The Evidence That 25(OH)D3 and VK2 MK-7 Vitamins Influence the Proliferative Potential and Gene Expression Profiles of Multiple Myeloma Cells and the Development of Resistance to Bortezomib
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. RNA Isolation
2.3. Proliferation Assay
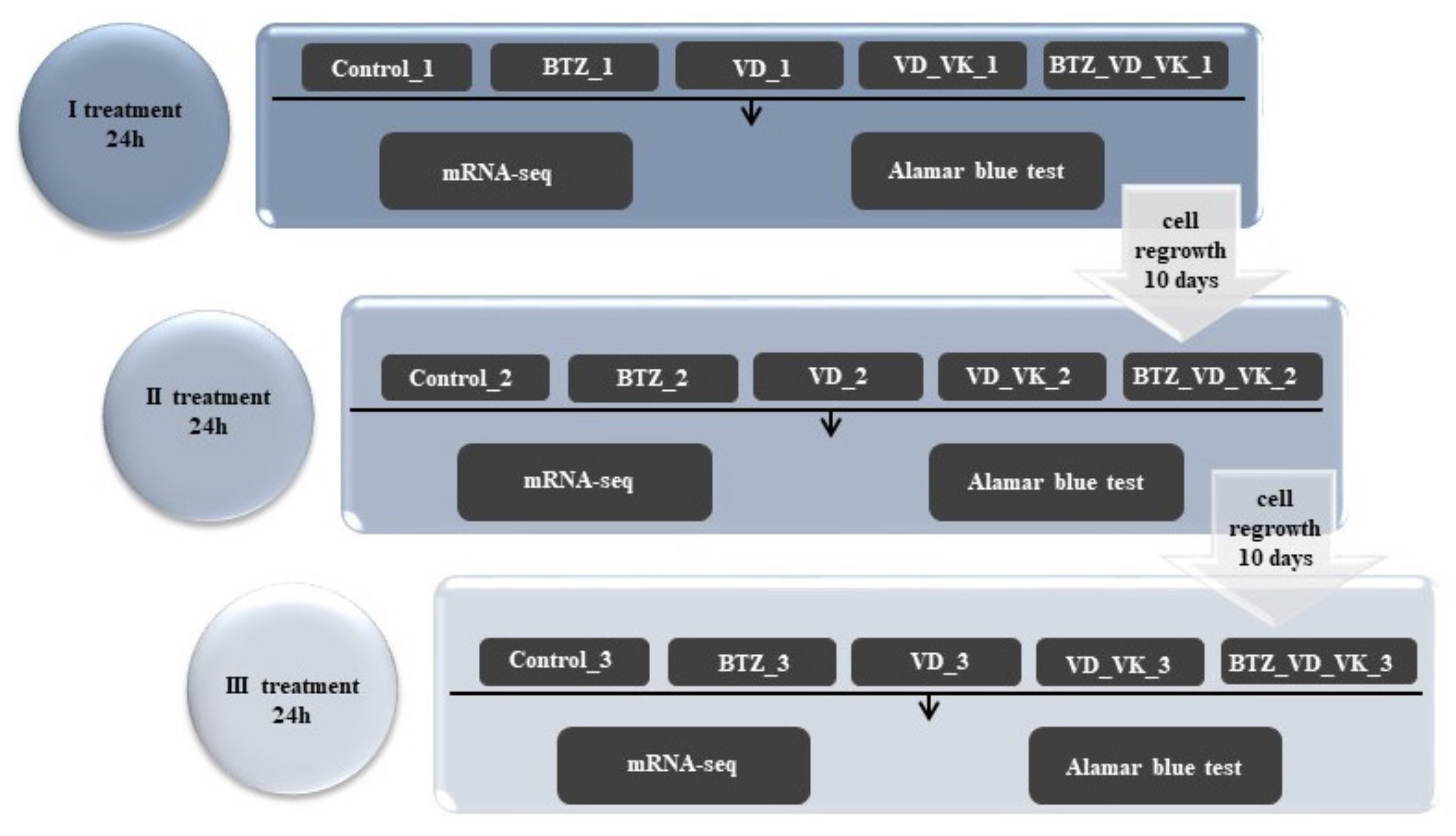
2.4. The Course of the Experiment and Determination of the Dose of BTZ and Vitamins
2.5. RNA-Seq
2.6. Bioinformatic Analysis
2.7. Statistical Methods
3. Results
3.1. Proliferation Assay
3.1.1. U266 Myeloma Cells
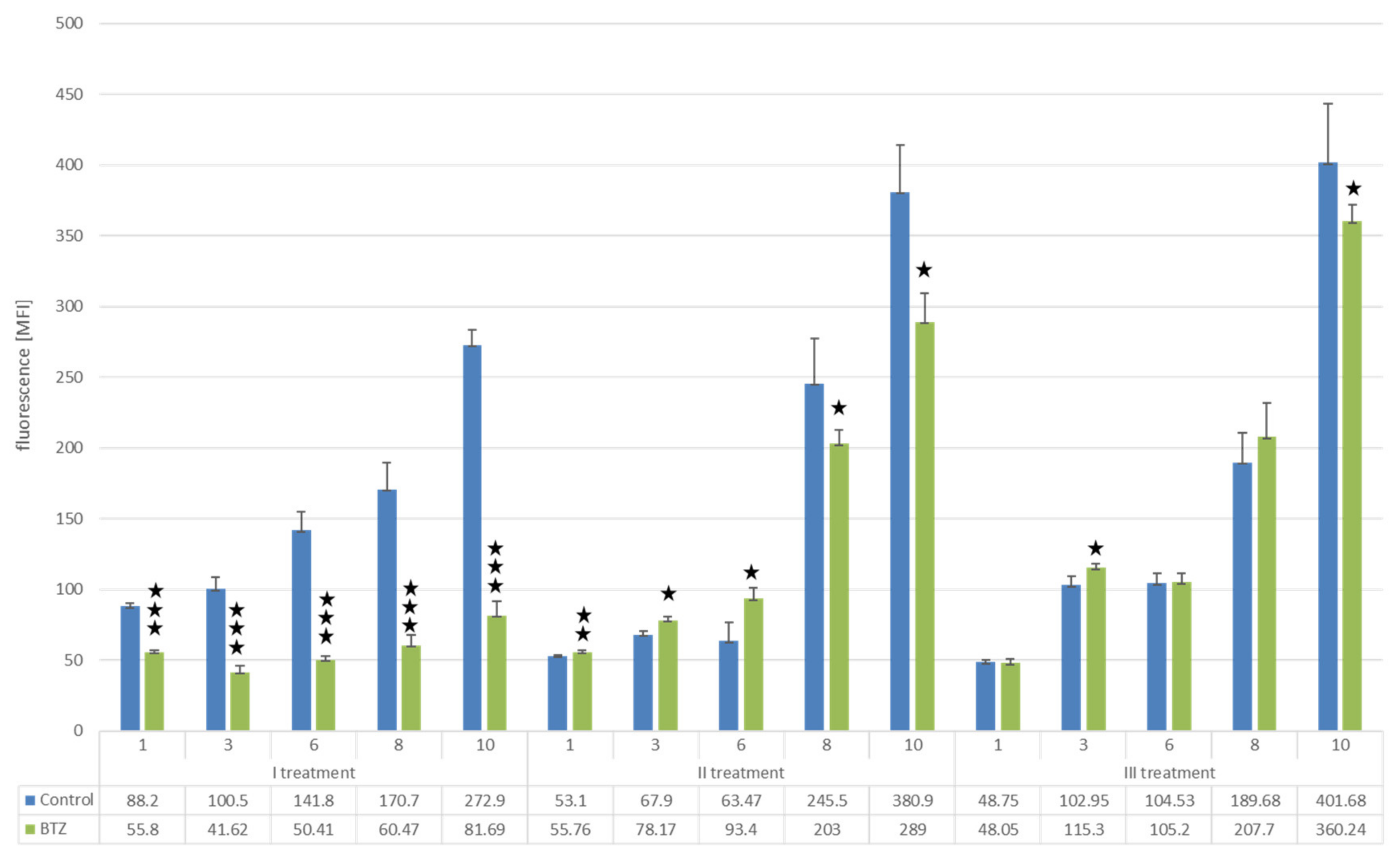
Changes in Proliferation Resulting from the Development of Resistance of U266 Myeloma Cells to BTZ
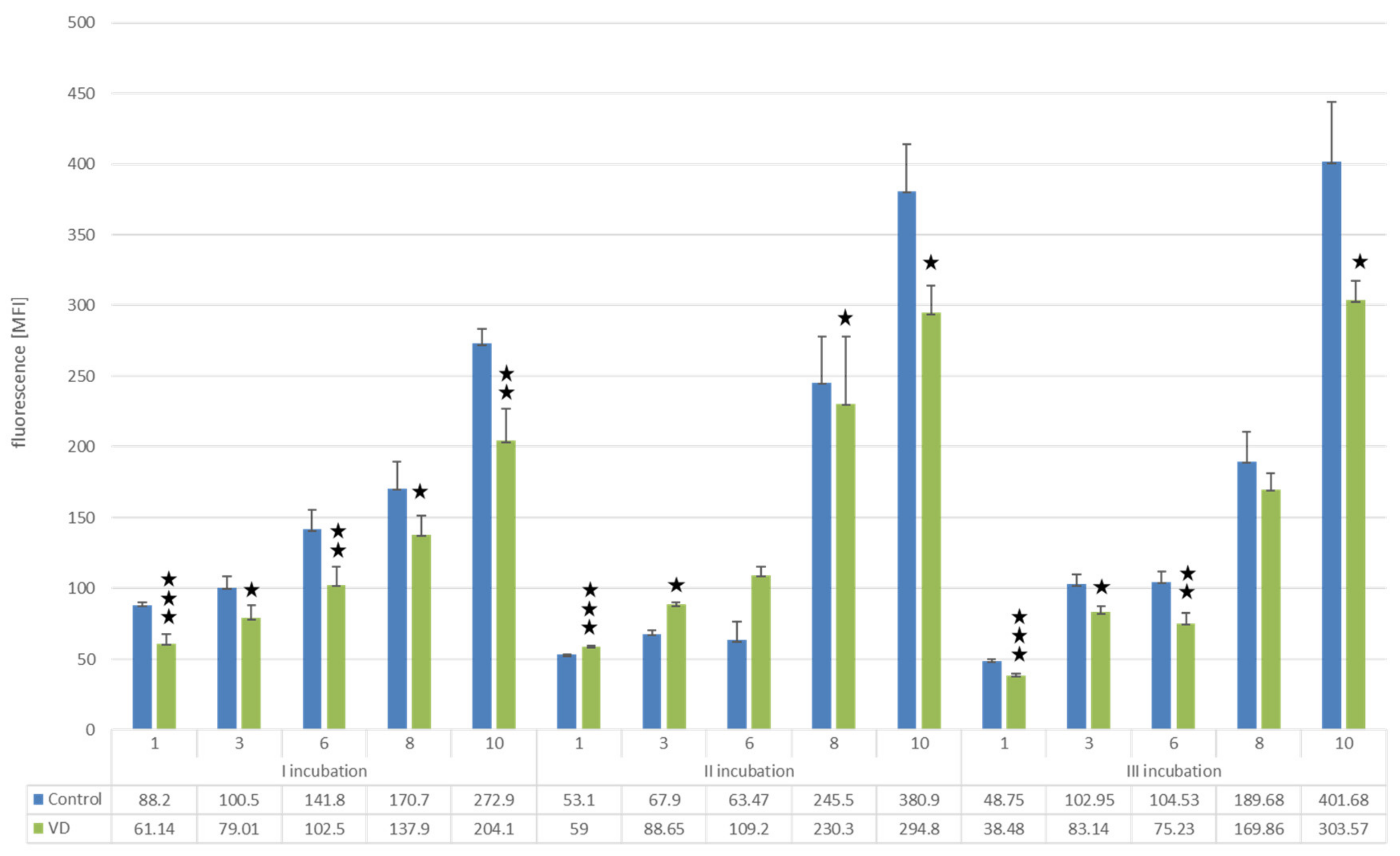
Effect of Vitamins D and K on Proliferation of U266 Myeloma Cells
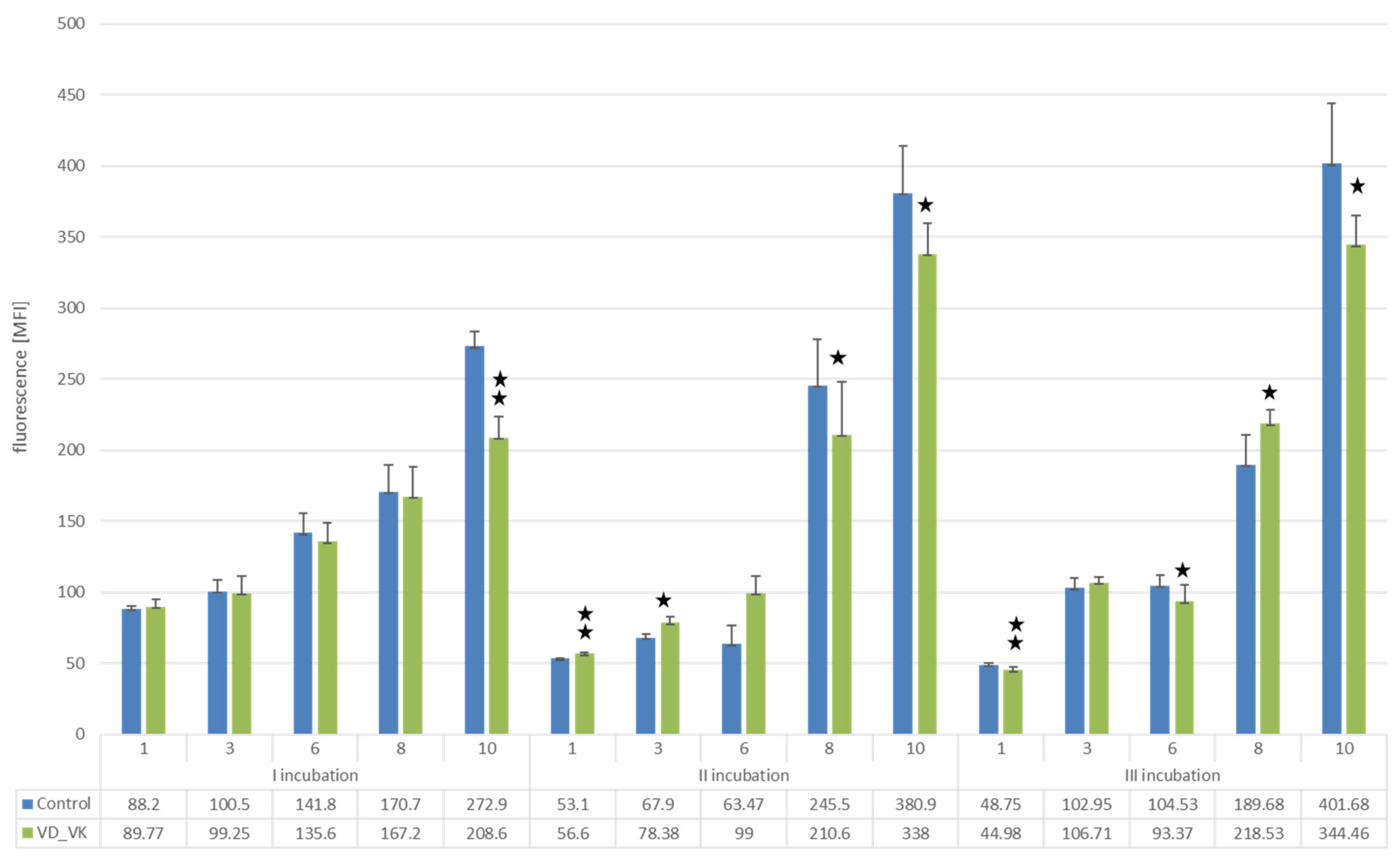
Changes in the Proliferation Level of U266 Myeloma Cells with a BTZ-Resistant Phenotype Induced by Vitamin D and K
3.1.2. L929 Fibroblast Cells
3.2. mRNA-Seq
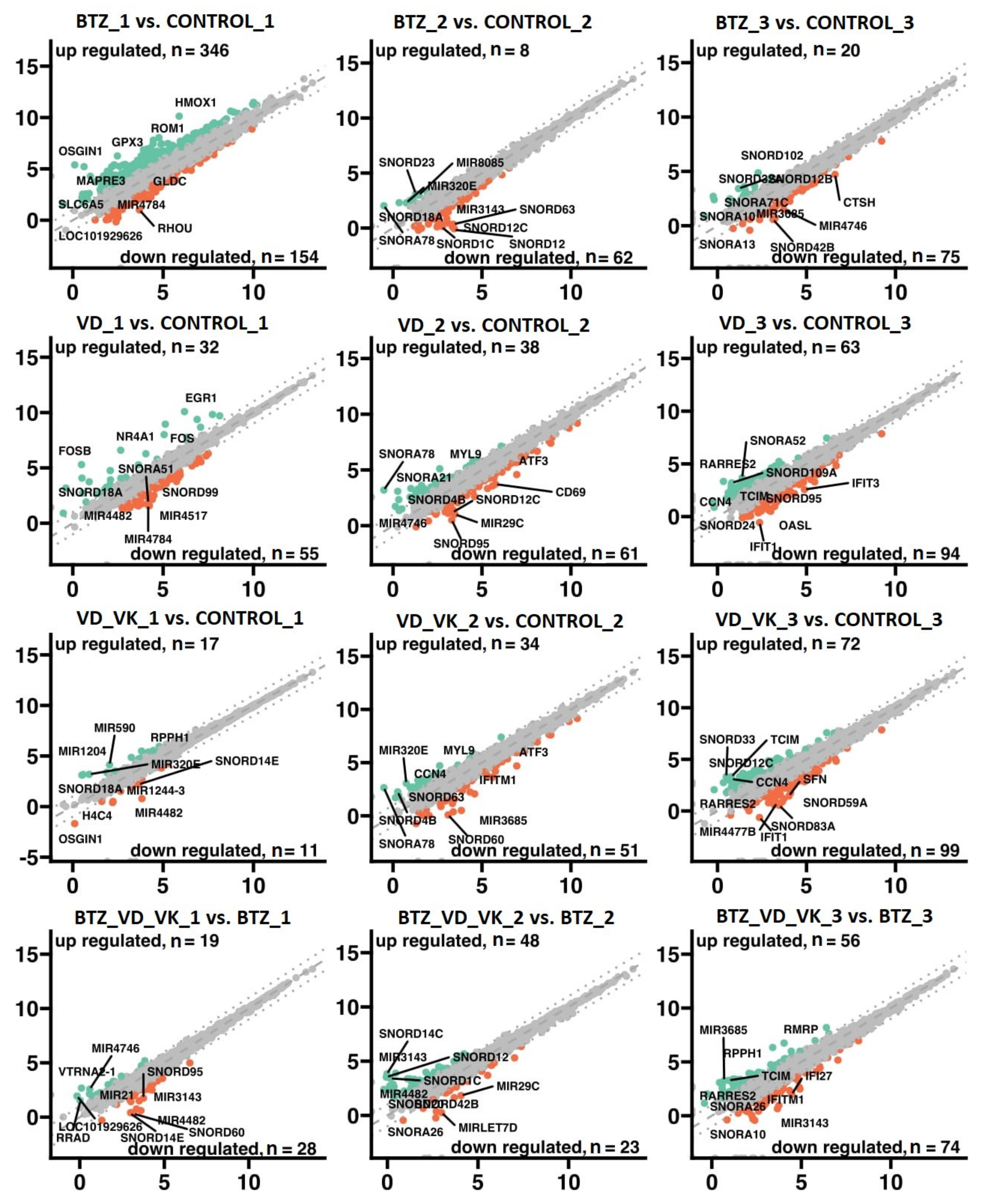
3.2.1. Transcriptional Changes Associated with the Development of Resistance of U266 Myeloma Cells to BTZ
3.2.2. Effect of VD and VK on Transcriptional Changes in Myeloma Cells U266
3.2.3. Transcriptional Changes Induced by VD and VK in U266 Myeloma Cells in BTZ-Resistant Phenotype
4. Discussion
5. Conclusions
6. Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kazandjian, D. Multiple myeloma epidemiology and survival: A unique malignancy. Semin. Oncol. 2016, 43, 676–681. [Google Scholar] [PubMed]
- Kumar, S.K.; Rajkumar, V.; Kyle, R.A.; van Duin, M.; Sonneveld, P.; Mateos, M.V.; Gay, F.; Anderson, K.C. Multiple myeloma. Nat. Rev. Dis. Prim. 2017, 3, 17046. [Google Scholar] [PubMed]
- Padala, S.A.; Barsouk, A.; Barsouk, A.; Rawla, P.; Vakiti, A.; Kolhe, R.; Kota, V.; Ajebo, G.H. Epidemiology, Staging, and Management of Multiple Myeloma. Med. Sci. 2021, 9, 3. [Google Scholar]
- Kristinsson, S.Y.; Anderson, W.F.; Landgren, O. Improved long-term survival in multiple myeloma up to the age of 80 years. Leukemia 2014, 28, 1346–1348. [Google Scholar] [CrossRef] [PubMed]
- Richardson, P.G.; Barlogie, B.; Berenson, J.; Singhal, S.; Jagannath, S.; Irwin, D.; Rajkumar, S.V.; Srkalovic, G.; Alsina, M.; Alexanian, R.; et al. A Phase 2 Study of Bortezomib in Relapsed, Refractory Myeloma. N. Engl. J. Med. 2003, 348, 2609–2617. [Google Scholar]
- Richardson, P.G.; Mitsiades, C.; Hideshima, T.; Anderson, K.C. Bortezomib: Proteasome Inhibition as an Effective Anticancer Therapy. Annu. Rev. Med. 2006, 57, 33–47. [Google Scholar] [CrossRef]
