Maternal Weight Gain during Pregnancy and the Developing Autonomic Nervous System—Possible Impact of GDM
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Measurements and Calculations
2.2.1. Children
2.2.2. Mothers
2.3. Statistical Analysis
3. Results
3.1. Maternal and Offspring Characteristics
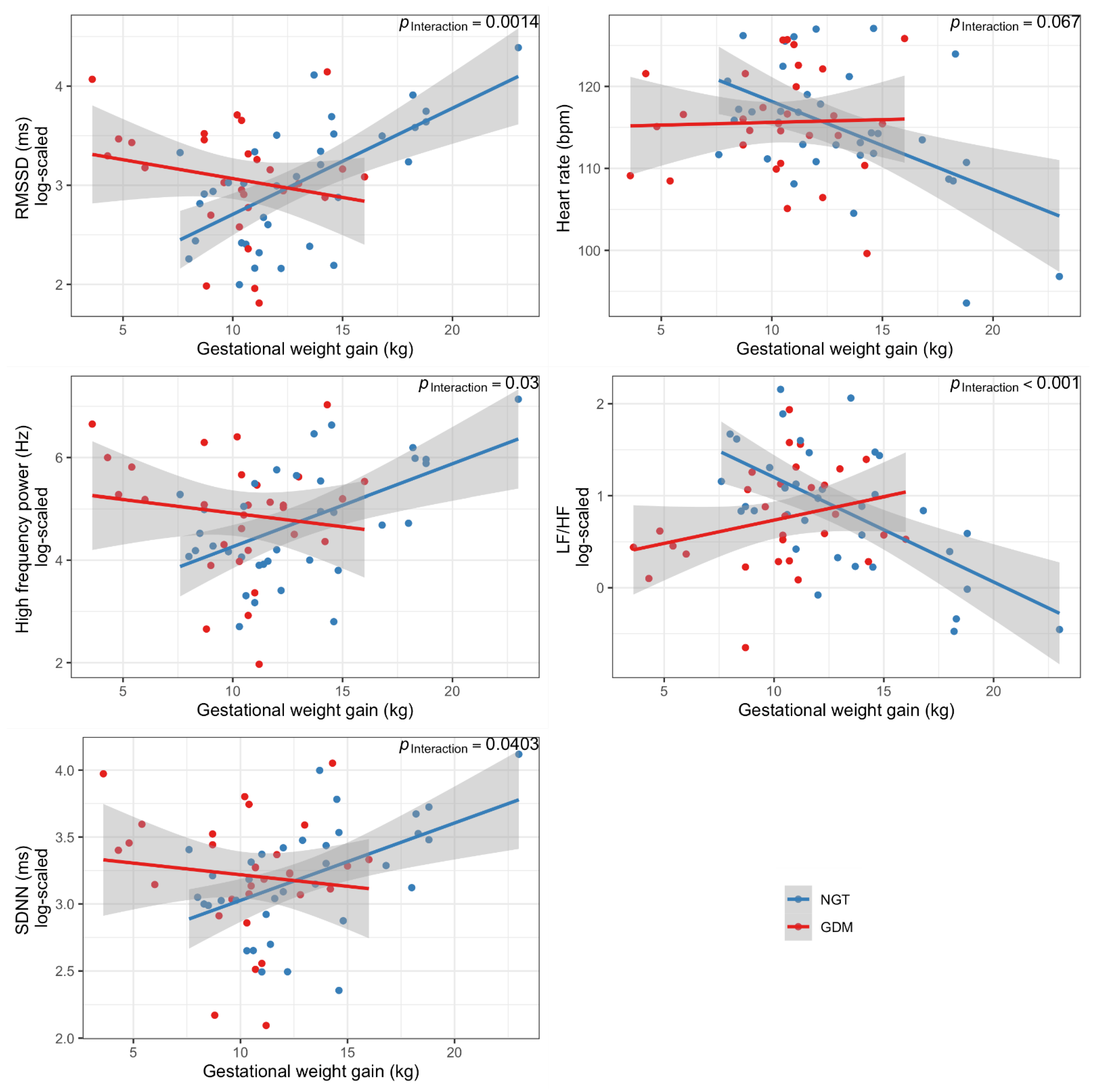
3.2. Maternal Gestational Weight Gain Is Associated with Offspring ANS Function
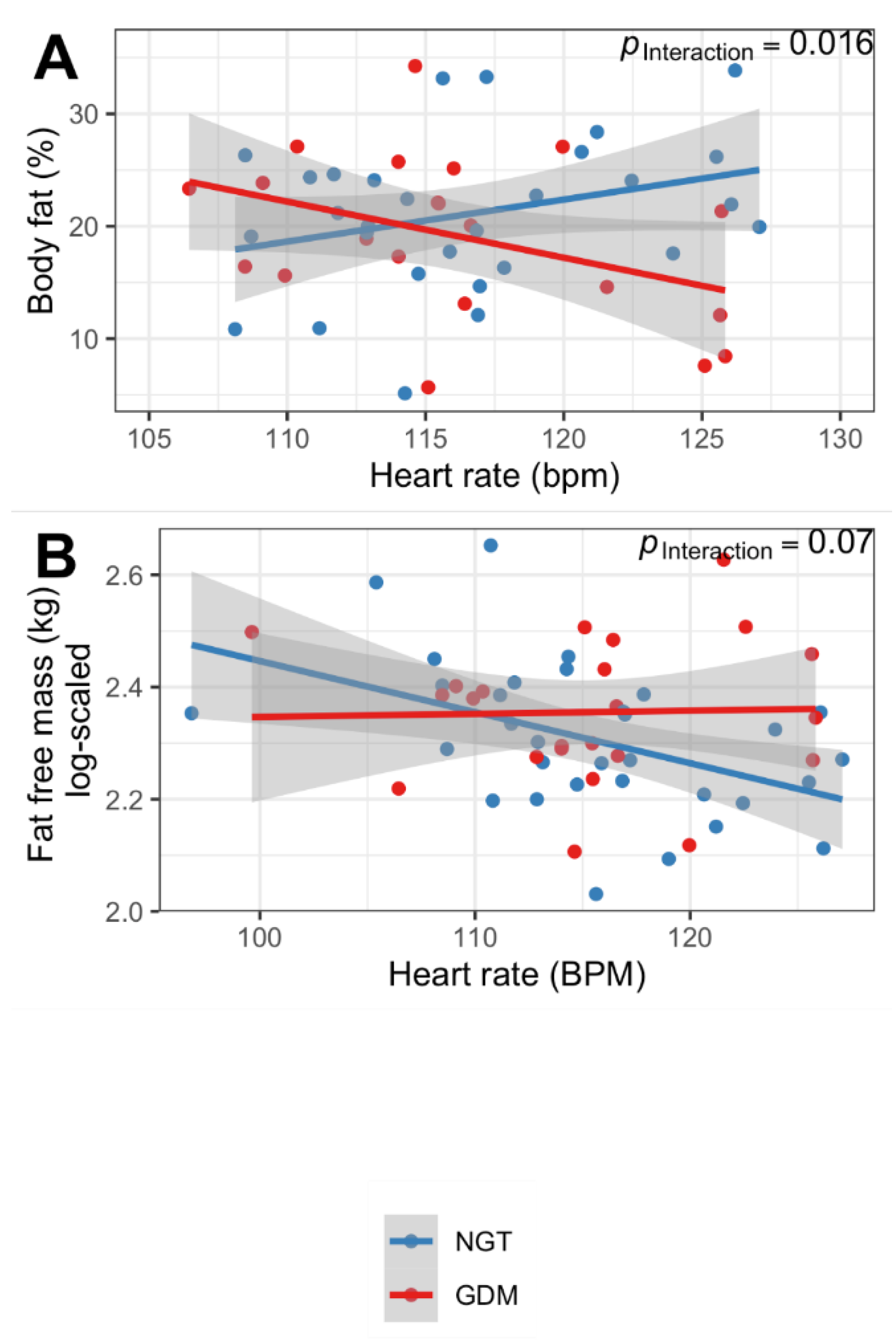
3.3. Offspring ANS Function Is Associated with Body Composition Only in Children from Mothers with Normoglycemic Pregnancy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Benichou, T.; Pereira, B.; Mermillod, M.; Tauveron, I.; Pfabigan, D.; Maqdasy, S.; Dutheil, F. Heart Rate Variability in Type 2 Diabetes Mellitus: A Systematic Review and Meta–Analysis. PLoS ONE 2018, 13, e0195166. [Google Scholar] [CrossRef]
- Porges, S.W.; Furman, S.A. The Early Development of the Autonomic Nervous System Provides a Neural Platform for Social Behaviour: A Polyvagal Perspective. Infant Child Dev. 2011, 20, 106–118. [Google Scholar] [CrossRef] [PubMed]
- Schneider, U.; Bode, F.; Schmidt, A.; Nowack, S.; Rudolph, A.; Doelcker, E.-M.; Schlattmann, P.; Götz, T.; Hoyer, D. Developmental Milestones of the Autonomic Nervous System Revealed via Longitudinal Monitoring of Fetal Heart Rate Variability. PLoS ONE 2018, 13, e0200799. [Google Scholar] [CrossRef]
- Longin, E.; Gerstner, T.; Schaible, T.; Lenz, T.; König, S. Maturation of the Autonomic Nervous System: Differences in Heart Rate Variability in Premature vs. Term Infants. J. Périnat. Med. 2006, 34, 303–308. [Google Scholar] [CrossRef]
- Hanson, M.A.; Gluckman, P.D. Early Developmental Conditioning of Later Health and Disease: Physiology or Pathophysiology? Physiol. Rev. 2014, 94, 1027–1076. [Google Scholar] [CrossRef]
- Barker, D.J.; Fall, C.H. Fetal and Infant Origins of Cardiovascular Disease. Arch. Dis. Child. 1993, 68, 797–799. [Google Scholar] [CrossRef]
- Kaseva, N.; Vääräsmäki, M.; Matinolli, H.-M.; Sipola-Leppänen, M.; Tikanmäki, M.; Heinonen, K.; Lano, A.; Wolke, D.; Andersson, S.; Järvelin, M.-R.; et al. Pre-Pregnancy Overweight or Obesity and Gestational Diabetes as Predictors of Body Composition in Offspring Twenty Years Later: Evidence from Two Birth Cohort Studies. Int. J. Obes. 2018, 42, 872–879. [Google Scholar] [CrossRef] [PubMed]
