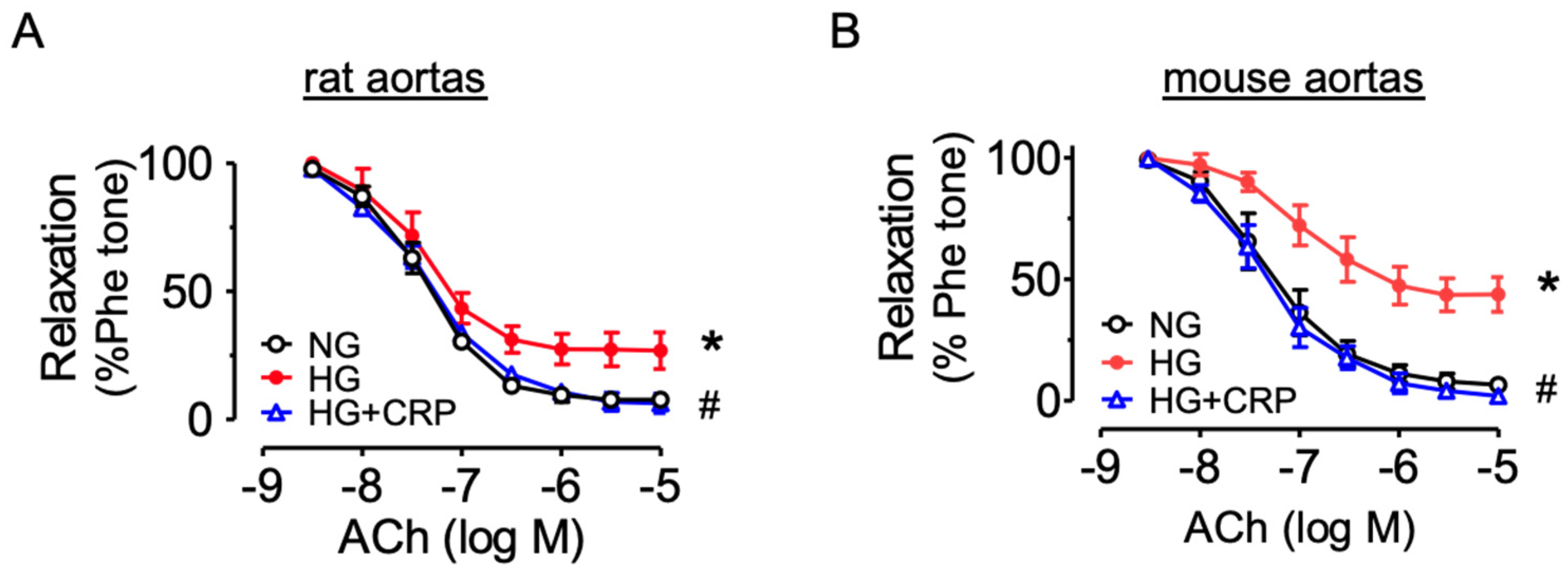
3.5. CRP Extract Protects against Endothelial dysfunction in Hyperglycemic conditions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Einarson, T.R.; Acs, A.; Ludwig, C.; Panton, U.H. Prevalence of cardiovascular disease in type 2 diabetes: A systematic literature review of scientific evidence from across the world in 2007–2017. Cardiovasc. Diabetol. 2018, 17, 83. [Google Scholar] [CrossRef] [PubMed]
- Emerging Risk Factors Collaboration. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: A collaborative meta-analysis of 102 prospective studies. Lancet 2010, 375, 2215–2222. [Google Scholar] [CrossRef] [PubMed]
- Bakker, W.; Eringa, E.C.; Sipkema, P.; van Hinsbergh, V.W.M. Endothelial dysfunction and diabetes: Roles of hyperglycemia, impaired insulin signaling and obesity. Cell Tissue Res. 2009, 335, 165–189. [Google Scholar] [CrossRef] [PubMed]
- Sena, C.M.; Carrilho, F.; Seiça, R. Endothelial Dysfunction in Type 2 Diabetes: Targeting Inflammation. Endothel. Dysfunct. Old Concepts New Chall. 2018, 24, 23110. [Google Scholar]
- Duncan, B.B.; Schmidt, M.I.; Pankow, J.S.; Ballantyne, C.M.; Couper, D.; Vigo, A.; Hoogeveen, R.; Folsom, A.R.; Heiss, G. Low-Grade Systemic Inflammation and the Development of Type 2 Diabetes: The Atherosclerosis Risk in Communities Study. Diabetes 2003, 52, 1799–1805. [Google Scholar] [CrossRef]
- Hingorani, A.D.; Cross, J.; Kharbanda, R.K.; Mullen, M.J.; Bhagat, K.; Taylor, M.; Donald, A.E.; Palacios, M.; Griffin, G.E.; Deanfield, J.E.; et al. Acute systemic inflammation impairs endothelium-dependent dilatation in humans. Circulation 2000, 102, 994–999. [Google Scholar] [CrossRef]
- Qi, J.; Wu, Q.; Cheng, Q.; Chen, X.; Zhu, M.; Miao, C. High Glucose Induces Endothelial COX2 and iNOS Expression via Inhibition of Monomethyltransferase SETD8 Expression. J. Diabetes Res. 2020, 2020, 2308520. [Google Scholar] [CrossRef]
- Theofilis, P.; Sagris, M.; Oikonomou, E.; Antonopoulos, A.S.; Siasos, G.; Tsioufis, C.; Tousoulis, D. Inflammatory Mechanisms Contributing to Endothelial Dysfunction. Biomedicines 2021, 9, 781. [Google Scholar] [CrossRef]
- Hardie, D.G. Adenosine monophosphate-activated protein kinase: A central regulator of metabolism with roles in diabetes, cancer, and viral infection. Cold Spring Harb. Symp. Quant. Biol. 2011, 76, 155–164. [Google Scholar] [CrossRef]
- Ewart, M.-A.; Kennedy, S. AMPK and vasculoprotection. Pharmacol. Ther. 2011, 131, 242–253. [Google Scholar] [CrossRef]
- Cheang, W.S.; Tian, X.Y.; Wong, W.T.; Lau, C.W.; Lee, S.S.; Chen, Z.Y.; Yao, X.; Wang, N.; Huang, Y. Metformin protects endothelial function in diet-induced obese mice by inhibition of endoplasmic reticulum stress through 5’ adenosine monophosphate-activated protein kinase-peroxisome proliferator-activated receptor delta pathway. Arter. Thromb. Vasc. Biol. 2014, 34, 830–836. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Tan, Y.; Xu, B.; Wang, Y.; Cheang, W.S. 3,4′,5-Trimethoxy-trans-stilbene Alleviates Endothelial Dysfunction in Diabetic and Obese Mice via Activation of the AMPK/SIRT1/eNOS Pathway. Antioxidants 2022, 11, 1286. [Google Scholar] [CrossRef]
