Liver-Derived Exosomes Induce Inflammation and Lipogenesis in Mice Fed High-Energy Diets
Abstract
1. Introduction
2. Materials and Methods
2.1. HF or HFS Diet–Fed Mice
2.2. Serum Biochemical Analysis
2.3. Histological Observation
2.4. Isolation and Quantification of Liver-Derived Exosoms
2.5. Cell Culture
2.6. Exosome Uptake Assay
2.7. Measurement of TG Content
2.8. Western Blotting
2.9. RNA Isolation and Quantitative Real-Time PCR (q-PCR)
2.10. Statistical Analysis
3. Results
3.1. Hematological and Histological Changes in HF or HFS Diet–Fed Mice
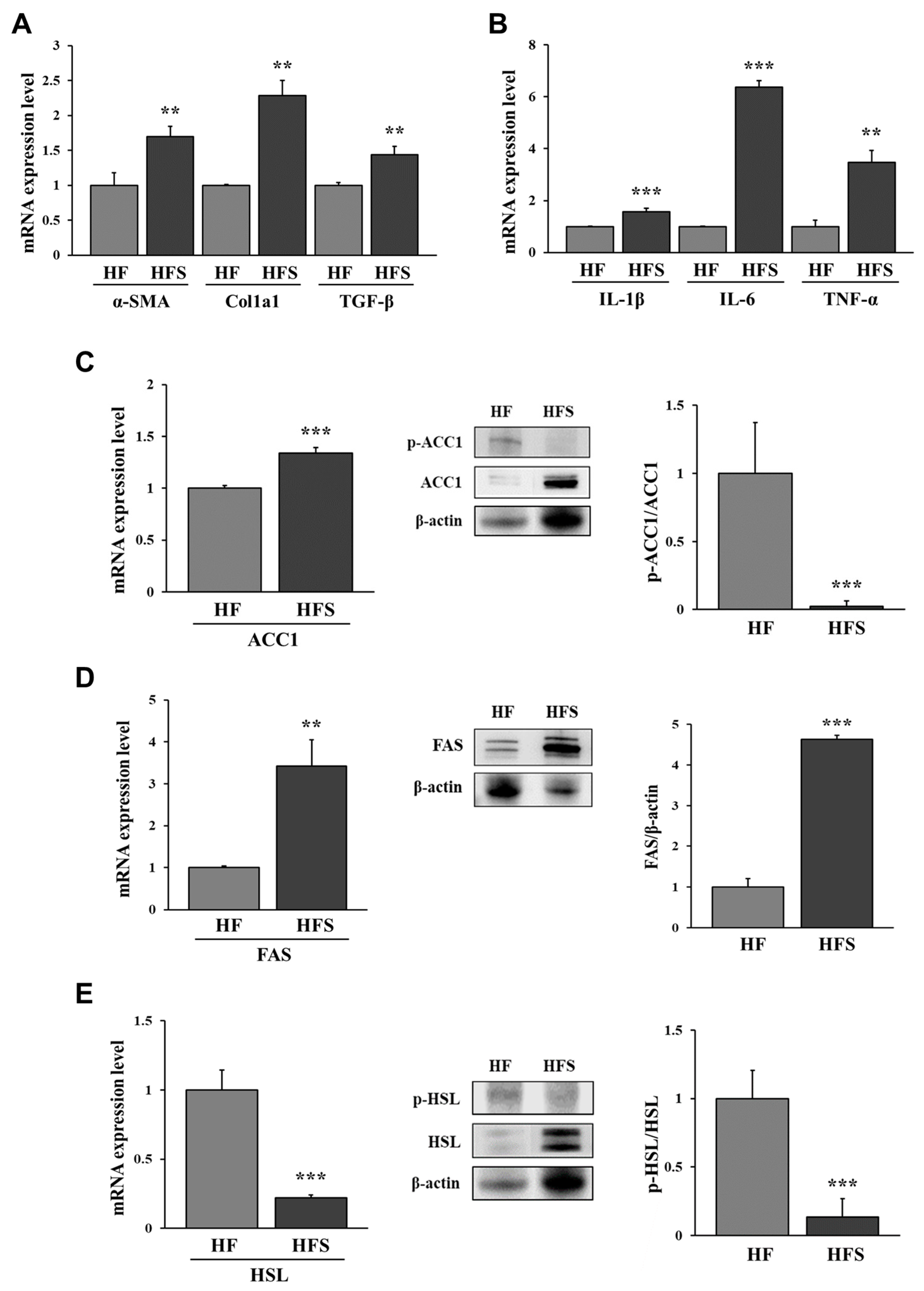
3.2. Lipid Metabolism-Related Factors in the Liver of HF or HFS Diet-Fed Mice
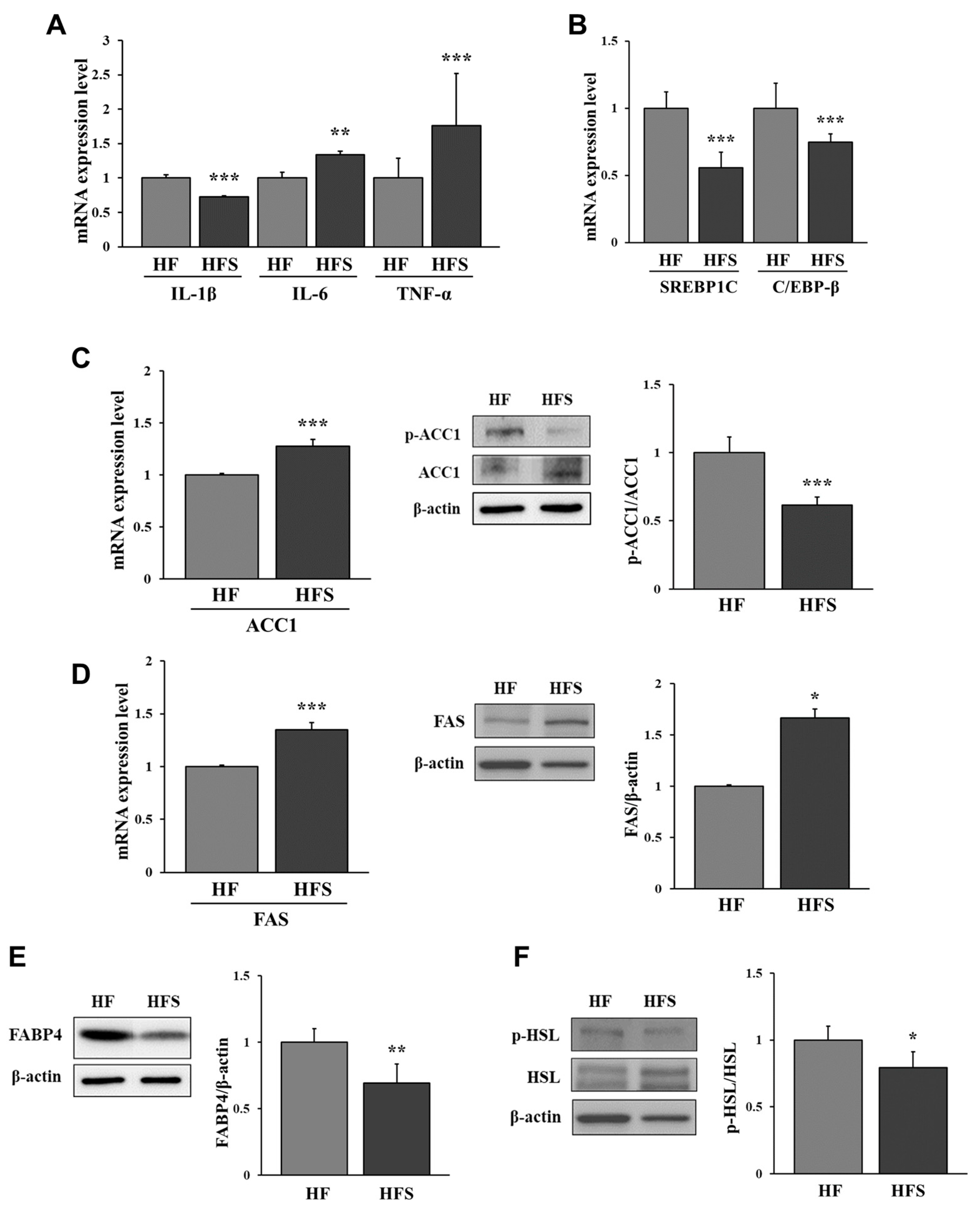
3.3. Lipid Metabolism-Related Factors in the Adipose Tissue of HF or HFS Diet-Fed Mice
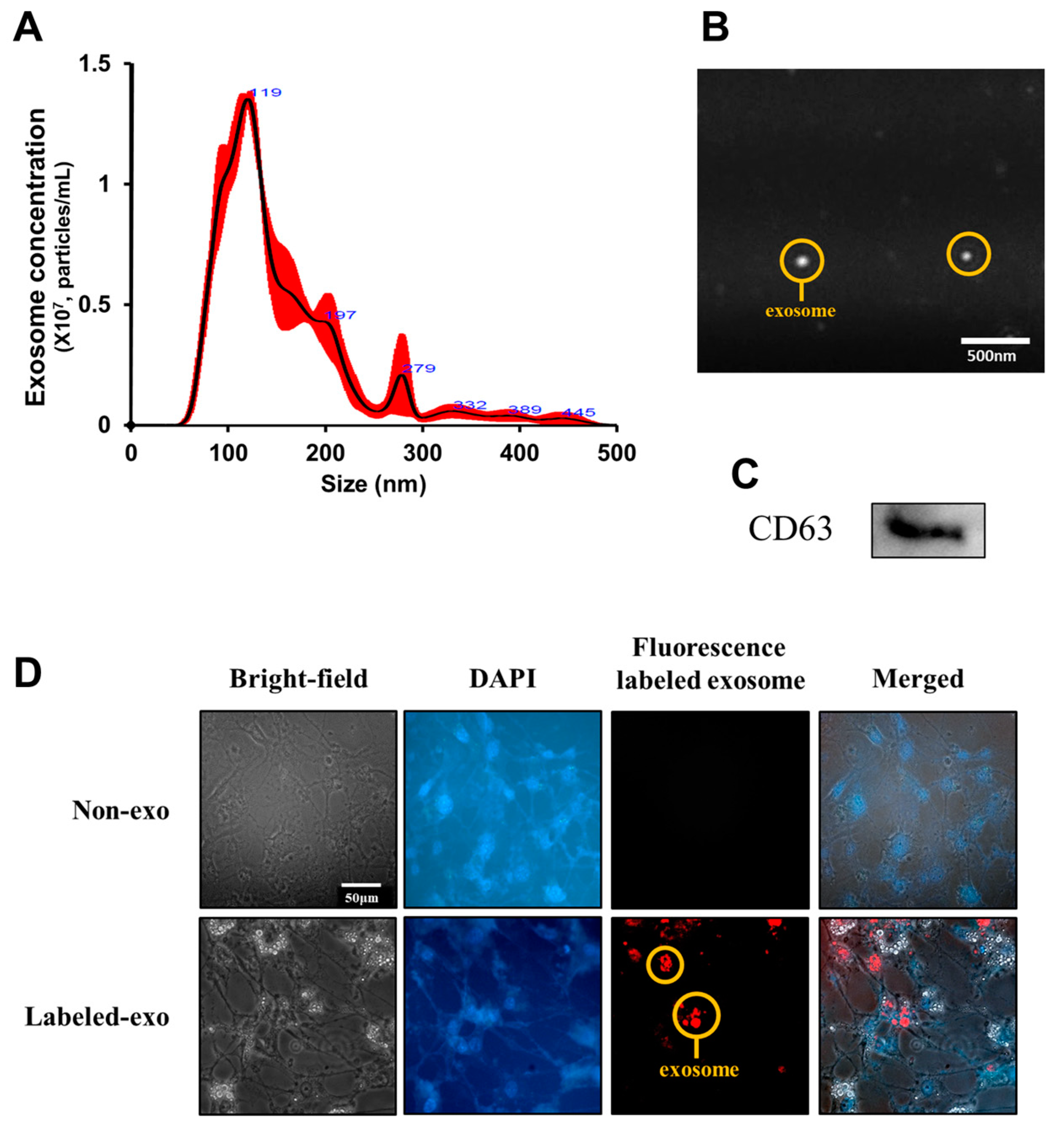
3.4. Uptake of Liver-Derived Exosomes by Adipocytes
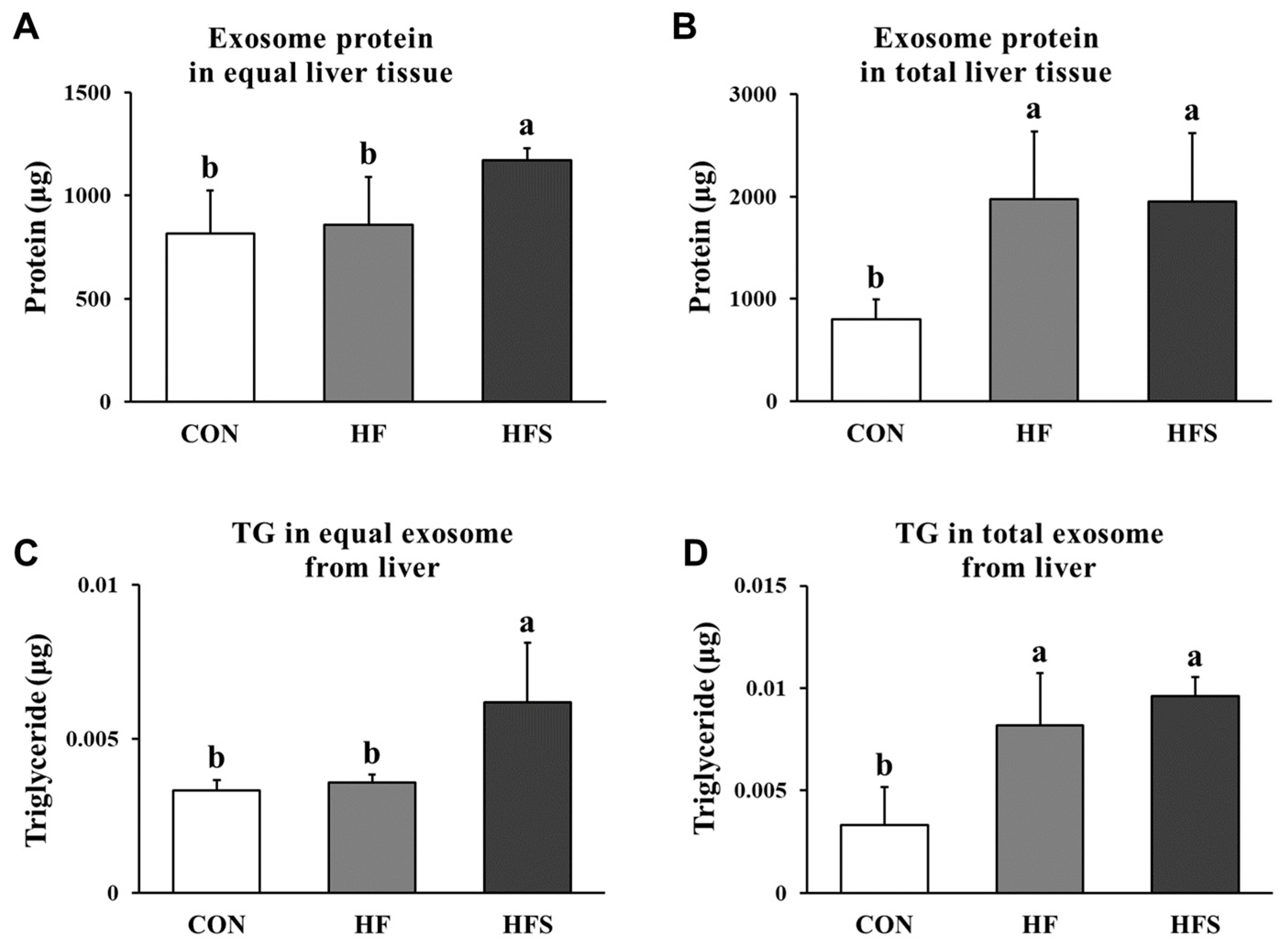
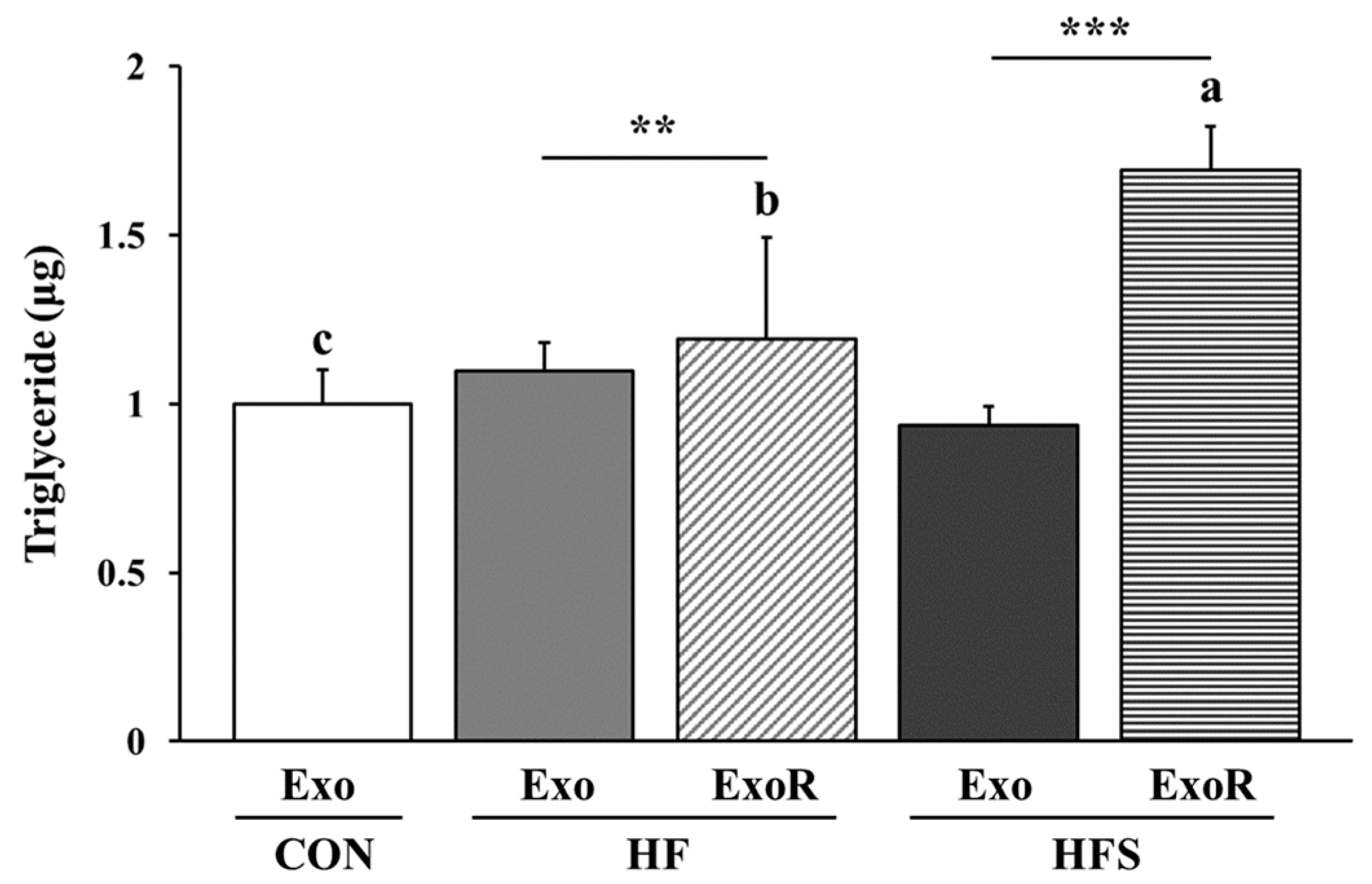
3.5. Amounts and TG Content of Liver-Derived Exosomes
3.6. TG Levels in Adipocytes Treated with Liver-Derived Exosomes
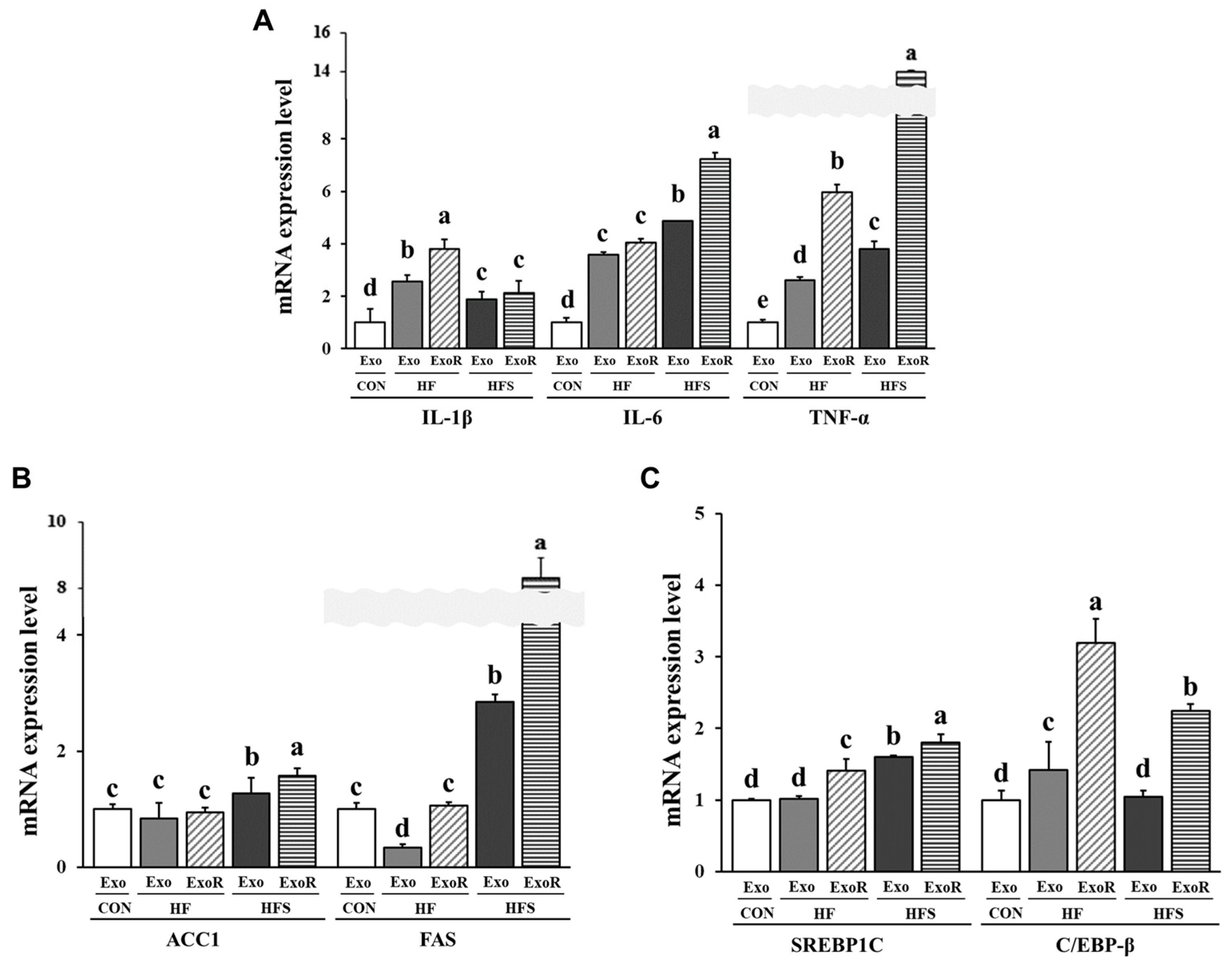
3.7. Lipid Metabolism-Related Factors in Adipocytes Treated with Liver-Derived Exosomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Esmaillzadeh, A.; Kimiagar, M.; Mehrabi, Y.; Azadbakht, L.; Hu, F.B.; Willett, W.C. Dietary patterns, insulin resistance, and prevalence of the metabolic syndrome in women. Am. J. Clin. Nutr. 2007, 85, 910–918. [Google Scholar] [CrossRef] [PubMed]
