Neck Circumference for NAFLD Assessment during a 2-Year Nutritional Intervention: The FLiO Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Variable Assessment
2.3. Imaging Techniques
2.4. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Graffigna, M.; Catoira, N.; Soutelo, J.; Azpelicueta, A.; Berg, G.; Perel, C.; Farias, J. Diagnóstico de esteatosis hepática por métodos clínicos, bioquímicos y por imágenes. Rev. Argent. Endocrinol. Metab. 2017, 54, 37–46. [Google Scholar] [CrossRef]
- Rovira, L.C.; Majeed, I.; Escudé, A.M.; Pillasagua, I.A.; Monserrat, P.T. Esteatosis hepática: Diagnóstico y seguimiento. FMC Form. Médica Contin En Atención Primaria 2017, 24, 378–389. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Venkatesan, C. A 2012 clinical update for internists in adult nonalcoholic fatty liver disease. Panminerva Med. 2012, 54, 29–37. [Google Scholar] [PubMed]
- Martinou, E.; Pericleous, M.; Stefanova, I.; Kaur, V.; Angelidi, A.M. Diagnostic Modalities of Non-Alcoholic Fatty Liver Disease: From Biochemical Biomarkers to Multi-Omics Non-Invasive Approaches. Diagnostics 2022, 12, 407. [Google Scholar] [CrossRef] [PubMed]
- Bravo, A.A.; Sheth, S.G.; Chopra, S. Liver biopsy. N. Engl. J. Med. 2001, 344, 495–500. [Google Scholar] [CrossRef]
- Ismaiel, A.; Leucuta, D.-C.; Popa, S.-L.; Fagoonee, S.; Pellicano, R.; Abenavoli, L.; Dumitrascu, D.L. Non-invasive biomarkers in predicting non-alcoholic steatohepatitis and assessing liver fibrosis: Systematic review and meta-analysis. Panminerva Med. 2020, 63, 508–518. [Google Scholar]
- Collantes, R.S.; Ong, J.P.; Younossi, Z.M. The metabolic syndrome and nonalcoholic fatty liver disease. Panminerva Med. 2006, 48, 41–48. [Google Scholar]
- Mennesson, N.; Dumortier, J.; Hervieu, V.; Milot, L.; Guillaud, O.; Scoazec, J.Y.; Pilleul, F. Liver steatosis quantification using magnetic resonance imaging: A prospective comparative study with liver biopsy. J. Comput. Assist. Tomogr. 2009, 33, 672–677. [Google Scholar] [CrossRef]
- Li, Q.; Dhyani, M.; Grajo, J.R. Current status of imaging in nonalcoholic fatty liver disease. World J. Hepatol. 2018, 10, 530–542. [Google Scholar] [CrossRef]