- Commission, C.P. Pharmacopoeia of the People’s Republic of China; China Medical Science Press: Beijing, China, 2020; p. 199. [Google Scholar]
- Hua, L.S.L. Advance of Main Chemical Components and Quality Control of Citri Reticulatae Pericarpium. Pharm. Today 2020, 30, 861–864. [Google Scholar]
- Wu, Y.; He, Y.; Wang, R.; Zhao, X. Preventive Effect of Flavonoid Extract from the Peel of Gonggan (Citrus reticulata Blanco Var. Gonggan) on CCl(4)-Induced Acute Liver Injury in Mice. J. Inflamm. Res. 2021, 14, 5111–5121. [Google Scholar] [CrossRef]
- Fu, M.; Zou, B.; An, K.; Yu, Y.; Tang, D.; Wu, J.; Xu, Y.; Ti, H. Anti-asthmatic activity of alkaloid compounds from Pericarpium Citri Reticulatae (Citrus reticulata ‘Chachi’). Food Funct. 2019, 10, 903–911. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Chen, L.; Chen, S.; Chen, H.; Liu, Y. Microbial biotransformation of Pericarpium Citri Reticulatae (PCR) by Aspergillus niger and effects on antioxidant activity. Food Sci. Nutr. 2021, 9, 855–865. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Phenolic composition, antioxidant potential and health benefits of citrus peel. Food Res. Int. 2020, 132, 109114. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, Y.; Cheong, M.S.; Khan, H.; Ruan, C.-C.; Fu, M.; Xiao, J.; Cheang, W.S. Citri Reticulatae Pericarpium extract and flavonoids reduce inflammation in RAW 264.7 macrophages by inactivation of MAPK and NF-κB pathways. Food Front. 2022, 3, 785–795. [Google Scholar] [CrossRef]
- Ling, Y.; Shi, Z.; Yang, X.; Cai, Z.; Wang, L.; Wu, X.; Ye, A.; Jiang, J. Hypolipidemic effect of pure total flavonoids from peel of Citrus (PTFC) on hamsters of hyperlipidemia and its potential mechanism. Exp. Gerontol. 2020, 130, 110786. [Google Scholar] [CrossRef]
- Mulvihill, E.E.; Burke, A.C.; Huff, M.W. Citrus Flavonoids as Regulators of Lipoprotein Metabolism and Atherosclerosis. Annu. Rev. Nutr. 2016, 36, 275–299. [Google Scholar] [CrossRef]
- Kaneda, H.; Otomo, R.; Sasaki, N.; Omi, T.; Sato, T.; Kaneda, T. Endothelium-independent vasodilator effects of nobiletin in rat aorta. J. Pharmacol. Sci. 2019, 140, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Lv, X.Y.; Li, J.; Xu, Z.G.; Chen, L. The characterization of high-fat diet and multiple low-dose streptozotocin induced type 2 diabetes rat model. Exp. Diabetes Res. 2008, 2008, 704045. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Tao, H.; Cao, Y.; Ho, C.-T.; Jin, S.; Huang, Q. Prevention of Obesity and Type 2 Diabetes with Aged Citrus Peel (Chenpi) Extract. J. Agric. Food Chem. 2016, 64, 2053–2061. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Xi, W.; Ding, X.; Fan, S.; Zhang, Y.; Jiang, D.; Li, Y.; Huang, C.; Zhou, Z. Citrange fruit extracts alleviate obesity-associated metabolic disorder in high-fat diet-induced obese C57BL/6 mouse. Int. J. Mol. Sci. 2013, 14, 23736–23750. [Google Scholar] [CrossRef]