- Ferron, A.J.T.; Francisqueti, F.V.; Minatel, I.O.; Silva, C.C.V.D.A.; Bazan, S.G.Z.; Kitawara, K.A.H.; Garcia, J.L.; Corrêa, C.R.; Moreto, F.; Ferreira, A.L.A. Association between cardiac remodeling and metabolic alteration in an experimental model of obesity induced by Western Diet. Nutrients 2018, 10, 1675. [Google Scholar] [CrossRef] [PubMed]
- Neves, F.A.; Cortez, E.; Bernardo, A.F.; Mattos, A.B.; Vieira, A.K.; Malafaia, T.D.O.; Moura, A.S. Heart energy metabolism impairment in Western-diet induced obese mice. J. Nutr. Biochem. 2014, 25, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Drewnowski, A.; Mennella, J.A.; Johnson, S.L.; Bellisle, F. Sweetness and food preference. J. Nutr. 2012, 142, 1142–1148. [Google Scholar] [CrossRef] [PubMed]
- Bellisle, F.; Drewnowski, A. Intense sweeteners, energy intake and the control of body weight. Eur. J. Clin. Nutr. 2007, 61, 691–700. [Google Scholar] [CrossRef]
- Freedman, D.S.; Khan, L.K.; Serdula, M.K.; Dietz, W.H.; Srinivasan, S.R.; Berenson, G.S. The relation of childhood BMI to adult adiposity: The Bogalusa Heart Study. Pediatrics 2005, 115, 22–27. [Google Scholar] [CrossRef]
- Lifshitz, F. Obesity in children. J. Clin. Res. Pediatr. Endocrinol. 2008, 1, 53–60. [Google Scholar] [CrossRef]
- Nguyen, P.; Leray, V.; Diez, M.; Serisier, S.; Le Bloc’h, J.; Siliart, B.; Dumon, H. Liver lipid metabolism. J. Anim. Physiol. Anim. Nutr. 2008, 92, 272–283. [Google Scholar] [CrossRef]
- Trayhurn, P.; Beattie, J.H. Physiological role of adipose tissue: White adipose tissue as an endocrine and secretory organ. Proc. Nutr. Soc. 2001, 60, 329–339. [Google Scholar] [CrossRef]