- Powe, C.E.; Huston Presley, L.P.; Locascio, J.J.; Catalano, P.M. Augmented Insulin Secretory Response in Early Pregnancy. Diabetologia 2019, 62, 1445–1452. [Google Scholar] [CrossRef]
- Silvetti, M.S.; Drago, F.; Ragonese, P. Heart Rate Variability in Healthy Children and Adolescents Is Partially Related to Age and Gender. Int. J. Cardiol. 2001, 81, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Birch, S.L.; Duncan, M.J.; Franklin, C. Overweight and Reduced Heart Rate Variability in British Children: An Exploratory Study. Prev. Med. 2012, 55, 430–432. [Google Scholar] [CrossRef]
- Husin, H.M.; Schleger, F.; Bauer, I.; Fehlert, E.; Kiefer-Schmidt, I.; Weiss, M.; Kagan, K.O.; Brucker, S.; Pauluschke-Fröhlich, J.; Eswaran, H.; et al. Maternal Weight, Weight Gain, and Metabolism Are Associated with Changes in Fetal Heart Rate and Variability. Obesity 2020, 28, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Christifano, D.N.; Taylor, M.K.; Carlson, S.E.; Colombo, J.; Gustafson, K.M. Higher Maternal Weight Is Related to Poorer Fetal Autonomic Function. J. Dev. Orig. Health Dis. 2021, 12, 354–356. [Google Scholar] [CrossRef] [PubMed]
- Fehlert, E.; Willmann, K.; Fritsche, L.; Linder, K.; Mat-Husin, H.; Schleger, F.; Weiss, M.; Kiefer-Schmidt, I.; Brucker, S.; Häring, H.-U.; et al. Gestational Diabetes Alters the Fetal Heart Rate Variability during an Oral Glucose Tolerance Test: A Fetal Magnetocardiography Study. BJOG Int. J. Obstet. Gynaecol. 2017, 124, 1891–1898. [Google Scholar] [CrossRef] [PubMed]
- Souza, L.V.; Oliveira, V.; Meneck, F.D.; Clemente, A.P.G.; Strufaldi, M.W.L.; Franco, M. do C. Birth Weight and Its Relationship with the Cardiac Autonomic Balance in Healthy Children. PLoS ONE 2017, 12, e0167328. [Google Scholar] [CrossRef] [PubMed]
- Fritsche, L.; Hummel, J.; Wagner, R.; Loeffler, D.; Hartkopf, J.; Machann, J.; Hilberath, J.; Kantartzis, K.; Jakubowski, P.; Pauluschke-Froehlich, J.; et al. The German Gestational Diabetes Study (PREG), a Prospective Multicentre Cohort Study:Rationale, Methodology and Design 2021. BMJ Open 2022, 12, e058268. [Google Scholar] [CrossRef] [PubMed]
