The Impacts of Combined Blood Flow Restriction Training and Betaine Supplementation on One-Leg Press Muscular Endurance, Exercise-Associated Lactate Concentrations, Serum Metabolic Biomarkers, and Hypoxia-Inducible Factor-1α Gene Expression
Abstract
1. Introduction
2. Materials and Methods
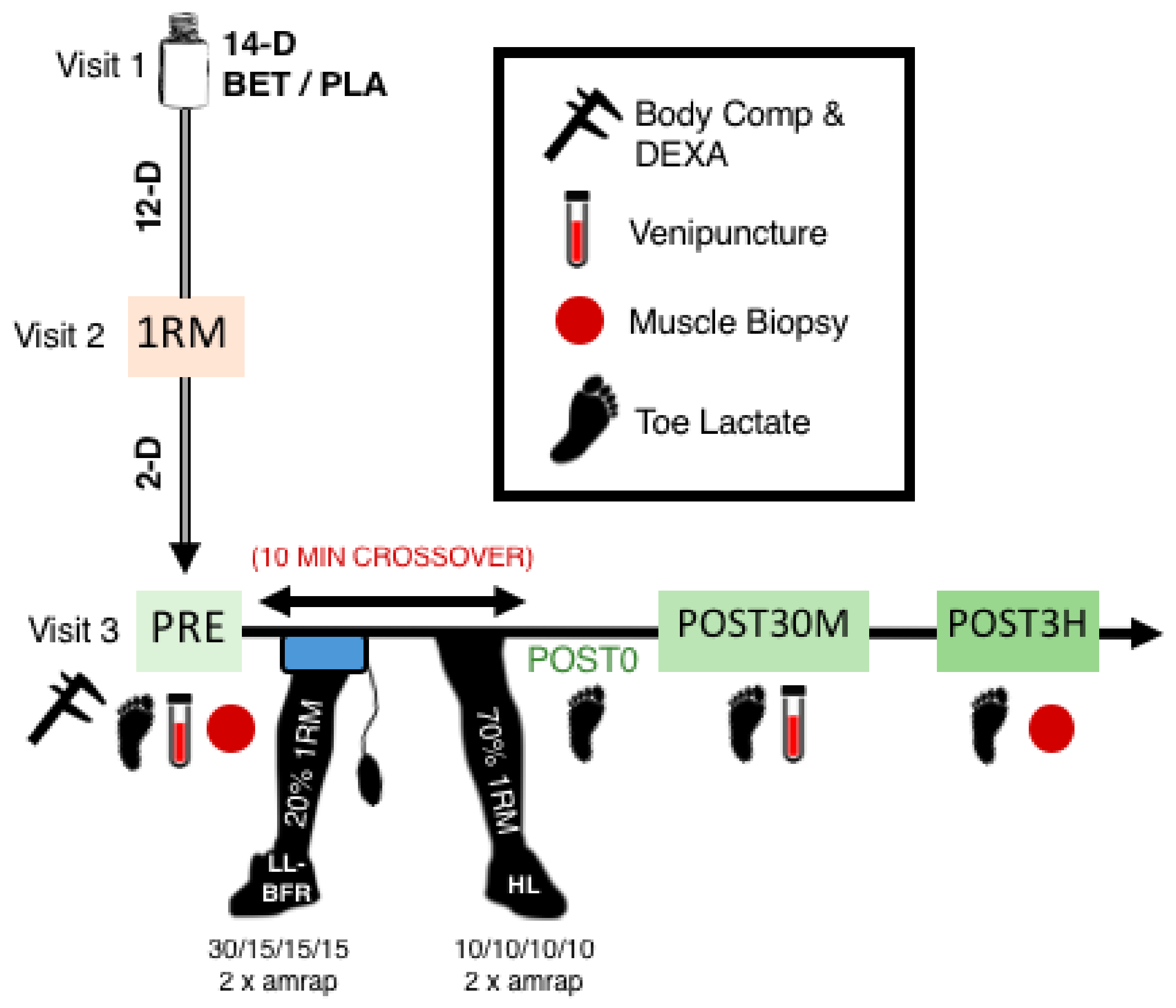
2.1. Experimental Approach to the Problem
2.2. Participants
2.3. Dietary Records
2.4. Betaine Supplementation Protocol
2.5. Body Composition Testing
2.6. One-Repetition Maximum (1RM) Testing
2.7. Blood Flow Restriction Cuff Application and Arterial Occlusion Pressure (AOP)
2.8. Resistance Exercise Protocol
2.9. Rated Perceived Exertion and Subjective Discomfort Scales
2.10. Toe-Tip Capillary Blood Lactate Analysis
2.11. Venipuncture
2.12. Skeletal Muscle Biopsy Procedures and Tissue Processing for cDNA Synthesis
2.13. Serum Growth Hormone (GH), Insulin-like Growth Factor-1 (IGF-1), and Homocysteine (HCY)
2.14. Skeletal Muscle HIF-1A Gene Expression Assessment
2.15. Statistical Analyses
3. Results
3.1. Subject Descriptives, Hematocrit/Packed Cell Volume (PCV%), Body Composition, and Aterial Occlusion Pressure (AOP)
3.2. Dietary Assessments
3.3. 1RM Determination and Repetitions to Failure
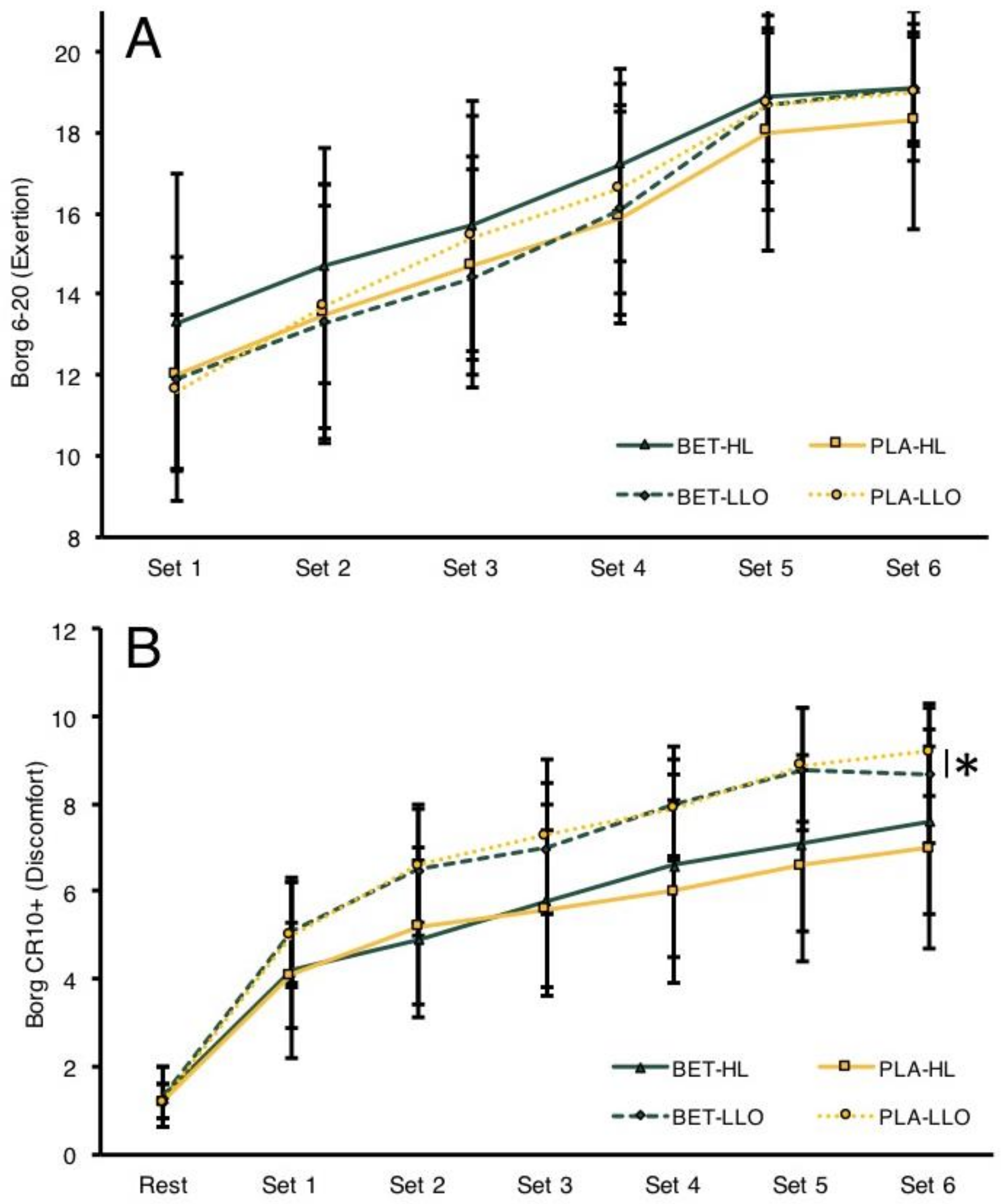
3.4. RPE and Subjective Discomfort
3.5. 1RM Toe-Tip Capillary Lactate
3.6. Serum GH, IGF-1, and HCY Assessment
3.7. HIF-1A Gene Expression Assessment
4. Discussion
5. Conclusions
Limitations and Final Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gundersen, K. Excitation-transcription coupling in skeletal muscle: The molecular pathways of exercise. Biol. Rev. 2011, 86, 564–600. [Google Scholar] [CrossRef] [PubMed]
- Machek, S.B.; Cardaci, T.D.; Willoughby, D.S. Blood Flow Restriction Training and Betaine Supplementation as a Novel Combined Modality to Augment Skeletal Muscle Adaptation: A Short Review. Strength Cond. J. 2021, 43, 50–63. [Google Scholar] [CrossRef]
- Nalbandian, M.; Takeda, M. Lactate as a Signaling Molecule That Regulates Exercise-Induced Adaptations. Biology 2016, 5, 38. [Google Scholar] [CrossRef] [PubMed]
- Patterson, S.D.; Hughes, L.; Warmington, S.; Burr, J.; Scott, B.R.; Owens, J.; Abe, T.; Nielsen, J.L.; Libardi, C.A.; Laurentino, G.; et al. Blood Flow Restriction Exercise: Considerations of Methodology, Application, and Safety. Front. Physiol. 2019, 10, 533. [Google Scholar] [CrossRef] [PubMed]
- Loenneke, J.; Fahs, C.A.; Rossow, L.M.; Abe, T.; Bemben, M.G. The anabolic benefits of venous blood flow restriction training may be induced by muscle cell swelling. Med. Hypotheses 2012, 78, 151–154. [Google Scholar] [CrossRef]
- Riebe, D.; Franklin, B.A.; Thompson, P.D.; Garber, C.E.; Whitefield, G.P.; Magal, M.; Pescatello, L.S. Updating ACSM’s Recommendations for Exercise Preparticipation Health Screening. Med. Sci. Sports Exerc. 2015, 47, 2473–2479. [Google Scholar] [CrossRef]
- Lauver, J.D.; Cayot, T.E.; Rotarius, T.R.; Scheuermann, B.W. Acute Neuromuscular and Microvascular Responses to Concentric and Eccentric Exercises With Blood Flow Restriction. J. Strength Cond. Res. 2020, 34, 2725–2733. [Google Scholar] [CrossRef]
