The Effect of a Comprehensive Life-Style Intervention Program of Diet and Exercise on Four Bone-Derived Proteins, FGF-23, Osteopontin, NGAL and Sclerostin, in Overweight or Obese Children and Adolescents
Abstract
:1. Introduction
2. Materials and Methods
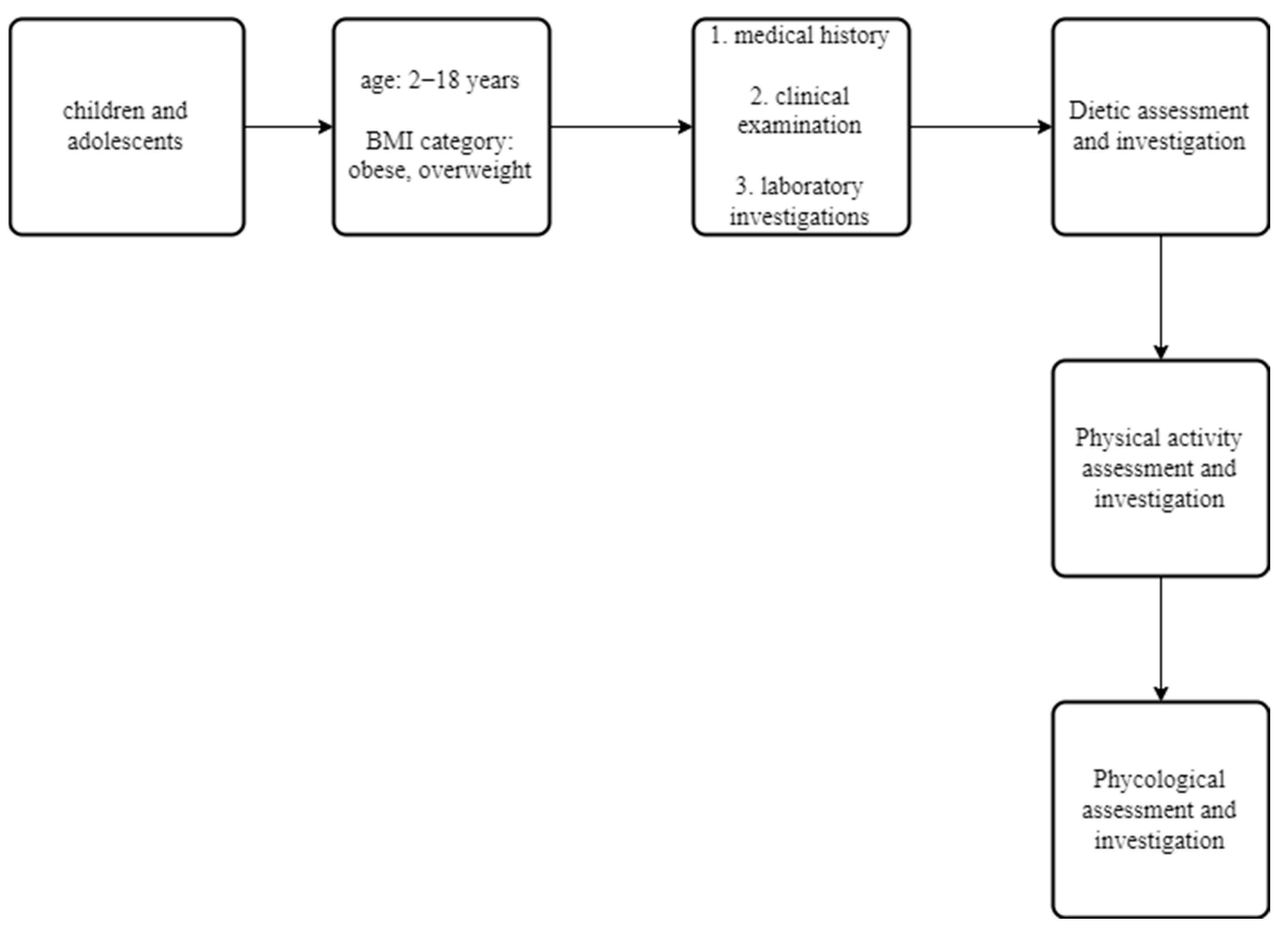
2.1. Patients
2.2. Methods
2.3. Assessment and Interventions
2.4. Assays
2.5. Statistical Analysis
3. Results
3.1. Clinical Characteristics, and Biochemical, Endocrinologic and Body Composition Parameters of All Subjects at Initial and Annual Assessment
3.2. Bone-Derived Proteins and Adipokines at Initial and Annual Assessment
3.3. Correlation Coefficient Analysis in Obese, Overweight and All Subjects
3.4. Multivariate Linear Regression Analysis of Anthropometric Parameters, Body Composition Parameters, Metabolic Syndrome Parameters, Glucose Metabolism Parameters, Adiposity Parameters and Bone Metabolism Parameters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saponaro, C.; Gaggini, M.; Carli, F.; Gastaldelli, A. The Subtle Balance between Lipolysis and Lipogenesis: A Critical Point in Metabolic Homeostasis. Nutrients 2015, 7, 9453–9474. [Google Scholar] [CrossRef]
- Greco, E.A.; Lenzi, A.; Migliaccio, S. The Obesity of Bone. Ther. Adv. Endocrinol. Metab. 2015, 6, 273–286. [Google Scholar] [CrossRef] [PubMed]
- Rutkowski, J.M.; Pastor, J.; Sun, K.; Park, S.C.; Alexandru, I.; Chen, C.T.; Moe, O.W.; Scherer, P.E. Adiponectin Alters Renal Calcium and Phosphate Excretion through Regulation of Klotho Expression. Kidney Int. 2018, 91, 324–337. [Google Scholar] [CrossRef] [PubMed]
- Bennett, J.H.; Joyner, C.J.; Triffitt, J.T.; Owen, M.E. Adipocytic Cells Cultured from Marrow Have Osteogenic Potential. J. Cell Sci. 1991, 99, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Aline, M.; Valentin, D.; Quarles, L.D. Regulation And Function Of The FGF23/Klotho Endocrine Pathways. Physiol. Rev. 2012, 23, 131–155. [Google Scholar] [CrossRef]
- Rodelo-Haad, C.; Santamaria, R.; Muñoz-Castañeda, J.R.; Pendón-Ruiz De Mier, M.V.; Martin-Malo, A.; Rodriguez, M. FGF23, Biomarker or Target? Toxins 2019, 11, 175. [Google Scholar] [CrossRef] [PubMed]
- Franzen, A.; Heinegard, D. Isolation and Characterization of Two Sialoproteins Present Only in Bone Calcified Matrix. Biochem. J. 1985, 232, 715–724. [Google Scholar] [CrossRef]
- Bailey, S.; Karsenty, G.; Gundberg, C.; Vashishth, D. Osteocalcin and Osteopontin Influence Bone Morphology and Mechanical Properties. Ann. N. Y. Acad. Sci. 2017, 1409, 79–84. [Google Scholar] [CrossRef]
- Saki, F.; Sheikhi, A.; Omrani, G.H.R.; Karimi, H.; Dabbaghmanesh, M.H.; Mousavinasab, S.N. Evaluation of Bone Mineral Density in Children with Type i Diabetes Mellitus and Relationship to Serum Levels of Osteopontin. Prog. Drug Res. 2017, 67, 527–533. [Google Scholar] [CrossRef]
- Filardi, T.; Carnevale, V.; Massoud, R.; Russo, C.; Nieddu, L.; Tavaglione, F.; Turinese, I.; Lenzi, A.; Romagnoli, E.; Morano, S. High Serum Osteopontin Levels Are Associated with Prevalent Fractures and Worse Lipid Profile in Post-Menopausal Women with Type 2 Diabetes. J. Endocrinol. Investig. 2019, 42, 295–301. [Google Scholar] [CrossRef] [Green Version]
- Min, S.; Shi, T.; Han, X.; Chen, D.; Xu, Z.; Shi, D.; Teng, H.; Jiang, Q. Serum Levels of Leptin, Osteopontin, and Sclerostin in Patients with and without Knee Osteoarthritis. Clin. Rheumatol. 2020, 40, 287–294. [Google Scholar] [CrossRef]
- Han, X.; Wang, W.; He, J.; Jiang, L.; Li, X. Osteopontin as a Biomarker for Osteosarcoma Therapy and Prognosis (Review). Oncol. Lett. 2019, 17, 2592–2598. [Google Scholar] [CrossRef]
