Extra-Virgin Olive Oil Enhances the Blood–Brain Barrier Function in Mild Cognitive Impairment: A Randomized Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects and Study Design
2.2. Inclusion/Exclusion Criteria
2.3. Neuropsychological Evaluation
2.4. MRI Data Acquisition
2.5. N-Back Task
2.6. MRI Data Preprocessing
2.7. Task fMRI Activation Analysis
2.8. Resting-State fMRI Connectivity Analysis
2.9. Contrast-Enhanced MRI for BBB Permeability Analysis
2.10. Measurements of Plasma Aβ40, Aβ42, Tau and p-tau181, and Serum NFL Using SIMOA
2.11. Statistical Analysis
3. Results
3.1. Baseline Demographic and Clinical Characteristics
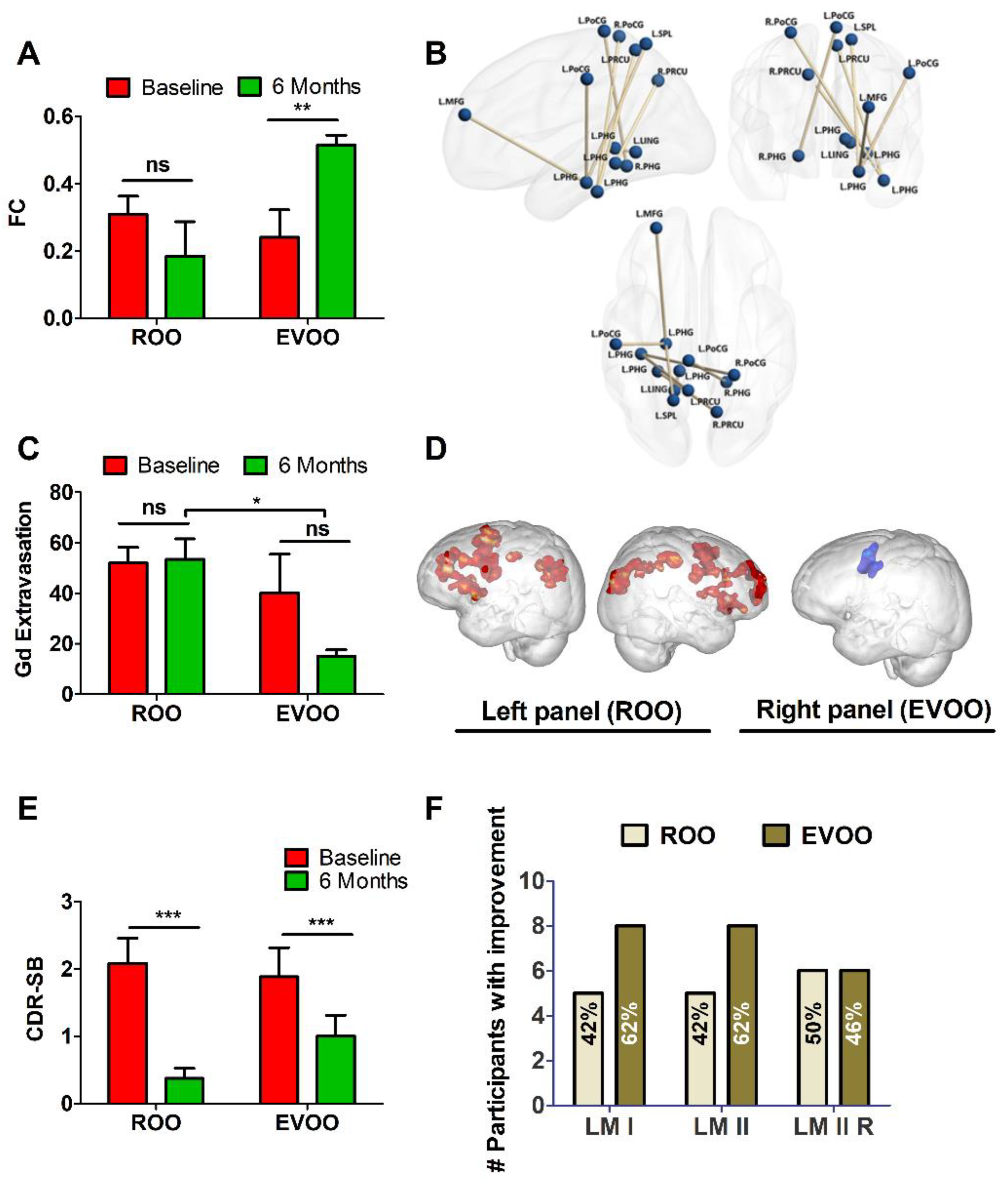
3.2. Effect of ROO and EVOO on Functional Connectivity and BBB Permeability
3.3. Functional Neuroimaging and N-Back Task
3.4. Effect of ROO and EVOO on Cognitive Measures
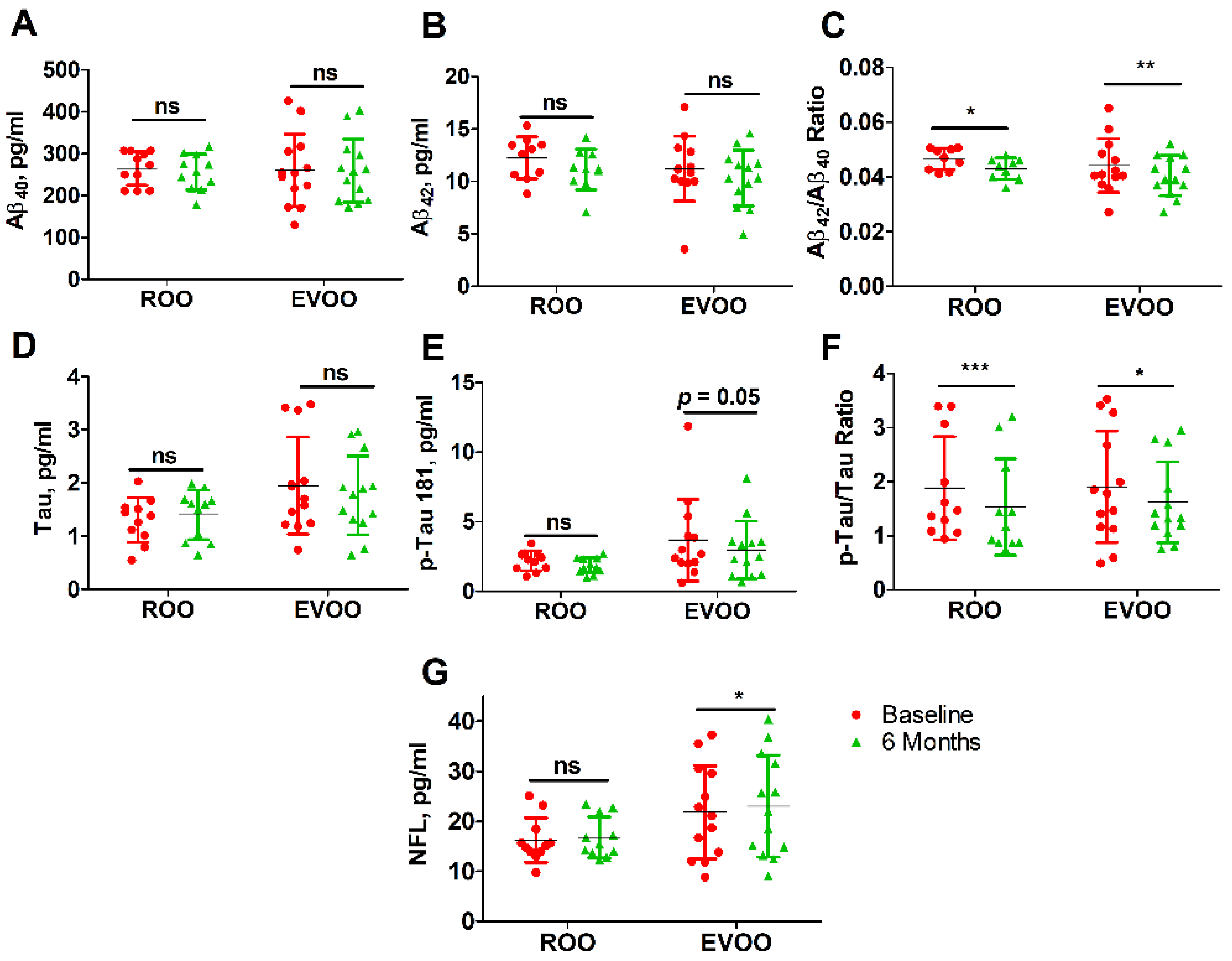
3.5. Effect of ROO and EVOO on Blood Biomarkers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zlokovic, B.V. Neurovascular mechanisms of Alzheimer’s neurodegeneration. Trends Neurosci. 2005, 28, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Kelleher, R.J.; Soiza, R.L. Evidence of endothelial dysfunction in the development of Alzheimer’s disease: Is Alzheimer’s a vascular disorder? Am. J. Cardiovasc. Dis. 2013, 3, 197–226. [Google Scholar] [PubMed]
- Cordonnier, C.; van der Flier, W.M. Brain microbleeds and Alzheimer’s disease: Innocent observation or key player? Brain 2011, 134, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Van Norden, A.G.W.; van Dijk, E.J.; de Laat, K.F.; Scheltens, P.; Olderikkert, M.G.M.; de Leeuw, F.E. Dementia: Alzheimer pathology and vascular factors: From mutually exclusive to interaction. Biochim. Biophys Acta 2012, 1822, 340–349. [Google Scholar] [CrossRef] [PubMed]
- De la Torre, J.C. Cerebral hemodynamics and vascular risk factors: Setting the stage for Alzheimer’s disease. J. Alzheimer’s Dis. 2012, 32, 553–567. [Google Scholar] [CrossRef]