- International Association of Diabetes and Pregnancy Study Groups Consensus Panel; Metzger, B.E.; Gabbe, S.G.; Persson, B.; Buchanan, T.A.; Catalano, P.A.; Damm, P.; Dyer, A.R.; de Leiva, A.; Hod, M.; et al. International Association of Diabetes and Pregnancy Study Groups Recommendations on the Diagnosis and Classification of Hyperglycemia in Pregnancy. Diabetes Care 2010, 33, 676–682. [Google Scholar] [CrossRef]
- Schäfer-Graf, U.; Laubner, K.; Hummel, S.; Gembruch, U.; Groten, T.; Kainer, F.; Grieshop, M.; Bancher-Todesca, D.; Cervar-Zivakovic, M.; Hösli, I.; et al. Gestationsdiabetes mellitus (GDM), Diagnostik, Therapie und Nachsorge: Praxisempfehlung—Kurzfassung der S3-Leitlinie (AWMF-Registernummer: 057-008). Diabetol. Stoffwechs. 2019, 14, S196–S206. [Google Scholar] [CrossRef]
- Goran, M.I.; Kaskoun, M.C.; Carpenter, W.H.; Poehlman, E.T.; Ravussin, E.; Fontvieille, A.M. Estimating Body Composition of Young Children by Using Bioelectrical Resistance. J. Appl. Physiol. 1993, 75, 1776–1780. [Google Scholar] [CrossRef]
- WHO Multicentre Growth Reference Study Group; WHO Multicentre Growth Reference Study Group. WHO Child Growth Standards: Length/Height-for-Age, Weight-for-Age, Weight-for-Length, Weight-for-Height and Body Mass Index-for-Age: Methods and Development; Geneva World Health Organization: Geneva, Switzerland, 2006; p. 312. [Google Scholar]
- Shaffer, F.; Ginsberg, J.P. An Overview of Heart Rate Variability Metrics and Norms. Front. Public Health 2017, 5, 258. [Google Scholar] [CrossRef]
- Institute of Medicine (US) and National Research Council (US) Committee to Reexamine IOM Pregnancy Weight Guidelines. Weight Gain during Pregnancy: Reexamining the Guidelines. In The National Academies Collection: Reports funded by National Institutes of Health; Rasmussen, K.M., Yaktine, A.L., Eds.; National Academies Press (US): Washington, DC, USA, 2009; ISBN 978-0-309-13113-1. [Google Scholar]
- Wagner, R.; Fritsche, L.; Heni, M.; Fehlert, E.; Stefan, N.; Staiger, H.; Häring, H.-U.; Fritsche, A. A Novel Insulin Sensitivity Index Particularly Suitable to Measure Insulin Sensitivity during Gestation. Acta Diabetol. 2016, 53, 1037–1044. [Google Scholar] [CrossRef]
- Baecke, J.A.; Burema, J.; Frijters, J.E. A Short Questionnaire for the Measurement of Habitual Physical Activity in Epidemiological Studies. Am. J. Clin. Nutr. 1982, 36, 936–942. [Google Scholar] [CrossRef] [PubMed]