- Popovics, P.; Jain, A.; Skalitzky, K.O.; Schroeder, E.; Ruetten, H.; Cadena, M.; Uchtmann, K.S.; Vezina, C.M.; Ricke, W.A. Osteopontin Deficiency Ameliorates Prostatic Fibrosis and Inflammation. Int. J. Mol. Sci. 2021, 22, 12461. [Google Scholar] [CrossRef]
- Tardelli, M.; Zeyda, K.; Moreno-Viedma, V.; Wanko, B.; Grün, N.G.; Staffler, G.; Zeyda, M.; Stulnig, T.M. Osteopontin Is a Key Player for Local Adipose Tissue Macrophage Proliferation in Obesity. Mol. Metab. 2016, 5, 1131–1137. [Google Scholar] [CrossRef]
- Qi, L.; Kash, J.C.; Dugan, V.G.; Jagger, B.W.; Lau, Y.; Crouch, E.C.; Hartshorn, K.L.; Taubenberger, J.K. An Osteopontin-Integrin Interaction Plays a Critical Role in Directing Adipogenesis and Osteogenesis by Mesenchymal Stem Cells. Stem. Cells 2012, 412, 426–434. [Google Scholar] [CrossRef]
- Kuchay, M.S.; Choudhary, N.S.; Mishra, S.K. Pathophysiological Mechanisms Underlying MAFLD. Diabetes Metab. Syndr. 2020, 14, 1875–1887. [Google Scholar] [CrossRef]
- Wu, J.; Wu, D.; Zhang, L.; Lin, C.; Liao, J.; Xie, R.; Li, Z.; Wu, S.; Liu, A.; Hu, W.; et al. NK Cells Induce Hepatic ER Stress to Promote Insulin Resistance in Obesity through Osteopontin Production. J. Leukoc. Biol. 2020, 107, 589–596. [Google Scholar] [CrossRef]
- Kjeldsen, L.; Johnsen, A.H.; Sengelov, H.; Borregaard, N. Isolation and Primary Structure of NGAL, a Novel Protein Associated with Human Neutrophil Gelatinase. J. Biol. Chem. 1993, 268, 10425–10432. [Google Scholar] [CrossRef]
- Jaberi, S.A.; Cohen, A.; D’Souza, C.; Abdulrazzaq, Y.M.; Ojha, S.; Bastaki, S.; Adeghate, E.A. Lipocalin-2: Structure, Function, Distribution and Role in Metabolic Disorders. Biomed. Pharmacother. 2021, 142, 112002. [Google Scholar] [CrossRef]
- Mosialou, I.; Shikhel, S.; Liu, J.M.; Maurizi, A.; Luo, N.; He, Z.; Huang, Y.; Zong, H.; Friedman, R.A.; Barasch, J.; et al. MC4R-Dependent Suppression of Appetite by Bone-Derived Lipocalin 2. Nature 2017, 543, 385–390. [Google Scholar] [CrossRef]
- Han, Y.; You, X.; Xing, W.; Zhang, Z.; Zou, W. Paracrine and Endocrine Actions of Bone—The Functions of Secretory Proteins from Osteoblasts, Osteocytes, and Osteoclasts. Bone Res. 2018, 6, 16. [Google Scholar] [CrossRef]
- Na, G.Y.; Yoon, S.R.; An, J.; Yeo, R.; Song, J.; Jo, M.N.; Han, S.; Kim, O.Y. The Relationship between Circulating Neutrophil Gelatinase-Associated Lipocalin and Early Alteration of Metabolic Parameteres Is Associated with Dietary Saturated Fat Intake in Non—Diabetic Korean Women. Endocr. J. 2017, 64, 303–314. [Google Scholar] [CrossRef]
- Luo, Y.; Ma, X.; Pan, X.; Xu, Y.; Xiong, Q.; Xiao, Y.; Bao, Y.; Jia, W. Serum Lipocalin-2 Levels Are Positively Associated with Not Only Total Body Fat but Also Visceral Fat Area in Chinese Men. Medicine 2016, 95, e4039. [Google Scholar] [CrossRef]
- Gayret, Ö.B.; Taşdemir, M.; Erol, M.; Nacaroğlu, H.T.; Zengi, O.; Yiğit, Ö. Are There Any New Reliable Markers to Detect Renal Injury in Obese Children? Ren. Fail 2018, 40, 416–422. [Google Scholar] [CrossRef]
- Brunkow, M.E.; Gardner, J.C.; Ness, J.; van Paeper, B.W.; Kovacevich, B.R.; Proll, S.; Skonier, J.E.; Zhao, L.; Sabo, P.J.; Fu, Y.-H.; et al. Bone Dysplasia Sclerosteosis Results from Loss of the SOST Gene Product, a Novel Cystine Knot-Containing Protein. Am. J. Hum. Genet 2001, 68, 577–589. [Google Scholar] [CrossRef]
- Moester, M.J.C.; Papapoulos, S.E.; Löwik, C.W.G.M.; Van Bezooijen, R.L. Sclerostin: Current knowledge and future perspectives. Calcif. Tissue Int. 2010, 87, 99–107. [Google Scholar] [CrossRef]