- Abete, P.; Della-Morte, D.; Gargiulo, G.; Basile, C.; Langellotto, A.; Galizia, G.; Testa, G.; Canonico, V.; Bonaduce, D.; Cacciatore, F. Cognitive impairment and cardiovascular diseases in the elderly. A heart-brain continuum hypothesis. Ageing Res. Rev. 2014, 18, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Butt, A.M.; Jones, H.C.; Abbott, N.J. Electrical resistance across the blood-brain barrier in anaesthetized rats: A developmental study. J. Physiol. 1990, 429, 47–62. [Google Scholar] [CrossRef]
- Beheshti, I.; Maikusa, N.; Daneshmand, M.; Matsuda, H.; Demirel, H.; Anbarjafari, G.; Japanese-Alzheimer’s Disease Neuroimaging Initiative. Classification of Alzheimer’s Disease and Prediction of Mild Cognitive Impairment Conversion Using Histogram-Based Analysis of Patient-Specific Anatomical Brain Connectivity Networks. J. Alzheimer’s Dis. 2017, 60, 295–304. [Google Scholar] [CrossRef]
- Scarmeas, N.; Stern, Y.; Tang, M.X.; Mayeux, R.; Luchsinger, J.A. Mediterranean diet and risk for Alzheimer’s disease. Ann. Neurol. 2006, 59, 912–921. [Google Scholar] [CrossRef]
- Gu, Y.; Nieves, J.W.; Stern, Y.; Luchsinger, J.A.; Scarmeas, N. Food combination and Alzheimer disease risk: A protective diet. Arch. Neurol. 2010, 67, 699–706. [Google Scholar] [CrossRef]
- Scarmeas, N.; Stern, Y.; Mayeux, R.; Manly, J.J.; Schupf, N.; Luchsinger, J.A. Mediterranean diet and mild cognitive impairment. Arch. Neurol. 2009, 66, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Tripoli, E.; Giammanco, M.; Tabacchi, G.; Di Majo, D.; Giammanco, S.; La Guardia, M. The phenolic compounds of olive oil: Structure, biological activity and beneficial effects on human health. Nutr. Res. Rev. 2005, 18, 98–112. [Google Scholar] [CrossRef] [PubMed]
- Cicerale, S.; Lucas, L.; Keast, R. Biological activities of phenolic compounds present in virgin olive oil. Int. J. Mol. Sci. 2010, 11, 458–479. [Google Scholar] [CrossRef] [PubMed]
- Tuck, K.L.; Hayball, P.J. Major phenolic compounds in olive oil: Metabolism and health effects. J. Nutr. Biochem. 2002, 13, 636–644. [Google Scholar] [CrossRef] [PubMed]
- Vissers, M.N.; Zock, P.L.; Katan, M.B. Bioavailability and antioxidant effects of olive oil phenols in humans: A review. Eur. J. Clin. Nutr. 2004, 58, 955–965. [Google Scholar] [CrossRef] [PubMed]