- Park, H.J.; Jung, U.J.; Cho, S.J.; Jung, H.K.; Shim, S.; Choi, M.S. Citrus unshiu peel extract ameliorates hyperglycemia and hepatic steatosis by altering inflammation and hepatic glucose- and lipid-regulating enzymes in db/db mice. J. Nutr. Biochem. 2013, 24, 419–427. [Google Scholar] [CrossRef]
- Lee, Y.S.; Cha, B.Y.; Saito, K.; Choi, S.S.; Wang, X.X.; Choi, B.K.; Yonezawa, T.; Teruya, T.; Nagai, K.; Woo, J.T. Effects of a Citrus depressa Hayata (shiikuwasa) extract on obesity in high-fat diet-induced obese mice. Phytomedicine 2011, 18, 648–654. [Google Scholar] [CrossRef]
- Boshtam, M.; Asgary, S.; Moshtaghian, J.; Naderi, G.; Jafari-Dinani, N. Impacts of fresh lime juice and peel on atherosclerosis progression in an animal model. ARYA Atheroscler. 2013, 9, 357–362. [Google Scholar]
- Parkar, N.A.; Bhatt, L.K.; Addepalli, V. Efficacy of nobiletin, a citrus flavonoid, in the treatment of the cardiovascular dysfunction of diabetes in rats. Food Funct. 2016, 7, 3121–3129. [Google Scholar] [CrossRef]
- Gagov, H.; Gribkova, I.V.; Serebryakov, V.N.; Schubert, R. Sodium Nitroprusside-Induced Activation of Vascular Smooth Muscle BK Channels Is Mediated by PKG Rather Than by a Direct Interaction with NO. Int. J. Mol. Sci. 2022, 23, 2798. [Google Scholar] [CrossRef]
- Boden, G. Role of fatty acids in the pathogenesis of insulin resistance and NIDDM. Diabetes 1997, 46, 3–10. [Google Scholar] [CrossRef]
- Berk, B.C.; Weintraub, W.S.; Alexander, R.W. Elevation of C-reactive protein in “active” coronary artery disease. Am. J. Cardiol. 1990, 65, 168–172. [Google Scholar] [CrossRef] [PubMed]
- Maedler, K.; Sergeev, P.; Ris, F.; Oberholzer, J.; Joller-Jemelka, H.I.; Spinas, G.A.; Kaiser, N.; Halban, P.A.; Donath, M.Y. Glucose-induced beta cell production of IL-1beta contributes to glucotoxicity in human pancreatic Isletsj. Clin. Investig. 2002, 110, 851–860. [Google Scholar] [CrossRef]
- Navarro-Gonzalez, J.F.; Mora-Fernandez, C. The role of inflammatory cytokines in diabetic nephropathy. J. Am. Soc. Nephrol. 2008, 19, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Cushman, M.; Stampfer, M.J.; Tracy, R.P.; Hennekens, C.H. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N. Engl. J. Med. 1997, 336, 973–979. [Google Scholar] [CrossRef] [PubMed]
- Wellen, K.E.; Hotamisligil, G.S. Inflammation, stress, and diabetes. J. Clin. Investig. 2005, 115, 1111–1119. [Google Scholar] [CrossRef]
- Steven, S.; Frenis, K.; Oelze, M.; Kalinovic, S.; Kuntic, M.; Bayo Jimenez, M.T.; Vujacic-Mirski, K.; Helmstadter, J.; Kroller-Schon, S.; Munzel, T.; et al. Vascular Inflammation and Oxidative Stress: Major Triggers for Cardiovascular Disease. Oxid. Med. Cell Longev. 2019, 2019, 7092151. [Google Scholar] [CrossRef]
- Ho, S.-C.; Kuo, C.-T. Hesperidin, nobiletin, and tangeretin are collectively responsible for the anti-neuroinflammatory capacity of tangerine peel (Citri reticulatae pericarpium). Food Chem. Toxicol. 2014, 71, 176–182. [Google Scholar] [CrossRef]