- Franco, C.N.; Noe, M.M.; Albrecht, L.V. Metabolism and Endocrine Disorders: What Wnt Wrong? Front. Endocrinol. 2022, 13, 887037. [Google Scholar] [CrossRef]
- Ominsky, M.S.; Li, C.; Li, X.; Tan, H.L.; Lee, E.; Barrero, M.; Asuncion, F.J.; Dwyer, D.; Han, C.Y.; Vlasseros, F.; et al. Inhibition of Sclerostin by Monoclonal Antibody Enhances Bone Healing and Improves Bone Density and Strength of Nonfractured Bones. J. Bone Miner. Res. 2011, 26, 1012–1021. [Google Scholar] [CrossRef]
- Colaianni, G.; Brunetti, G.; Faienza, M.F.; Colucci, S.; Grano, M. Osteoporosis and Obesity: Role of Wnt Pathway in Human and Murine Models. World J. Orthop. 2014, 5, 242–246. [Google Scholar] [CrossRef]
- Muschitz, C.; Kocijan, R.; Marterer, C.; Nia, A.R.; Muschitz, G.K.; Resch, H.; Pietschmann, P. Sclerostin Levels and Changes in Bone Metabolism after Bariatric Surgery. J. Clin. Endocrinol. Metab. 2015, 100, 891–901. [Google Scholar] [CrossRef]
- Armamento-Villareal, R.; Sadler, C.; Napoli, N.; Shah, K.; Chode, S.; Sinacore, D.R.; Qualls, C.; Villareal, D.T. Weight Loss in Obese Older Adults Increases Serum Sclerostin and Impairs Hip Geometry but both Are Prevented by Exercise Training. J. Bone Miner. Res. 2012, 27, 1215–1221. [Google Scholar] [CrossRef] [PubMed]
- del Toro, R.; Cavallari, I.; Tramontana, F.; Park, K.; Strollo, R.; Valente, L.; de Pascalis, M.; Grigioni, F.; Pozzilli, P.; Buzzetti, R.; et al. Association of Bone Biomarkers with Advanced Atherosclerotic Disease in People with Overweight/Obesity. Endocrine 2021, 73, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Carbone, F.; Migliola, E.N.; Bonaventura, A.; Vecchié, A.; de Vuono, S.; Ricci, M.A.; Vaudo, G.; Boni, M.; Ministrini, S.; Lupattelli, G.; et al. Circulating Levels of Sclerostin Predict Glycemic Improvement after Sleeve Gastrectomy. Nutrients 2021, 13, 623. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Choi, Y.J.; Ahn, M.B.; Cho, W.K.; Cho, K.S.; Jung, M.H.; Suh, B.K. Associations between Sclerostin and Anthropometric and Metabolic Parameters in Children and Adolescents. Children 2021, 8, 788. [Google Scholar] [CrossRef]
- Cole, T.J.; Lobstein, T. Extended International (IOTF) Body Mass Index Cut-Offs for Thinness, Overweight and Obesity. Pediatr. Obes. 2012, 7, 284–294. [Google Scholar] [CrossRef]
- Karampatsou, S.I.; Genitsaridi, S.M.; Michos, A.; Kourkouni, E.; Kourlaba, G.; Kassari, P.; Manios, Y.; Charmandari, E. The Effect of a Life-Style Intervention Program of Diet and Exercise on Irisin and Fgf-21 Concentrations in Children and Adolescents with Overweight and Obesity. Nutrients 2021, 13, 1274. [Google Scholar] [CrossRef]
- World Health Organisation (WHO). Waist Circumference and Waist–Hip Ratio: Report of a WHO Expert Consultation. Geneva, 8–11 December 2008. World Health Organ. 2008, No. December, 1–39. Available online: https://www.who.int/publications/i/item/9789241501491 (accessed on 16 June 2022).
- Genitsaridi, S.M.; Giannios, C.; Karampatsou, S.; Papageorgiou, I.; Papadopoulos, G.; Farakla, I.; Koui, E.; Georgiou, A.; Romas, S.; Terzioglou, E.; et al. A Comprehensive Multidisciplinary Management Plan Is Effective in Reducing the Prevalence of Overweight and Obesity in Childhood and Adolescence. Horm. Res. Paediatr. 2020, 93, 94–107. [Google Scholar] [CrossRef]
- Conway, J.M.; Ingwersen, L.A.; Moshfegh, A.J. Accuracy of dietary recall using the USDA fivestep multiple-pass method in men: An observational validation study. J. Am. Diet Assoc. 2004, 104, 595–603. [Google Scholar] [CrossRef]
- USDA; CNPP. My Plate Background. 2011. Available online: https://www.myplate.gov (accessed on 25 August 2022).