- Al Rihani, S.B.; Darakjian, L.I.; Kaddoumi, A. Oleocanthal-Rich Extra-Virgin Olive Oil Restores the Blood-Brain Barrier Function through NLRP3 Inflammasome Inhibition Simultaneously with Autophagy Induction in TgSwDI Mice. ACS Chem. Neurosci. 2019, 10, 3543–3554. [Google Scholar] [CrossRef] [PubMed]
- Batarseh, Y.S.; Kaddoumi, A. Oleocanthal-rich extra-virgin olive oil enhances donepezil effect by reducing amyloid-β load and related toxicity in a mouse model of Alzheimer’s disease. J. Nutr. Biochem. 2018, 55, 113–123. [Google Scholar] [CrossRef]
- Qosa, H.; Mohamed, L.A.; Batarseh, Y.S.; Alqahtani, S.; Ibrahim, B.; LeVine H 3rd Keller, J.N.; Kaddoumi, A. Extra-virgin olive oil attenuates amyloid-β and tau pathologies in the brains of TgSwDI mice. J. Nutr. Biochem. 2015, 26, 1479–1490. [Google Scholar] [CrossRef]
- Lauretti, E.; Iuliano, L.; Praticò, D. Extra-virgin olive oil ameliorates cognition and neuropathology of the 3xTg mice: Role of autophagy. Ann. Clin. Transl. Neurol. 2017, 4, 564–574. [Google Scholar] [CrossRef]
- Lauretti, E.; Nenov, M.; Dincer, O.; Iuliano, L.; Praticò, D. Extra virgin olive oil improves synaptic activity, short-term plasticity, memory, and neuropathology in a tauopathy model. Aging Cell 2020, 19, e13076. [Google Scholar] [CrossRef]
- Esteban, O.; Markiewicz, C.J.; Blair, R.W.; Moodie, C.A.; Isik, A.I.; Erramuzpe, A.; Kent, J.D.; Goncalves, M.; DuPre, E.; Snyder, M.; et al. fMRIPrep: A robust preprocessing pipeline for functional MRI. Nat. Methods 2019, 16, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Esteban, O.; Markiewicz, C.J.; Goncalves, M.; Provins, C.; Kent, J.D.; DuPre, E.; Salo, T.; Ciric, R.; Pinsard, B.; Blair, R.W.; et al. fMRIPrep: A robust preprocessing pipeline for functional MRI. Zenodo 2018. [Google Scholar] [CrossRef] [PubMed]
- Gorgolewski, K.; Burns, C.D.; Madison, C.; Clark, D.; Halchenko, Y.O.; Waskom, M.L.; Ghosh, S. Nipype: A Flexible, Lightweight and Extensible Neuroimaging Data Processing Framework in Python. Front. Neuroinformatics 2011, 5, 13. [Google Scholar] [CrossRef] [PubMed]