- Zhou, W.; Yang, Y.; Xia, J.; Wang, H.; Salama, M.E.; Xiong, W.; Xu, H.; Shetty, S.; Chen, T.; Zeng, Z.; et al. NEK2 Induces Drug Resistance Mainly through Activation of Efflux Drug Pumps and Is Associated with Poor Prognosis in Myeloma and Other Cancers. Cancer Cell 2013, 23, 48–62. [Google Scholar] [CrossRef]
- Xia, J.; He, Y.; Meng, B.; Chen, S.; Zhang, J.; Wu, X.; Zhu, Y.; Shen, Y.; Feng, X.; Guan, Y.; et al. NEK2 induces autophagy-mediated bortezomib resistance by stabilizing Beclin-1 in multiple myeloma. Mol. Oncol. 2020, 14, 763–778. [Google Scholar] [CrossRef]
- Franqui-Machin, R.; Hao, M.; Bai, H.; Gu, Z.; Zhan, X.; Habelhah, H.; Jethava, Y.; Qiu, L.; Frech, I.; Tricot, G.; et al. Destabilizing NEK2 overcomes resistance to proteasome inhibition in multiple myeloma. J. Clin. Investig. 2018, 128, 2877–2893. [Google Scholar] [CrossRef]
- Lipchick, B.C.; Utley, A.; Han, Z.; Moparthy, S.; Yun, D.H.; Bianchi-Smiraglia, A.; Wolff, D.W.; Fink, E.; Liu, L.; Furdui, C.M.; et al. The fatty acid elongase ELOVL6 regulates bortezomib resistance in multiple myeloma. Blood Adv. 2021, 5, 1933–1946. [Google Scholar] [CrossRef]
- Dosani, T.; Carlsten, M.; Maric, I.; Landgren, O. The cellular immune system in myelomagenesis: NK cells and T cells in the development of MM and their uses in immunotherapies. Blood Cancer, J. 2015, 5, e306. [Google Scholar] [CrossRef] [PubMed]
- Beyar-Katz, O.; Magidey, K.; Reiner-Benaim, A.; Barak, N.; Avivi, I.; Cohen, Y.; Timaner, M.; Avraham, S.; Hayun, M.; Lavi, N.; et al. Proinflammatory Macrophages Promote Multiple Myeloma Resistance to Bortezomib Therapy. Mol. Cancer Res. 2019, 17, 2331–2340. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; He, D.; Chen, Q.; Guo, X.; Yang, L.; Lin, X.; Li, Y.; Wu, W.; Yang, Y.; He, J.; et al. BAFF is involved in macrophage-induced bortezomib resistance in myeloma. Cell Death Dis. 2017, 8, e3161. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hendrix, A.; Hernot, S.; Lemaire, M.; de Bruyne, E.; van Valckenborgh, E.; Lahoutte, T.; de Wever, O.; Vanderkerken, K.; Menu, E.; et al. Bone marrow stromal cell–derived exosomes as communicators in drug resistance in multiple myeloma cells. Blood 2014, 124, 555–566. [Google Scholar] [PubMed]
- Boullata, J.I. Vitamin D supplementation: A pharmacologic perspective. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 677–684. [Google Scholar] [PubMed]
- Mak, R.H.; Querfeld, U.; Gonzalez, A.; Gunta, S.; Cheung, W.W. Differential Effects of 25-Hydroxyvitamin D3 versus 1α 25-Dihydroxyvitamin D3 on Adipose Tissue Browning in CKD-Associated Cachexia. Cells 2021, 10, 3382. [Google Scholar] [CrossRef]
