The Anti-Cancer Activity of Lycopene: A Systematic Review of Human and Animal Studies
Abstract
1. Introduction
2. Methods
2.1. Eligibility Criteria and Search Strategy
2.2. Data Extraction
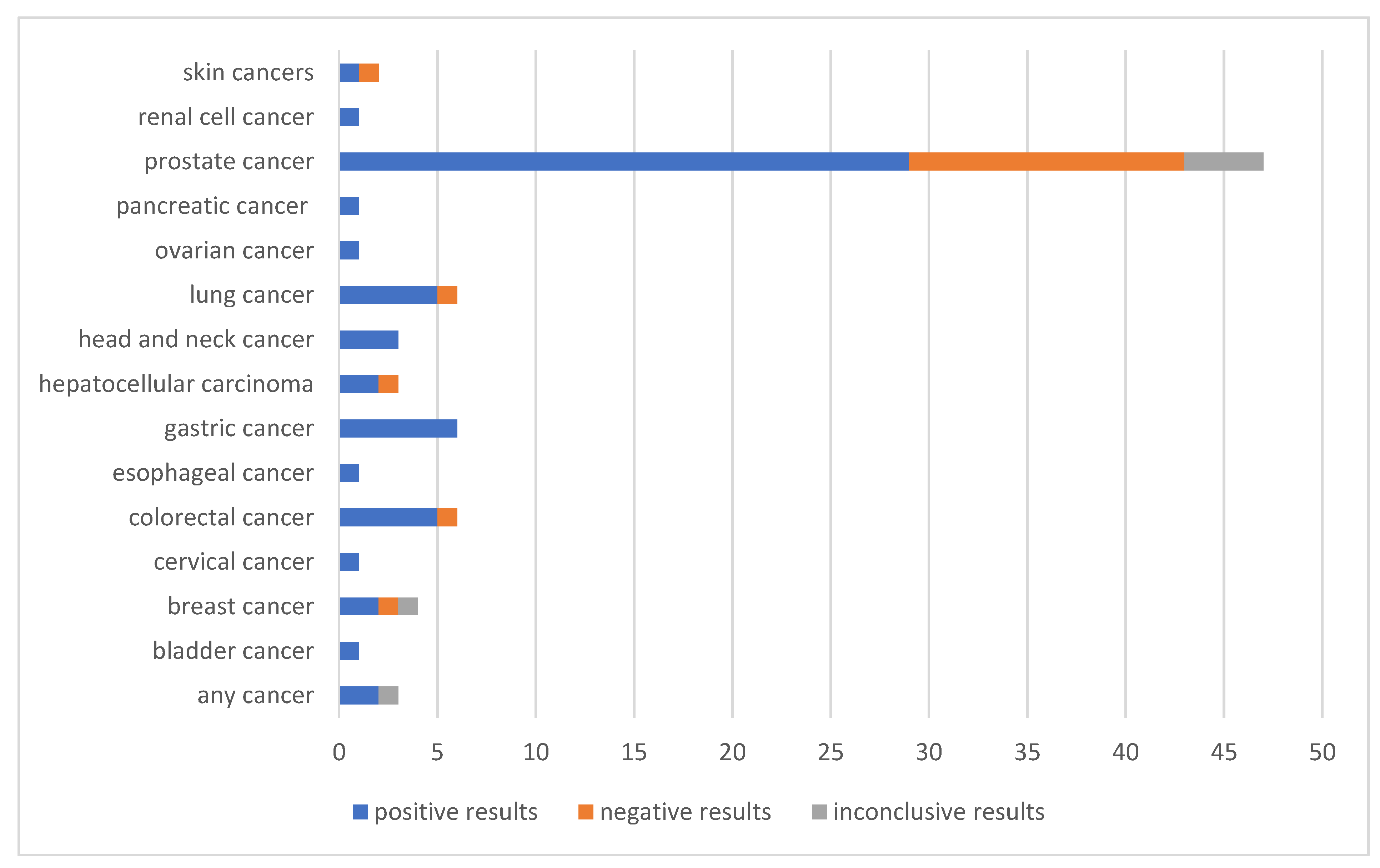
3. Results
3.1. Prostate Cancer
3.2. Other Cancers
3.2.1. Positive Results That Confirm the Anti-Cancer Effect of Lycopene
3.2.2. Negative Results That Deny the Anti-Cancer Effect of Lycopene
| Mechanism | Pathway | Cancer | Model | Reference |
|---|---|---|---|---|
| Inhibition of angiogenesis | Lower degree of angiogenesis in the tumour | Prostate cancer | Human | [24] |
| ↓ vascular endothelial growth factor (VEGF) | Prostate cancer | Animal | [27] | |
| ↓ VEGF and CD31 | Hepatocellular cancer | Animal | [62,88] | |
| Reduction of oxidative DNA damage | ↓ oxidative injury (↓ HIF-1α, Cyr61, NDRG1, BNIP3, STAT2 mRNA); ↑ antioxidant enzyme activities (SOD, CAT and GPx) | Gastric cancer | Animal | [73,83] |
| ↑ GSH, GPx, GST and GR | Gastric cancer | Animal | [71,93] | |
| ↑ activity of enzymic antioxidants (superoxide dismutase, catalase, and glutathione peroxidase) and reduced levels of nonenzymic antioxidants (glutathione, vitamins E and C) | Lung cancer | Animal | [80] | |
| ↓ level of malondialdehyde, ↑ NRF2 and its major target protein (heme oxygenase 1) | Ovarian cancer | Animal | [94] | |
| ↓ serum thiobarbituric acid-reactive substances | Any cancer | Human | [77] | |
| ↑ serum protein thiol levels | Prostate cancer | Human | [46] | |
| Reduction of inflammation | ↓ COX-2 and PGE2 | Colon cancer | Animal | [69] |
| ↓ NF-κB i COX-2 | Oesophageal cancer | Animal | [81] | |
| ↑IL-4 and IL-10; ↓ IL-6 and TNFα | Gastric cancer | Animal | [73,83] | |
| Hypermethylation of CD40LG and TEC | Head and neck cancer | Human | [66] | |
| ↓ NF-κB | Ovarian cancer | Animal | [94] | |
| Enhanced cytotoxicity | Hypomethylation of CD8A and ↑ CD8 and CD8+ T cells | Head and neck cancer | Human | [66] |
| Enhanced apoptosis | ↑ PPARγ, caspase-3 | Oesophageal cancer | Animal | [81] |
| Accumulation of tumour cells in the G(0)/G(1) phase and further apoptosis | Prostate cancer | Animal | [22,56] | |
| ↓ FOXO3a | Skin cancers | Animal | [86] | |
| ↓ phosphorylation of BAD | Lung cancer | Animal | [91] | |
| ↑ caspase-3, Bax1 | Gastric cancer | Animal | [72] | |
| Inhibition of proliferation: downregulation of insulin-like growth factor (IGF) | ↓ IGF-1 | Breast cancer | Human | [65] |
| ↓ IGF-1; ↑ IGF-BP | Colorectal cancer | Human | [61,76] | |
| ↑ IGF-BP3 | Lung cancer Prostate cancer | Animal | [91] [27] | |
| Inhibition of cell cycle | ↑ cell cycle inhibitors p21(CIP1/WAF1) and p27(Kip1); ↓ factors for DNA division (PCNA, β-catenin, cyclin D1, and c-Myc proteins) | Colon cancer | Animal | [63,69] |
| ↑ p27(Kip1) | Prostate cancer | Animal | [34] | |
| ↓ STAT3 by inducing the protein inhibitor | Ovarian cancer | Animal | [94] | |
| ↓ CDK2 and CDK4 | Skin cancers | Animal | [86] | |
| ↑ p21 and Bax1; ↓ factors for DNA division (cyclin 1, PCNA) | Gastric cancer | Animal | [72] | |
| ↓ PCNA | Hepatocellular cancer | Animal | [62] | |
| ↓ PCNA | Prostate cancer | Animal | [27,34] | |
| Inhibition of hormone-dependent carcinogenesis | ↓ steroid target genes (cystatin-related protein 1 and 2, prostatic spermine-binding protein, prostatic steroid-binding protein C1, C2, and C3 chain, probasin) | Prostate cancer | Animal | [36] |
| Inhibition of metastasis | ↓ MMP-7 and MMP-9 | Colon cancer | Animal | [69] |
| ↓ MMP-2 and MMP-9 | Hepatocellular cancer | Animal | [62,88] |
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Khan, U.M.; Sevindik, M.; Zarrabi, A.; Nami, M.; Ozdemir, B.; Kaplan, D.N.; Selamoglu, Z.; Hasan, M.; Kumar, M.; Alshehri, M.M.; et al. Lycopene: Food Sources, Biological Activities, and Human Health Benefits. Oxid. Med. Cell. Longev. 2021, 2021, 2713511. [Google Scholar] [CrossRef]
- Zechmeister, L.; Lerosen, A.L.; Went, F.W.; Pauling, L. Prolycopene, a Naturally Occuring Stereoisomer of Lycopene. Proc. Natl. Acad. Sci. USA 1941, 27, 468–474. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.V.; Waseem, Z.; Agarwal, S. Lycopene content of tomatoes and tomato products and their contribution to dietary lycopene. Food Res. Int. 1998, 31, 737–741. [Google Scholar] [CrossRef]
- Unlu, N.Z.; Bohn, T.; Francis, D.M.; Nagaraja, H.N.; Clinton, S.K.; Schwartz, S.J. Lycopene from heat-induced cis-isomer-rich tomato sauce is more bioavailable than from all-trans-rich tomato sauce in human subjects. Br. J. Nutr. 2007, 98, 140–146. [Google Scholar] [CrossRef]
- Bohn, T.; Desmarchelier, C.; Dragsted, L.O.; Nielsen, C.S.; Stahl, W.; Rühl, R.; Keijer, J.; Borel, P. Host-related factors explaining interindividual variability of carotenoid bioavailability and tissue concentrations in humans. Mol. Nutr. Food Res. 2017, 61, 1600685. [Google Scholar] [CrossRef]