- Li, Q.; Wang, N.; Han, B.; Chen, Y.; Zhu, C.; Chen, Y.; Lu, Y. Neck circumference as an independent indicator of non-alcoholic fatty liver disease in non-obese men. Nutr. Metab. 2015, 12, 1–8. [Google Scholar] [CrossRef]
- Jian, C.; Xu, Y.; Ma, X.; Shen, Y.; Wang, Y.; Bao, Y. Neck Circumference is an Effective Supplement for Nonalcoholic Fatty Liver Disease Screening in a Community-Based Population. Int. J. Endrocrin. 2020, 2020, 7982107. [Google Scholar] [CrossRef]
- Marin-Alejandre, B.A.; Cantero, I.; Perez-Diaz-Del-Campo, N.; Monreal, J.I.; Elorz, M.; Herrero, J.I.; Benito-Boillos, A.; Quiroga, J.; Martinez-Echeverria, A.; Uriz-Otano, J.I.; et al. Effects of two personalized dietary strategies during a 2-year intervention in subjects with nonalcoholic fatty liver disease: A randomized trial. Liver Int. 2021, 7, 1532–1544. [Google Scholar] [CrossRef]
- Marin-Alejandre, B.A.; Abete, I.; Cantero, I.; Monreal, J.I.; Elorz, M.; Herrero, J.I.; Benito-Boillos, A.; Quiroga, J.; Martinez-Echeverria, A.; Uriz-Otano, J.I.; et al. The metabolic and hepatic impact of two personalized dietary strategies in subjects with obesity and nonalcoholic fatty liver disease: The fatty liver in obesity (FLiO) randomized controlled trial. Nutrients 2019, 11, 2543. [Google Scholar] [CrossRef] [PubMed]
- Wallace, T.M.; Levy, J.C. Use and abuse of HOMA modelling. Diabetes Care 2004, 27, 1487–1495. [Google Scholar] [CrossRef] [PubMed]
- Kinner, S.; Reeder, S.B.; Yokoo, T. Quantitative Imaging Biomarkers of NAFLD. Dig Dis. Sci. 2016, 61, 1337–1347. [Google Scholar] [CrossRef] [PubMed]
- Tibshirani, R.; Knight, K. Model Search by Bootstrap “Bumping”. J. Comput. Graph. Stat. 1999, 8, 71–86. [Google Scholar]
- Boemeke, L.; Raimundo, F.V.; Bopp, M.; Leonhardt, L.R.; Fernandes, S.A.; Marroni, C.A. The correlation of neck circumference and insulin resistance in NAFLD patients. Arq. Gastroenterol. 2019, 56, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.-X.; Zhu, M.-F.; Wu, T.; Zhou, J.-Y.; Liu, Y.; Chen, X.-L.; Zhou, R.-F.; Wang, L.-J.; Chen, Y.-M.; Zhu, H.-L. Neck circumference, along with other anthropometric indices, has an independent and additional contribution in predicting fatty liver disease. PLoS ONE 2015, 10, e0118071. [Google Scholar] [CrossRef]