- Dretsch, M.N.; Daniel, T.A.; Goodman, A.M.; Katz, J.S.; Denney, T.; Deshpande, G.; Robinson, J.L. Differential neural activation when voluntarily regulating emotions in service members with chronic mild traumatic brain injury. Appl. Neuropsychol. Adult 2019, 26, 76–88. [Google Scholar] [CrossRef] [PubMed]
- Jenkinson, M.; Beckmann, C.F.; Behrens, T.E.; Woolrich, M.W.; Smith, S.M. FSL. Neuroimage 2012, 62, 782–790. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.M.; Jenkinson, M.; Woolrich, M.W.; Beckmann, C.F.; Behrens, T.E.; Johansen-Berg, H.; Bannister, P.R.; De Luca, M.; Drobnjak, I.; Flitney, D.E.; et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 2004, 23 (Suppl. S1), S208–S219. [Google Scholar] [CrossRef]
- Mencarelli, L.; Neri, F.; Momi, D.; Menardi, A.; Rossi, S.; Rossi, A.; Santarnecchi, E. Stimuli, presentation modality, and load-specific brain activity patterns during n-back task. Hum. Br. Mapp. 2019, 40, 3810–3831. [Google Scholar] [CrossRef]
- Knight, R.A.; Nagaraja, T.N.; Ewing, J.R.; Nagesh, V.; Whitton, P.A.; Bershad, E.; Fagan, S.C.; Fenstermacher, J.D. Quantitation and localization of blood-to-brain influx by magnetic resonance imaging and quantitative autoradiography in a model of transient focal ischemia. J. Magn. Reson. Med. 2005, 54, 813–821. [Google Scholar] [CrossRef]
- Sourbron, S.P.; Buckley, D.L. Tracer kinetic modelling in MRI: Estimating perfusion and capillary permeability. Phys. Med. Biol. 2012, 57, R1–R33. [Google Scholar] [CrossRef]
- Chassidim, Y.; Veksler, R.; Lublinsky, S.; Pell, G.S.; Friedman, A.; Shelef, I. Quantitative imaging assessment of blood-brain barrier permeability in humans. Fluids Barriers CNS 2013, 10, 9. [Google Scholar] [CrossRef]
- Bar-Klein, G.; Lublinsky, S.; Kamintsky, L.; Noyman, I.; Veksler, R.; Dalipaj, H.; Senatorov, V.V., Jr.; Swissa, E.; Rosenbach, D.; Elazary, N.; et al. Imaging blood-brain barrier dysfunction as a biomarker for epileptogenesis. Brain 2017, 140, 1692–1705. [Google Scholar] [CrossRef] [PubMed]