- Schwartz, G.J.; Work, D.F. Measurement and Estimation of GFR in Children and Adolescents. Clin. J. Am. Soc. Nephrol. 2009, 4, 1832–1843. [Google Scholar] [CrossRef]
- Alberti, G.; Zimmet, P.; Kaufman, F.; Tajima, N.; Silink, M.; Arslanian, S.; Wong, G.; Bennett, P.; Shaw, J.; Caprio, S. The metabolic syndrome in children andadolescents—An IDF consensus report. Pediatr. Diabetes 2007, 8, 299–306. [Google Scholar] [CrossRef]
- Dai, C.; Wang, C.; Xia, F.; Liu, Z.; Mo, Y.; Shan, X.; Zhou, Y. Age and Gender-Specific Reference Intervals for Uric Acid Level in Children Aged 5–14 Years in Southeast Zhejiang Province of China: Hyperuricemia in Children May Need Redefinition. Front. Pediatr. 2021, 9, 788–790. [Google Scholar] [CrossRef]
- Tsimikas, S. A Test in Context: Lipoprotein (a) Diagnosis, Prognosis, Controversies, and Emerging Therapies. J. Am. Coll. Cardiol. 2017, 69, 692–711. [Google Scholar] [CrossRef]
- Xu, Y.; Ma, X.; Pan, X.; He, X.; Xiao, Y.; Bao, Y. Correlations between Serum Concentration of Three Bone-Derived Factors and Obesity and Visceral Fat Accumulation in a Cohort of Middle Aged Men and Women. Cardiovasc. Diabetol. 2018, 17, 143. [Google Scholar] [CrossRef]
- Zaheer, S.; de Boer, I.H.; Allison, M.; Brown, J.M.; Psaty, B.M.; Robinson-cohen, C.; Michos, E.D.; Ix, J.H.; Kestenbaum, B.; Siscovick, D.; et al. Fibroblast Growth Factor 23, Mineral Metabolism, and Adiposity in Normal Kidney Function. J. Clin. Endocrinol. Metab. 2017, 102, 1387–1395. [Google Scholar] [CrossRef]
- Mirza, M.A.I.; Alsio, J.; Hammarstedt, A.; Erben, R.G.; Michae, K.; Tivesten, Å.; Mellstro, D.; Marsell, R.; Orwoll, E.; Karlsson, M.K.; et al. Circulating Fibroblast Growth Factor-23 Is Associated with Fat Mass and Dyslipidemia in Two Independent Cohorts of Elderly Individuals. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 219–227. [Google Scholar] [CrossRef]
- Falkner, B.; Keith, S.W.; Gidding, S.S.; Langman, C.B. Fibroblast Growth Factor-23 Is Independently Associated with Cardiac Mass in African American Adolescent Males. J. Am. Soc. Hypertens. 2017, 11, 480–487. [Google Scholar] [CrossRef]
- Hu, X.; Ma, X.; Luo, Y.; Xu, Y.; Xiong, Q.; Pan, X.; Xiao, Y.; Bao, Y.; Jia, W. Associations of Serum Fibroblast Growth Factor 23 Levels with Obesity and Visceral Fat Accumulation. Clin. Nutr. 2018, 37, 223–228. [Google Scholar] [CrossRef]
- Ali, F.N.; Falkner, B.; Gidding, S.S.; Price, H.E.; Keith, S.W.; Langman, C.B. Fibroblast Growth Factor-23 in Obese, Normotensive Adolescents Is Associated with Adverse Cardiac Structure. J. Pediatr. 2015, 165, 738–743. [Google Scholar] [CrossRef]
- Tsuji, K.; Maeda, T.; Kawane, T.; Matsunuma, A.; Horiuchi, N. Leptin stimulates fibroblast growth factor 23 expression in bone and suppresses renal 1α,25-dihydroxyvitamin D3 synthesis in leptin-deficient mice. J. Bone Miner. Res. 2010, 25, 1711–1723. [Google Scholar] [CrossRef]
- Bar, L.; Feger, M.; Fajor, A.; Klotz, L.; Zeng, S.; Lang, F.; Hocher, B.; Foller, M. Insulin suppresses the production of fibroblast growth factor 23 (FGF23). Proc. Natl. Acad. Sci. USA 2018, 115, 5804–5809. [Google Scholar] [CrossRef] [Green Version]
- Kutluturk, Y.; Akinci, A.; Ozerol, I.H.; Yologlu, S. The Relationship between Serum FGF-23 Concentration and Insulin Resistance, Prediabetes and Dyslipidemia in Obese Children and Adolescents. J. Pediatr. Endocrinol. Metab. 2019, 32, 707–714. [Google Scholar] [CrossRef]
- Hanks, L.J.; Casazza, K.; Judd, S.E.; Jenny, N.S.; Gutiérrez, O.M. Associations of Fibroblast Growth Factor-23 with Markers of Inflammation, Insulin Resistance and Obesity in Adults. PLoS ONE 2015, 10, e0122885. [Google Scholar] [CrossRef]
- Aztatzi-Aguilar, O.G.; Sierra-Vargas, M.P.; Ortega-Romero, M.; Jimenez-Corona, A.E. Osteopontin’s Relationship with Malnutrition and Oxidative Stress in Adolescents. A Pilot Study. PLoS ONE 2021, 16, e0249057. [Google Scholar] [CrossRef]
- Schreier, M.; Schwartze, J.T.; Landgraf, K.; Scheuermann, K.; Erbs, S.; Herberth, G.; Pospisilik, J.A.; Kratzsch, J.; Kiess, W.; Körner, A. Osteopontin Is BMI-Independently Related to Early Endothelial Dysfunction in Children. J. Clin. Endocrinol. Metab. 2016, 101, 4161–4169. [Google Scholar] [CrossRef] [PubMed]
- Weghuber, D.; Mangge, H.; Hochbrugger, E.; Stulnig, T.M. Impact of Age and Metabolic Syndrome on the Adipokine Profile in Childhood and Adult Obesity. Exp. Clin. Endocrinol. Diabetes 2014, 122, 363–367. [Google Scholar] [CrossRef] [PubMed]
- Shinoda, Y.; Yamaguchi, M.; Ogata, N.; Akune, T.; Kubota, N.; Yamauchi, T.; Terauchi, Y.; Kadowaki, T.; Takeuchi, Y.; Fukumoto, S.; et al. Regulation of Bone Formation by Adiponectin through Autocrine/Paracrine and Endocrine Pathways. J. Cell Biochem. 2006, 99, 196–208. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Ambrosi, J.; Catalán, V.; Ramírez, B.; Rodríguez, A.; Colina, I.; Silva, C.; Rotellar, F.; Mugueta, C.; Gil, M.J.; Cienfuegos, J.A.; et al. Plasma Osteopontin Levels and Expression in Adipose Tissue Are Increased in Obesity. J. Clin. Endocrinol. Metab. 2007, 92, 3719–3727. [Google Scholar] [CrossRef]
- Qian, J.; Xu, L.; Sun, X.; Wang, Y.; Xuan, W.; Zhang, Q.; Zhao, P.; Wu, Q.; Liu, R.; Che, N.; et al. Adiponectin Aggravates Bone Erosion by Promoting Osteopontin Production in Synovial Tissue of Rheumatoid Arthritis. Arthritis Res. Ther. 2018, 20, 26. [Google Scholar] [CrossRef]
