Longitudinal Association of Telomere Dynamics with Obesity and Metabolic Disorders in Young Children
Abstract
1. Introduction
2. Methods
2.1. Patients
2.2. Clinical Examination
2.3. Assessment and Interventions
2.4. Assays and Scores
2.5. BMI Score and BMI Profiles
2.6. LTL Measurements by Southern Blot
2.7. Statistical Analyses
3. Results
3.1. Characteristics of the Participants and Classification According to BMI Profiles
3.2. LTL Dynamics in the Three BMI Profiles
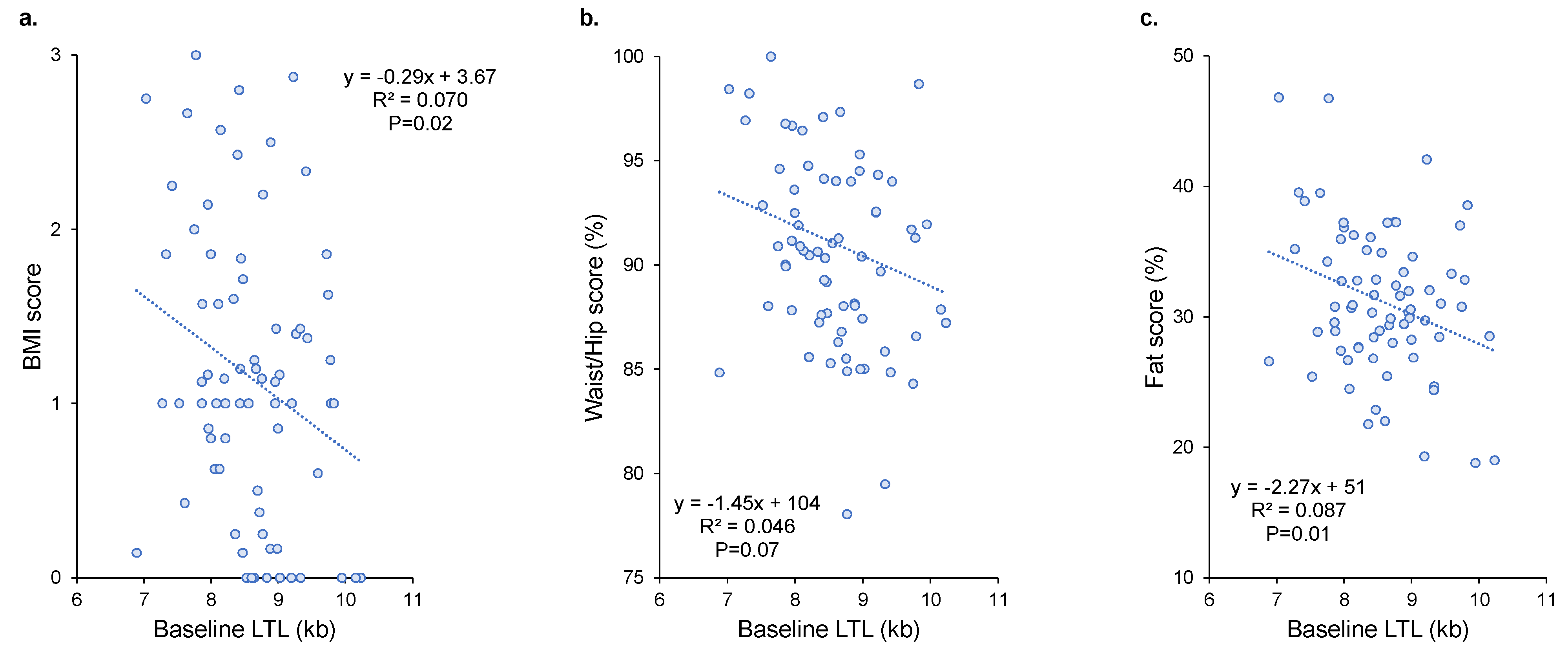
3.3. Association between LTL Dynamics and Obesity Indicators
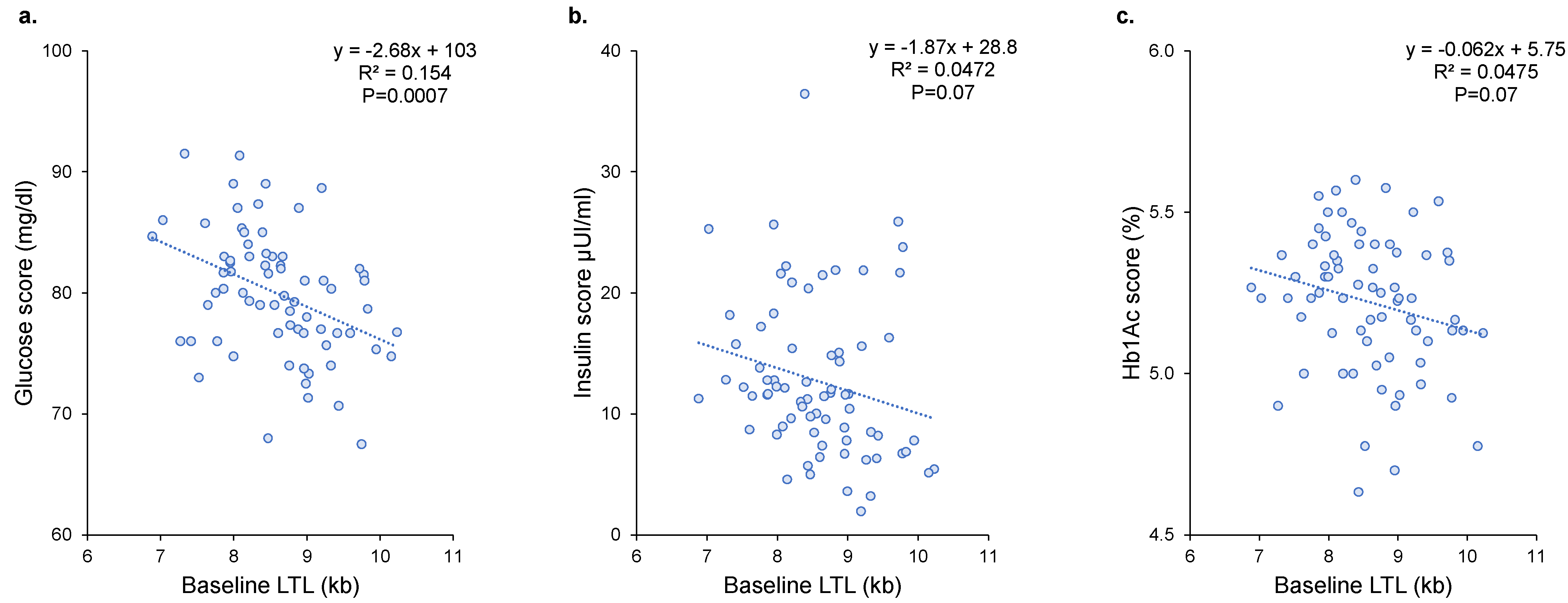
3.4. Association between LTL Dynamics and Metabolic Disorders
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The Hallmarks of Aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [PubMed]
- Rossiello, F.; Jurk, D.; Passos, J.F.; d’Adda di Fagagna, F. Telomere Dysfunction in Ageing and Age-Related Diseases. Nat. Cell Biol. 2022, 24, 135–147. [Google Scholar] [CrossRef]
- Wang, J.; Dong, X.; Cao, L.; Sun, Y.; Qiu, Y.; Zhang, Y.; Cao, R.; Covasa, M.; Zhong, L. Association between Telomere Length and Diabetes Mellitus: A Meta-Analysis. J. Int. Med. Res. 2016, 44, 1156–1173. [Google Scholar] [CrossRef]
- Gielen, M.; Hageman, G.J.; Antoniou, E.E.; Nordfjall, K.; Mangino, M.; Balasubramanyam, M.; de Meyer, T.; Hendricks, A.E.; Giltay, E.J.; Hunt, S.C.; et al. Body Mass Index Is Negatively Associated with Telomere Length: A Collaborative Cross-Sectional Meta-Analysis of 87 Observational Studies. Am. J. Clin. Nutr. 2018, 108, 453–475. [Google Scholar] [CrossRef]
- Weischer, M.; Bojesen, S.E.; Nordestgaard, B.G. Telomere Shortening Unrelated to Smoking, Body Weight, Physical Activity, and Alcohol Intake: 4,576 General Population Individuals with Repeat Measurements 10 Years Apart. PLoS Genet. 2014, 10, e1004191. [Google Scholar] [CrossRef]
- Verhulst, S.; Dalgård, C.; Labat, C.; Kark, J.D.; Kimura, M.; Christensen, K.; Toupance, S.; Aviv, A.; Kyvik, K.O.; Benetos, A. A Short Leucocyte Telomere Length Is Associated with Development of Insulin Resistance. Diabetologia 2016, 59, 1258–1265. [Google Scholar] [CrossRef]
- Haycock, P.C.; Burgess, S.; Nounu, A.; Zheng, J.; Okoli, G.N.; Bowden, J.; Wade, K.H.; Timpson, N.J.; Evans, D.M.; Willeit, P.; et al. Association Between Telomere Length and Risk of Cancer and Non-Neoplastic Diseases: A Mendelian Randomization Study. JAMA Oncol. 2017, 3, 636–651. [Google Scholar] [CrossRef]
- Zhan, Y.; Karlsson, I.K.; Karlsson, R.; Tillander, A.; Reynolds, C.A.; Pedersen, N.L.; Hägg, S. Exploring the Causal Pathway From Telomere Length to Coronary Heart Disease: A Network Mendelian Randomization Study. Circ. Res. 2017, 121, 214–219. [Google Scholar] [CrossRef]