- Veldman, C.M.; Cantorna, M.T.; De Luca, H.F. Expression of 1,25-Dihydroxyvitamin D3 Receptor in the Immune System. Arch. Biochem. Biophys. 2000, 374, 334–338. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, J.; DeLuca, H.F. Where is the vitamin D receptor? Arch. Biochem. Biophys. 2012, 523, 123–133. [Google Scholar]
- Martens, P.J.; Gysemans, C.; Verstuyf, A.; Mathieu, C. Vitamin D’s Effect on Immune Function. Nutrients 2020, 12, 1248. [Google Scholar] [CrossRef]
- Latic, N.; Erben, R.G. Vitamin D and Cardiovascular Disease, with Emphasis on Hypertension, Atherosclerosis, and Heart Failure. Int. J. Mol. Sci. 2020, 21, 6483. [Google Scholar] [CrossRef]
- Guillot, X.; Semerano, L.; Saidenberg-Kermanac’h, N.; Falgarone, G.; Boissier, M.C. Vitamin D and inflammation. Jt. Bone Spine 2010, 77, 552–557. [Google Scholar]
- Mantovani, A.; Allavena, P.; Sica, A.; Balkwill, F. Cancer-related inflammation. Nature 2008, 454, 436–444. [Google Scholar] [CrossRef] [PubMed]
- El-Sharkawy, A.; Malki, A. Vitamin D Signaling in Inflammation and Cancer: Molecular Mechanisms and Therapeutic Implications. Molecules 2020, 25, 3219. [Google Scholar] [PubMed]
- Halder, M.; Petsophonsakul, P.; Akbulut, A.; Pavlic, A.; Bohan, F.; Anderson, E.; Maresz, K.; Kramann, R.; Schurgers, L. Vitamin K: Double Bonds beyond Coagulation Insights into Differences between Vitamin K1 and K2 in Health and Disease. Int. J. Mol. Sci. 2019, 20, 896. [Google Scholar] [CrossRef] [PubMed]
- Ohsaki, Y.; Shirakawa, H.; Miura, A.; Giriwono, P.E.; Sato, S.; Ohashi, A.; Iribe, M.; Goto, T.; Komai, M. Vitamin K suppresses the lipopolysaccharide-induced expression of inflammatory cytokines in cultured macrophage-like cells via the inhibition of the activation of nuclear factor κB through the repression of IKKα/β phosphorylation. J. Nutr. Biochem. 2010, 21, 1120–1126. [Google Scholar] [PubMed]
- Reddi, K.; Henderson, B.; Meghji, S.; Wilson, M.; Poole, S.; Hopper, C.; Harris, M.; Hodges, S.J. Interleukin 6 production by lipopolysaccharide-stimulated human fibroblasts is potently inhibited by Naphthoquinone (vitamin K) compounds. Cytokine 1995, 7, 287–290. [Google Scholar] [CrossRef]
- Ohsaki, Y.; Shirakawa, H.; Hiwatashi, K.; Furukawa, Y.; Mizutani, T.; Komai, M. Vitamin K Suppresses Lipopolysaccharide-Induced Inflammation in the Rat. Biosci. Biotechnol. Biochem. 2006, 70, 926–932. [Google Scholar]