- van Het Hof, K.H.; West, C.E.; Weststrate, J.A.; Hautvast, J.G. Dietary factors that affect the bioavailability of carotenoids. J. Nutr. 2000, 130, 503–506. [Google Scholar] [CrossRef] [PubMed]
- Al-Yafeai, A.; Böhm, V. In Vitro Bioaccessibility of Carotenoids and Vitamin E in Rosehip Products and Tomato Paste as Affected by Pectin Contents and Food Processing. J. Agric. Food Chem. 2018, 66, 3801–3809. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Maguer, M. Le Lycopene in Tomatoes: Chemical and Physical Properties Affected by Food Processing. Crit. Rev. Food Sci. Nutr. 2000, 40, 1–42. [Google Scholar] [CrossRef]
- Saini, R.K.; Rengasamy, K.R.R.; Mahomoodally, F.M.; Keum, Y.-S. Protective effects of lycopene in cancer, cardiovascular, and neurodegenerative diseases: An update on epidemiological and mechanistic perspectives. Pharmacol. Res. 2020, 155, 104730. [Google Scholar] [CrossRef]
- Jian, L.; Lee, A.H.; Binns, C.W. Tea and lycopene protect against prostate cancer. Asia Pac. J. Clin. Nutr. 2007, 16 (Suppl. S1), 453–457. [Google Scholar]
- Jian, L.; Du, C.-J.; Lee, A.H.; Binns, C.W. Do dietary lycopene and other carotenoids protect against prostate cancer? Int. J. Cancer 2005, 113, 1010–1014. [Google Scholar] [CrossRef]
- Tang, L.; Jin, T.; Zeng, X.; Wang, J.-S. Lycopene inhibits the growth of human androgen-independent prostate cancer cells in vitro and in BALB/c nude mice. J. Nutr. 2005, 135, 287–290. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, E.; Uchio, E.; Lilly, M.; Zi, X.; Fruehauf, J.P. A phase II study of docetaxel plus lycopene in metastatic castrate resistant prostate cancer. Biomed. Pharmacother. 2021, 143, 112226. [Google Scholar] [CrossRef] [PubMed]
- Graff, R.E.; Pettersson, A.; Lis, R.T.; Ahearn, T.U.; Markt, S.C.; Wilson, K.M.; Rider, J.R.; Fiorentino, M.; Finn, S.; Kenfield, S.A.; et al. Dietary lycopene intake and risk of prostate cancer defined by ERG protein expression. Am. J. Clin. Nutr. 2016, 103, 851–860. [Google Scholar] [CrossRef] [PubMed]
- Schwenke, C.; Ubrig, B.; Thürmann, P.; Eggersmann, C.; Roth, S. Lycopene for advanced hormone refractory prostate cancer: A prospective, open phase II pilot study. J. Urol. 2009, 181, 1098–1103. [Google Scholar] [CrossRef]
- Kirsh, V.A.; Mayne, S.T.; Peters, U.; Chatterjee, N.; Leitzmann, M.F.; Dixon, L.B.; Urban, D.A.; Crawford, E.D.; Hayes, R.B. A prospective study of lycopene and tomato product intake and risk of prostate cancer. Cancer Epidemiol. Biomark. Prev. 2006, 15, 92–98. [Google Scholar] [CrossRef]
- Canene-Adams, K.; Lindshield, B.L.; Wang, S.; Jeffery, E.H.; Clinton, S.K.; Erdman, J.W.J. Combinations of tomato and broccoli enhance antitumor activity in dunning r3327-h prostate adenocarcinomas. Cancer Res. 2007, 67, 836–843. [Google Scholar] [CrossRef]
- Beynon, R.A.; Richmond, R.C.; Santos Ferreira, D.L.; Ness, A.R.; May, M.; Smith, G.D.; Vincent, E.E.; Adams, C.; Ala-Korpela, M.; Würtz, P.; et al. Investigating the effects of lycopene and green tea on the metabolome of men at risk of prostate cancer: The ProDiet randomised controlled trial. Int. J. Cancer 2019, 144, 1918–1928. [Google Scholar] [CrossRef]
- Tang, Y.; Parmakhtiar, B.; Simoneau, A.R.; Xie, J.; Fruehauf, J.; Lilly, M.; Zi, X. Lycopene enhances docetaxel’s effect in castration-resistant prostate cancer associated with insulin-like growth factor I receptor levels. Neoplasia 2011, 13, 108–119. [Google Scholar] [CrossRef]
- Lu, Y.; Edwards, A.; Chen, Z.; Tseng, T.-S.; Li, M.; Gonzalez, G.V.; Zhang, K. Insufficient Lycopene Intake Is Associated With High Risk of Prostate Cancer: A Cross-Sectional Study From the National Health and Nutrition Examination Survey (2003–2010). Front. Public Heal. 2021, 9, 792572. [Google Scholar] [CrossRef]
- Moran, N.E.; Thomas-Ahner, J.M.; Fleming, J.L.; McElroy, J.P.; Mehl, R.; Grainger, E.M.; Riedl, K.M.; Toland, A.E.; Schwartz, S.J.; Clinton, S.K. Single Nucleotide Polymorphisms in β-Carotene Oxygenase 1 are Associated with Plasma Lycopene Responses to a Tomato-Soy Juice Intervention in Men with Prostate Cancer. J. Nutr. 2019, 149, 381–397. [Google Scholar] [CrossRef] [PubMed]
- Boileau, T.W.-M.; Liao, Z.; Kim, S.; Lemeshow, S.; Erdman, J.W.J.; Clinton, S.K. Prostate carcinogenesis in N-methyl-N-nitrosourea (NMU)-testosterone-treated rats fed tomato powder, lycopene, or energy-restricted diets. J. Natl. Cancer Inst. 2003, 95, 1578–1586. [Google Scholar] [CrossRef] [PubMed]
- Konijeti, R.; Henning, S.; Moro, A.; Sheikh, A.; Elashoff, D.; Shapiro, A.; Ku, M.; Said, J.W.; Heber, D.; Cohen, P.; et al. Chemoprevention of prostate cancer with lycopene in the TRAMP model. Prostate 2010, 70, 1547–1554. [Google Scholar] [CrossRef] [PubMed]
- Conlon, L.E.; Wallig, M.A.; Erdman, J.W.J. Low-lycopene containing tomato powder diet does not protect against prostate cancer in TRAMP mice. Nutr. Res. 2015, 35, 882–890. [Google Scholar] [CrossRef]