- Liang, X.; Liu, L.; Fu, T.; Zhou, Q.; Zhou, D.; Xiao, L.; Liu, J.; Kong, Y.; Xie, H.; Yi, F.; et al. Exercise Inducible Lactate Dehydrogenase B Regulates Mitochondrial Function in Skeletal Muscle. J. Biol. Chem. 2016, 291, 25306–25318. [Google Scholar] [CrossRef]
- Powers, S.K.; Howley, E.T.; Quindry, J. Exercise Physiology: Theory and Application to Fitness and Performance, 11th ed.; McGraw Hill LLC: New York, NY, USA, 2021. [Google Scholar]
- Godfrey, R.J.; Whyte, G.P.; Buckley, J.; Quinlivan, R. The role of lactate in the exercise-induced human growth hormone response: Evidence from McArdle disease. Br. J. Sports Med. 2009, 43, 521–525. [Google Scholar] [CrossRef]
- Kim, E.; Gregg, L.D.; Kim, L.; Sherk, V.D.; Bemben, M.G.; Bemben, D.A. Hormone responses to an acute bout of low intensity blood flow restricted resistance exercise in college-aged females. J. Sports Sci. Med. 2014, 13, 91–96. [Google Scholar]
- Manini, T.M.; Yarrow, J.F.; Buford, T.W.; Clark, B.C.; Conover, C.F.; Borst, S.E. Growth hormone responses to acute resistance exercise with vascular restriction in young and old men. Growth Horm. IGF Res. 2012, 22, 167–172. [Google Scholar] [CrossRef]
- Fujita, S.; Abe, T.; Drummond, M.K.; Cadenas, J.G.; Dreyer, H.C.; Sato, Y.; Volpi, E.; Rasmussen, B.B. Blood flow restriction during low-intensity resistance exercise increases S6K1 phosphorylation and muscle protein synthesis. J. Appl. Physiol. 2007, 103, 903–910. [Google Scholar] [CrossRef]
- Tanimoto, M.; Madarame, H.; Ishii, N. Muscle oxygenation and plasma growth hormone concentration durign and after resistance exercise: Comparison between KAATSU and other types of regimen. Int. J. Kaatsu Train. Res. 2005, 1, 51–56. [Google Scholar] [CrossRef]
- Takano, H.; Morita, T.; Iida, H.; Asada, K.I.; Kato, M.; Uno, K.; Hirose, K.; Matsumoto, A.; Takenaka, K.; Hirata, Y.; et al. Hemodynamic and hormonal responses to a short-term low-intensity resistance exercise with the reduction of muscle blood flow. Eur. J. Appl. Physiol. 2005, 95, 65–73. [Google Scholar] [CrossRef]
- Behringer, M.; Behlau, D.; Montag, J.C.K.; McCourt, M.L.; Mester, J. Low-Intensity Sprint Training With Blood Flow Restriction Improves 100-m Dash. J. Strength Cond. Res. 2017, 31, 2462–2472. [Google Scholar] [CrossRef]
- Apicella, J.M.; Lee, E.C.; Bailey, B.L.; Saenz, C.; Anderson, J.M.; Craig, S.A.; Kraemer, W.J.; Volek, J.S.; Maresh, C.M. Betaine supplementation enhances anabolic endocrine and Akt signaling in response to acute bouts of exercise. Eur. J. Appl. Physiol. 2013, 113, 793–802. [Google Scholar] [CrossRef]
- Cholewa, J.M.; Hudson, A.; Cicholski, T.; Cervenka, A.; Barreno, K.; Broom, K.; Barch, M.; Craig, S.A. The effects of chronic betaine supplementation on body composition and performance in collegiate females: A double-blind, randomized, placebo controlled trial. J. Int. Soc. Sports Nutr. 2018, 15, 37. [Google Scholar] [CrossRef]
- Hoffman, J.R.; Ratamess, N.A.; Kang, J.; Rashti, S.L.; Faigenbaum, A.D. Effect of betaine supplementation on power performance and fatigue. J. Int. Soc. Sports Nutr. 2009, 6, 7. [Google Scholar] [CrossRef]