- Bhupathiraju, S.N.; Hu, F.B. Epidemiology of Obesity and Diabetes and Their Cardiovascular Complications. Circ. Res. 2016, 118, 1723–1735. [Google Scholar] [CrossRef]
- Ng, A.C.T.; Delgado, V.; Borlaug, B.A.; Bax, J.J. Diabesity: The Combined Burden of Obesity and Diabetes on Heart Disease and the Role of Imaging. Nat. Rev. Cardiol. 2021, 18, 291–304. [Google Scholar] [CrossRef]
- Jebeile, H.; Kelly, A.S.; O’Malley, G.; Baur, L.A. Obesity in Children and Adolescents: Epidemiology, Causes, Assessment, and Management. Lancet Diabetes Endocrinol. 2022, 10, 351–365. [Google Scholar] [CrossRef]
- Clemente, D.B.P.; Maitre, L.; Bustamante, M.; Chatzi, L.; Roumeliotaki, T.; Fossati, S.; Grazuleviciene, R.; Gützkow, K.B.; Lepeule, J.; Martens, D.S.; et al. Obesity Is Associated with Shorter Telomeres in 8 Year-Old Children. Sci. Rep. 2019, 9, 18739. [Google Scholar] [CrossRef]
- Cole, T.J.; Lobstein, T. Extended International (IOTF) Body Mass Index Cut-Offs for Thinness, Overweight and Obesity. Pediatr. Obes. 2012, 7, 284–294. [Google Scholar] [CrossRef]
- World Health Organization. Waist Circumference and Waist-Hip Ratio: Report of a WHO Expert Consultation; World Health Organization: Geneva, Switzerland, 2008. [Google Scholar]
- Genitsaridi, S.-M.; Giannios, C.; Karampatsou, S.; Papageorgiou, I.; Papadopoulos, G.; Farakla, I.; Koui, E.; Georgiou, A.; Romas, S.; Terzioglou, E.; et al. A Comprehensive Multidisciplinary Management Plan Is Effective in Reducing the Prevalence of Overweight and Obesity in Childhood and Adolescence. Horm. Res. Paediatr. 2020, 93, 94–107. [Google Scholar] [CrossRef]
- Tragomalou, A.; Moschonis, G.; Kassari, P.; Papageorgiou, I.; Genitsaridi, S.-M.; Karampatsou, S.; Manios, Y.; Charmandari, E. A National E-Health Program for the Prevention and Management of Overweight and Obesity in Childhood and Adolescence in Greece. Nutrients 2020, 12, 2858. [Google Scholar] [CrossRef]
- Conway, J.M.; Ingwersen, L.A.; Moshfegh, A.J. Accuracy of Dietary Recall Using the USDA Five-Step Multiple-Pass Method in Men: An Observational Validation Study. J. Am. Diet. Assoc. 2004, 104, 595–603. [Google Scholar] [CrossRef]
- Tragomalou, A.; Moschonis, G.; Manios, Y.; Kassari, P.; Ioakimidis, I.; Diou, C.; Stefanopoulos, L.; Lekka, E.; Maglaveras, N.; Delopoulos, A.; et al. Novel E-Health Applications for the Management of Cardiometabolic Risk Factors in Children and Adolescents in Greece. Nutrients 2020, 12, 1380. [Google Scholar] [CrossRef]