- Barnard, R.J.; Roberts, C.K.; Varon, S.M.; Berger, J.J. Diet-induced insulin resistance precedes other aspects of the metabolic syndrome. J. Appl. Physiol. 1985, 84, 1311–1315. [Google Scholar] [CrossRef]
- McCarthy, E.M.; Rinella, M.E. The role of diet and nutrient composition in nonalcoholic fatty liver disease. J. Acad. Nutr. Diet. 2012, 112, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Bell, E.A.; Rolls, B.J. Energy density of foods affects energy intake across multiple levels of fat content in lean and obese women. Am. J. Clin. Nutr. 2001, 73, 1010–1018. [Google Scholar] [CrossRef] [PubMed]
- Fabbrini, E.; Sullivan, S.; Klein, S. Obesity and nonalcoholic fatty liver disease: Biochemical, metabolic, and clinical implications. Hepatology 2010, 51, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Anstee, Q.M.; Targher, G.; Day, C.P. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 330–344. [Google Scholar] [CrossRef]
- Engelmann, C.; Tacke, F. The Potential Role of Cellular Senescence in Non-Alcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2022, 23, 652. [Google Scholar] [CrossRef]
- Margini, C.; Dufour, J.F. The story of HCC in NAFLD: From epidemiology, across pathogenesis, to prevention and treatment. Liver Int. 2016, 36, 317–324. [Google Scholar] [CrossRef]
- Lee, Y.A.; Friedman, S.L. Inflammatory and fibrotic mechanisms in NAFLD—Implications for new treatment strategies. J. Intern. Med. 2022, 291, 11–31. [Google Scholar] [CrossRef]
- Marchesini, G.; Petta, S.; Dalle Grave, R. Diet, weight loss, and liver health in nonalcoholic fatty liver disease: Pathophysiology, evidence, and practice. Hepatology 2016, 63, 2032–2043. [Google Scholar] [CrossRef]