- Kurata, M.; Okura, T.; Irita, J.; Enomoto, D.; Nagao, T.; Jotoku, M.; Miyoshi, K.I.; Higaki, J. The Relationship between Osteopontin and Adiponectin in Patients with Essential Hypertension. Clin. Exp. Hypertens. A 2010, 32, 358–363. [Google Scholar] [CrossRef]
- Albassam, R.S.; Sabico, S.; Alnaami, A.M.; Khattak, M.N.K.; Lei, K.Y.; Al-Daghri, N.M.; Reginster, J.Y.; Alokail, M.S. Bone Metabolism Markers Are Associated with Neck Circumference in Adult Arab Women. Osteoporos. Int. 2019, 30, 845–852. [Google Scholar] [CrossRef]
- Kiefer, F.W.; Zeyda, M.; Gollinger, K.; Pfau, B.; Neuhofer, A.; Weichhart, T.; Säemann, M.D.; Geyeregger, R.; Schlederer, M.; Kenner, L.; et al. Neutralization of Osteopontin Inhibits Obesity-Induced Inflammation and Insulin Resistance. Diabetes 2010, 59, 935–946. [Google Scholar] [CrossRef]
- Kiefer, F.W.; Neschen, S.; Pfau, B.; Legerer, B.; Neuhofer, A.; Kahle, M.; De Angelis, M.H.; Schlederer, M.; Mair, M.; Kenner, L.; et al. Osteopontin Deficiency Protects against Obesity-Induced Hepatic Steatosis and Attenuates Glucose Production in Mice. Diabetologia 2011, 54, 2132–2142. [Google Scholar] [CrossRef]
- Hamilcıkan, S.; Erol, M.; Gayret, O.B.; Buke, O.; Can, E. The Association of Serum Osteopontin Levels with Insulin Resistance in Obese, Dyslipidemic Children. Iran. J. Pediatr. 2017, 27, e7483. [Google Scholar] [CrossRef]
- Daniele, G.; Winnier, D.; Mari, A.; Bruder, J.; Fourcaudot, M.; Pengou, Z.; Hansis-Diarte, A.; Jenkinson, C.; Tripathy, D.; Folli, F. The Potential Role of the Osteopontin–Osteocalcin–Osteoprotegerin Triad in the Pathogenesis of Prediabetes in Humans. Acta Diabetol. 2018, 55, 139–148. [Google Scholar] [CrossRef]
- Yan, Q.; Yang, Q.; Mody, N.; Graham, T.; Hsu, C.; Houstis, N.; Kahn, B.; Xu, Z.; Rosen, E. The adipokine lipocalin 2 is regulated by obesity and promotes insulin resistance. Diabetes 2007, 56, 2533–2540. [Google Scholar] [CrossRef]
- Wu, C.; Wang, Q.; Lv, C.; Qin, N.; Lei, S.; Yuan, Q.; Wang, G. The Changes of Serum SKlotho and NGAL Levels and Their Correlation in Type 2 Diabetes Mellitus Patients with Different Stages of Urinary Albumin. Diabetes Res. Clin. Pract. 2014, 106, 343–350. [Google Scholar] [CrossRef]
- Polidori, N.; Giannini, C.; Salvatore, R.; Pelliccia, P.; Parisi, A.; Chiarelli, F.; Mohn, A. Role of Urinary NGAL and KIM-1 as Biomarkers of Early Kidney Injury in Obese Prepubertal Children. J. Pediatr. Endocrinol. Metab. 2020, 33, 1183–1189. [Google Scholar] [CrossRef]
- Akelma, A.Z.; Abaci, A.; Ozdemir, O.; Celik, A.; Avci, Z.; Razi, C.H.; Hizli, S.; Akin, O. The Association of Serum Lipocalin-2 Levels with Metabolic and Clinical Parameters in Obese Children: A Pilot Study. J. Pediatr. Endocrinol. Metab. 2012, 25, 525–528. [Google Scholar] [CrossRef]
- Morell-Azanza, L.; Ojeda-Rodríguez, A.; Giuranna, J.; Azcona-Sanjulián, M.C.; Hebebrand, J.; Marti, A.; Hinney, A. Melanocortin-4 Receptor and Lipocalin 2 Gene Variants in Spanish Children with Abdominal Obesity: Effects on BMI-SDS after a Lifestyle Intervention. Nutrients 2019, 11, 960. [Google Scholar] [CrossRef]
- Huang, Y.; Yang, Z.; Ye, Z.; Li, Q.; Wen, J.; Tao, X.; Chen, L.; He, M.; Wang, X.; Lu, B.; et al. Lipocalin-2, Glucose Metabolism and Chronic Low-Grade Systemic Inflammation in Chinese People. Cardiovasc. Diabetol. 2012, 11, 11. [Google Scholar] [CrossRef] [Green Version]
- Jin, D.; Guo, H.; Bu, S.Y.; Zhang, Y.; Hannaford, J.; Mashek, D.G.; Chen, X. Lipocalin 2 Is a Selective Modulator of Peroxisome Proliferator-activated Receptor-γ Activation and Function in Lipid Homeostasis and Energy Expenditure. FASEB J. 2011, 25, 754–764. [Google Scholar] [CrossRef]
- Şen, S.; Kızılay, D.Ö.; Taneli, F.; Özen, Ç.; Ertan, P.; Özunan, İ.; Yıldız, R.; Ersoy, B. Urinary NGAL Is a Potential Biomarker for Early Renal Injury in Insulin Resistant Obese Non-Diabetic Children. J. Clin. Res. Pediatr. Endocrinol. 2021, 13, 400–407. [Google Scholar] [CrossRef]
- Thrailkill, K.M.; Moreau, C.S.; Cockrell, G.E.; Jo, C.H.; Bunn, R.C.; Morales-Pozzo, A.E.; Lumpkin, C.K.; Fowlkes, J.L. Disease and Gender-Specific Dysregulation of NGAL and MMP-9 in Type 1 Diabetes Mellitus. Endocrine 2010, 37, 336–343. [Google Scholar] [CrossRef]
- Azzam, E.Z.; Ata, M.N.; Younan, D.N.; Salem, T.M.; Abdul-Aziz, A.A. DObesity: Relationship between Vitamin D Deficiency, Obesity and Sclerostin as a Novel Biomarker of Bone Metabolism. J. Clin. Transl. Endocrinol. 2019, 17, 100197. [Google Scholar] [CrossRef]
- Radetti, G.; Franceschi, R.; Adami, S.; Longhi, S.; Rossini, M.; Gatti, D. Higher Circulating Parathormone Is Associated with Smaller and Weaker Bones in Obese Children. Calcif. Tissue Int. 2014, 95, 1–7. [Google Scholar] [CrossRef]
- Wędrychowicz, A.; Sztefko, K.; Starzyk, J.B. Sclerostin and Its Association with Insulin Resistance in Children and Adolescents. Bone 2019, 120, 232–238. [Google Scholar] [CrossRef]
- Catalano, A.; Pintaudi, N.; Morabito, N.; Di Vieste, G.; Guina, L.; Bruno, M.L.; Cucinotta, D.; Di Benedetto, A. Gender differences in sclerostin and clinical characteristics in type 1 diabetes mellitus. Eur. J. Endocrinol. 2014, 171, 293–300. [Google Scholar] [CrossRef]
- Lombardi, G.; Galeazzi, I.O.; Mariotti, M.; Banfi, G. Sclerostin concentrations in athletes: Role of load and gender. J. Biol. Regul. Homeost. Agents 2012, 26, 157–163. Available online: https://www.researchgate.net/publication/223957015 (accessed on 26 August 2022).