- Cholewa, J.M.; Wyszczelska-Rokiel, M.; Glowacki, R.; Jakubowski, H.; Matthews, T.; Wood, R.; Craig, S.A.; Paolone, V. Effects of betaine on body composition, performance, and homocysteine thiolactone. J. Int. Soc. Sports Nutr. 2013, 10, 39. [Google Scholar] [CrossRef]
- Cholewa, J.M.; Guimaraes-Ferreira, L.; Zanchi, N.E. Effects of betaine on performance and body composition: A review of recent findings and potential mechanisms. Amino Acids 2014, 46, 1785–1793. [Google Scholar] [CrossRef]
- Gropper, S.; Smith, J. Advanced Nutrition and Human Metabolism, 6th ed.; Cengage Learning: Belmont, CA, USA, 2013. [Google Scholar]
- Veeranki, S.; Tyagi, S.C. Defective homocysteine metabolism: Potential implications for skeletal muscle malfunction. Int. J. Mol. Sci. 2013, 14, 15074–15091. [Google Scholar] [CrossRef] [PubMed]
- Kempson, S.A.; Vovor-Dassu, K.; Day, C. Betaine transport in kidney and liver: Use of betaine in liver injury. Cell. Physiol. Biochem. 2013, 32, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Kempson, S.A.; Zhou, Y.; Danbolt, N.C. The betaine/GABA transporter and betaine: Roles in brain, kidney, and liver. Front. Physiol. 2014, 5, 159. [Google Scholar] [CrossRef] [PubMed]
- Willingham, B.D.; Ragland, T.J.; Ormsbee, M.J. Betaine Supplementation May Improve Heat Tolerance: Potential Mechanisms in Humans. Nutrients 2020, 12, 2939. [Google Scholar] [CrossRef] [PubMed]
- Burg, M.B.; Ferraris, J.D. Intracellular organic osmolytes: Function and regulation. J. Biol. Chem. 2008, 283, 7309–7313. [Google Scholar] [CrossRef]
- Ortiz-Costa, S.; Sorenson, M.M.; Sola-Penna, M. Betaine protects urea-induced denaturation of myosin subfragment-1. FEBS J. 2008, 275, 3388–3396. [Google Scholar] [CrossRef]
- Pearson, S.J.; Hussain, S.R. A review on the mechanisms of blood-flow restriction resistance training-induced muscle hypertrophy. Sports Med. 2015, 45, 187–200. [Google Scholar] [CrossRef]
- Scott, B.R.; Loenneke, J.P.; Slattery, K.M.; Dascombe, B.J. Exercise with blood flow restriction: An updated evidence-based approach for enhanced muscular development. Sports Med. 2015, 45, 313–325. [Google Scholar] [CrossRef]
- Slow, S.; Lever, M.; Chambers, S.T.; George, P.M. Plasma dependent and independent accumulation of betaine in male and female rat tissues. Physiol. Res. 2009, 58, 403–410. [Google Scholar] [CrossRef]
- Manini, T.M.; Vincent, K.R.; Leewenburgh, C.L.; Lees, H.A.; Kavazis, A.N.; Borst, S.E.; Clark, B.C. Myogenic and proteolytic mRNA expression following blood flow restricted exercise. Acta Physiol. 2011, 201, 255–263. [Google Scholar] [CrossRef]
- Buckner, S.L.; Dankel, S.J.; Counts, B.R.; Jessee, M.B.; Mouser, J.G.; Mattocks, K.T.; Laurentino, G.C.; Abe, T.; Loenneke, J.P. Influence of cuff material on blood flow restriction stimulus in the upper body. J. Physiol. Sci. 2017, 67, 207–215. [Google Scholar] [CrossRef]
- Machek, S.B.; Zawieja, E.E.; Heileson, J.L.; Harris, D.R.; Wilburn, D.T.; Fletcher, E.A.; Cholewa, J.M.; Szwengiel, A.; Chmurzynska, A.; Willoughby, D.S. Human Serum Betaine and Associated Biomarker Concentrations Following a 14 Day Supplemental Betaine Loading Protocol and during a 28 Day Washout Period: A Pilot Investigation. Nutrients 2022, 14, 498. [Google Scholar] [CrossRef]