- Kristal, A.R.; Till, C.; Platz, E.A.; Song, X.; King, I.B.; Neuhouser, M.L.; Ambrosone, C.B.; Thompson, I.M. Serum lycopene concentration and prostate cancer risk: Results from the Prostate Cancer Prevention Trial. Cancer Epidemiol. Biomark. Prev. 2011, 20, 638–646. [Google Scholar] [CrossRef]
- Peters, U.; Leitzmann, M.F.; Chatterjee, N.; Wang, Y.; Albanes, D.; Gelmann, E.P.; Friesen, M.D.; Riboli, E.; Hayes, R.B. Serum lycopene, other carotenoids, and prostate cancer risk: A nested case-control study in the prostate, lung, colorectal, and ovarian cancer screening trial. Cancer Epidemiol. Biomark. Prev. 2007, 16, 962–968. [Google Scholar] [CrossRef]
- Vaishampayan, U.; Hussain, M.; Banerjee, M.; Seren, S.; Sarkar, F.H.; Fontana, J.; Forman, J.D.; Cher, M.L.; Powell, I.; Pontes, J.E.; et al. Lycopene and soy isoflavones in the treatment of prostate cancer. Nutr. Cancer 2007, 59, 1–7. [Google Scholar] [CrossRef]
- Gann, P.H.; Ma, J.; Giovannucci, E.; Willett, W.; Sacks, F.M.; Hennekens, C.H.; Stampfer, M.J. Lower prostate cancer risk in men with elevated plasma lycopene levels: Results of a prospective analysis. Cancer Res. 1999, 59, 1225–1230. [Google Scholar]
- Fraser, G.E.; Jacobsen, B.K.; Knutsen, S.F.; Mashchak, A.; Lloren, J.I. Tomato consumption and intake of lycopene as predictors of the incidence of prostate cancer: The Adventist Health Study-2. Cancer Causes Control 2020, 31, 341–351. [Google Scholar] [CrossRef]
- Morgia, G.; Voce, S.; Palmieri, F.; Gentile, M.; Iapicca, G.; Giannantoni, A.; Blefari, F.; Carini, M.; Vespasiani, G.; Santelli, G.; et al. Association between selenium and lycopene supplementation and incidence of prostate cancer: Results from the post-hoc analysis of the procomb trial. Phytomedicine 2017, 34, 1–5. [Google Scholar] [CrossRef]
- Zu, K.; Mucci, L.; Rosner, B.A.; Clinton, S.K.; Loda, M.; Stampfer, M.J.; Giovannucci, E. Dietary lycopene, angiogenesis, and prostate cancer: A prospective study in the prostate-specific antigen era. J. Natl. Cancer Inst. 2014, 106, djt430. [Google Scholar] [CrossRef] [PubMed]
- Kucuk, O.; Sarkar, F.H.; Sakr, W.; Khachik, F.; Djuric, Z.; Banerjee, M.; Pollak, M.N.; Bertram, J.S.; Wood, D.P. Lycopene in the treatment of prostate cancer. Pure Appl. Chem. 2002, 74, 1443–1450. [Google Scholar] [CrossRef]
- Vogt, T.M.; Mayne, S.T.; Graubard, B.I.; Swanson, C.A.; Sowell, A.L.; Schoenberg, J.B.; Swanson, G.M.; Greenberg, R.S.; Hoover, R.N.; Hayes, R.B.; et al. Serum lycopene, other serum carotenoids, and risk of prostate cancer in US Blacks and Whites. Am. J. Epidemiol. 2002, 155, 1023–1032. [Google Scholar] [CrossRef]
- Yang, C.-M.; Yen, Y.-T.; Huang, C.-S.; Hu, M.-L. Growth inhibitory efficacy of lycopene and β-carotene against androgen-independent prostate tumor cells xenografted in nude mice. Mol. Nutr. Food Res. 2011, 55, 606–612. [Google Scholar] [CrossRef]
- Limpens, J.; Schröder, F.H.; de Ridder, C.M.A.; Bolder, C.A.; Wildhagen, M.F.; Obermüller-Jevic, U.C.; Krämer, K.; van Weerden, W.M. Combined lycopene and vitamin E treatment suppresses the growth of PC-346C human prostate cancer cells in nude mice. J. Nutr. 2006, 136, 1287–1293. [Google Scholar] [CrossRef] [PubMed]
- Bowen, P.; Chen, L.; Stacewicz-Sapuntzakis, M.; Duncan, C.; Sharifi, R.; Ghosh, L.; Kim, H.-S.; Christov-Tzelkov, K.; van Breemen, R. Tomato sauce supplementation and prostate cancer: Lycopene accumulation and modulation of biomarkers of carcinogenesis. Exp. Biol. Med. 2002, 227, 886–893. [Google Scholar] [CrossRef]
- Wang, Y.; Jacobs, E.J.; Newton, C.C.; McCullough, M.L. Lycopene, tomato products and prostate cancer-specific mortality among men diagnosed with nonmetastatic prostate cancer in the Cancer Prevention Study II Nutrition Cohort. Int. J. Cancer 2016, 138, 2846–2855. [Google Scholar] [CrossRef]
- Bunker, C.H.; McDonald, A.C.; Evans, R.W.; de la Rosa, N.; Boumosleh, J.M.; Patrick, A.L. A randomized trial of lycopene supplementation in Tobago men with high prostate cancer risk. Nutr. Cancer 2007, 57, 130–137. [Google Scholar] [CrossRef]
- Chan, J.M.; Weinberg, V.; Magbanua, M.J.; Sosa, E.; Simko, J.; Shinohara, K.; Federman, S.; Mattie, M.; Hughes-Fulford, M.; Haqq, C.; et al. Nutritional supplements, COX-2 and IGF-1 expression in men on active surveillance for prostate cancer. Cancer Causes Control 2011, 22, 141–150. [Google Scholar] [CrossRef]
- Venkateswaran, V.; Fleshner, N.E.; Sugar, L.M.; Klotz, L.H. Antioxidants block prostate cancer in lady transgenic mice. Cancer Res. 2004, 64, 5891–5896. [Google Scholar] [CrossRef]