- Fossati, S.; Ramos Cejudo, J.; Debure, L.; Pirraglia, E.; Sone, J.Y.; Li, Y.; Chen, J.; Butler, T.; Zetterberg, H.; Blennow, K.; et al. Plasma tau complements CSF tau and P-tau in the diagnosis of Alzheimer’s disease. Alzheimer’s Dement. 2019, 11, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Scheltens, P.; Kamphuis, P.J.; Verhey, F.R.; Olde Rikkert, M.G.; Wurtman, R.J.; Wilkinson, D.; Twisk, J.W.; Kurz, A. Efficacy of a medical food in mild Alzheimer’s disease: A randomized, controlled trial. Alzheimer’s Dement. 2010, 6, 1.e1–10.e1. [Google Scholar] [CrossRef] [PubMed]
- Guasch-Ferré, M.; Li, Y.; Willett, W.C.; Sun, Q.; Sampson, L.; Salas-Salvadó, J.; Martínez-González, M.A.; Stampfer, M.J.; Hu, F.B. Consumption of Olive Oil and Risk of Total and Cause-Specific Mortality Among U.S. Adults. J. Am. Coll. Cardiol. 2022, 79, 101–112. [Google Scholar] [CrossRef]
- Tsolaki, M.; Lazarou, E.; Kozori, M.; Petridou, N.; Tabakis, I.; Lazarou, I.; Karakota, M.; Saoulidis, I.; Melliou, E.; Magiatis, P. A Randomized clinical trial of Greek high phenolic early harvest extra virgin olive oil in mild cognitive impairment: The MICOIL pilot study. J. Alzheimer’s Dis. 2020, 78, 801–817. [Google Scholar] [CrossRef]
- Tzekaki, E.E.; Tsolaki, M.; Geromichalos, G.D.; Pantazaki, A.A. Extra Virgin Olive Oil consumption from Mild Cognitive Impairment patients attenuates oxidative and nitrative stress reflecting on the reduction of the PARP levels and DNA damage. Exp. Gerontol. 2021, 156, 111621. [Google Scholar] [CrossRef]
- Montagne, A.; Barnes, S.R.; Sweeney, M.D.; Halliday, M.R.; Sagare, A.P.; Zhao, Z.; Toga, A.W.; Jacobs, R.E.; Liu, C.Y.; Amezcua, L.; et al. Blood-Brain barrier breakdown in the aging human hippocampus. Neuron 2015, 85, 296–302. [Google Scholar] [CrossRef]
- van de Haar, H.J.; Jansen, J.F.; van Osch, M.J.; van Buchem, M.A.; Muller, M.; Wong, S.M.; Hofman, P.A.; Burgmans, S.; Verhey, F.R.; Backes, W.H. Neurovascular unit impairment in early Alzheimer’s disease measured with magnetic resonance imaging. Neurobiol. Aging 2016, 45, 190–196. [Google Scholar] [CrossRef]
- Kalaria, R.N. Cerebral vessels in ageing and Alzheimer’s disease. Pharmacol. Ther. 1996, 72, 193–214. [Google Scholar] [CrossRef]
- Kalaria, R. Similarities between Alzheimer’s disease and vascular dementia. J. Neurol. Sci. 2002, 15, 29–34. [Google Scholar] [CrossRef]