- Clow, K.A.; Treberg, J.R.; Brosnan, M.E.; Brosnan, J.T. Elevated tissue betaine contents in developing rats are due to dietary betaine, not to synthesis. J. Nutr. 2008, 138, 1641–1646. [Google Scholar] [CrossRef]
- Schwab, U.; Alfthan, G.; Aro, A.; Uusitupa, M. Long-term effect of betaine on risk factors associated with the metabolic syndrome in healthy subjects. Eur. J. Clin. Nutr. 2011, 65, 70–76. [Google Scholar] [CrossRef]
- Moir, G.L. NSCA’s Guide to Tests and Assessments; Muscular Strength; Human Kinetics: Champaign, IL, USA, 2010. [Google Scholar]
- Ramsey, J. Bilateral Deficit: A Comparison between Upper-Body and Lower-Body Maximal Strength. In Health Promotion and Human Performane; Eastern Michigan University: Ypsilanti, MI, USA, 2018. [Google Scholar]
- Wilburn, D.T.; Machek, S.B.; Cardaci, T.D.; Hwang, P.S.; Willoughby, D.S. Acute Maltodextrin Supplementation During Resistance Exercise. J. Sports Sci. Med. 2020, 19, 282–288. [Google Scholar]
- Hoffman, J. Norms for Fitness, Performance, and Health; Human Kinetics: Champaign, IL, USA, 2007. [Google Scholar]
- Wallace, B.J.; Winchester, J.B.; McGuigan, M.R. Effects of elastic bands on force and power characteristics during the back squat exercise. J. Strength Cond. Res. 2006, 20, 268–272. [Google Scholar]
- Machek, S.B.; Cardaci, T.D.; Wilburn, D.T.; Cholewinski, M.C.; Latt, S.L.; Harris, D.R.; Willoughby, D.S. Neoprene Knee Sleeves of Varying Tightness Augment Barbell Squat One Repetition Maximum Performance Without Improving Other Indices of Muscular Strength, Power, or Endurance. J. Strength Cond. Res. 2021, 35 (Suppl. 1), S6–S15. [Google Scholar] [CrossRef]
- Laurentino, G.C.; Loenneke, J.P.; Mouser, J.G.; Buckner, S.L.; Counts, B.R.; Dankel, S.J.; Jessee, M.B.; Mattocks, K.T.; Iared, W.; Tavares, L.D.; et al. Validity of the Handheld Doppler to Determine Lower-Limb Blood Flow Restriction Pressure for Exercise Protocols. J. Strength Cond. Res. 2020, 34, 2693–2696. [Google Scholar] [CrossRef]
- Cardaci, T.D.; Machek, S.B.; Wilburn, D.T.; Heileson, J.L.; Willoughby, D.S. High-Load Resistance Exercise Augments Androgen Receptor-DNA Binding and Wnt/beta-Catenin Signaling without Increases in Serum/Muscle Androgens or Androgen Receptor Content. Nutrients 2020, 12, 3829. [Google Scholar] [CrossRef]
- Clark, N.C.; Reilly, L.J.; Davies, S.C. Intra-rater reliability, measurement precision, and inter-test correlations of 1RM single-leg leg-press, knee-flexion, and knee-extension in uninjured adult agility-sport athletes: Considerations for right and left unilateral measurements in knee injury control. Phys. Ther. Sport 2019, 40, 128–136. [Google Scholar]
- Wilburn, D.T.; Machek, S.B.; Zechmann, B.; Willoughby, D.S. Comparison of skeletal muscle ultrastructural changes between normal and blood flow restricted resistance exercise: A case report. Exp. Physiol. 2021, 106, 2177–2184. [Google Scholar] [CrossRef] [PubMed]