- Toupance, S.; Simoncini, S.; Labat, C.; Dumoulin, C.; Lai, T.-P.; Lakomy, C.; Regnault, V.; Lacolley, P.; Dignat George, F.; Sabatier, F.; et al. Number and Replating Capacity of Endothelial Colony-Forming Cells Are Telomere Length Dependent: Implication for Human Atherogenesis. J. Am. Heart Assoc. 2021, 10, e020606. [Google Scholar] [CrossRef]
- Chen, S.; Yeh, F.; Lin, J.; Matsuguchi, T.; Blackburn, E.; Lee, E.T.; Howard, B.V.; Zhao, J. Short Leukocyte Telomere Length Is Associated with Obesity in American Indians: The Strong Heart Family Study. Aging 2014, 6, 380–389. [Google Scholar] [CrossRef]
- Müezzinler, A.; Zaineddin, A.K.; Brenner, H. Body Mass Index and Leukocyte Telomere Length in Adults: A Systematic Review and Meta-Analysis. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2014, 15, 192–201. [Google Scholar] [CrossRef]
- Rode, L.; Nordestgaard, B.G.; Weischer, M.; Bojesen, S.E. Increased Body Mass Index, Elevated C-Reactive Protein, and Short Telomere Length. J. Clin. Endocrinol. Metab. 2014, 99, E1671–E1675. [Google Scholar] [CrossRef] [PubMed]
- Mundstock, E.; Sarria, E.E.; Zatti, H.; Mattos Louzada, F.; Kich Grun, L.; Herbert Jones, M.; Guma, F.T.C.R.; Mazzola In Memoriam, J.; Epifanio, M.; Stein, R.T.; et al. Effect of Obesity on Telomere Length: Systematic Review and Meta-Analysis. Obesity 2015, 23, 2165–2174. [Google Scholar] [CrossRef] [PubMed]
- D’Mello, M.J.J.; Ross, S.A.; Briel, M.; Anand, S.S.; Gerstein, H.; Paré, G. Association between Shortened Leukocyte Telomere Length and Cardiometabolic Outcomes: Systematic Review and Meta-Analysis. Circ. Cardiovasc. Genet. 2015, 8, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Carroll, L.; Joglekar, M.V.; Januszewski, A.S.; Wong, K.K.; Hardikar, A.A.; Jenkins, A.J.; Ma, R.C.W. Diabetes, Metabolic Disease, and Telomere Length. Lancet Diabetes Endocrinol. 2021, 9, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Babizhayev, M.A.; Savel’yeva, E.L.; Moskvina, S.N.; Yegorov, Y.E. Telomere Length Is a Biomarker of Cumulative Oxidative Stress, Biologic Age, and an Independent Predictor of Survival and Therapeutic Treatment Requirement Associated with Smoking Behavior. Am. J. Ther. 2011, 18, e209–e226. [Google Scholar] [CrossRef]
- Gavia-García, G.; Rosado-Pérez, J.; Arista-Ugalde, T.L.; Aguiñiga-Sánchez, I.; Santiago-Osorio, E.; Mendoza-Núñez, V.M. Telomere Length and Oxidative Stress and Its Relation with Metabolic Syndrome Components in the Aging. Biology 2021, 10, 253. [Google Scholar] [CrossRef]
- Azcona-Sanjulian, M.C. Telomere Length and Pediatric Obesity: A Review. Genes 2021, 12, 946. [Google Scholar] [CrossRef]