- Wang, H.; Golob, E.J.; Su, M.Y. Vascular volume and blood-brain barrier permeability measured by dynamic contrast enhanced MRI in hippocampus and cerebellum of patients with MCI and normal controls. J. Magn. Reson. Imaging 2006, 24, 695–700. [Google Scholar] [CrossRef] [PubMed]
- Marchi, N.; Bazarian, J.J.; Puvenna, V.; Janigro, M.; Ghosh, C.; Zhong, J.; Zhu, T.; Blackman, E.; Stewart, D.; Ellis, J.; et al. Consequences of repeated blood-brain barrier disruption in football players. PLoS ONE 2013, 8, e56805. [Google Scholar] [CrossRef] [PubMed]
- Horn, A.; Ostwald, D.; Reisert, M.; Blankenburg, F. The structural-functional connectome and the default mode network of the human brain. Neuroimage 2014, 102 Pt 1, 142–151. [Google Scholar] [CrossRef] [PubMed]
- O’Bryant, S.E.; Waring, S.C.; Cullum, C.M.; Hall, J.; Lacritz, L.; Massman, P.J.; Lupo, P.J.; Reisch, J.S.; Doody, R.; Texas Alzheimer’s Research Consortium. Staging dementia using Clinical Dementia Rating Scale Sum of Boxes scores: A Texas Alzheimer’s research consortium study. Arch. Neurol. 2008, 65, 1091–1095. [Google Scholar] [CrossRef] [PubMed]
- Graff-Radford, N.R.; Crook, J.E.; Lucas, J.; Boeve, B.F.; Knopman, D.S.; Ivnik, R.J.; Smith, G.E.; Younkin, L.H.; Petersen, R.C.; Younkin, S.G. Association of low plasma Aβ42/Aβ40 ratios with increased imminent risk for mild cognitive impairment and Alzheimer disease. Arch. Neurol. 2007, 64, 354–362. [Google Scholar] [CrossRef]
- Mohamed, L.A.; Kaddoumi, A. In vitro investigation of amyloid-β hepatobiliary disposition in sandwich-cultured primary rat hepatocytes. Drug Metab. Dispos. 2013, 41, 1787–1796. [Google Scholar] [CrossRef]
- Mohamed, L.A.; Qosa, H.; Kaddoumi, A. Age-related decline in brain and hepatic clearance of amyloid-beta is rectified by the cholinesterase inhibitors donepezil and rivastigmine in rats. ACS Chem. Neurosci. 2015, 6, 725–736. [Google Scholar] [CrossRef]
- Tian, D.Y.; Cheng, Y.; Zhuang, Z.Q.; He, C.Y.; Pan, Q.G.; Tang, M.Z.; Hu, X.L.; Shen, Y.Y.; Wang, Y.R.; Chen, S.H.; et al. Physiological clearance of amyloid-beta by the kidney and its therapeutic potential for Alzheimer’s disease. Mol. Psychiatry 2021, 26, 6074–6082. [Google Scholar] [CrossRef]