- Imaida, K.; Tamano, S.; Kato, K.; Ikeda, Y.; Asamoto, M.; Takahashi, S.; Nir, Z.; Murakoshi, M.; Nishino, H.; Shirai, T. Lack of chemopreventive effects of lycopene and curcumin on experimental rat prostate carcinogenesis. Carcinogenesis 2001, 22, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Siler, U.; Barella, L.; Spitzer, V.; Schnorr, J.; Lein, M.; Goralczyk, R.; Wertz, K. Lycopene and vitamin E interfere with autocrine/paracrine loops in the Dunning prostate cancer model. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2004, 18, 1019–1021. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.Y.; Hung, J.C.; Heber, D.; Go, V.L.; Reuter, V.E.; Cordon-Cardo, C.; Scher, H.I.; Marshall, J.R.; Zhang, Z.F. Inverse associations between plasma lycopene and other carotenoids and prostate cancer. Cancer Epidemiol. Biomark. Prev. 2001, 10, 749–756. [Google Scholar]
- Goodman, M.; Bostick, R.M.; Ward, K.C.; Terry, P.D.; van Gils, C.H.; Taylor, J.A.; Mandel, J.S. Lycopene intake and prostate cancer risk: Effect modification by plasma antioxidants and the XRCC1 genotype. Nutr. Cancer 2006, 55, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Rowles, J.L.; Smith, J.W.; Applegate, C.C.; Miller, R.J.; Wallig, M.A.; Kaur, A.; Sarol, J.N.; Musaad, S.; Clinton, S.K.; O’Brien, W.D.; et al. Dietary Tomato or Lycopene Do Not Reduce Castration-Resistant Prostate Cancer Progression in a Murine Model. J. Nutr. 2020, 150, 1808–1817. [Google Scholar] [CrossRef]
- Mariani, S.; Lionetto, L.; Cavallari, M.; Tubaro, A.; Rasio, D.; De Nunzio, C.; Hong, G.M.; Borro, M.; Simmaco, M. Low prostate concentration of lycopene is associated with development of prostate cancer in patients with high-grade prostatic intraepithelial neoplasia. Int. J. Mol. Sci. 2014, 15, 1433–1440. [Google Scholar] [CrossRef]
- Beilby, J.; Ambrosini, G.L.; Rossi, E.; de Klerk, N.H.; Musk, A.W. Serum levels of folate, lycopene, β-carotene, retinol and vitamin E and prostate cancer risk. Eur. J. Clin. Nutr. 2010, 64, 1235–1238. [Google Scholar] [CrossRef]
- Magbanua, M.J.M.; Roy, R.; Sosa, E.V.; Weinberg, V.; Federman, S.; Mattie, M.D.; Hughes-Fulford, M.; Simko, J.; Shinohara, K.; Haqq, C.M.; et al. Gene expression and biological pathways in tissue of men with prostate cancer in a randomized clinical trial of lycopene and fish oil supplementation. PLoS ONE 2011, 6, e24004. [Google Scholar] [CrossRef]
- van Breemen, R.B.; Sharifi, R.; Viana, M.; Pajkovic, N.; Zhu, D.; Yuan, L.; Yang, Y.; Bowen, P.E.; Stacewicz-Sapuntzakis, M. Antioxidant effects of lycopene in African American men with prostate cancer or benign prostate hyperplasia: A randomized, controlled trial. Cancer Prev. Res. 2011, 4, 711–718. [Google Scholar] [CrossRef]
- Clark, P.E.; Hall, M.C.; Borden, L.S.J.; Miller, A.A.; Hu, J.J.; Lee, W.R.; Stindt, D.; D’Agostino, R.J.; Lovato, J.; Harmon, M.; et al. Phase I-II prospective dose-escalating trial of lycopene in patients with biochemical relapse of prostate cancer after definitive local therapy. Urology 2006, 67, 1257–1261. [Google Scholar] [CrossRef]
- Rao, A.V.; Fleshner, N.; Agarwal, S. Serum and tissue lycopene and biomarkers of oxidation in prostate cancer patients: A case-control study. Nutr. Cancer 1999, 33, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Giovannucci, E.; Rimm, E.B.; Liu, Y.; Stampfer, M.J.; Willett, W.C. A prospective study of tomato products, lycopene, and prostate cancer risk. J. Natl. Cancer Inst. 2002, 94, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Jatoi, A.; Burch, P.; Hillman, D.; Vanyo, J.M.; Dakhil, S.; Nikcevich, D.; Rowland, K.; Morton, R.; Flynn, P.J.; Young, C.; et al. A Tomato-Based, Lycopene-Containing Intervention for Androgen-Independent Prostate Cancer: Results of a Phase II Study from the North Central Cancer Treatment Group. Urology 2007, 69, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Kucuk, O.; Sarkar, F.H.; Djuric, Z.; Sakr, W.; Pollak, M.N.; Khachik, F.; Banerjee, M.; Bertram, J.S.; Wood, D.P. Effects of lycopene supplementation in patients with localized prostate cancer. Exp. Biol. Med. 2002, 227, 881–885. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.S.; Gupta, N.P. Lycopene: A novel drug therapy in hormone refractory metastatic prostate cancer. Urol. Oncol. 2004, 22, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.S.; Gupta, N.P. A comparison of lycopene and orchidectomy vs orchidectomy alone in the management of advanced prostate cancer. BJU Int. 2003, 92, 375–378, discussion 378. [Google Scholar] [CrossRef] [PubMed]
- Aizawa, K.; Liu, C.; Tang, S.; Veeramachaneni, S.; Hu, K.-Q.; Smith, D.E.; Wang, X.-D. Tobacco carcinogen induces both lung cancer and non-alcoholic steatohepatitis and hepatocellular carcinomas in ferrets which can be attenuated by lycopene supplementation. Int. J. Cancer 2016, 139, 1171–1181. [Google Scholar] [CrossRef]
- Sahin, K.; Cross, B.; Sahin, N.; Ciccone, K.; Suleiman, S.; Osunkoya, A.O.; Master, V.; Harris, W.; Carthon, B.; Mohammad, R.; et al. Lycopene in the prevention of renal cell cancer in the TSC2 mutant Eker rat model. Arch. Biochem. Biophys. 2015, 572, 36–39. [Google Scholar] [CrossRef]