- Duan, F.; Mei, C.; Yang, L.; Zheng, J.; Lu, H.; Xia, Y.; Hsu, S.; Liang, H.; Hong, L. Vitamin K2 promotes PI3K/AKT/HIF-1α-mediated glycolysis that leads to AMPK-dependent autophagic cell death in bladder cancer cells. Sci. Rep. 2020, 10, 7714. [Google Scholar] [CrossRef]
- Xia, J.; Matsuhashi, S.; Hamajima, H.; Iwane, S.; Takahashi, H.; Eguchi, Y.; Mizuta, T.; Fujimoto, K.; Kuroda, S.; Ozaki, I. The role of PKC isoforms in the inhibition of NF-κB activation by vitamin K2 in human hepatocellular carcinoma cells. J. Nutr. Biochem. 2012, 23, 1668–1675. [Google Scholar] [CrossRef]
- Enomoto, M.; Tsuchida, A.; Miyazawa, K.; Yokoyama, T.; Kawakita, H.; Tokita, H.; Naito, M.; Itoh, M.; Ohyashiki, K.; Aoki, T.; et al. Vitamin K2-induced cell growth inhibition via autophagy formation in cholangiocellular carcinoma cell lines. Int. J. Mol. Med. 2007, 20, 801–808. [Google Scholar] [CrossRef]
- Yokoyama, T.; Miyazawa, K.; Naito, M.; Toyotake, J.; Tauchi, T.; Itoh, M.; Yuo, A.; Hayashi, Y.; Georgescu, M.M.; Kondo, Y.; et al. Vitamin K2 induces autophagy and apoptosis simultaneously in leukemia cells. Autophagy 2008, 4, 629–640. [Google Scholar] [CrossRef] [PubMed]
- Yaguchi, M.; Miyazawa, K.; Katagiri, T.; Nishimaki, J.; Kizaki, M.; Tohyama, K.; Toyama, K. Vitamin K2 and its derivatives induce apoptosis in leukemia cells and enhance the effect of all-trans retinoic acid. Leukemia 1997, 11, 779–787. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Xu, W.; Wu, H.; Chen, S.; Wang, X.; Tanaka, S.; Sugiyama, K.; Yamada, H.; Hirano, T. Cytotoxic effects of vitamins K1, K2, and K3 against human T lymphoblastoid leukemia cells through apoptosis induction and cell cycle arrest. Chem. Biol. Drug Des. 2020, 96, 1134–1147. [Google Scholar] [CrossRef]
- Tsujioka, T.; Miura, Y.; Otsuki, T.; Nishimura, Y.; Hyodoh, F.; Wada, H.; Sugihara, T. The mechanisms of vitamin K2-induced apoptosis of myeloma cells. Haematologica 2006, 91, 613–619. [Google Scholar]
- Sandur, S.K.; Pandey, M.K.; Sung, B.; Aggarwal, B.B. 5-Hydroxy-2-Methyl-1,4-Naphthoquinone, a Vitamin K3 Analogue, Suppresses STAT3 Activation Pathway through Induction of Protein Tyrosine Phosphatase, SHP-1: Potential Role in Chemosensitization. Mol. Cancer Res. 2010, 8, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Kuang, X.; Liu, C.; Guo, X.; Li, K.; Deng, Q.; Li, D. The combination effect of vitamin K and vitamin D on human bone quality: A meta-analysis of randomized controlled trials. Food Funct. 2020, 11, 3280–3297. [Google Scholar] [CrossRef] [PubMed]