- Jastrzebski, S.; Kalinowski, J.; Stolina, M.; Mirza, F.; Torreggiani, E.; Kalajzic, I.; Won, H.; Lee, S.; Lorenzo, J. Changes in bone sclerostin levels in mice after ovariectomy vary independently of changes in serum sclerostin levels. J. Bone Miner. Res. 2013, 28, 618–626. [Google Scholar] [CrossRef] [Green Version]
| Initial Assessment | Annual Assessment | |
|---|---|---|
| Gender | ||
| Male | 172 (49.9%) | |
| Female | 173 (50.1%) | |
| Pubertal status | ||
| Prepubertal | 181 (52.5%) | 115 (33.3%) |
| Pubertal | 164 (47.5%) | 230 (66.7%) |
| BMI category | ||
| Morbidly obese | 89 (25.8%) | 60 (17.4%) |
| Obese | 131 (38%) | 79 (22.9%) |
| Overweight | 125 (36.2%) | 153 (44.3%) |
| Normal BMI | - | 53 (15.4%) |
| Obese (n = 220) | Overweight (n = 125) | p | |
|---|---|---|---|
| Age (years) | 10.26 ± 0.21 | 10.53 ± 0.24 | NS |
| Height (cm) | 145.24 ± 1.30 | 145.56 ± 1.41 | NS |
| BW (kg) | 64.53 ± 1.68 | 52.82 ± 1.38 | <0.01 |
| BMI (kg/m2) | 28.71 ± 0.32 | 24.25 ± 0.20 | <0.01 |
| BMI z-score | 3.17 ± 0.07 | 1.55 ± 0.02 | <0.01 |
| TMI (kg/m3) | 19.91 ± 0.23 | 16.74 ± 0.10 | <0.01 |
| Waist circumference (cm) | 87.58 ± 1.05 | 78.03 ± 1.03 | <0.01 |
| Hip circumference (cm) | 93.20 ± 1.14 | 84.98 ± 1.09 | <0.01 |
| WHR | 0.95 ± 0.01 | 0.92 ± 0.01 | NS |
| WHtR | 0.60 ± 0.01 | 0.54 ± 0.01 | <0.01 |
| SBP (mmHg) | 112.67 ± 1.33 | 109.02 ± 1.05 | NS |
| DBP (mmHg) | 65.35 ± 1.08 | 60.76 ± 0.97 | <0.01 |
| (A) | |||
|---|---|---|---|
| Initial Assessment | Annual Assessment | p-Value | |
| Age (years) | 10.36 ± 0.16 | 11.46 ± 0.16 | <0.01 |
| BW (kg) | 60.27 ± 1.22 | 62.18 ± 1.18 | <0.01 |
| Height (cm) | 145.36 ± 0.97 | 151.43 ± 0.98 | <0.01 |
| BMI (kg/m2) | 27.09 ± 0.24 | 25.84 ± 0.24 | <0.01 |
| BMI z-score | 2.58 ± 0.06 | 2.02 ± 0.06 | <0.01 |
| TMI (kg/m2) | 18.76 ± 0.17 | 17.24 ± 0.21 | <0.01 |
| SBP (mmHg) | 111.29 ± 0.92 | 113.10 ± 0.88 | NS |
| DBP (mmHg) | 63.62 ± 0.78 | 67.88 ± 0.75 | <0.01 |
| Waist circumference (cm) | 84.34 ± 0.82 | 85.51 ± 1.17 | NS |
| Hip circumference (cm) | 90.44 ± 0.87 | 92.94 ± 1.10 | <0.01 |
| WHR | 0.94 ± 0.01 | 0.92 ± 0.01 | <0.01 |
| WHtR | 0.58 ± 0.01 | 0.56 ± 0.01 | <0.01 |
| (B) | |||
| Initial Assessment | Annual Assessment | p-Value | |
| ESR (mm/hr) | 19.64 ± 0.77 | 16.36 ± 0.67 | <0.01 |
| Ferritin (μg/L) | 59.07 ± 1.77 | 58.29 ± 2.20 | NS |
| Folic acid (ng/mL) | 12.88 ± 0.51 | 11.04 ± 0.33 | <0.01 |
| Iron (μg/dL) | 85.61 ± 2.07 | 79.36 ± 6.34 | NS |
| Glucose (mg/dL) | 78.69 ± 0.48 | 82.80 ± 2.54 | NS |
| Urea (mg/dL) | 29.17 ± 0.34 | 27.87 ± 0.34 | <0.01 |
| Creatinine (mg/dL) | 0.52 ± 0.05 | 0.53 ± 0.01 | NS |
| eGFR (mL/min/1.73 m2) | 132.07 ± 1.50 | 123.51 ± 1.42 | <0.01 |
| AST (U/L) | 24.22 ± 0.34 | 22.25 ± 0.36 | <0.01 |
| ALT (U/L) | 22.80 ± 0.88 | 19.70 ± 0.63 | <0.01 |
| γGT (U/L) | 15.04 ± 0.31 | 13.84 ± 0.30 | <0.01 |
| ALP (U/L) | 234.45 ± 4.19 | 213.77 ± 4.46 | <0.01 |
| Phosphorus (mg/dL) | 4.80 ± 0.05 | 4.67 ± 0.02 | <0.01 |
| Albumin (g/dL) | 4.65 ± 0.02 | 4.66 ± 0.02 | NS |
| Cholesterol (mg/dL) | 159.67 ± 1.62 | 152.60 ± 2.10 | <0.01 |
| Triglycerides (mg/dL) | 82.90 ± 2.38 | 80.90 ± 2.55 | NS |
| HDL (mg/dL) | 50.08 ± 0.68 | 53.41 ± 0.75 | <0.01 |
| LDL (mg/dL) | 94.35 ± 1.28 | 88.28 ± 1.30 | <0.01 |
| Uric acid (mg/dL) | 4.89 ± 0.06 | 6.19 ± 0.56 | <0.05 |
| K (mmol/L) | 4.39 ± 0.02 | 4.40 ± 0.02 | NS |
| Na (mmol/L) | 140.73 ± 0.08 | 140.38 ± 0.13 | NS |
| Cl (mmol/L) | 102.66 ± 0.16 | 101.10 ± 0.83 | NS |
| Ca (mmol/L) | 9.95 ± 0.02 | 9.81 ± 0.02 | <0.01 |
| Apo-A1 (mg/dL) | 141.67 ± 1.30 | 141.83 ± 1.23 | NS |
| Apo-B (mg/dL) | 76.09 ± 1.07 | 71.77 ± 0.93 | <0.01 |