- Sesso, H.D.; Buring, J.E.; Zhang, S.M.; Norkus, E.P.; Gaziano, J.M. Dietary and plasma lycopene and the risk of breast cancer. Cancer Epidemiol. Biomark. Prev. 2005, 14, 1074–1081. [Google Scholar] [CrossRef]
- Watanabe, S.; Kitade, Y.; Masaki, T.; Nishioka, M.; Satoh, K.; Nishino, H. Effects of lycopene and Sho-saiko-to on hepatocarcinogenesis in a rat model of spontaneous liver cancer. Nutr. Cancer 2001, 39, 96–101. [Google Scholar] [CrossRef]
- Vrieling, A.; Voskuil, D.W.; Bonfrer, J.M.; Korse, C.M.; van Doorn, J.; Cats, A.; Depla, A.C.; Timmer, R.; Witteman, B.J.; van Leeuwen, F.E.; et al. Lycopene supplementation elevates circulating insulin-like growth factor binding protein-1 and -2 concentrations in persons at greater risk of colorectal cancer. Am. J. Clin. Nutr. 2007, 86, 1456–1462. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-S.; Liao, J.-W.; Hu, M.-L. Lycopene inhibits experimental metastasis of human hepatoma SK-Hep-1 cells in athymic nude mice. J. Nutr. 2008, 138, 538–543. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.-Y.; Pai, M.-H.; Wang, X.-D. Consumption of lycopene inhibits the growth and progression of colon cancer in a mouse xenograft model. J. Agric. Food Chem. 2011, 59, 9011–9021. [Google Scholar] [CrossRef] [PubMed]
- Mayne, S.T.; Cartmel, B.; Lin, H.; Zheng, T.; Goodwin, W.J.J. Low plasma lycopene concentration is associated with increased mortality in a cohort of patients with prior oral, pharynx or larynx cancers. J. Am. Coll. Nutr. 2004, 23, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Voskuil, D.W.; Vrieling, A.; Korse, C.M.; Beijnen, J.H.; Bonfrer, J.M.G.; van Doorn, J.; Kaas, R.; Oldenburg, H.S.A.; Russell, N.S.; Rutgers, E.J.T.; et al. Effects of lycopene on the insulin-like growth factor (IGF) system in premenopausal breast cancer survivors and women at high familial breast cancer risk. Nutr. Cancer 2008, 60, 342–353. [Google Scholar] [CrossRef] [PubMed]
- Moody, L.; Crowder, S.L.; Fruge, A.D.; Locher, J.L.; Demark-Wahnefried, W.; Rogers, L.Q.; Delk-Licata, A.; Carroll, W.R.; Spencer, S.A.; Black, M.; et al. Epigenetic stratification of head and neck cancer survivors reveals differences in lycopene levels, alcohol consumption, and methylation of immune regulatory genes. Clin. Epigenetics 2020, 12, 138. [Google Scholar] [CrossRef]
- De Stefani, E.; Oreggia, F.; Boffetta, P.; Deneo-Pellegrini, H.; Ronco, A.; Mendilaharsu, M. Tomatoes, tomato-rich foods, lycopene and cancer of the upper aerodigestive tract: A case-control in Uruguay. Oral Oncol. 2000, 36, 47–53. [Google Scholar] [CrossRef]
- Sahin, K.; Tuzcu, M.; Sahin, N.; Akdemir, F.; Ozercan, I.; Bayraktar, S.; Kucuk, O. Inhibitory effects of combination of lycopene and genistein on 7,12- dimethyl benz(a)anthracene-induced breast cancer in rats. Nutr. Cancer 2011, 63, 1279–1286. [Google Scholar] [CrossRef]
- Tang, F.-Y.; Pai, M.-H.; Kuo, Y.-H.; Wang, X.-D. Concomitant consumption of lycopene and fish oil inhibits tumor growth and progression in a mouse xenograft model of colon cancer. Mol. Nutr. Food Res. 2012, 56, 1520–1531. [Google Scholar] [CrossRef]
- Palan, P.R.; Mikhail, M.S.; Goldberg, G.L.; Basu, J.; Runowicz, C.D.; Romney, S.L. Plasma levels of beta-carotene, lycopene, canthaxanthin, retinol, and alpha- and tau-tocopherol in cervical intraepithelial neoplasia and cancer. Clin. Cancer Res. 1996, 2, 181–185. [Google Scholar]
- Velmurugan, B.; Bhuvaneswari, V.; Burra, U.K.; Nagini, S. Prevention of N-methyl-N’-nitro-N-nitrosoguanidine and saturated sodium chloride-induced gastric carcinogenesis in Wistar rats by lycopene. Eur. J. Cancer Prev. 2002, 11, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Russell, R.M.; Wang, X.-D. Lycopene supplementation prevents smoke-induced changes in p53, p53 phosphorylation, cell proliferation, and apoptosis in the gastric mucosa of ferrets. J. Nutr. 2006, 136, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.F.; Zhou, S.K.; Feng, X.J.; Jiang, L.X. Modulatory effect of lycopene on oxidative injury, related proteins and gene expression in gastric cancer tissue. J. Food Agric. Environ. 2013, 11, 511–515. [Google Scholar]
- Kune, G.; Watson, L. Colorectal cancer protective effects and the dietary micronutrients folate, methionine, vitamins B6, B12, C, E, selenium, and lycopene. Nutr. Cancer 2006, 56, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Di Franco, R.; Calvanese, M.; Murino, P.; Manzo, R.; Guida, C.; Di Gennaro, D.; Anania, C.; Ravo, V. Skin toxicity from external beam radiation therapy in breast cancer patients: Protective effects of Resveratrol, Lycopene, Vitamin C and anthocianin (Ixor®). Radiat. Oncol. 2012, 7, 12. [Google Scholar] [CrossRef] [PubMed]