- Loenneke, J.P.; Thiebaud, R.S.; Fahs, C.A.; Rossow, L.M.; Abe, T.; Bemben, M.G. Blood flow restriction: Effects of cuff type on fatigue and perceptual responses to resistance exercise. Acta Physiol. Hung. 2014, 101, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Forsyth, J.J.; Farrally, M.R. A comparison of lactate concentration in plasma collected from the toe, ear, and fingertip after a simulated rowing exercise. Br. J. Sports Med. 2000, 34, 35–38. [Google Scholar] [CrossRef] [PubMed]
- Tanner, R.K.; Fuller, K.L.; Ross, M.L. Evaluation of three portable blood lactate analysers: Lactate Pro, Lactate Scout and Lactate Plus. Eur. J. Appl. Physiol. 2010, 109, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Newmire, D.E.; Willoughby, D.S. The skeletal muscle microbiopsy method in exercise and sports science research: A narrative and methodological review. Scand. J. Med. Sci. Sports 2022, 32, 1550–1568. [Google Scholar] [CrossRef]
- O’Connell, N.S.; Dai, L.; Jiang, Y.; Speiser, J.L.; Ward, R.; Wei, W.; Carroll, R.; Gebregziabher, M. Methods for Analysis of Pre-Post Data in Clinical Research: A Comparison of Five Common Methods. J. Biom. Biostat. 2017, 8, 1–8. [Google Scholar]
- O’Connor, K.; Stip, E.; Pélissier, M.C.; Aardema, F.; Guay, S.; Gaudette, G.; Van Haaster, I.; Robillard, S.; Grenier, S.; Careau, Y.; et al. Treating delusional disorder: A comparison of cognitive-behavioural therapy and attention placebo control. Can. J. Psychiatry 2007, 52, 182–190. [Google Scholar] [CrossRef]
- Kubera, B.; Hubold, C.; Otte, S.; Lindenberg, A.S.; Zeiß, I.; Krause, R.; Steinkamp, M.; Klement, J.; Entringer, S.; Pellerin, L.; et al. Rise in plasma lactate concentrations with psychosocial stress: A possible sign of cerebral energy demand. Obes. Facts 2012, 5, 384–392. [Google Scholar] [CrossRef]
- Tibana, R.; de Sousa, N.; Prestes, J.; Nascimento, D.; Ernesto, C.; Neto, J.; Kennedy, M.; Voltarelli, F. Is perceived exertion a useful indicator of the metabolic and cardiovascular responses to a metabolic conditioning session of functional fitness? Sports 2019, 7, 1–12. [Google Scholar]
- Mazzeo, R.S.; Marshall, P. Influence of plasma catecholamines on the lactate threshold during graded exercise. J. Appl. Physiol. 1989, 67, 1319–1322. [Google Scholar] [CrossRef]
- Bocek, R.M.; Young, M.D.; Beatty, C.H. Effect of insulin and epinephrine on the carbohydrate metabolism and adenylate cyclase activity of rhesus fetal muscle. Pediatr. Res. 1973, 7, 787–793. [Google Scholar] [CrossRef]
- Baker, J.S.; Bailey, D.M.; Hullin, D.; Young, I.; Davies, B. Metabolic implications of resistive force selection for oxidative stress and markers of muscle damage during 30 s of high-intensity exercise. Eur. J. Appl. Physiol. 2004, 92, 321–327. [Google Scholar] [CrossRef]
- Arazi, H.; Mirzaei, B.; Heidari, N. Neuromuscular and metabolic responses to three different resistance exercise methods. Asian J. Sports Med. 2014, 5, 30–38. [Google Scholar] [CrossRef]
- Mahmoodi, N.; Harijan, R.K.; Schramm, V.L. Transition-State Analogues of Phenylethanolamine N-Methyltransferase. J. Am. Chem. Soc. 2020, 142, 14222–14233. [Google Scholar] [CrossRef]
- Patterson, S.D.; Leggate, M.; Nimmo, M.A.; Ferguson, R.A. Circulating hormone and cytokine response to low-load resistance training with blood flow restriction in older men. Eur. J. Appl. Physiol. 2013, 113, 713–719. [Google Scholar] [CrossRef]