- Gauthier, M.S.; Favier, R.; Lavoie, J.M. Time course of the development of non-alcoholic hepatic steatosis in response to high-fat diet-induced obesity in rats. Br. J. Nutr. 2006, 95, 273–281. [Google Scholar] [CrossRef]
- Ishimoto, T.; Lanaspa, M.A.; Rivard, C.J.; Roncal-Jimenez, C.A.; Orlicky, D.J.; Cicerchi, C.; McMahan, R.H.; Abdelmalek, M.F.; Rosen, H.R.; Jackman, M.R.; et al. High-fat and high-sucrose (western) diet induces steatohepatitis that is dependent on fructokinase. Hepatology 2013, 58, 1632–1643. [Google Scholar] [CrossRef]
- Torres-Villalobos, G.; Hamdan-Pérez, N.; Tovar, A.R.; Ordaz-Nava, G.; Martínez-Benítez, B.; Torre-Villalvazo, I.; Torres, N. Combined high-fat diet and sustained high sucrose consumption promotes NAFLD in a murine model. Ann. Hepatol. 2015, 14, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yang, F.J.; Shang, L.C.; Zhang, Y.H.; Zhou, Y.; Shi, X.L. Puerarin protects against high-fat high-sucrose diet-induced non-alcoholic fatty liver disease by modulating PARP-1/PI3K/AKT signaling pathway and facilitating mitochondrial homeostasis. Phytother. Res. 2019, 33, 2347–2359. [Google Scholar] [CrossRef] [PubMed]
- Stoorvogel, W.; Kleijmeer, M.J.; Geuze, H.J.; Raposo, G. The biogenesis and functions of exosomes. Traffic 2002, 3, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhao, M.F.; Jiang, S.; Wu, J.; Liu, J.; Yuan, X.W.; Li, C.J. Liver governs adipose remodelling via extracellular vesicles in response to lipid overload. Nat. Commun. 2020, 11, 719. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Son, T.; Park, J.; Jun, W.; Kim, O.K. Role of Exosomes Derived from Adipose Tissue under Obese Conditions in Skeletal Muscle and Liver Cells: Commonalities and Differences. Mol. Nutr. Food Res. 2022, 16, 2200358. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Lee, M.; Oh, D.H.; Kim, J.L.; Park, M.R.; Kim, T.G.; Kim, O.K.; Lee, J. Emblica officinalis and Hordeum vulgare L. Mixture Regulates Lipolytic Activity in Differentiated 3T3-L1 Cells. J. Med. Food 2021, 24, 172–179. [Google Scholar] [CrossRef]
- Zhao, X.Y.; Xiong, X.; Liu, T.; Mi, L.; Peng, X.; Rui, C.; Lin, J.D. Long noncoding RNA licensing of obesity-linked hepatic lipogenesis and NAFLD pathogenesis. Nat. Commun. 2018, 9, 2986. [Google Scholar]
- Mundi, M.S.; Velapati, S.; Patel, J.; Kellogg, T.A.; Abu Dayyeh, B.K.; Hurt, R.T. Evolution of NAFLD and its management. Nutr. Clin. Pract. 2020, 35, 72–84. [Google Scholar] [CrossRef]
- Morrison, M.C.; Kleemann, R. Role of macrophage migration inhibitory factor in obesity, insulin resistance, type 2 diabetes, and associated hepatic co-morbidities: A comprehensive review of human and rodent studies. Front. Immunol. 2015, 6, 308. [Google Scholar] [CrossRef]
- Ann, J.Y.; Eo, H.; Lim, Y. Mulberry leaves (Morus alba L.) ameliorate obesity-induced hepatic lipogenesis, fibrosis, and oxidative stress in high-fat diet-fed mice. Genes Nutr. 2015, 10, 46. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, M.; Tian, W. Physiological and pathological impact of exosomes of adipose tissue. Cell Prolif. 