- Kurtzhals, L.L.; Nørgaard, S.K.; Secher, A.L.; Nichum, V.L.; Ronneby, H.; Tabor, A.; McIntyre, H.D.; Damm, P.; Mathiesen, E.R. The Impact of Restricted Gestational Weight Gain by Dietary Intervention on Fetal Growth in Women with Gestational Diabetes Mellitus. Diabetologia 2018, 61, 2528–2538. [Google Scholar] [CrossRef]
- Andersson-Hall, U.K.; Järvinen, E.A.J.; Bosaeus, M.H.; Gustavsson, C.E.; Hårsmar, E.J.; Niklasson, C.A.; Albertsson-Wikland, K.G.; Holmäng, A.B. Maternal Obesity and Gestational Diabetes Mellitus Affect Body Composition through Infancy: The PONCH Study. Pediatr. Res. 2019, 85, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Kearney, M.; Perron, J.; Marc, I.; Weisnagel, S.J.; Tchernof, A.; Robitaille, J. Association of Prenatal Exposure to Gestational Diabetes with Offspring Body Composition and Regional Body Fat Distribution. Clin. Obes. 2018, 8, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Gillman, M.W.; Oakey, H.; Baghurst, P.A.; Volkmer, R.E.; Robinson, J.S.; Crowther, C.A. Effect of Treatment of Gestational Diabetes Mellitus on Obesity in the Next Generation. Diabetes Care 2010, 33, 964–968. [Google Scholar] [CrossRef]
- Landon, M.B.; Rice, M.M.; Varner, M.W.; Casey, B.M.; Reddy, U.M.; Wapner, R.J.; Rouse, D.J.; Biggio, J.R.; Thorp, J.M.; Chien, E.K.; et al. Mild Gestational Diabetes Mellitus and Long-Term Child Health. Diabetes Care 2015, 38, 445–452. [Google Scholar] [CrossRef]
- Crume, T.L.; Ogden, L.; Daniels, S.; Hamman, R.F.; Norris, J.M.; Dabelea, D. The Impact of In Utero Exposure to Diabetes on Childhood Body Mass Index Growth Trajectories: The EPOCH Study. J. Pediatr. 2011, 158, 941–946. [Google Scholar] [CrossRef]
- Krzeczkowski, J.E.; Boylan, K.; Arbuckle, T.E.; Muckle, G.; Poliakova, N.; Séguin, J.R.; Favotto, L.A.; Savoy, C.; Amani, B.; Mortaji, N.; et al. Maternal Pregnancy Diet Quality Is Directly Associated with Autonomic Nervous System Function in 6-Month-Old Offspring. J. Nutr. 2020, 150, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Torloni, M.R.; Betrán, A.P.; Horta, B.L.; Nakamura, M.U.; Atallah, A.N.; Moron, A.F.; Valente, O. Prepregnancy BMI and the Risk of Gestational Diabetes: A Systematic Review of the Literature with Meta-Analysis. Obes. Rev. 2009, 10, 194–203. [Google Scholar] [CrossRef]
- Koletzko, B.; Bauer, C.P.; Bung, P.; Cremer, M.; Flothkötter, M.; Hellmers, C.; Kersting, M.; Krawinkel, M.; Przyrembel, H.; Rasenack, R.; et al. German National Consensus Recommendations on Nutrition and Lifestyle in Pregnancy by the ‘Healthy Start—Young Family Network’. Ann. Nutr. Metab. 2013, 63, 311–322. [Google Scholar] [CrossRef]