- Zanuncio, V.V.; Sediyama, C.M.N.O.; Dias, M.M.; Nascimento, G.M.; Pessoa, M.C.; Pereira, P.F.; Silva, M.R.I.; Segheto, K.J.; Longo, G.Z. Neck circumference and the burden of metabolic syndrome disease: A population-based sample. J. Public Health 2021, 44, 753–760. [Google Scholar] [CrossRef]
- Salmanroghani, H.; Salmanroghani, R.; Nourian, M.; Khayarn, K.; Lahmi, F.; Iravani, S. Evaluation of neck circumference as an easy and reliable predictor for non-alcoholic fatty liver disease. Turk. J. Gastroenterol. 2019, 30, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, H.; Mahmoudi, P.; Zamani, F.; Moradi, S. Neck circumference and metabolic syndrome: A cross-sectional population-based study. Prim. Care Diabetes 2021, 15, 582–587. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Wang, Z.; Zhang, W.; Niu, Y.; Lin, N.; Li, X.; Zhang, H.; Ning, G.; Fan, J.; Qin, L.; et al. Neck circumference as an independent predictor for NAFLD among postmenopausal women with normal body mass index. Nutr. Metab. 2021, 18, 30. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.-H.; Cai, J.-J.; She, Z.-G.; Li, H.-L. Noninvasive evaluation of nonalcoholic fatty liver disease: Current evidence and practice. World J. Gastroenterol. 2019, 25, 1307–1326. [Google Scholar] [CrossRef] [PubMed]
- Preis, S.R.; Massaro, J.M.; Hoffmann, U.; D’Agostino Sr, R.B.; Levy, D.; Robins, S.J.; Fox, C.S. Neck circumference as a novel measure of cardiometabolic risk: The Framingham heart study. J. Clin. Endocrinol. Metab. 2010, 95, 3701–3710. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.; Zou, L.; Yin, X.; Wu, J.; Zhang, S.; Mao, J.; Cao, S.; Li, W.; Gan, Y.; Yan, S.; et al. Association between neck circumference and cardiometabolic disease in Chinese adults: A community-based cross-sectional study. BMJ Open 2019, 9, e026253. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Avendaño, G. Elastografía hepática cuantitativa en la valoración de sujetos normales y con esteatosis hepática no alcohólica. Correlación interobservador. Radiol. México 2013, 12, 21–28. [Google Scholar]