- Müezzinler, A.; Mons, U.; Dieffenbach, A.K.; Butterbach, K.; Saum, K.-U.; Schick, M.; Stammer, H.; Boukamp, P.; Holleczek, B.; Stegmaier, C.; et al. Body Mass Index and Leukocyte Telomere Length Dynamics among Older Adults: Results from the ESTHER Cohort. Exp. Gerontol. 2016, 74, 1–8. [Google Scholar] [CrossRef]
- Himbert, C.; Thompson, H.; Ulrich, C.M. Effects of Intentional Weight Loss on Markers of Oxidative Stress, DNA Repair and Telomere Length—A Systematic Review. Obes. Facts 2017, 10, 648–665. [Google Scholar] [CrossRef]
- Bethancourt, H.J.; Kratz, M.; Beresford, S.A.A.; Hayes, M.G.; Kuzawa, C.W.; Duazo, P.L.; Borja, J.B.; Eisenberg, D.T.A. No Association between Blood Telomere Length and Longitudinally Assessed Diet or Adiposity in a Young Adult Filipino Population. Eur. J. Nutr. 2017, 56, 295–308. [Google Scholar] [CrossRef]
- Pérez, L.M.; Amaral, M.A.; Mundstock, E.; Barbé-Tuana, F.M.; Guma, F.T.C.R.; Jones, M.H.; Machado, D.C.; Sarria, E.E.; Marques, E.; Marques, M.; et al. Effects of Diet on Telomere Length: Systematic Review and Meta-Analysis. Public Health Genom. 2017, 20, 286–292. [Google Scholar] [CrossRef]
- De Meyer, T.; Bekaert, S.; De Buyzere, M.L.; De Bacquer, D.D.; Langlois, M.R.; Shivappa, N.; Hébert, J.R.; Gillebert, T.C.; Rietzschel, E.R.; Huybrechts, I. Leukocyte Telomere Length and Diet in the Apparently Healthy, Middle-Aged Asklepios Population. Sci. Rep. 2018, 8, 6540. [Google Scholar] [CrossRef]
- Milte, C.M.; Russell, A.P.; Ball, K.; Crawford, D.; Salmon, J.; McNaughton, S.A. Diet Quality and Telomere Length in Older Australian Men and Women. Eur. J. Nutr. 2018, 57, 363–372. [Google Scholar] [CrossRef]
- Meinilä, J.; Perälä, M.-M.; Kautiainen, H.; Männistö, S.; Kanerva, N.; Shivappa, N.; Hébert, J.R.; Iozzo, P.; Guzzardi, M.A.; Eriksson, J.G. Healthy Diets and Telomere Length and Attrition during a 10-Year Follow-Up. Eur. J. Clin. Nutr. 2019, 73, 1352–1360. [Google Scholar] [CrossRef]
- Galiè, S.; Canudas, S.; Muralidharan, J.; García-Gavilán, J.; Bulló, M.; Salas-Salvadó, J. Impact of Nutrition on Telomere Health: Systematic Review of Observational Cohort Studies and Randomized Clinical Trials. Adv. Nutr. Bethesda Md. 2020, 11, 576–601. [Google Scholar] [CrossRef]
- Lamprokostopoulou, A.; Moschonis, G.; Manios, Y.; Critselis, E.; Nicolaides, N.C.; Stefa, A.; Koniari, E.; Gagos, S.; Charmandari, E. Childhood Obesity and Leucocyte Telomere Length. Eur. J. Clin. Investig. 2019, 49, e13178. [Google Scholar] [CrossRef]