- Takarada, Y.; Nakamura, Y.; Aruga, S.; Onda, T.; Miyazaki, S.; Ishii, N. Rapid increase in plasma growth hormone after low-intensity resistance exercise with vascular occlusion. J. Appl. Physiol. 2000, 88, 61–65. [Google Scholar] [CrossRef]
- Takarada, Y.; Tsuruta, T.; Ishii, N. Cooperative effects of exercise and occlusive stimuli on muscular function in low-intensity resistance exercise with moderate vascular occlusion. Jpn. J. Physiol. 2004, 54, 585–592. [Google Scholar] [CrossRef]
- Drummond, M.J.; Fujita, S.; Abe, T.; Dreyer, H.C.; Volpi, E.; Rasmussen, B.B. Human muscle gene expression following resistance exercise and blood flow restriction. Med. Sci. Sports Exerc. 2008, 40, 691–698. [Google Scholar] [CrossRef]
- Laurentino, G.; Aoki, M.; Fernandez, R.; Soares, A.; Ugrinowitsch, C.; Hoschel, H.; Tricoli, V. Low-load resistance exercise with blood flow restriction changes hypoxia-induced genes expression. FASEB J. 2018, 32 (Suppl. 1), 855.23. [Google Scholar] [CrossRef]
- Li, J.; Li, Y.; Atakan, M.M.; Kuang, J.; Hu, Y.; Bishop, D.J.; Yan, X. The Molecular Adaptive Responses of Skeletal Muscle to High-Intensity Exercise/Training and Hypoxia. Antioxidants 2020, 9, 656. [Google Scholar] [CrossRef]
- Lindholm, M.E.; Fischer, H.; Poellinger, L.; Johnson, R.S.; Gustafsson, T.; Sundberg, C.J.; Rundqvist, H. Negative regulation of HIF in skeletal muscle of elite endurance athletes: A tentative mechanism promoting oxidative metabolism. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014, 307, R248–R255. [Google Scholar] [CrossRef] [PubMed]
- Bjornsen, T.; Wernbom, M.; Kirketeig, A.; Paulsen, G.; Samnoy, L.; Baekken, L.; Cameron-Smith, D.; Berntsen, S.; Raastad, T. Type 1 Muscle Fiber Hypertrophy after Blood Flow-restricted Training in Powerlifters. Med. Sci. Sports Exerc. 2019, 51, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Wooten, S.V.; Stray-Gundersen, S.; Tanaka, H. Hemodynamic and Pressor Responses to Combination of Yoga and Blood Flow Restriction. Int. J. Sports Med. 2020, 41, 759–765. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Han, T.; Wener, H.; Rosen, C.J.; Schaffller, M.B.; Yakar, S. Reduced Serum IGF-1 Associated With Hepatic Osteodystrophy Is a Main Determinant of Low Cortical but Not Trabecular Bone Mass. J. Bone Miner. Res. 2018, 33, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Wang, W.; Tang, X.; Chen, R.; Chen, Z.; Lu, Y.; Yuan, H. Association between homocysteine and non-alcoholic fatty liver disease in Chinese adults: A cross-sectional study. Nutr. J. 2016, 15, 102. [Google Scholar] [CrossRef] [PubMed]
- Yosef, A.; Noha, D.; Khaled, G. Correlations among insulin-like growth factor-1, homocysteine, vitamin B12, and folic acid plasma levels following isoretinoin therapy in acne vulgaris patients. J. Egypt. Women Dermatol. Soc. 2018, 15, 47–53. [Google Scholar] [CrossRef]
- Alvarez-Nava, F.; Lanes, R. GH/IGF-1 Signaling and Current Knowledge of Epigenetics; a Review and Considerations on Possible Therapeutic Options. Int. J. Mol. Sci. 2017, 18, 1624. [Google Scholar] [CrossRef]