- Walfisch, S.; Walfisch, Y.; Kirilov, E.; Linde, N.; Mnitentag, H.; Agbaria, R.; Sharoni, Y.; Levy, J. Tomato lycopene extract supplementation decreases insulin-like growth factor-I levels in colon cancer patients. Eur. J. Cancer Prev. 2007, 16, 298–303. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.V.; Agarwal, S. Bioavailability and in vivo antioxidant properties of lycopene from tomato products and their possible role in the prevention of cancer. Nutr. Cancer 1998, 31, 199–203. [Google Scholar] [CrossRef]
- Nkondjock, A.; Ghadirian, P.; Johnson, K.C.; Krewski, D. Dietary intake of lycopene is associated with reduced pancreatic cancer risk. J. Nutr. 2005, 135, 592–597. [Google Scholar] [CrossRef]
- Jiang, X.; Wu, H.; Zhao, W.; Ding, X.; You, Q.; Zhu, F.; Qian, M.; Yu, P. Lycopene improves the efficiency of anti-PD-1 therapy via activating IFN signaling of lung cancer cells. Cancer Cell Int. 2019, 19, 68. [Google Scholar] [CrossRef]
- Radhakrishnan, V.K.; Ravikumar, V.; Shivashangari, K.; Sattu, K.; Devaki, T. Chemopreventive Role of Lycopene and D-arginine in Benzo(a) Pyrene Induced Lung Cancer with Reference to Lipid Peroxidation, Antioxidant and Tumor Marker Enzymes. Int. J. Cancer Res. 2006, 2, 224–233. [Google Scholar] [CrossRef][Green Version]
- Cui, L.; Xu, F.; Wu, K.; Li, L.; Qiao, T.; Li, Z.; Chen, T.; Sun, C. Anticancer effects and possible mechanisms of lycopene intervention on N-methylbenzylnitrosamine induced esophageal cancer in F344 rats based on PPARγ(1). Eur. J. Pharmacol. 2020, 881, 173230. [Google Scholar] [CrossRef] [PubMed]
- Sengngam, K.; Hoc, T.H.; Hang, D.V.; Tran Ngoan, L. Trans-Lycopene and β-Cryptoxanthin Intake and Stomach Cancer in Vietnamese Men: A Pilot Case-Control Study. Asian Pac. J. Cancer Prev. 2022, 23, 861–865. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Wu, X.-G. Lycopene enhances antioxidant enzyme activities and immunity function in N-methyl-N’-nitro-N-nitrosoguanidine-enduced gastric cancer rats. Int. J. Mol. Sci. 2011, 12, 3340–3351. [Google Scholar] [CrossRef] [PubMed]
- Mazidi, M.; Ferns, G.A.; Banach, M. A high consumption of tomato and lycopene is associated with a lower risk of cancer mortality: Results from a multi-ethnic cohort. Public Health Nutr. 2020, 23, 1569–1575. [Google Scholar] [CrossRef]
- Kim, D.J.; Takasuka, N.; Nishino, H.; Tsuda, H. Chemoprevention of lung cancer by lycopene. Biofactors 2000, 13, 95–102. [Google Scholar] [CrossRef]
- Chen, P.; Xu, S.; Qu, J. Lycopene Protects Keratinocytes Against UVB Radiation-Induced Carcinogenesis via Negative Regulation of FOXO3a through the mTORC2/AKT Signaling Pathway. J. Cell. Biochem. 2018, 119, 366–377. [Google Scholar] [CrossRef]
- Breslow, R.A.; Alberg, A.J.; Helzlsouer, K.J.; Bush, T.L.; Norkus, E.P.; Morris, J.S.; Spate, V.E.; Comstock, G.W. Serological precursors of cancer: Malignant melanoma, basal and squamous cell skin cancer, and prediagnostic levels of retinol, beta- carotene, lycopene, alpha-tocopherol, and selenium. Cancer Epidemiol. Biomark. Prev. 1995, 4, 837–842. [Google Scholar]
- Bhatia, N.; Gupta, P.; Singh, B.; Koul, A. Lycopene Enriched Tomato Extract Inhibits Hypoxia, Angiogenesis, and Metastatic Markers in early Stage N-Nitrosodiethylamine Induced Hepatocellular Carcinoma. Nutr. Cancer 2015, 67, 1268–1275. [Google Scholar] [CrossRef]
- Karppi, J.; Kurl, S.; Nurmi, T.; Rissanen, T.H.; Pukkala, E.; Nyyssönen, K. Serum lycopene and the risk of cancer: The Kuopio Ischaemic Heart Disease Risk Factor (KIHD) study. Ann. Epidemiol. 2009, 19, 512–518. [Google Scholar] [CrossRef]
- Moroni, M.; Pirovano, M.; Brugnatelli, S.; Zucca, M.; Morreale, M.; Rizzo, V.; Ferrari, A.; Tinelli, C.; De Silvestri, A.; Meregalli, M.; et al. Lycopene minimizes skin toxicity and oxidative stress in patients treated with panitumumab-containing therapy for metastatic colorectal cancer. J. Funct. Foods 2021, 83, 104533. [Google Scholar] [CrossRef]
- Liu, C.; Lian, F.; Smith, D.E.; Russell, R.M.; Wang, X.-D. Lycopene supplementation inhibits lung squamous metaplasia and induces apoptosis via up-regulating insulin-like growth factor-binding protein 3 in cigarette smoke-exposed ferrets. Cancer Res. 2003, 63, 3138–3144. [Google Scholar] [PubMed]
- Helzlsouer, K.J.; Comstock, G.W.; Morris, J.S. Selenium, lycopene, alpha-tocopherol, beta-carotene, retinol, and subsequent bladder cancer. Cancer Res. 1989, 49, 6144–6148. [Google Scholar] [PubMed]
- Velmurugan, B.; Nagini, S. Combination chemoprevention of experimental gastric carcinogenesis by s-allylcysteine and lycopene: Modulatory effects on glutathione redox cycle antioxidants. J. Med. Food 2005, 8, 494–501. [Google Scholar] [CrossRef]
- Sahin, K.; Yenice, E.; Tuzcu, M.; Orhan, C.; Mizrak, C.; Ozercan, I.H.; Sahin, N.; Yilmaz, B.; Bilir, B.; Ozpolat, B.; et al. Lycopene Protects Against Spontaneous Ovarian Cancer Formation in Laying Hens. J. Cancer Prev. 2018, 23, 25–36. [Google Scholar] [CrossRef]