- Todendi, P.F.; Martínez, J.A.; Reuter, C.P.; Matos, W.L.; Franke, S.I.R.; Razquin, C.; Milagro, F.I.; Kahl, V.F.S.; Fiegenbaum, M.; de Moura Valim, A.R. Biochemical Profile, Eating Habits, and Telomere Length among Brazilian Children and Adolescents. Nutrion 2020, 71, 110645. [Google Scholar] [CrossRef]
- Baskind, M.J.; Hawkins, J.; Heyman, M.B.; Wojcicki, J.M. Obesity at Age 6 Months Is Associated with Shorter Preschool Leukocyte Telomere Length Independent of Parental Telomere Length. J. Pediatr. 2021, 233, 141–149. [Google Scholar] [CrossRef]
- García-Calzón, S.; Gea, A.; Razquin, C.; Corella, D.; Lamuela-Raventós, R.M.; Martínez, J.A.; Martínez-González, M.A.; Zalba, G.; Marti, A. Longitudinal Association of Telomere Length and Obesity Indices in an Intervention Study with a Mediterranean Diet: The PREDIMED-NAVARRA Trial. Int. J. Obes. 2014, 38, 177–182. [Google Scholar] [CrossRef]
- Njajou, O.T.; Cawthon, R.M.; Blackburn, E.H.; Harris, T.B.; Li, R.; Sanders, J.L.; Newman, A.B.; Nalls, M.; Cummings, S.R.; Hsueh, W.-C. Shorter Telomeres Are Associated with Obesity and Weight Gain in the Elderly. Int. J. Obes. 2012, 36, 1176–1179. [Google Scholar] [CrossRef]
- You, N.-C.Y.; Chen, B.H.; Song, Y.; Lu, X.; Chen, Y.; Manson, J.E.; Kang, M.; Howard, B.V.; Margolis, K.L.; Curb, J.D.; et al. A Prospective Study of Leukocyte Telomere Length and Risk of Type 2 Diabetes in Postmenopausal Women. Diabetes 2012, 61, 2998–3004. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Han, L.; Chang, D.; Ye, Y.; Shen, J.; Daniel, C.R.; Gu, J.; Chow, W.-H.; Wu, X. Social-Demographics, Health Behaviors, and Telomere Length in the Mexican American Mano a Mano Cohort. Oncotarget 2017, 8, 96553–96567. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Luk, A.O.; Shi, M.; Huang, C.; Jiang, G.; Yang, A.; Wu, H.; Lim, C.K.P.; Tam, C.H.T.; Fan, B.; et al. Shortened Leukocyte Telomere Length Is Associated With Glycemic Progression in Type 2 Diabetes: A Prospective and Mendelian Randomization Analysis. Diabetes Care 2022, 45, 701–709. [Google Scholar] [CrossRef] [PubMed]
- Morell-Azanza, L.; Ojeda-Rodríguez, A.; Azcona-SanJulián, M.C.; Zalba, G.; Marti, A. Associations of Telomere Length with Anthropometric and Glucose Changes after a Lifestyle Intervention in Abdominal Obese Children. Nutr. Metab. Cardiovasc. Dis. NMCD 2020, 30, 694–700. [Google Scholar] [CrossRef] [PubMed]
- García-Calzón, S.; Moleres, A.; Marcos, A.; Campoy, C.; Moreno, L.A.; Azcona-Sanjulián, M.C.; Martínez-González, M.A.; Martínez, J.A.; Zalba, G.; Marti, A.; et al. Telomere Length as a Biomarker for Adiposity Changes after a Multidisciplinary Intervention in Overweight/Obese Adolescents: The EVASYON Study. PLoS ONE 2014, 9, e89828. [Google Scholar] [CrossRef]