- Groves, A.M.; Price, A.N.; Russell-Webster, T.; Jhaveri, S.; Yang, Y.; Battersby, E.E.; Shahid, S.; Vieira, M.C.; Hughes, E.; Miller, F.; et al. Impact of Maternal Obesity on Neonatal Heart Rate and Cardiac Size. Arch. Dis. Child. Fetal Neonatal Ed. 2021, 107, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Plaza-Florido, A.; Migueles, J.H.; Mora-Gonzalez, J.; Molina-Garcia, P.; Rodriguez-Ayllon, M.; Cadenas-Sanchez, C.; Esteban-Cornejo, I.; Navarrete, S.; Maria Lozano, R.; Michels, N.; et al. The Role of Heart Rate on the Associations Between Body Composition and Heart Rate Variability in Children With Overweight/Obesity: The ActiveBrains Project. Front. Physiol. 2019, 10, 895. [Google Scholar] [CrossRef]
- Martini, G.; Riva, P.; Rabbia, F.; Molini, V.; Ferrero, G.B.; Cerutti, F.; Carra, R.; Veglio, F. Heart Rate Variability in Childhood Obesity. Clin. Auton. Res. 2001, 11, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Harteveld, L.M.; Nederend, I.; ten Harkel, A.D.J.; Schutte, N.M.; de Rooij, S.R.; Vrijkotte, T.G.M.; Oldenhof, H.; Popma, A.; Jansen, L.M.C.; Suurland, J.; et al. Maturation of the Cardiac Autonomic Nervous System Activity in Children and Adolescents. J. Am. Heart Assoc. 2021, 10, e017405. [Google Scholar] [CrossRef]
- Stein, P.K.; Barzilay, J.I.; Domitrovich, P.P.; Chaves, P.M.; Gottdiener, J.S.; Heckbert, S.R.; Kronmal, R.A. The Relationship of Heart Rate and Heart Rate Variability to Non-Diabetic Fasting Glucose Levels and the Metabolic Syndrome: The Cardiovascular Health Study. Diabet. Med. 2007, 24, 855–863. [Google Scholar] [CrossRef] [PubMed]
- Vrijkotte, T.G.M.; van den Born, B.-J.H.; Hoekstra, C.M.C.A.; Gademan, M.G.J.; van Eijsden, M.; de Rooij, S.R.; Twickler, M.T.B. Cardiac Autonomic Nervous System Activation and Metabolic Profile in Young Children: The ABCD Study. PLoS ONE 2015, 10, e0138302. [Google Scholar] [CrossRef]
- HAPO Study Cooperative Research Group Hyperglycemia and Adverse Pregnancy Outcomes. N. Engl. J. Med. 2008, 358, 1991–2002. [CrossRef] [PubMed]
- Licht, C.M.M.; de Geus, E.J.C.; Penninx, B.W.J.H. Dysregulation of the Autonomic Nervous System Predicts the Development of the Metabolic Syndrome. J. Clin. Endocrinol. Metab. 2013, 98, 2484–2493. [Google Scholar] [CrossRef]