- Jia, Y.; Song, H.; Gao, G.; Cai, D.; Yang, X.; Zhao, R. Maternal Betaine Supplementation during Gestation Enhances Expression of mtDNA-Encoded Genes through D-Loop DNA Hypomethylation in the Skeletal Muscle of Newborn Piglets. J. Agric. Food Chem. 2015, 63, 10152–10160. [Google Scholar] [CrossRef]
- Egerman, M.A.; Glass, D.J. Signaling pathways controlling skeletal muscle mass. Crit. Rev. Biochem. Mol. Biol. 2014, 49, 59–68. [Google Scholar] [CrossRef]
- Bagley, J.; Rosengarten, J.; Galpin, J. Is blood flow restriction training beneficial for athletes? Strength Cond. J. 2015, 37, 48–53. [Google Scholar] [CrossRef]
| Mean ± SD | BET (n = 9) | PLA (n = 9) |
|---|---|---|
| Age (years) | 23 ± 3 | 22 ± 3 |
| Height (cm) | 177.9 ± 6.5 | 179.5 ± 7.7 |
| Weight (kg) | 87.6 ± 5.4 | 84.2 ± 17.3 |
| BF% | 15.6 ± 3.0 | 13.5 ± 3.6 |
| Resting HR (bpm) | 68 ± 8 | 68 ± 7 |
| Resting SBP/DBP (mmHg) | 125 ± 10/74 ± 13 | 128 ± 9/69 ± 9 |
| Mean ± SD | BET (n = 9) | PLA (n = 9) | p-Value (<0.05) |
|---|---|---|---|
| 1RM Testing (Supplementation Day 12) | |||
| 1RM RL (kg) | 263 ± 46 | 251 ± 72 | Main Leg Effect = 0.802 Interaction Effect = 0.819 |
| 1RM LL (kg) | 260 ± 53 | 251 ± 81 | |
| Total 1RM Attempts RL | 5 ± 1 | 6 ± 2 | 0.200 |
| Total 1RM Attempts LL | 5 ± 2 | 6 ± 2 | 0.200 |
| Experimental Protocol (12 h Post Supplementation) | |||
| AOP (mmHg) | 318 ± 82 | 310 ± 62 | 0.833 |
| PCV% | 45.9 ± 1.6 | 46.9 ± 2.9 | 0.384 |
| Mean ± SD | BET (n = 9) | PLA (n = 9) | p-Value (<0.05); ES (ηp2) |
|---|---|---|---|
| Dietary CHO (g·kg−1 bw) | Main Supplement Effect = 0.388; ηp2 = 0.042 | ||
| 1RM Testing | 3.0 ± 0.9 | 3.2 ± 1.0 | Main Time Effect = 0.416; ηp2 = 0.042 |
| Experimental Testing | 2.7 ± 1.0 | 3.2 ± 1.3 | Interaction Effect = 0.451; ηp2 = 0.036 |
| Dietary PRO (g·kg−1 bw) | Main Supplement Effect = 0.502; ηp2 = 0.936 | ||
| 1RM Testing | 1.6 ± 0.6 | 1.7 ± 0.4 | Main Time Effect = 0.334; ηp2 = 0.058 |
| Experimental Testing | 1.6 ± 0.6 | 1.8 ± 0.5 | Interaction Effect = 0.863; ηp2 = 0.002 |
| Dietary FAT (g·kg−1 bw) | Main Supplement Effect = 0.078; ηp2 = 0.181 | ||
| 1RM Testing | 0.9 ± 0.2 | 1.3 ± 0.4 | Main Time Effect = 0.666; ηp2 = 0.012 |
| Experimental Testing | 1.0 ± 0.4 | 1.3 ± 0.8 | Interaction Effect = 0.666; ηp2 = 0.012 |
| Dietary Fiber (g·kg−1 bw) | Main Supplement Effect = 1.00; ηp2 = 0.835 | ||
| 1RM Testing | 0.3 ± 0.1 | 0.2 ± 0.1 | Main Time Effect = 0.429; ηp2 = 0.040 |
| Experimental Testing | 0.2 ± 0.1 | 0.2 ± 0.1 | Interaction Effect = 0.124; ηp2 = 0.142 |
| Mean ± SD Lactate (mmol·L−1) | BET | PLA | ||||||
|---|---|---|---|---|---|---|---|---|
| PRE | POST0 | POST30M | POST3H | PRE | POST0 | POST30M | POST3H | |
| HL | 2.7 ± 0.7 | 6.9 ± 2.3 | 6.4 ± 1.9 | 2.9 ± 1.0 | 2.4 ± 0.7 | 6.1 ± 1.2 | 6.2 ± 1.7 | 2.7 ± 1.6 |
| LL-BFR | 2.3 ± 0.8 | 6.1 ± 1.2 | 5.0 ± 1.6 | 2.7 ± 1.1 | 2.5 ± 0.8 | 5.9 ± 1.6 | 5.3 ± 1.6 | 2.7 ± 1.1 |
| ∆POST0 | ∆POST30M | ∆POST3H | ∆POST0 | ∆POST30M | ∆POST3H | |||
| HL | 4.1 ± 2.2 * | 3.7 ± 2.1 * | 0.2 ± 0.9 *,† | 3.7 ± 1.3 * | 3.8 ± 1.7 * | 0.3 ± 1.1 † | ||
| LL-BFR | 3.8 ± 2.6 | 2.6 ± 1.7 | 0.6 ± 0.5 † | 3.4 ± 1.4 | 2.8 ± 1.4 | 0.2 ± 0.8 † | ||
| Mean ± SD | BET | PLA | Supplement-Collapsed | |||