- Kjaer, T.W.; Faurholt-Jepsen, D.; Mehta, K.M.; Christensen, V.B.; Epel, E.; Lin, J.; Blackburn, E.; Wojcicki, J.M. Shorter Preschool, Leukocyte Telomere Length Is Associated with Obesity at Age 9 in Latino Children. Clin. Obes. 2018, 8, 88–94. [Google Scholar] [CrossRef]
- Michels, N.; van Aart, C.J.C.; Martens, D.S.; De Henauw, S.; Nawrot, T.S. Telomere Length and Cardiovascular Disease Precursors: A 7-Year Follow-up from Childhood to Early Adolescence. Eur. J. Prev. Cardiol. 2022, 29, e22–e24. [Google Scholar] [CrossRef]
- Wojcicki, J.M.; Lustig, R.H.; Jacobs, L.M.; Mason, A.E.; Hartman, A.; Leung, C.; Stanhope, K.; Lin, J.; Schmidt, L.A.; Epel, E.S. Longer Leukocyte Telomere Length Predicts Stronger Response to a Workplace Sugar-Sweetened Beverage Sales Ban: An Exploratory Study. Curr. Dev. Nutr. 2021, 5, nzab084. [Google Scholar] [CrossRef]
- Elks, C.E.; Scott, R.A. The Long and Short of Telomere Length and Diabetes. Diabetes 2014, 63, 65–67. [Google Scholar] [CrossRef]
- Burton, D.G.A.; Faragher, R.G.A. Obesity and Type-2 Diabetes as Inducers of Premature Cellular Senescence and Ageing. Biogerontology 2018, 19, 447–459. [Google Scholar] [CrossRef]
- Jacome Burbano, M.S.; Gilson, E. Long-Lived Post-Mitotic Cell Aging: Is a Telomere Clock at Play? Mech. Ageing Dev. 2020, 189, 111256. [Google Scholar] [CrossRef]
- Entringer, S.; de Punder, K.; Buss, C.; Wadhwa, P.D. The Fetal Programming of Telomere Biology Hypothesis: An Update. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2018, 373, 20170151. [Google Scholar] [CrossRef]
- Lazarides, C.; Epel, E.S.; Lin, J.; Blackburn, E.H.; Voelkle, M.C.; Buss, C.; Simhan, H.N.; Wadhwa, P.D.; Entringer, S. Maternal Pro-Inflammatory State during Pregnancy and Newborn Leukocyte Telomere Length: A Prospective Investigation. Brain. Behav. Immun. 2019, 80, 419–426. [Google Scholar] [CrossRef]
- Wei, H.; Zhang, C.; Silveyra, P. The Relationships Between Prenatal Smoking Exposure and Telomere Lengths in Fetuses, Infants, and Children: A Systematic Literature Review. J. Addict. Nurs. 2020, 31, 243–252. [Google Scholar] [CrossRef]
- Martens, D.S.; Plusquin, M.; Gyselaers, W.; De Vivo, I.; Nawrot, T.S. Maternal Pre-Pregnancy Body Mass Index and Newborn Telomere Length. BMC Med. 2016, 14, 148. [Google Scholar] [CrossRef]
- Wei, B.; Shao, Y.; Liang, J.; Tang, P.; Mo, M.; Liu, B.; Huang, H.; Tan, H.J.J.; Huang, D.; Liu, S.; et al. Maternal Overweight but Not Paternal Overweight before Pregnancy Is Associated with Shorter Newborn Telomere Length: Evidence from Guangxi Zhuang Birth Cohort in China. BMC Pregnancy Childbirth 2021, 21, 283. [Google Scholar] [CrossRef]