| Lp(a) (mg/dL) | 16.74 ± 1.31 | 17.93 ± 1.49 | <0.05 |
| (C) | |||
| Initial Assessment | Annual Assessment | p-Value | |
| TSH (μUI/mL) | 2.97 ± 0.08 | 2.91 ± 0.09 | NS |
| FT4 (mg/dL) | 1.15 ± 0.03 | 1.12 ± 0.01 | NS |
| T3 (ng/dL) | 147.63 ± 1.43 | 141.07 ± 1.56 | <0.01 |
| Anti-TG (IU/mL) | 20.80 ± 0.40 | 23.64 ± 1.98 | NS |
| Anti-TPO (IU/mL) | 15.78 ± 1.45 | 16.39 ± 2.18 | NS |
| IGF-1 (ng/mL) | 307.27 ± 9.25 | 389.39 ± 10.89 | <0.01 |
| IGFBP-3 (μg/mL) | 5.14 ± 0.06 | 5.47 ± 0.07 | NS |
| Androstenedione (ng/mL) | 1.07 ± 0.08 | 2.44 ± 1.11 | NS |
| Testosterone (ng/mL) | 56.36 ± 4.79 | 89.56 ± 6.91 | <0.01 |
| DHEA-s (μg/dl) | 113.26 ± 4.92 | 139.97 ± 5.76 | <0.01 |
| Prolactin (ng/mL) | 11.75 ± 0.39 | 12.18 ± 0.38 | <0.05 |
| LH (mUI/mL) | 2.30 ± 0.32 | 2.96 ± 0.19 | <0.01 |
| FSH (mUI/mL) | 5.22 ± 2.54 | 3.32 ± 0.14 | NS |
| E2 (pg/mL) | 14.79 ± 1.67 | 17.47 ± 1.25 | <0.01 |
| PTH (pg/mL) | 33.84 ± 0.70 | 36.93 ± 0.68 | <0.01 |
| Vitamin D (ng/mL) | 22.79 ± 0.54 | 24.52 ± 0.52 | <0.01 |
| ACTH (pg/mL) | 34.09 ± 3.96 | 27.94 ± 1.08 | NS |
| Cortisol (μg/dL) | 15.85 ± 0.44 | 13.81 ± 0.34 | <0.01 |
| Insulin (μUI/mL) | 16.18 ± 0.54 | 15.80 ± 0.56 | NS |
| SHBG (nmol/L) | 45.72 ± 1.79 | 46.79 ± 1.64 | NS |
| HbA1c (%) | 7.01 ± 1.59 | 5.27 ± 0.01 | NS |
| HOMA-IR | 3.13 ± 0.11 | 3.15 ± 0.13 | NS |
| (D) | |||
| Initial Assessment | Annual Assessment | p-Value | |
| Adiponectin (pg/mL) | 21.61 ± 0.95 | 25.91 ± 1.07 | <0.01 |
| Osteopontin (ng/mL) | 30.63 ± 1.80 | 21.22 ± 1.15 | <0.01 |
| NGAL (ng/mL) | 19.97 ± 1.41 | 10.76 ± 0.77 | <0.01 |
| Sclerostin (pg/mL) | 2.00 ± 0.39 | 4.82 ± 0.59 | <0.01 |
| FGF-23 (pg/mL) | 10.38 ± 2.63 | 7.50 ± 1.76 | <0.05 |
| Leptin (ng/mL) | 29.79 ± 1.32 | 23.94 ± 0.99 | <0.01 |
| TNF-α (pg/mL) | 3.80 ± 0.12 | 3.99 ± 0.09 | NS |
| (E) | |||
| Initial Assessment | Annual Assessment | p-Value | |
| Fat Percentage (%) | 35.03 ± 0.35 | 32.85 ± 0.33 | <0.01 |
| Fat Mass (kg) | 21.48 ± 0.60 | 20.63 ± 0.54 | NS |
| Muscle Mass Percentage (%) | 36.65 ± 0.74 | 38.68 ± 0.66 | <0.01 |
| Bone Mass (kg) | 1.99 ± 0.04 | 2.17 ± 0.08 | <0.01 |
| Fat-Free Mass (kg) | 38.65 ± 0.78 | 40.75 ± 0.70 | <0.01 |
| Total Body Water | 28.29 ± 0.57 | 29.88 ± 0.51 | <0.01 |
| BMR (Kilojoule) | 6428.30 ± 86.51 | 6585.94 ± 77.27 | <0.01 |
| (A) | |||||||
|---|---|---|---|---|---|---|---|
| Initial Assessment | Annual Assessment | ||||||
| Obese | Overweight | pwithin | Obese | Overweight | pwithin | pbetween timepoints (obese/overweight) | |
| Adiponectin (pg/mL) | 20.77 ± 1.16 | 22.85 ± 1.58 | NS | 24.07 ± 1.23 | 29.29 ± 1.89 | 0.05 | 0.05/0.01 |
| Osteopontin (ng/mL) | 30.91 ± 2.24 | 30.21 ± 2.93 | NS | 21.52 ± 1.49 | 20.42 ± 1.69 | NS | 0.01/0.01 |
| NGAL (ng/mL) | 21.16 ± 1.83 | 16.62 ± 1.82 | NS | 10.68 ± 0.86 | 10.76 ± 1.52 | NS | 0.01/0.05 |
| Sclerostin (pg/mL) | 2.06 ± 0.49 | 1.75 ± 0.55 | NS | 5.01 ± 0.68 | 4.08 ± 1.05 | NS | 0.01/0.01 |
| TNF-α (pg/mL) | 3.67 ± 0.15 | 4.04 ± 0.17 | NS | 4.05 ± 0.10 | 3.90 ± 0.18 | 0.05 | 0.05/NS |
| FGF-23 (pg/mL) | 9.44 ± 2.73 | 11.92 ± 5.25 | NS | 7.63 ± 2.36 | 7.30 ± 2.52 | NS | NS/0.05 |
| Leptin (ng/mL) | 32.62 ± 1.71 | 24.33 ± 1.86 | 0.01 | 24.96 ± 1.21 | 21.84 ± 1.61 | 0.05 | 0.01/NS |
| (B) | |||||||
| Initial Assessment | Annual Assessment | ||||||
| Boys | Girls | pwithin | Boys | Girls | pwithin | pbetween timepoints (Boys/Girls) | |
| Adiponectin (pg/mL) | 18.71 ± 1.14 | 24.48 ± 1.49 | 0.05 | 24.97 ± 1.50 | 26.87 ± 1.51 | NS | 0.01/NS |
| Osteopontin (ng/mL) | 31.76 ± 2.51 | 29.58 ± 2.59 | NS | 23.18 ± 1.83 | 19.38 ± 1.40 | NS | 0.01/0.01 |
| NGAL (ng/mL) | 18.61 ± 1.97 | 21.31 ± 2.02 | NS | 9.93 ± 1.04 | 11.58 ± 1.13 | NS | 0.01/0.01 |