2016, 49, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Shang, Q.; Pan, Z.; Bai, Y.; Li, Z.; Zhang, H.; Wang, Q. Exosomes from adipose-derived stem cells attenuate adipose inflammation and obesity through polarizing M2 macrophages and beiging in white adipose tissue. Diabetes 2018, 67, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Li, Z.; Qi, M.; Zhao, P.; Duan, Y.; Yang, G.; Yuan, L. Brown adipose tissue-derived exosomes mitigate the metabolic syndrome in high fat diet mice. Theranostics 2020, 10, 8197. [Google Scholar] [CrossRef] [PubMed]
- Sung, S.; Kim, J.; Jung, Y. Liver-derived exosomes and their implications in liver pathobiology. Int. J. Mol. Sci. 2018, 19, 3715. [Google Scholar] [CrossRef]
- Younossi, Z.; Tacke, F.; Arrese, M.; Chander Sharma, B.; Mostafa, I.; Bugianesi, E.; Vos, M.B. Global perspectives on nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Hepatology 2019, 69, 2672–2682. [Google Scholar] [CrossRef]
- Chen, H.; Wang, J.; Li, Z.; Lam, C.W.K.; Xiao, Y.; Wu, Q.; Zhang, W. Consumption of sugar-sweetened beverages has a dose-dependent effect on the risk of non-alcoholic fatty liver disease: An updated systematic review and dose-response meta-analysis. Int. J. Environ. Res. Public Health 2019, 1166, 2192. [Google Scholar] [CrossRef]

| Gene | Primer Sequences |
|---|---|
| ACC1 | F: 5′-GATGAACCATCTCCGTTG-3′ |
| R: 5′-CCCAATTATGAATCGGGA-3′ | |
| FAS | F: 5′-ACTGCCTTCGGTTCAGTCTC-3′ |
| R: 5′-CACCCTCCAAGGAGTCTCAC-3′ | |
| SREBP1C | F: 5′-TGAAGACAGATGCAGGAG-3′ |
| R: 5′-ATGGTCCCTCCACTCACC-3′ | |
| C/EBP-β | F: 5′-GACAAGCTGAGCGACGAG-3′ |
| R: 5′-GTCAGCTCCAGCACCTTG-3′ | |