- Busch, L.; Mougiakakos, D.; Büttner-Herold, M.; Müller, M.J.; Volmer, D.A.; Bach, C.; Fabri, M.; Bittenbring, J.T.; Neumann, F.; Boxhammer, R.; et al. Lenalidomide enhances MOR202-dependent macrophage-mediated effector functions via the vitamin D pathway. Leukemia 2018, 32, 2445–2458. [Google Scholar] [PubMed]
- Wigington, D.P.; Strugnell, S.A.; Knutson, J.C. Pamidronate and 1,24(S)-dihydroxyvitamin D2 synergistically inhibit the growth of myeloma, breast and prostate cancer cells. Anticancer. Res. 2005, 25, 1909–1917. [Google Scholar]
- Eicher, F.; Mansouri Taleghani, B.; Schild, C.; Bacher, U.; Pabst, T. Reduced survival after autologous stem cell transplantation in myeloma and lymphoma patients with low vitamin D serum levels. Hematol. Oncol. 2020, 38, 523–530. [Google Scholar]
- Raoufinejad, K.; Shamshiri, A.R.; Pezeshki, S.; Chahardouli, B.; Hadjibabaie, M.; Jahangard-Rafsanjani, Z.; Gholami, K.; Rajabi, M.; Vaezi, M. Oral calcitriol in hematopoietic recovery and survival after autologous stem cell transplantation: A randomized clinical trial. DARU J. Pharm. Sci. 2019, 27, 709–720. [Google Scholar] [CrossRef]
- Wang, J.; Udd, K.A.; Vidisheva, A.; Swift, R.A.; Spektor, T.M.; Bravin, E.; Ibrahim, E.; Treisman, J.; Masri, M.; Berenson, J.R.; et al. Low serum vitamin D occurs commonly among multiple myeloma patients treated with bortezomib and/or thalidomide and is associated with severe neuropathy. Support. Care Cancer 2016, 24, 3105–3110. [Google Scholar] [CrossRef] [PubMed]
- Díaz, G.D.; Paraskeva, C.; Thomas, M.G.; Binderup, L.; Hague, A. Apoptosis is induced by the active metabolite of vitamin D3 and its analogue EB1089 in colorectal adenoma and carcinoma cells: Possible implications for prevention and therapy. Cancer Res. 2000, 60, 2304–2312. [Google Scholar] [PubMed]
- Park, W.H.; Seol, J.G.; Kim, E.S.; Hyun, J.M.; Jung, C.W.; Lee, C.C.; Binderup, L.; Koeffler, H.P.; Kim, B.K.; Lee, Y.Y.; et al. Induction of apoptosis by vitamin D3 analogue EB1089 in NCI-H929 myeloma cells via activation of caspase 3 and p38 MAP kinase: Induction of Apoptosis by EB1089 in NCI-H929 Cells. Br. J. Haematol. 2000, 109, 576–583. [Google Scholar] [CrossRef] [PubMed]
- Leggett, R.M.; Ramirez-Gonzalez, R.H.; Clavijo, B.J.; Waite, D.; Davey, R.P. Sequencing quality assessment tools to enable data-driven informatics for high throughput genomics. Front. Genet. 2013, 4, 288. [Google Scholar] [CrossRef]
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet. J. 2011, 17, 10. [Google Scholar] [CrossRef]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef]
- Liao, Y.; Smyth, G.K.; Shi, W. featureCounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef]