| Sclerostin (pg/mL) | 1.68 ± 0.62 | 2.32 ± 0.49 | NS | 3.80 ± 0.83 | 5.82 ± 0.80 | <0.05 | 0.01/0.01 |
| TNF-α (pg/mL) | 3.88 ± 0.20 | 3.72 ± 0.12 | NS | 4.02 ± 0.14 | 3.96 ± 0.13 | NS | NS/NS |
| FGF-23 (pg/mL) | 8.16 ± 2.29 | 12.56 ± 4.70 | NS | 6.31 ± 1.69 | 8.66 ± 3.07 | NS | NS/0.05 |
| Leptin (ng/mL) | 27.88 ± 1.85 | 31.72 ± 1.86 | NS | 20.19 ± 1.33 | 27.73 ± 1.41 | 0.01 | 0.01/NS |
| (C) | |||||||
| Initial Assessment | Annual Assessment | ||||||
| Boys | Girls | pwithin | Boys | Girls | pwithin | pbetween timepoints (Boys/Girls) | |
| Adiponectin (pg/mL) | 21.34 ± 1.71 | 27.23 ± 2.24 | 0.05 | 27.94 ± 2.03 | 29.01 ± 2.12 | NS | 0.01/NS |
| Osteopontin (ng/mL) | 33.99 ± 3.90 | 29.60 ± 3.90 | NS | 23.25 ± 2.66 | 21.21 ± 2.06 | NS | 0.01/0.05 |
| NGAL (ng/mL) | 17.90 ± 2.31 | 22.26 ± 2.75 | NS | 10.84 ± 1.89 | 9.91 ± 1.39 | NS | 0.05/0.01 |
| Sclerostin (pg/mL) | 0.89 ± 0.39 | 2.57 ± 1.07 | NS | 2.88 ± 1.03 | 4.95 ± 1.37 | NS | 0.05/0.05 |
| TNF-α (pg/mL) | 3.87 ± 0.21 | 3.69 ± 0.15 | NS | 4.07 ± 0.21 | 4.07 ± 0.17 | NS | NS/NS |
| FGF-23 (pg/mL) | 9.93 ± 3.50 | 13.21 ± 8.08 | NS | 6.98 ± 2.67 | 7.36 ± 3.75 | NS | NS/0.05 |
| Leptin (ng/mL) | 26.12 ± 2.68 | 26.62 ± 2.30 | NS | 20.90 ± 1.61 | 23.90 ± 1.62 | NS | NS/NS |
| (D) | |||||||
| Initial Assessment | Annual Assessment | ||||||
| Boys | Girls | pwithin | Boys | Girls | pwithin | pbetween timepoints (Boys/Girls) | |
| Adiponectin (pg/mL) | 15.55 ± 1.35 | 21.41 ± 1.87 | 0.05 | 21.31 ± 2.16 | 24.65 ± 2.15 | NS | 0.01/NS |
| Osteopontin (ng/mL) | 29.48 ± 3.16 | 29.56 ± 3.45 | NS | 23.12 ± 2.55 | 17.59 ± 1.88 | NS | NS/0.01 |
| NGAL (ng/mL) | 19.34 ± 3.26 | 20.66 ± 2.86 | NS | 8.99 ± 0.81 | 12.70 ± 1.62 | NS | 0.01/0.01 |
| Sclerostin (pg/mL) | 2.38 ± 1.11 | 2.17 ± 0.48 | NS | 4.62 ± 1.27 | 6.33 ± 0.99 | 0.05 | 0.05/0.01 |
| TNF-α (pg/mL) | 3.90 ± 0.37 | 3.74 ± 0.19 | NS | 3.97 ± 0.17 | 3.85 ± 0.19 | NS | NS/NS |
| FGF-23 (pg/mL) | 6.42 ± 2.99 | 11.92 ± 4.98 | NS | 5.61 ± 2.07 | 9.92 ± 4.86 | NS | NS/NS |
| Leptin (ng/mL) | 29.99 ± 2.51 | 37.08 ± 2.85 | 0.05 | 19.33 ± 2.21 | 31.70 ± 2.24 | 0.01 | NS/NS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karampatsou, S.I.; Paltoglou, G.; Genitsaridi, S.M.; Kassari, P.; Charmandari, E. The Effect of a Comprehensive Life-Style Intervention Program of Diet and Exercise on Four Bone-Derived Proteins, FGF-23, Osteopontin, NGAL and Sclerostin, in Overweight or Obese Children and Adolescents. Nutrients 2022, 14, 3772. https://doi.org/10.3390/nu14183772
Karampatsou SI, Paltoglou G, Genitsaridi SM, Kassari P, Charmandari E. The Effect of a Comprehensive Life-Style Intervention Program of Diet and Exercise on Four Bone-Derived Proteins, FGF-23, Osteopontin, NGAL and Sclerostin, in Overweight or Obese Children and Adolescents. Nutrients. 2022; 14(18):3772. https://doi.org/10.3390/nu14183772
Chicago/Turabian StyleKarampatsou, Sofia I., George Paltoglou, Sofia M. Genitsaridi, Penio Kassari, and Evangelia Charmandari. 2022. "The Effect of a Comprehensive Life-Style Intervention Program of Diet and Exercise on Four Bone-Derived Proteins, FGF-23, Osteopontin, NGAL and Sclerostin, in Overweight or Obese Children and Adolescents" Nutrients 14, no. 18: 3772. https://doi.org/10.3390/nu14183772
APA StyleKarampatsou, S. I., Paltoglou, G., Genitsaridi, S. M., Kassari, P., & Charmandari, E. (2022). The Effect of a Comprehensive Life-Style Intervention Program of Diet and Exercise on Four Bone-Derived Proteins, FGF-23, Osteopontin, NGAL and Sclerostin, in Overweight or Obese Children and Adolescents. Nutrients, 14(18), 3772. https://doi.org/10.3390/nu14183772