| IL-1β | F: 5′-GCCACCTTTTGACAGTGATG-3′ |
| R: 5′-ATCAGGACAGCCCAGGTCAA-3′ | |
| IL-6 | F: 5′-CCAAGAGATAAGCTGGAGTCA-3′ |
| R: 5′-GCACTAGGTTTGCCGAGTAGA-3′ | |
| TNF-α | F: 5′ AAGTTCCCAAATGGCCTCCC 3′ |
| R: 5′-TTTGCTACGACGTGGGCTAC-3′ | |
| α-SMA | F: 5′-TCACCATTGGAAACGAACGC-3′ |
| R: 5′-GCTGTTATAGGTGGTTTCGT-3′ | |
| Col1a1 | F: 5′-AGCACGTCTGGTTTGGAGAG-3′ |
| R: 5′-GACATTAGGCGCAGGAAGGT-3′ | |
| TGF-β | F: 5′-CATCCATGACATGAACCGGC-3′ |
| R: 5′-GTTGGTATCCAGGGCTCTCC-3′ | |
| ACS1 | F: 5′-CCGCGACTCCTTAAATAGCA-3′ |
| R: 5′-GGGTTGGTGGTTCTCTATGC-3′ | |
| HSL | F: 5′-GTGAATGAGATGGCGAGGGT-3′ |
| R: 5′-GTGCCCTCACAGCAGGAATA-3′ | |
| FABP4 | F: 5′-TGGGATGGAAAGTCGACCAC-3′ |
| R: 5′-TTCTTTGGCTCATGCCCTT-3′ | |
| GAPDH | F: 5′-AACTTGGCATTGTGGAAGG-3′ |
| R: 5′-CACATTGGGGGTAGGAACAC-3′ |
| CON (n = 8) | HF (n = 8) | HFS (n = 8) | ||
|---|---|---|---|---|
| BW (g) | Initial (0 weeks) | 20.88 ± 0.6 NS | 20.46 ± 0.9 | 20.14 ± 0.7 |
| Final (12 weeks) | 32.16 ± 2.0 c | 46.30 ± 2.7 a | 41.65 ± 4.1 b | |
| Weight gain (g) | 11.27 ± 2.4 c | 25.83 ± 2.5 a | 21.51 ± 3.8 b | |
| Diet intake (g) | 239.91 ± 3.1 NS | 230.56 ± 2.6 | 218.76 ± 3.3 | |
| Sucrose water intake (mL) | - | - | 251.69 ± 4.9 | |
| Total calorie intake (kcal) 1 | 904.4 ± 44.58 b | 1164.9 ± 48.63 a | 1154.8 ± 60.99 a | |
| FER 2 | 1.24 ± 0.06 a | 2.22 ± 0.09 c | 1.87 ± 0.1 b | |
| Liver (g) | 1.01 ± 0.1 c | 2.09 ± 0.3 a | 1.59 ± 0.4 b | |
| Total WAT (g) | 2.94 ± 0.8 b | 6.74 ± 1.4 a | 6.02 ± 0.8 a | |
| Epididymal WAT (g) | 1.12 ± 0.2 b | 2.03 ± 0.3 a | 2.15 ± 0.2 a | |
| Visceral WAT (g) | 0.52 ± 0.2 b | 1.14 ± 0.3 a | 1.00 ± 0.2 a | |