| Characteristics | Control (n = 37) | GDM (n = 30) | p | padjusted |
|---|---|---|---|---|
| Maternal age | 34.92 (3.75) | 35.33 (5.21) | 0.707 | - |
| Parity (%) Nulliparous Multiparous | 20 (54.1) 17 (45.9) | 23 (76.7) 7 (23.3) | 0.096 | - |
| Mode of delivery (%) Spontaneous C-section | 29 (78.4) 8 (21.6) | 13 (43.3) 17 (56.7) | 0.007 | - |
| Pre-gestational BMI (kg/m2) | 22.7 [20.7, 27.7] | 25.1 [22.6, 31.0] | 0.041 | - |
| Total gestational weight gain (kg) | 12.0 [10.5, 14.6] | 10.6 [8.9, 12.2] | 0.002 | - |
| Third trimester gestational weight gain (kg) | 4.9 [3.5, 6.7] | 3.8 [2.1, 4.9] | 0.021 | - |
| GWG category (%) Insufficient Appropriate Excessive | 11 (31.4) 13 (37.1) 11 (31.4) | 13 (43.3) 10 (33.3) 7 (23.3) | 0.586 | - |
| Educational level (%) A-level Secondary school diploma None | 29 (78.4) 8 (21.6) 0 (0) | 15 (50.0) 13 (43.3) 2 (6.7) | 0.021 | - |
| GDM Treatment (%) None Nutritional therapy Insulin therapy | - | 1 (3.3) 21 (70.0) 8 (26.7) | - | - |
| Gestational age at OGTT # | 27.4 (2.1) | 27 (1.6) | 0.508 | - |
| Insulin sensitivity (NEFA-ISI) # | 3.49 [3.11, 4.57] | 2.48 [2.11, 3.26] | 0.003 | - |
| Triglycerides (mg/dL) # | 178 (86) | 245 (104) | 0.013 | - |
| Habitual physical activity index # | 8.16 (1.51) | 7.38 (1.44) | 0.075 | - |
| Birth outcome | ||||
| Sex (%) Female Male | 20 (54.1) 17 (45.9) | 13 (43.3) 17 (56.7) | 0.464 | - |
| Gestational age at birth (weeks) | 39.37 (1.55) | 38.61 (1.99) | 0.086 | - |
| Birth weight (g) | 3360 [3100, 3610] | 3360 [2985, 3633] | 0.940 | 0.683 † |
| Birth length (cm) | 51 [49, 53] | 51 [49, 53] | 0.804 | 0.524 † |
| Macrosomia (%) yes no | 4 (10.8) 33 (89.2) | 1 (3.3) 29 (96.7) | 0.49 | - |
| Breastfeeding (%) yes no | 32 (86.7) 5 (13.5) | 26 (86.7) 4 (13.3) | 1.000 | - |
| Follow up | ||||
| Offspring age (months) | 25.19 (1.73) | 25.70 (1.64) | 0.223 | - |
| Weight (kg) | 12.54 (1.36) | 13.10 (1.80) | 0.149 | 0.43 ‡ |
| BMI (kg/m2) | 16.41 (1.11) | 16.11 (1.23) | 0.310 | 0.14 ‡ |
| Body fat (%) | 21.11 (6.8) | 19.13 (7.3) | 0.334 | 0.46 ‡ |
| BMI-for-age z-score | 0.42 (0.81) | 0.13 (0.97) | 0.179 | - |
| Heart Rate (BPM) | RMSSD (ms) | SDNN (ms) | Low Frequency (LF) Power (m2) | High Frequency (HF) Power (m2) | LF_n. u. | HF_n. u. | LF/HF | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B (95% CI) | p | B (95% CI) | p | B (95% CI) | p | B (95% CI) | p | B (95% CI) | p | B (95% CI) | p | B (95% CI) | p | B (95% CI) | p | |