- Xu, J.; Ye, J.; Wu, Y.; Zhang, H.; Luo, Q.; Han, C.; Ye, X.; Wang, H.; He, J.; Huang, H.; et al. Reduced Fetal Telomere Length in Gestational Diabetes. PLoS ONE 2014, 9, e86161. [Google Scholar] [CrossRef]
- Garcia-Martin, I.; Penketh, R.J.A.; Janssen, A.B.; Jones, R.E.; Grimstead, J.; Baird, D.M.; John, R.M. Metformin and Insulin Treatment Prevent Placental Telomere Attrition in Boys Exposed to Maternal Diabetes. PLoS ONE 2018, 13, e0208533. [Google Scholar] [CrossRef]
- Hjort, L.; Vryer, R.; Grunnet, L.G.; Burgner, D.; Olsen, S.F.; Saffery, R.; Vaag, A. Telomere Length Is Reduced in 9- to 16-Year-Old Girls Exposed to Gestational Diabetes in Utero. Diabetologia 2018, 61, 870–880. [Google Scholar] [CrossRef]
- McAninch, D.; Bianco-Miotto, T.; Gatford, K.L.; Leemaqz, S.Y.; Andraweera, P.H.; Garrett, A.; Plummer, M.D.; Dekker, G.A.; Roberts, C.T.; Smithers, L.G.; et al. The Metabolic Syndrome in Pregnancy and Its Association with Child Telomere Length. Diabetologia 2020, 63, 2140–2149. [Google Scholar] [CrossRef]


| All | Profile 1 | Profile 2 | Profile 3 | One Way ANOVA p | Trend p | |
|---|---|---|---|---|---|---|
| Number | 73 | 12 | 48 | 13 | ||
| Females (%) | 66% | 58% | 75% | 38% # | 0.04 | 0.26 |
| Age at baseline (years) | 7.6 ± 2.0 | 7.1 ± 2.2 | 7.7 ± 1.9 | 7.8 ± 2.0 | 0.64 | 0.38 |
| Age at follow-up (years) | 10.3 ± 2.1 | 9.9 ± 2.3 | 10.4 ± 2.1 | 10.5 ± 1.9 | 0.74 | 0.47 |
| Follow-up (years) | 2.8 ± 0.7 | 2.8 ± 0.7 | 2.8 ± 0.8 | 2.7 ± 0.5 | 0.91 | 0.75 |
| Baseline LTL (kb) | 8.58 ± 0.75 | 9.06 ± 0.74 | 8.46 ± 0.72 * | 8.61 ± 0.71 | 0.04 | 0.13 |
| Follow-up LTL (kb) | 8.40 ± 0.78 | 8.85 ± 0.72 | 8.29 ± 0.78 | 8.41 ± 0.78 | 0.08 | 0.16 |
| LTL attrition (bp/year) | 67 ± 69 | 83 ± 56 | 63 ± 70 | 69 ± 80 | 0.67 | 0.60 |
| Baseline BMIc | 1.70 ± 1.13 | 0.00 | 2.13 ± 0.89 | 1.69 ± 0.95 | - | - |
| Baseline glucose (mg/dl) | 78 ± 9 | 76 ± 7 | 78 ± 9 | 78 ± 13 | 0.78 | 0.63 |
| Baseline insulin (µUI/mL) | 10.1 ± 5.6 | 6.5 ± 2.8 | 11.1 ± 5.6 * | 10.2 ± 6.5 | 0.049 | 0.09 |
| Baseline HbA1c (%) | 5.22 ± 0.24 | 5.09 ± 0.34 | 5.24 ± 0.23 | 5.28 ± 0.16 | 0.14 | 0.06 |
| Baseline waist (cm) | 72 ± 11 | 58 ± 8 | 74 ± 9 *** | 79 ± 9 *** | <0.0001 | <0.0001 |
| Baseline hip (cm) | 78 ± 12 | 65 ± 10 | 79 ± 11 *** | 83 ± 10 *** | <0.0001 | <0.0001 |
| Baseline waist/hip (%) | 93 ± 8 | 90 ± 5 | 93 ± 9 | 94 ± 7 | 0.24 | 0.17 |
| Baseline fat percentage (%) | 31.5 ± 6.6 | 24.3 ± 4.6 | 33.3 ± 5.2 *** | 32.9 ± 8.2 ** | 0.0003 | 0.002 |
| Mother diabetes mellitus during pregnancy | 23% | 10% | 27% | 20% | 0.51 | 0.60 |