- Cafforio, P.; D’Oronzo, S.; Felici, C.; Sigala, S.; Fragni, M.; Silvestris, F. 1,25(OH)2 vitamin D(3) contributes to osteoclast-like trans-differentiation of malignant plasma cells. Exp. Cell Res. 2017, 358, 260–268. [Google Scholar] [CrossRef]
- Park, W.H.; Seol, J.G.; Kim, E.S.; Jung, C.W.; Lee, C.C.; Binderup, L.; Koeffler, H.P.; Kim, B.K.; Lee, Y.Y. Cell Cycle Arrest Induced by the Vitamin D3 Analog EB1089 in NCI-H929 Myeloma Cells Is Associated with Induction of the Cyclin-Dependent Kinase Inhibitor p27. Exp. Cell Res. 2000, 254, 279–286. [Google Scholar]
- Puthier, D.; Bataille, R.; Barille, S.; Mellerin, M.; Harousseau, J.; Ponzio, A.; Robillard, N.; Wijdenes, J.; Amiot, M. Myeloma cell growth arrest, apoptosis, and interleukin-6 receptor modulation induced by EB1089, a vitamin D3 derivative, alone or in association with dexamethasone. Blood 1996, 88, 4659–4666. [Google Scholar]
- Park, W.H.; Seol, J.G.; Kim, E.S.; Binderup, L.; Koeffler, H.P.; Kim, B.K.; Lee, Y.Y. The induction of apoptosis by a combined 1,25(OH)2D3 analog, EB1089 and TGF-beta1 in NCI-H929 multiple myeloma cells. Int. J. Oncol. 2002, 20, 533–542. [Google Scholar] [PubMed]
- Kumagai, T.; O’Kelly, J.; Said, J.W.; Koeffler, H.P. Vitamin D2 Analog 19-nor-1,25-Dihydroxyvitamin D2: Antitumor Activity Against Leukemia, Myeloma, and Colon Cancer Cells. JNCI J. Natl. Cancer Inst. 2003, 95, 896–905. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Giltnane, J.M.; Balko, J.M.; Schwarz, L.J.; Guerrero-Zotano, A.L.; Hutchinson, K.E.; Nixon, M.J.; Estrada, M.V.; Sánchez, V.; Sanders, M.E.; et al. MYC and MCL1 Cooperatively Promote Chemotherapy-Resistant Breast Cancer Stem Cells via Regulation of Mitochondrial Oxidative Phosphorylation. Cell Metab. 2017, 26, 633–647. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Zhou, L.; Xia, M.; Lin, N.; Ma, J.; Dong, D.; Sun, L. PGC1α regulates mitochondrial oxidative phosphorylation involved in cisplatin resistance in ovarian cancer cells via nucleo-mitochondrial transcriptional feedback. Exp. Cell Res. 2021, 398, 112369. [Google Scholar] [PubMed]
- Matula, Z.; Mikala, G.; Lukácsi, S.; Matkó, J.; Kovács, T.; Monostori, É.; Uher, F.; Vályi-Nagy, I. Stromal Cells Serve Drug Resistance for Multiple Myeloma via Mitochondrial Transfer: A Study on Primary Myeloma and Stromal Cells. Cancers 2021, 13, 3461. [Google Scholar] [CrossRef]
- Jang, J.S.; Li, Y.; Mitra, A.K.; Bi, L.; Abyzov, A.; van Wijnen, A.J.; Baughn, L.B.; van Ness, B.; Rajkumar, V.; Kumar, S.; et al. Molecular signatures of multiple myeloma progression through single cell RNA-Seq. Blood Cancer, J. 2019, 9, 2. [Google Scholar] [CrossRef]