- Mielke, M.M.; Hagen, C.E.; Xu, J.; Chai, X.; Vemuri, P.; Lowe, V.J.; Airey, D.C.; Knopman, D.S.; Roberts, R.O.; Machulda, M.M.; et al. Plasma phospho-tau181 increases with Alzheimer’s disease clinical severity and is associated with tau- and amyloid-positron emission tomography. Alzheimer’s Dement. 2018, 14, 989–997. [Google Scholar] [CrossRef]
- Karikari, T.K.; Pascoal, T.A.; Ashton, N.J.; Janelidze, S.; Benedet, A.L.; Rodriguez, J.L.; Chamoun, M.; Savard, M.; Kang, M.S.; Therriault, J.; et al. Blood phosphorylated tau 181 as a biomarker for Alzheimer’s disease: A diagnostic performance and prediction modelling study using data from four prospective cohorts. Lancet Neurol. 2020, 19, 422–433. [Google Scholar] [CrossRef]
- Janelidze, S.; Mattsson, N.; Palmqvist, S.; Smith, R.; Beach, T.G.; Serrano, G.E.; Chai, X.; Proctor, N.K.; Eichenlaub, U.; Zetterberg, H.; et al. Plasma P-tau181 in Alzheimer’s disease: Relationship to other biomarkers, differential diagnosis, neuropathology and longitudinal progression to Alzheimer’s dementia. Nat. Med. 2020, 26, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Barro, C.; Chitnis, T.; Weiner, H.L. Blood neurofilament light: A critical review of its application to neurologic disease. Ann. Clin. Transl. Neurol. 2020, 7, 2508–2523. [Google Scholar] [CrossRef] [PubMed]

| Variable | ROO (n = 12) | EVOO (n = 13) | p Value |
|---|---|---|---|
| Male | 3 (25%) | 5 (38%) | |
| Female | 9 (75%) | 8 (62%) | 0.47 |
| Age (years) | 65.5 ± 4.4 | 67.5 ± 5.5 | 0.31 |
| Bodyweight (kg) | 74.± 3 ± 12.4 | 85.1 ± 19.9 | 0.11 |
| Education (years) | 15.8 ± 2.7 | 14.2 ± 3.7 | 0.25 |
| MMSE | 27.83 ± 2.17 | 27.15 ± 2.64 | 0.49 |
| CDR | 0.42 ± 0.19 | 0.38 ± 0.22 | 0.87 |
| Functional Connection | Pair-Wise p-Values | ||||
|---|---|---|---|---|---|
| EVOO2 > EVOO1 | ROO2 vs. ROO1 | EVOO1 vs. ROO2 | EVOO1 vs. ROO1 | EVOO2 > ROO2 | |
| L Precuneus ↔ L Parahippocampal Gyrus | 0.02 | 0.46 | 0.83 | 0.66 | 0.01 |
| R Precuneus ↔ L Parahippocampal Gyrus | 0.02 | 0.41 | 0.78 | 0.50 | 0.05 |
| L Postcentral Gyrus ↔ R Parahippocampal Gyrus | 0.03 | 0.93 | 0.80 | 0.77 | 0.04 |