- Jansen, T.; Kvandova, M.; Daiber, A.; Stamm, P.; Frenis, K.; Schulz, E.; Munzel, T.; Kroller-Schon, S. The AMP-Activated Protein Kinase Plays a Role in Antioxidant Defense and Regulation of Vascular Inflammation. Antioxidants 2020, 9, 525. [Google Scholar] [CrossRef]
- Cheng, Y.F.; Young, G.H.; Lin, J.T.; Jang, H.H.; Chen, C.C.; Nong, J.Y.; Chen, P.K.; Kuo, C.Y.; Kao, S.H.; Liang, Y.J.; et al. Activation of AMP-Activated Protein Kinase by Adenine Alleviates TNF-Alpha-Induced Inflammation in Human Umbilical Vein Endothelial Cells. PLoS ONE 2015, 10, e0142283. [Google Scholar] [CrossRef]
- Zhang, Y.; Qiu, J.; Wang, X.; Zhang, Y.; Xia, M. AMP-activated protein kinase suppresses endothelial cell inflammation through phosphorylation of transcriptional coactivator p300. Arter. Thromb. Vasc. Biol. 2011, 31, 2897–2908. [Google Scholar] [CrossRef]
- Thornton, C.C.; Al-Rashed, F.; Calay, D.; Birdsey, G.M.; Bauer, A.; Mylroie, H.; Morley, B.J.; Randi, A.M.; Haskard, D.O.; Boyle, J.J.; et al. Methotrexate-mediated activation of an AMPK-CREB-dependent pathway: A novel mechanism for vascular protection in chronic systemic inflammation. Ann. Rheum. Dis. 2016, 75, 439. [Google Scholar] [CrossRef] [PubMed]
- Gelinas, R.; Mailleux, F.; Dontaine, J.; Bultot, L.; Demeulder, B.; Ginion, A.; Daskalopoulos, E.P.; Esfahani, H.; Dubois-Deruy, E.; Lauzier, B.; et al. AMPK activation counteracts cardiac hypertrophy by reducing O-GlcNAcylation. Nat. Commun. 2018, 9, 374. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.K.; Shang, W.; Liu, J.; Cheang, W.S.; Wang, Y.; Xiang, L.; Lau, C.W.; Luo, J.Y.; Ng, C.F.; Huang, Y.; et al. Activation of AMPK/miR-181b Axis Alleviates Endothelial Dysfunction and Vascular Inflammation in Diabetic Mice. Antioxidants 2022, 11, 1137. [Google Scholar] [CrossRef] [PubMed]
- Miao, L.; Liu, C.; Cheong, M.S.; Zhong, R.; Tan, Y.; Rengasamy, K.R.R.; Leung, S.W.S.; Cheang, W.S.; Xiao, J. Exploration of natural flavones’ bioactivity and bioavailability in chronic inflammation induced-type-2 diabetes mellitus. Crit. Rev. Food Sci. Nutr. 2022, 1–28. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Zhang, X.; Zhou, C.; Khan, H.; Fu, M.; Cheang, W.S. Citri Reticulatae Pericarpium (Chenpi) Protects against Endothelial Dysfunction and Vascular Inflammation in Diabetic Rats. Nutrients 2022, 14, 5221. https://doi.org/10.3390/nu14245221
Wang Y, Zhang X, Zhou C, Khan H, Fu M, Cheang WS. Citri Reticulatae Pericarpium (Chenpi) Protects against Endothelial Dysfunction and Vascular Inflammation in Diabetic Rats. Nutrients. 2022; 14(24):5221. https://doi.org/10.3390/nu14245221
Chicago/Turabian StyleWang, Yuehan, Xutao Zhang, Chunxiu Zhou, Haroon Khan, Manqin Fu, and Wai San Cheang. 2022. "Citri Reticulatae Pericarpium (Chenpi) Protects against Endothelial Dysfunction and Vascular Inflammation in Diabetic Rats" Nutrients 14, no. 24: 5221. https://doi.org/10.3390/nu14245221
APA StyleWang, Y., Zhang, X., Zhou, C., Khan, H., Fu, M., & Cheang, W. S. (2022). Citri Reticulatae Pericarpium (Chenpi) Protects against Endothelial Dysfunction and Vascular Inflammation in Diabetic Rats. Nutrients, 14(24), 5221. https://doi.org/10.3390/nu14245221