| Dependent Variable | Independent Variable | Regression Coefficient ± Standard Error | R2 | p |
|---|---|---|---|---|
| BMI score | LTL baseline (1 kb) | −0.29 ± 0.13 | 7.0% | 0.02 |
| Age (1 year) | - | - | 0.18 | |
| Female (yes) | - | - | 0.26 | |
| Model | 7.0% | |||
| Waist/hip score (%) | LTL baseline (1 kb) | −2.22 ± 0.77 | 9.8% | 0.005 |
| Age (1 year) | −0.99 ± 0.29 | 13.8% | 0.001 | |
| Female (yes) | - | - | 0.13 | |
| Model | 18.3% | |||
| Fat score (%) | LTL baseline (1 kb) | −2.60 ± 0.87 | 11.1% | 0.004 |
| Age (1 year) | - | - | 0.62 | |
| Female (yes) | 3.01 ± 1.34 | 6.2% | 0.03 | |
| Model | 14.9% |
| Dependent Variable | Independent Variable | Regression Coefficient ± Standard Error | R2 | p |
|---|---|---|---|---|
| Glucose score | LTL baseline (1 kb) | −2.68 ± 0.76 | 15.4% | 0.0007 |
| Age (1 year) | - | - | 0.62 | |
| Female (yes) | - | - | 0.71 | |
| Model | 15.4% | |||
| Insulin score | LTL baseline (1 kb) | - | - | 0.11 |
| Age (1 year) | 1.17 ± 0.36 | 13.0% | 0.002 | |
| Female (yes) | 3.16 ± 1.48 | 5.4% | 0.04 | |
| Model | 17.9% | |||
| Hb1Ac score | LTL baseline (1 kb) | −0.061 ± 0.033 | 4.7% | 0.07 |
| Age (1 year) | - | - | 0.81 | |
| Female (yes) | - | - | 0.59 | |
| Model | 4.7% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toupance, S.; Karampatsou, S.I.; Labat, C.; Genitsaridi, S.-M.; Tragomalou, A.; Kassari, P.; Soulis, G.; Hollander, A.; Charmandari, E.; Benetos, A. Longitudinal Association of Telomere Dynamics with Obesity and Metabolic Disorders in Young Children. Nutrients 2022, 14, 5191. https://doi.org/10.3390/nu14235191
Toupance S, Karampatsou SI, Labat C, Genitsaridi S-M, Tragomalou A, Kassari P, Soulis G, Hollander A, Charmandari E, Benetos A. Longitudinal Association of Telomere Dynamics with Obesity and Metabolic Disorders in Young Children. Nutrients. 2022; 14(23):5191. https://doi.org/10.3390/nu14235191
Chicago/Turabian StyleToupance, Simon, Sofia I. Karampatsou, Carlos Labat, Sofia-Maria Genitsaridi, Athanasia Tragomalou, Penio Kassari, George Soulis, Allyson Hollander, Evangelia Charmandari, and Athanase Benetos. 2022. "Longitudinal Association of Telomere Dynamics with Obesity and Metabolic Disorders in Young Children" Nutrients 14, no. 23: 5191. https://doi.org/10.3390/nu14235191
APA StyleToupance, S., Karampatsou, S. I., Labat, C., Genitsaridi, S.-M., Tragomalou, A., Kassari, P., Soulis, G., Hollander, A., Charmandari, E., & Benetos, A. (2022). Longitudinal Association of Telomere Dynamics with Obesity and Metabolic Disorders in Young Children. Nutrients, 14(23), 5191. https://doi.org/10.3390/nu14235191