- Saneei, P.; Shahdadian, F.; Moradi, S.; Ghavami, A.; Mohammadi, H.; Rouhani, M.H. Neck circumference in relation to glycemic parameters: A systematic review and meta-analysis of observational studies. Diabetol. Metab. Syndr. 2019, 11, 1–16. [Google Scholar] [CrossRef]
- Cho, N.H.; Oh, T.J.; Kim, K.M.; Choi, S.H.; Lee, J.H.; Park, K.S.; Lim, S. Neck Circumference and Incidence of Diabetes Mellitus over 10 Years in the Korean Genome and Epidemiology Study (KoGES). Sci. Rep. 2015, 5, 18565. [Google Scholar] [CrossRef]
- Volaco, A.; Cavalcanti, A.M.; Précoma, R.P.F. Socioeconomic Status: The Missing Link Between Obesity and Diabetes Mellitus? Curr. Diabetes Rev. 2018, 14, 321–326. [Google Scholar] [CrossRef]
- Bochaliya, R.K.; Sharma, A.; Saxena, P.; Ramchandani, G.D.; Mathur, G. To evaluate the association of neck circumference with metabolic syndrome and cardiovascular risk factors. J. Assoc. Physicians India 2019, 67, 60–62. [Google Scholar]

| Study Time-Points | |||||
|---|---|---|---|---|---|
| Variables | Basal (n = 98) | 6 m (n = 76) | 12 m (n = 72) | 24 m (n = 58) | p-Mixed Model |
| Anthropometric variables | |||||
| Weight (kg) | 94.9 ± 13.9 | 85.4 ± 13.1 * | 86.8 ± 14.2 * | 89.4 ± 14.8 * | <0.0001 |
| BMI (kg/m2) | 33.4 ± 3.7 | 30.1 ± 3.8 * | 30.7 ± 4.3 * | 31.5 ± 4.8 * | <0.0001 |
| Waist circ (cm) | 109.1 ± 8.8 | 99.7 ± 9.7 * | 96.9 ± 19.2 * | 105.0 ± 11.9 * | 0.001 |
| Total body fat (kg) | 38.4 ± 8.6 | 31.1 ± 9.0 * | 32.2 ± 9.4 * | 34.6 ± 10.1 * | <0.0001 |
| Visceral fat (kg) | 2.2 ± 0.9 | 1.5 ± 0.7 * | 1.7 ± 1.0 * | 1.9 ± 1.0 * | <0.0001 |
| Neck circ (cm) | 39.6 ± 3.7 | 38.0 ± 3.5 * | 38.2 ± 3.5 * | 39.4 ± 4.0 | 0.172 |
| NHtR | 23.4 ± 1.8 | 22.6 ± 1.8 * | 22.8 ± 1.8 * | 23.4 ± 2.1 | 0.216 |
| NWtR | 0.41 ± 0.04 | 0.45 ± 0.05 * | 0.44 ± 0.04 * | 0.44 ± 0.05 * | <0.0001 |
| Biochemical variables | |||||