- Satia, J.A.; Littman, A.; Slatore, C.G.; Galanko, J.A.; White, E. Long-term use of beta-carotene, retinol, lycopene, and lutein supplements and lung cancer risk: Results from the VITamins And Lifestyle (VITAL) study. Am. J. Epidemiol. 2009, 169, 815–828. [Google Scholar] [CrossRef] [PubMed]
- Nishida, N.; Yano, H.; Nishida, T.; Kamura, T.; Kojiro, M. Angiogenesis in cancer. Vasc. Health Risk Manag. 2006, 2, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Moran, N.E.; Erdman, J.W.J.; Clinton, S.K. Complex interactions between dietary and genetic factors impact lycopene metabolism and distribution. Arch. Biochem. Biophys. 2013, 539, 171–180. [Google Scholar] [CrossRef]
- Guttenplan, J.B.; Chen, M.; Kosinska, W.; Thompson, S.; Zhao, Z.; Cohen, L.A. Effects of a lycopene-rich diet on spontaneous and benzo[a]pyrene-induced mutagenesis in prostate, colon and lungs of the lacZ mouse. Cancer Lett. 2001, 164, 1–6. [Google Scholar] [CrossRef]
- Tjahjodjati; Sugandi, S.; Umbas, R.; Satari, M. The Protective Effect of Lycopene on Prostate Growth Inhibitory Efficacy by Decreasing Insulin Growth Factor-1 in Indonesian Human Prostate Cancer Cells. Res. Reports Urol. 2020, 12, 137–143. [Google Scholar] [CrossRef]
- Talvas, J.; Caris-Veyrat, C.; Guy, L.; Rambeau, M.; Lyan, B.; Minet-Quinard, R.; Lobaccaro, J.-M.A.; Vasson, M.-P.; Georgé, S.; Mazur, A.; et al. Differential effects of lycopene consumed in tomato paste and lycopene in the form of a purified extract on target genes of cancer prostatic cells. Am. J. Clin. Nutr. 2010, 91, 1716–1724. [Google Scholar] [CrossRef]
- Rinaldi, S.; Cleveland, R.; Norat, T.; Biessy, C.; Rohrmann, S.; Linseisen, J.; Boeing, H.; Pischon, T.; Panico, S.; Agnoli, C.; et al. Serum levels of IGF-I, IGFBP-3 and colorectal cancer risk: Results from the EPIC cohort, plus a meta-analysis of prospective studies. Int. J. Cancer 2010, 126, 1702–1715. [Google Scholar] [CrossRef] [PubMed]
- Key, T.J.; Appleby, P.N.; Reeves, G.K.; Roddam, A.W. Insulin-like growth factor 1 (IGF1), IGF binding protein 3 (IGFBP3), and breast cancer risk: Pooled individual data analysis of 17 prospective studies. Lancet Oncol. 2010, 11, 530–542. [Google Scholar] [CrossRef] [PubMed]
- Allen, N.E.; Key, T.J.; Appleby, P.N.; Travis, R.C.; Roddam, A.W.; Rinaldi, S.; Egevad, L.; Rohrmann, S.; Linseisen, J.; Pischon, T.; et al. Serum insulin-like growth factor (IGF)-I and IGF-binding protein-3 concentrations and prostate cancer risk: Results from the European Prospective Investigation into Cancer and Nutrition. Cancer Epidemiol. Biomark. Prev. 2007, 16, 1121–1127. [Google Scholar] [CrossRef]
- Weroha, S.J.; Haluska, P. The insulin-like growth factor system in cancer. Endocrinol. Metab. Clin. N. Am. 2012, 41, 335–350. [Google Scholar] [CrossRef] [PubMed]
- Puah, B.P.; Jalil, J.; Attiq, A.; Kamisah, Y. New insights into molecular mechanism behind anti-cancer activities of lycopene. Molecules 2021, 26, 3888. [Google Scholar] [CrossRef]
- Kirkegård, J.; Mortensen, F.V.; Cronin-Fenton, D. Chronic Pancreatitis and Pancreatic Cancer Risk: A Systematic Review and Meta-analysis. Am. J. Gastroenterol. 2017, 112, 1366–1372. [Google Scholar] [CrossRef] [PubMed]
- Alison, M.R.; Nicholson, L.J.; Lin, W.-R. Chronic inflammation and hepatocellular carcinoma. Recent Results Cancer Res. 2011, 185, 135–148. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, R.; Shimizu, T.; Kumagai, K.; Takai, A.; Marusawa, H. Genetic Pathogenesis of Inflammation-Associated Cancers in Digestive Organs. Pathogens 2021, 10, 453. [Google Scholar] [CrossRef]
- Stice, C.P.; Xia, H.; Wang, X.-D. Tomato lycopene prevention of alcoholic fatty liver disease and hepatocellular carcinoma development. Chronic Dis. Transl. Med. 2018, 4, 211–224. [Google Scholar] [CrossRef]
- Jiang, W.; Guo, M.-H.; Hai, X. Hepatoprotective and antioxidant effects of lycopene on non-alcoholic fatty liver disease in rat. World J. Gastroenterol. 2016, 22, 10180–10188. [Google Scholar] [CrossRef]
- Seren, S.; Mutchnick, M.; Hutchinson, D.; Harmanci, O.; Bayraktar, Y.; Mutchnick, S.; Sahin, K.; Kucuk, O. Potential role of lycopene in the treatment of hepatitis C and prevention of hepatocellular carcinoma. Nutr. Cancer 2008, 60, 729–735. [Google Scholar] [CrossRef] [PubMed]
- Sheriff, S.A.; Devaki, T. Lycopene stabilizes lipoprotein levels during D-galactosamine/lipopolysaccharide induced hepatitis in experimental rats. Asian Pac. J. Trop. Biomed. 2012, 2, 975–980. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Wu, J.; Li, J.; Bai, Y.; Luo, Y.; Ji, B.; Xia, B.; Liu, Z.; Tan, X.; Lv, J.; et al. Lycopene Alleviates DSS-Induced Colitis and Behavioral Disorders via Mediating Microbes-Gut-Brain Axis Balance. J. Agric. Food Chem. 2020, 68, 3963–3975. [Google Scholar] [CrossRef]