|---|---|---|---|---|---|---|
| PRE | POST30M | PRE | POST30M | |||
| GH (ng·mL−1) | ||||||
| HL | 3.64 ± 3.01 | 5.51 ± 5.23 | PRE | 1.36 ± 2.13 | ||
| LL-BFR | 4.32 ± 2.94 | 7.16 ± 12.01 | HL-POST30M | 4.52 ± 4.17 | ||
| Condition-Collapsed | 1.76 ± 2.62 | 3.98 ± 2.91 | 0.96 ± 1.53 | 4.40 ± 7.63 | LL-BFR-POST30M | 5.66 ± 8.34 |
| IGF-1 (ng·mL−1) | ||||||
| HL | 111.1 ± 35.2 | 122.7 ± 34.2 | PRE | 120.0 ± 42.0 | ||
| LL-BFR | 118.1 ± 31.5 | 134.7 ± 41.8 | HL-POST30M | 116.5 ± 34.2 | ||
| Condition-Collapsed | 105.4 ± 34.0 | 114.6 ± 32.6 * | 136.6 ± 45.1 | 129.1 ± 37.7 | LL-BFR-POST30M | 126.4 ± 36.9 |
| HCY (μmol·L−1) | ||||||
| HL | 29.1 ± 4.92 | 30.4 ± 3.85 | PRE | 28.2 ± 2.96 | ||
| LL-BFR | 27.9 ± 4.17 | 27.2 ± 3.12 | HL-POST30M | 29.7 ± 4.34 † | ||
| Condition-Collapsed | 27.7 ± 2.50 | 28.5 ± 4.47 | 28.8 ± 3.42 | 28.9 ± 3.79 | LL-BFR-POST30M | 27.6 ± 3.61 |
| HIF-1A transcript levels | ||||||
| HL | 0.42 ± 0.28 | 0.62 ± 0.49 | 1.40 ± 2.67 | 3.76 ± 9.21 | HL-PRE | 0.86 ± 1.90 |
| LL-BFR | 0.45 ± 0.23 | 0.45 ± 0.23 | 1.48 ± 3.40 | 0.73 ± 0.68 | LLO-PRE | 0.96 ± 2.40 |
| Condition-Collapsed | 0.43 ± 0.25 | 0.76 ± 0.58 | 1.44 ± 2.98 | 2.24 ± 6.52 | HL-POST30M | 2.19 ± 6.53 |
| LL-BFR-POST30M | 0.82 ± 0.70 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Machek, S.B.; Harris, D.R.; Zawieja, E.E.; Heileson, J.L.; Wilburn, D.T.; Radziejewska, A.; Chmurzynska, A.; Cholewa, J.M.; Willoughby, D.S. The Impacts of Combined Blood Flow Restriction Training and Betaine Supplementation on One-Leg Press Muscular Endurance, Exercise-Associated Lactate Concentrations, Serum Metabolic Biomarkers, and Hypoxia-Inducible Factor-1α Gene Expression. Nutrients 2022, 14, 5040. https://doi.org/10.3390/nu14235040
Machek SB, Harris DR, Zawieja EE, Heileson JL, Wilburn DT, Radziejewska A, Chmurzynska A, Cholewa JM, Willoughby DS. The Impacts of Combined Blood Flow Restriction Training and Betaine Supplementation on One-Leg Press Muscular Endurance, Exercise-Associated Lactate Concentrations, Serum Metabolic Biomarkers, and Hypoxia-Inducible Factor-1α Gene Expression. Nutrients. 2022; 14(23):5040. https://doi.org/10.3390/nu14235040
Chicago/Turabian StyleMachek, Steven B., Dillon R. Harris, Emilia E. Zawieja, Jeffery L. Heileson, Dylan T. Wilburn, Anna Radziejewska, Agata Chmurzynska, Jason M. Cholewa, and Darryn S. Willoughby. 2022. "The Impacts of Combined Blood Flow Restriction Training and Betaine Supplementation on One-Leg Press Muscular Endurance, Exercise-Associated Lactate Concentrations, Serum Metabolic Biomarkers, and Hypoxia-Inducible Factor-1α Gene Expression" Nutrients 14, no. 23: 5040. https://doi.org/10.3390/nu14235040
APA StyleMachek, S. B., Harris, D. R., Zawieja, E. E., Heileson, J. L., Wilburn, D. T., Radziejewska, A., Chmurzynska, A., Cholewa, J. M., & Willoughby, D. S. (2022). The Impacts of Combined Blood Flow Restriction Training and Betaine Supplementation on One-Leg Press Muscular Endurance, Exercise-Associated Lactate Concentrations, Serum Metabolic Biomarkers, and Hypoxia-Inducible Factor-1α Gene Expression. Nutrients, 14(23), 5040. https://doi.org/10.3390/nu14235040