| Glucose (mg/dL) | 103.2 ± 17.1 | 93.8 ± 12.6 * | 94.2 ± 17.6 * | 96.2 ± 19.4 * | <0.0001 |
| Insulin (mU/L) | 17.3 ± 8.2 | 11.2 ± 7.2 * | 12.5 ± 7.2 * | 12.0 ± 0.9 * | <0.0001 |
| HOMA-IR | 4.5 ± 2.4 | 2.6 ± 2.0 * | 3.1 ± 2.5 * | 3.0 ± 2.0 * | <0.0001 |
| Total cholesterol (mg/dL) | 191.1 ± 36.5 | 180.9 ± 41.9 * | 180.0 ± 34.1 * | 188.6 ± 41.7 | 0.299 |
| Triglycerides (mg/dL) | 129.8 ± 61.1 | 94.5 ± 50.6 * | 105.8 ± 46.9 * | 125.9 ± 79.0 | 0.240 |
| HDL-c (mg/dL) | 51.8 ± 13.0 | 53.8 ± 12.8 * | 54.8 ± 13.2 * | 53.5 ± 13.6 | 0.237 |
| LDL-c (mg/dL) | 113.2 ± 32.2 | 107.7 ± 36.0 | 104.2 ± 29.4 * | 109.9 ± 32.5 | 0.353 |
| Hepatic variables | |||||
| ALT (IU/L) | 33.2 ± 17.1 | 22.2 ± 8.8 * | 25.0 ± 12.0 * | 26.9 ± 15.1 * | 0.001 |
| AST (IU/L) | 25.3 ± 10.1 | 21.7 ± 7.3 * | 22.9 ± 8.7 | 24.4 ± 7.7 | 0.577 |
| GGT (IU/L) | 38.6 ± 28.6 | 27.3 ± 34.3 * | 28.4 ± 19.6 * | 29.4 ± 31.3 | 0.025 |
| Hepatic fat (hist) (%) | 10.5 ± 6.3 | 5.8 ± 4.0 * | 6.7 ± 5.7 * | 7.5 ± 6.1 * | <0.0001 |
| Hepatic fat (dix) (%) | 7.8 ± 8.2 | 3.2 ± 3.2 * | 5.3 ± 4.8 * | 5.7 ± 4.5 | 0.043 |
| Hepatic volumen (cm3) | 1757.6 ± 399.9 | 1591.2 ± 318.5 * | 1620.2 ± 380.3 * | 1660.1 ± 493.4 * | <0.0001 |
| Hepatic Fat Content (MRI-Dixon) | Hepatic Fat Content (MRI-Histo) | |||||||
|---|---|---|---|---|---|---|---|---|
| Combination Panels | Time-Point | Lroc | Sensitivity | Specificity | Time-Point | Lroc | Sensitivity | Specificity |
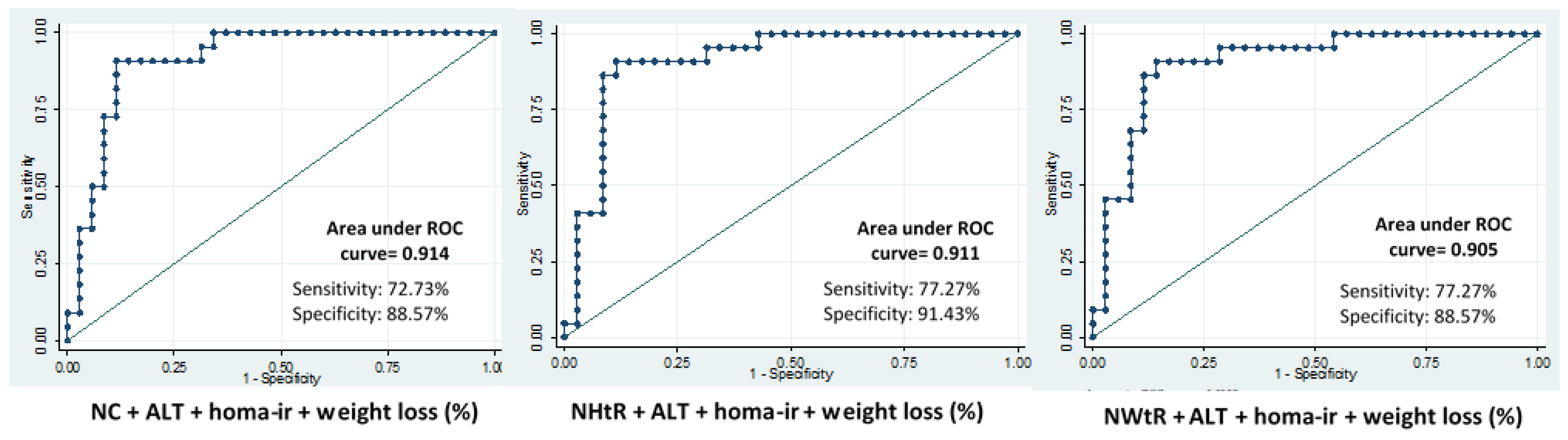
| NC + ALT + HOMA-IR | Baseline | 0.79 | 63.6 | 74.5 | Baseline | 0.79 | 85 | 70.5 |
| 6 m | 0.79 | 28.5 | 98.4 | 6 m | 0.83 | 42.1 | 94.2 | |
| 12 m | 0.75 | 38.8 | 95.9 | 12 m | 0.79 | 47.3 | 95.9 | |
| 24 m | 0.85 | 56.2 | 95.0 | 24 m | 0.89 | 68.1 | 91.4 | |
| NHtR + ALT + HOMA-IR | Baseline | 0.81 | 70.4 | 78.4 | Baseline | 0.81 | 83.3 | 61.7 |
| 6 m | 0.82 | 28.5 | 98.3 | 6 m | 0.87 | 52.6 | 94.2 | |
| 12 m | 0.78 | 61.1 | 95.9 | 12 m | 0.79 | 47.3 | 95.9 | |
| 24 m | 0.88 | 56.2 | 95.0 | 24 m | 0.90 | 68.1 | 88.5 | |
| NWtR + ALT + HOMA-IR | Baseline | 0.79 | 95.9 | 80.3 | Baseline | 0.80 | 83.3 | 58.8 |
| 6 m | 0.79 | 28.5 | 100 | 6 m | 0.81 | 47.3 | 96.1 | |