| Subcutaneous WAT (g) | 1.29 ± 0.3 c | 3.56 ± 0.7 a | 2.88 ± 0.3 b | |
| Liver weight/BW (%) | 3.18 ± 0.2 b | 4.5 ± 0.6 a | 3.78 ± 0.9 b | |
| Total WAT weight/BW (%) | 9.06 ± 1.9 b | 14.62 ± 2.6 a | 14.56 ± 1.1 a | |
| CON (n = 8) | HF (n = 8) | HFS (n = 8) | |
|---|---|---|---|
| Triglyceride (nmol/μL) | 6.52 ± 0.30 c | 8.39 ± 0.45 b | 10.35 ± 0.32 a |
| Total cholesterol (μg/μL) | 82.97 ± 4.9 c | 109.98 ± 4.71 a | 98.83 ± 6.04 b |
| LDL (μg/μL) | 0.15 ± 0.01 c | 0.32 ± 0.01 a | 0.23 ± 0.01 b |
| HDL (μg/μL) | 0.12 ± 0.02 b | 0.27 ± 0.02 a | 0.26 ± 0.02 a |
| Glucose (nmol/μL) | 1.8 ± 0.08 c | 2.9 ± 0.1 b | 3.5 ± 0.02 a |
| AST (U/mL) | 2.9 ± 1.0 c | 4.3 ± 1.1 ab | 5.4 ± 1.5 a |
| ALT (mU/mL) | 0.46 ± 0.05 b | 1.7 ± 0.11 a | 1.59 ± 0.05 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.; Jeong, I.; Kim, O.-K. Liver-Derived Exosomes Induce Inflammation and Lipogenesis in Mice Fed High-Energy Diets. Nutrients 2022, 14, 5124. https://doi.org/10.3390/nu14235124
Lee J, Jeong I, Kim O-K. Liver-Derived Exosomes Induce Inflammation and Lipogenesis in Mice Fed High-Energy Diets. Nutrients. 2022; 14(23):5124. https://doi.org/10.3390/nu14235124
Chicago/Turabian StyleLee, Jihee, Inae Jeong, and Ok-Kyung Kim. 2022. "Liver-Derived Exosomes Induce Inflammation and Lipogenesis in Mice Fed High-Energy Diets" Nutrients 14, no. 23: 5124. https://doi.org/10.3390/nu14235124
APA StyleLee, J., Jeong, I., & Kim, O.-K. (2022). Liver-Derived Exosomes Induce Inflammation and Lipogenesis in Mice Fed High-Energy Diets. Nutrients, 14(23), 5124. https://doi.org/10.3390/nu14235124