- Gul Baykalir, B.; Aksit, D.; Dogru, M.S.; Hanım Yay, A.; Aksit, H.; Seyrek, K.; Attesahin, A. Lycopene Ameliorates Experimental Colitis in Rats via Reducing Apoptosis and Oxidative Stress. Int. J. Vitam. Nutr. Res. 2016, 86, 27–35. [Google Scholar] [CrossRef]
- Kim, M.J.; Kim, H. Anticancer Effect of Lycopene in Gastric Carcinogenesis. J. Cancer Prev. 2015, 20, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.H.; Lim, J.W.; Morio, T.; Kim, H. Lycopene inhibits Helicobacter pylori-induced ATM/ATR-dependent DNA damage response in gastric epithelial AGS cells. Free Radic. Biol. Med. 2012, 52, 607–615. [Google Scholar] [CrossRef]
- Giri, A.K.; Rawat, J.K.; Singh, M.; Gautam, S.; Kaithwas, G. Effect of lycopene against gastroesophageal reflux disease in experimental animals. BMC Complement. Altern. Med. 2015, 15, 110. [Google Scholar] [CrossRef]
- Pernar, C.H.; Ebot, E.M.; Wilson, K.M.; Mucci, L.A. The Epidemiology of Prostate Cancer. Cold Spring Harb. Perspect. Med. 2018, 8, a030361. [Google Scholar] [CrossRef]
- Casso, D.; White, E.; Patterson, R.E.; Agurs-Collins, T.; Kooperberg, C.; Haines, P.S. Correlates of serum lycopene in older women. Nutr. Cancer 2000, 36, 163–169. [Google Scholar] [CrossRef]
- Kitamura, Y.; Tanaka, K.; Kiyohara, C.; Hirohata, T.; Tomita, Y.; Ishibashi, M.; Kido, K. Relationship of alcohol use, physical activity and dietary habits with serum carotenoids, retinol and alpha-tocopherol among male Japanese smokers. Int. J. Epidemiol. 1997, 26, 307–314. [Google Scholar] [CrossRef]
- van der Gaag, M.S.; van den Berg, R.; van den Berg, H.; Schaafsma, G.; Hendriks, H.F. Moderate consumption of beer, red wine and spirits has counteracting effects on plasma antioxidants in middle-aged men. Eur. J. Clin. Nutr. 2000, 54, 586–591. [Google Scholar] [CrossRef] [PubMed]
- Rydén, M.; Leanderson, P.; Kastbom, K.-O.; Jonasson, L. Effects of simvastatin on carotenoid status in plasma. Nutr. Metab. Cardiovasc. Dis. 2012, 22, 66–71. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Walmsley, C.M.; Bates, C.J.; Prentice, A.; Cole, T.J. Relationship between cigarette smoking and nutrient intakes and blood status indices of older people living in the UK: Further analysis of data from the National Diet and Nutrition Survey of people aged 65 years and over, 1994/95. Public Health Nutr. 1999, 2, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Ferrucci, L.; Perry, J.R.B.; Matteini, A.; Perola, M.; Tanaka, T.; Silander, K.; Rice, N.; Melzer, D.; Murray, A.; Cluett, C.; et al. Common variation in the beta-carotene 15,15’-monooxygenase 1 gene affects circulating levels of carotenoids: A genome-wide association study. Am. J. Hum. Genet. 2009, 84, 123–133. [Google Scholar] [CrossRef]
- Tomlins, S.A.; Laxman, B.; Varambally, S.; Cao, X.; Yu, J.; Helgeson, B.E.; Cao, Q.; Prensner, J.R.; Rubin, M.A.; Shah, R.B.; et al. Role of the TMPRSS2-ERG gene fusion in prostate cancer. Neoplasia 2008, 10, 177–188. [Google Scholar] [CrossRef]
- Caldecott, K.W. XRCC1 protein; Form and function. DNA Repair 2019, 81, 102664. [Google Scholar] [CrossRef]
- Shao, A.; Hathcock, J.N. Risk assessment for the carotenoids lutein and lycopene. Regul. Toxicol. Pharmacol. 2006, 45, 289–298. [Google Scholar] [CrossRef]
- Trumbo, P.R. Are there Adverse Effects of Lycopene Exposure? J. Nutr. 2005, 135, 2060S–2061S. [Google Scholar] [CrossRef]
- Diwadkar-Navsariwala, V.; Novotny, J.A.; Gustin, D.M.; Sosman, J.A.; Rodvold, K.A.; Crowell, J.A.; Stacewicz-Sapuntzakis, M.; Bowen, P.E. A physiological pharmacokinetic model describing the disposition of lycopene in healthy men. J. Lipid Res. 2003, 44, 1927–1939. [Google Scholar] [CrossRef]
- Richelle, M.; Bortlik, K.; Liardet, S.; Hager, C.; Lambelet, P.; Baur, M.; Applegate, L.A.; Offord, E.A. A Food-Based Formulation Provides Lycopene with the Same Bioavailability to Humans as That from Tomato Paste. J. Nutr. 2002, 132, 404–408. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kapała, A.; Szlendak, M.; Motacka, E. The Anti-Cancer Activity of Lycopene: A Systematic Review of Human and Animal Studies. Nutrients 2022, 14, 5152. https://doi.org/10.3390/nu14235152
Kapała A, Szlendak M, Motacka E. The Anti-Cancer Activity of Lycopene: A Systematic Review of Human and Animal Studies. Nutrients. 2022; 14(23):5152. https://doi.org/10.3390/nu14235152
Chicago/Turabian StyleKapała, Aleksandra, Małgorzata Szlendak, and Emilia Motacka. 2022. "The Anti-Cancer Activity of Lycopene: A Systematic Review of Human and Animal Studies" Nutrients 14, no. 23: 5152. https://doi.org/10.3390/nu14235152
APA StyleKapała, A., Szlendak, M., & Motacka, E. (2022). The Anti-Cancer Activity of Lycopene: A Systematic Review of Human and Animal Studies. Nutrients, 14(23), 5152. https://doi.org/10.3390/nu14235152