| 12 m | 0.77 | 38.8 | 95.9 | 12m | 0.81 | 42.1 | 95.9 | |
| 24 m | 0.84 | 50.0 | 92.5 | 24 m | 0.88 | 59.1 | 88.5 | |
| Combination Panels | Steatosis Degree | |||
|---|---|---|---|---|
| Time-Point | Lroc | Sensitivity | Specificity | |
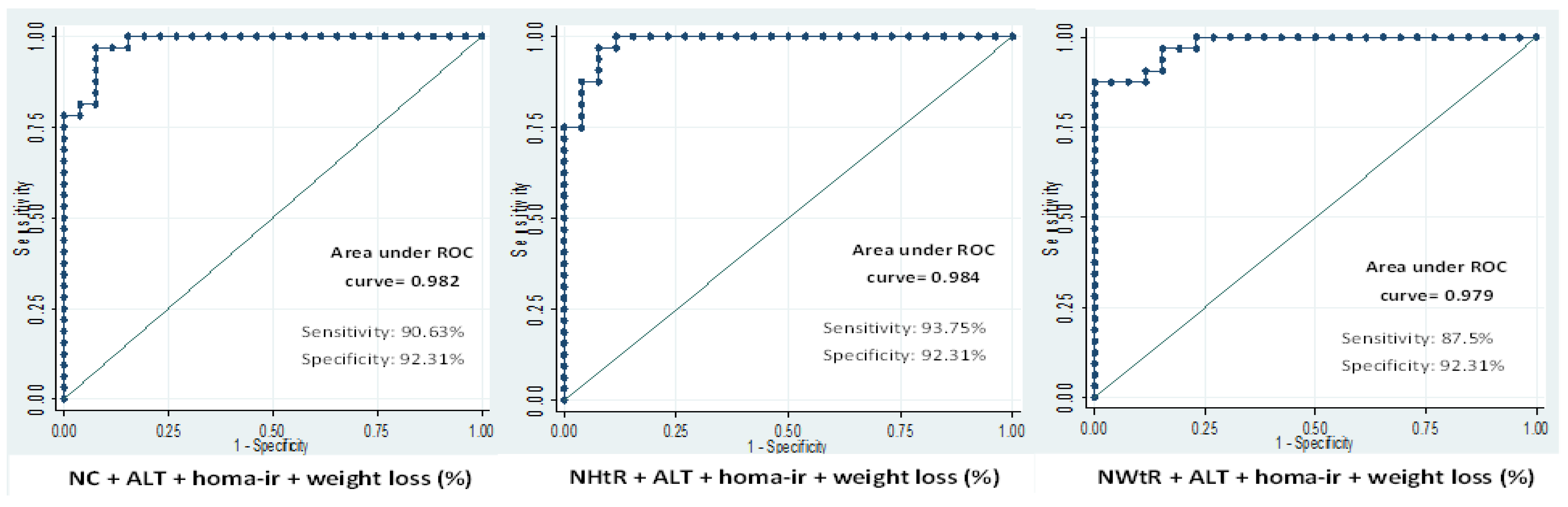
| NC + ALT + HOMA-IR | Baseline | 0.78 | 59.4 | 83.0 |
| 6 m | 0.70 | 83.3 | 29.1 | |
| 12 m | 0.74 | 79.0 | 57.1 | |
| 24 m | 0.95 | 84.3 | 84.6 | |
| NHtR + ALT + HOMA-IR | Baseline | 0.78 | 59.4 | 83.0 |
| 6 m | 0.73 | 85.7 | 50.0 | |
| 12 m | 0.77 | 76.7 | 53.5 | |
| 24 m | 0.97 | 93.7 | 92.3 | |
| NWtR + ALT + HOMA-IR | Baseline | 0.76 | 45.9 | 86.4 |
| 6 m | 0.71 | 80.9 | 29.1 | |
| 12 m | 0.75 | 76.7 | 57.1 | |
| 24 m | 0.95 | 81.2 | 84.6 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elorz, M.; Benito-Boilos, A.; Marin, B.A.; Pérez Díaz del Campo, N.; Herrero, J.I.; Monreal, J.I.; Tur, J.A.; Martínez, J.A.; Zulet, M.A.; Abete, I. Neck Circumference for NAFLD Assessment during a 2-Year Nutritional Intervention: The FLiO Study. Nutrients 2022, 14, 5160. https://doi.org/10.3390/nu14235160
Elorz M, Benito-Boilos A, Marin BA, Pérez Díaz del Campo N, Herrero JI, Monreal JI, Tur JA, Martínez JA, Zulet MA, Abete I. Neck Circumference for NAFLD Assessment during a 2-Year Nutritional Intervention: The FLiO Study. Nutrients. 2022; 14(23):5160. https://doi.org/10.3390/nu14235160
Chicago/Turabian StyleElorz, Mariana, Alberto Benito-Boilos, Bertha Araceli Marin, Nuria Pérez Díaz del Campo, Jose Ignacio Herrero, Jose Ignacio Monreal, Josep A. Tur, J. Alfredo Martínez, Maria Angeles Zulet, and Itziar Abete. 2022. "Neck Circumference for NAFLD Assessment during a 2-Year Nutritional Intervention: The FLiO Study" Nutrients 14, no. 23: 5160. https://doi.org/10.3390/nu14235160
APA StyleElorz, M., Benito-Boilos, A., Marin, B. A., Pérez Díaz del Campo, N., Herrero, J. I., Monreal, J. I., Tur, J. A., Martínez, J. A., Zulet, M. A., & Abete, I. (2022). Neck Circumference for NAFLD Assessment during a 2-Year Nutritional Intervention: The FLiO Study. Nutrients, 14(23), 5160. https://doi.org/10.3390/nu14235160