- García, V.; Lara-Chica, M.; Cantarero, I.; Sterner, O.; Calzado, M.A.; Muñoz, E. Galiellalactone induces cell cycle arrest and apoptosis through the ATM/ATR pathway in prostate cancer cells. Oncotarget 2016, 7, 4490–4506. [Google Scholar]
- Chen, X.X.; Xie, F.F.; Zhu, X.J.; Lin, F.; Pan, S.S.; Gong, L.H.; Qiu, J.G.; Zhang, W.J.; Jiang, Q.W.; Mei, X.L.; et al. Cyclin-dependent kinase inhibitor dinaciclib potently synergizes with cisplatin in preclinical models of ovarian cancer. Oncotarget 2015, 6, 14926–14939. [Google Scholar]
- Ajayi-Smith, A.; van der Watt, P.; Mkwanazi, N.; Carden, S.; Trent, J.O.; Leaner, V.D. Novel small molecule inhibitor of Kpnβ1 induces cell cycle arrest and apoptosis in cancer cells. Exp. Cell Res. 2021, 404, 112637. [Google Scholar] [CrossRef]
- Recasens, A.; Munoz, L. Targeting Cancer Cell Dormancy. Trends Pharmacol. Sci. 2019, 40, 128–141. [Google Scholar]
- Cole, A.J.; Iyengar, M.; Panesso-Gómez, S.; O’Hayer, P.; Chan, D.; Delgoffe, G.M.; Aird, K.M.; Yoon, E.; Bai, S.; Buckanovich, R.J.; et al. NFATC4 promotes quiescence and chemotherapy resistance in ovarian cancer. JCI Insight 2020, 5, e131486. [Google Scholar] [CrossRef] [PubMed]
- Jia, M.; Zheng, D.; Wang, X.; Zhang, Y.; Chen, S.; Cai, X.; Mo, L.; Hu, Z.; Li, H.; Zhou, Z.; et al. Cancer Cell enters reversible quiescence through Intracellular Acidification to resist Paclitaxel Cytotoxicity. Int. J. Med. Sci. 2020, 17, 1652–1664. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Ding, Y.F.; Jia, W.H.; Liu, X.L.; Feng, J.Y.; Zhu, Q.; Cai, S.L.; Yang, Y.S.; Lu, Q.Y.; Huang, X.T.; et al. SET Domain–Containing Protein 4 Epigenetically Controls Breast Cancer Stem Cell Quiescence. Cancer Res. 2019, 79, 4729–4743. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Lin, T.C.; Bi, X.; Lu, G.; Dawson, B.C.; Miranda, R.; Medeiros, L.J.; McNiece, I.; McCarty, N. TRIM44 promotes quiescent multiple myeloma cell occupancy and survival in the osteoblastic niche via HIF-1α stabilization. Leukemia 2019, 33, 469–486. [Google Scholar] [PubMed]
- Zang, M.; Guo, J.; Liu, L.; Jin, F.; Feng, X.; An, G.; Qin, X.; Wu, Y.; Lei, Q.; Meng, B.; et al. Cdc37 suppression induces plasma cell immaturation and bortezomib resistance in multiple myeloma via Xbp1s. Oncogenesis 2020, 9, 31. [Google Scholar]
- Watkins, N.J.; Bohnsack, M.T. The box C/D and H/ACA snoRNPs: Key players in the modification, processing and the dynamic folding of ribosomal RNA: Box C/D and H/ACA snoRNPs. Wiley Interdiscip. Rev. RNA 2012, 3, 397–414. [Google Scholar]