| All children Interaction GWG x GDM status | 0.99 (−0.07, 2.05) | 0.0668 | −0.14 (−0.22, −0.05) | 0.0014 | −0.07 (−0.13, 0.00) | 0.0403 | −0.03 (−0.16, 0.09) | 0.6054 | −0.19 (−0.35, −0.02) | 0.0307 | 3.34 (1.62, 5.07) | 0.0003 | −3.30 (−5.00, −1.59) | 0.0003 | 0.15 (0.07, 0.24) | 0.0006 |
| Children whose mothers had NGT | −1.02 (−1.67, −0.37) | 0.0031 | 0.11 (0.06, 0.15) | 0.0001 | 0.06 (0.02, 0.09) | 0.0030 | 0.05 (−0.02, 0.11) | 0.1795 | 0.15 (0.06, 0.25) | 0.0019 | −2.39 (−3.43, −1.35) | 0.0001 | 2.34 (1.31, 3.37) | 0.0001 | −0.11 (−0.16, −0.06) | 0.0002 |
| Children whose mothers had GDM | 0.02 (−0.90, 0.94) | 0.9721 | −0.03 (−0.10, 0.05) | 0.4420 | −0.01 (−0.08, 0.05) | 0.6947 | 0.01 (−0.11, 0.13) | 0.8344 | −0.03 (−0.19, 0.13) | 0.7187 | 0.88 (−0.65, 2.41) | 0.2482 | −0.88 (−2.39, 0.64) | 0.2466 | 0.04 (−0.03, 0.11) | 0.2551 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fritsche, L.; Hartkopf, J.; Hummel, J.; Löffler, D.S.; Yamazaki, H.; Häring, H.-U.; Peter, A.; Birkenfeld, A.L.; Wagner, R.; Fritsche, A.; et al. Maternal Weight Gain during Pregnancy and the Developing Autonomic Nervous System—Possible Impact of GDM. Nutrients 2022, 14, 5220. https://doi.org/10.3390/nu14245220
Fritsche L, Hartkopf J, Hummel J, Löffler DS, Yamazaki H, Häring H-U, Peter A, Birkenfeld AL, Wagner R, Fritsche A, et al. Maternal Weight Gain during Pregnancy and the Developing Autonomic Nervous System—Possible Impact of GDM. Nutrients. 2022; 14(24):5220. https://doi.org/10.3390/nu14245220
Chicago/Turabian StyleFritsche, Louise, Julia Hartkopf, Julia Hummel, Dorina S. Löffler, Hajime Yamazaki, Hans-Ulrich Häring, Andreas Peter, Andreas L. Birkenfeld, Robert Wagner, Andreas Fritsche, and et al. 2022. "Maternal Weight Gain during Pregnancy and the Developing Autonomic Nervous System—Possible Impact of GDM" Nutrients 14, no. 24: 5220. https://doi.org/10.3390/nu14245220
APA StyleFritsche, L., Hartkopf, J., Hummel, J., Löffler, D. S., Yamazaki, H., Häring, H.-U., Peter, A., Birkenfeld, A. L., Wagner, R., Fritsche, A., Preissl, H., & Heni, M. (2022). Maternal Weight Gain during Pregnancy and the Developing Autonomic Nervous System—Possible Impact of GDM. Nutrients, 14(24), 5220. https://doi.org/10.3390/nu14245220