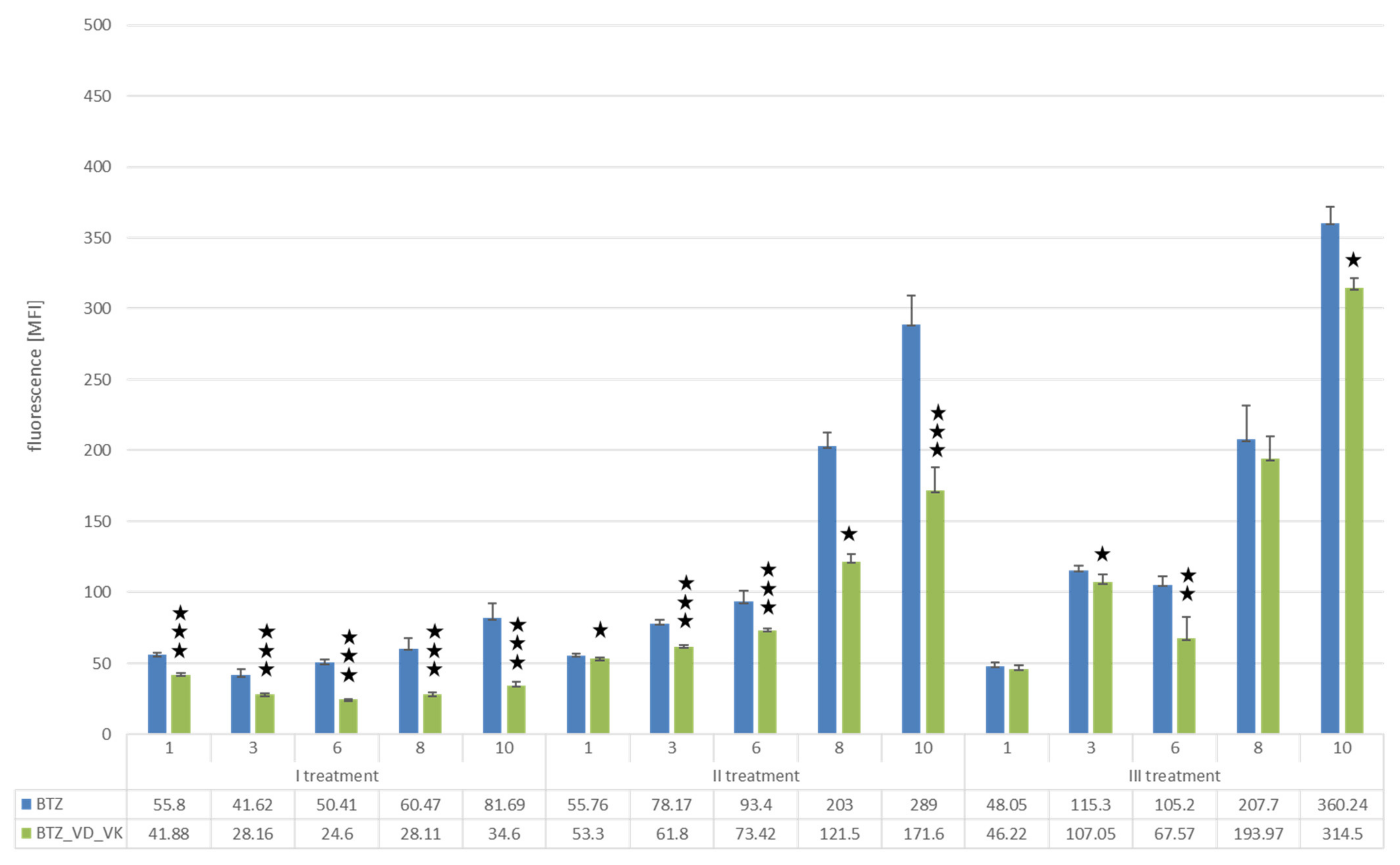

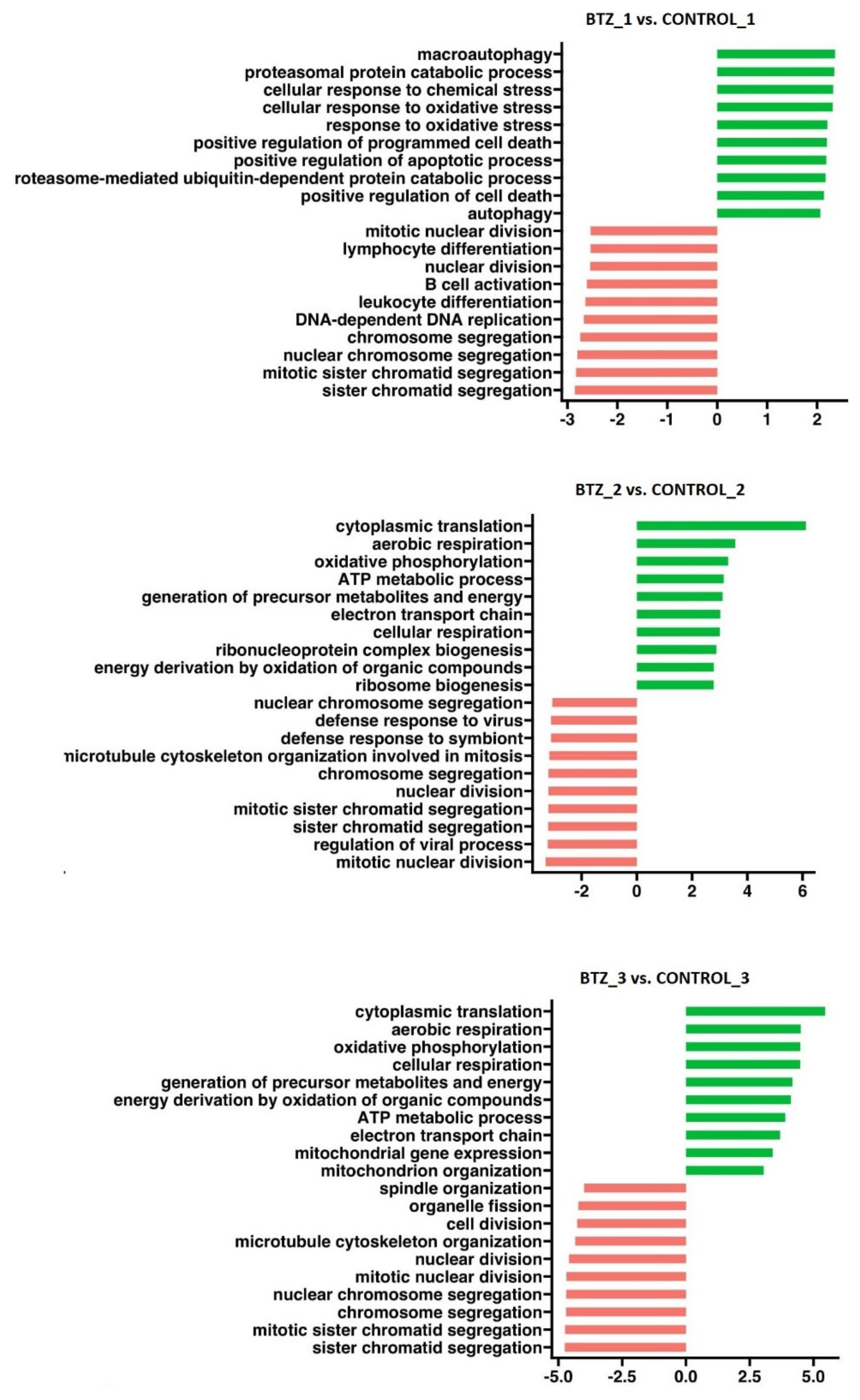


| BTZ Treatment II | BTZ Treatment III | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Symbol GO | Process Name | The Number of Genes Involved | NES | p-Value | p.adj | The Number of Genes Involved | NES | p-Value | p.adj |
| GO:0002181 | cytoplasmic translation | 136 | 6.098 | <0.0001 | <0.0001 | 136 | 5.427 | <0.0001 | <0.0001 |
| GO:0009060 | aerobic respiration | 153 | 3.601 | <0.0001 | <0.0001 | 153 | 4.611 | <0.0001 | <0.0001 |
| GO:0006119 | oxidative phosphorylation | 112 | 3.337 | <0.0001 | <0.0001 | 112 | 4.433 | <0.0001 | <0.0001 |
| GO:0006091 | generation of precursor metabolites and energy | 334 | 3.200 | <0.0001 | <0.0001 | 334 | 4.164 | <0.0001 | <0.0001 |
| GO:0046034 | ATP metabolic process | 195 | 3.156 | <0.0001 | <0.0001 | 195 | 3.956 | <0.0001 | <0.0001 |
| GO:0045333 | cellular respiration | 182 | 3.080 | <0.0001 | <0.0001 | 182 | 4.548 | <0.0001 | <0.0001 |
| GO:0022900 | electron transport chain | 128 | 2.993 | <0.0001 | <0.0001 | 128 | 3.653 | <0.0001 | <0.0001 |
| GO:0015980 | energy derivation by oxidation of organic compounds | 226 | 2.835 | <0.0001 | <0.0001 | 226 | 4.117 | <0.0001 | <0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Łuczkowska, K.; Kulig, P.; Baumert, B.; Machaliński, B. The Evidence That 25(OH)D3 and VK2 MK-7 Vitamins Influence the Proliferative Potential and Gene Expression Profiles of Multiple Myeloma Cells and the Development of Resistance to Bortezomib. Nutrients 2022, 14, 5190. https://doi.org/10.3390/nu14235190
Łuczkowska K, Kulig P, Baumert B, Machaliński B. The Evidence That 25(OH)D3 and VK2 MK-7 Vitamins Influence the Proliferative Potential and Gene Expression Profiles of Multiple Myeloma Cells and the Development of Resistance to Bortezomib. Nutrients. 2022; 14(23):5190. https://doi.org/10.3390/nu14235190
Chicago/Turabian StyleŁuczkowska, Karolina, Piotr Kulig, Bartłomiej Baumert, and Bogusław Machaliński. 2022. "The Evidence That 25(OH)D3 and VK2 MK-7 Vitamins Influence the Proliferative Potential and Gene Expression Profiles of Multiple Myeloma Cells and the Development of Resistance to Bortezomib" Nutrients 14, no. 23: 5190. https://doi.org/10.3390/nu14235190
APA StyleŁuczkowska, K., Kulig, P., Baumert, B., & Machaliński, B. (2022). The Evidence That 25(OH)D3 and VK2 MK-7 Vitamins Influence the Proliferative Potential and Gene Expression Profiles of Multiple Myeloma Cells and the Development of Resistance to Bortezomib. Nutrients, 14(23), 5190. https://doi.org/10.3390/nu14235190