| L Postcentral Gyrus ↔ L Parahippocampal Gyrus | 0.05 | 0.76 | 0.98 | 0.80 | 0.04 |
| R Postcentral Gyrus ↔ L Parahippocampal Gyrus | 0.05 | 0.31 | 0.50 | 0.91 | 0.01 |
| L Lingual Gyrus ↔ L Parahippocampal Gyrus | 0.04 | 0.22 | 0.84 | 0.34 | 0.05 |
| L Middle Frontal Gyrus ↔ L Parahippocampal Gyrus | 0.05 | 0.65 | 0.36 | 0.52 | 0.01 |
| L Superior Parietal Lobule ↔ L Parahippocampal Gyrus | 0.04 | 0.06 | 0.52 | 0.35 | 0.01 |
| BBB permeability | |||||
| L Parahippocampal Gyrus | 0.00 | 0.60 | 0.69 | 0.27 | 0.00 |
| R Parahippocampal Gyrus | 0.01 | 0.85 | 0.34 | 0.40 | 0.04 |
| L Hippocampus | 0.03 | 0.87 | 0.15 | 0.21 | 0.05 |
| R Hippocampus | 0.04 | 0.99 | 0.70 | 0.71 | 0.03 |
| Variable | ROO (n = 12) | EVOO (n = 13) | ||
|---|---|---|---|---|
| Baseline (SD) | 6 Months (SD) | Baseline (SD) | 6 Months (SD) | |
| Bodyweight (kg) | 74.3 (12.4) | 74.4 (13.1) | 85.1 (19.9) | 85.7 (21.3) |
| MMSE | 27.83 (2.17) | 28.83 (1.47) | 27.15 (2.64) | 26.92 (2.78) |
| CDR | 0.42 (0.19) | 0.13 (0.23) * | 0.38 (0.22) | 0.23 (0.26) * |
| % with CDR 0.5 | 83% | 25% | 77% | 46% |
| CDR-SOB | 2.1 (1.29) | 0.38 (0.53) *** | 1.88 (1.56) | 1.00 (1.14) *** |
| WMS-IV Logical Memory | ||||
| LM overall score | 16.3 (4.9) | 15.9 (4.4) | 12.5 (4.5) | 14.5 (5.3) * |
| %Correct | ||||
| LM I | 59.3 (18.9) | 56.3 (20.8) | 45.8 (19.6) | 52.4 (20.4) |
| LM II | 55.2 (27.0) | 50.4 (17.7) | 31.5 (24.1) # | 44.9 (31.2) * |
| LM II Recognition | 83.7 (12.3) | 88.1 (11.7) | 80.3 (16.2) | 82.5 (15.6) |
| VR I | 37.9 (5.2) | 41.3 (3.5) * | 38.9 (6.5) | 41.2 (2.7) |
| VR II | 41.3 (2.9) | 40.2 (4.6) | 42.8 (0.8) | 42.4 (2.2) |
| VR II Recognition | 3.8 (1.2) | 3.8 (1.4) | 2.2 (1.5) # | 3.7 (1.6) * |
| Blood biomarkers (pg/mL) | ||||
| Aβ40 | 265 (40) | 256 (43) | 260 (86) | 259 (75) |
| Aβ42 | 12.2 (2.0) | 11.1 (1.9) | 11.2 (3.1) | 10.3 (2.7) |
| Aβ42/Aβ40 | 0.047 (0.004) | 0.044 (0.005) * | 0.044 (0.009) | 0.040 (0.007) ** |
| Tau | 1.30 (0.42) | 1.40 (0.46) | 1.95 (0.91) # | 1.76 (0.74) |
| p-Tau181 | 2.19 (0.70) | 1.85 (0.56) | 3.67 (2.94) | 2.97 (2.08) * |
| p-Tau/t-Tau | 1.88 (0.95) | 1.53 (0.89) ** | 1.91 (1.03) | 1.62 (0.75) * |
| NFL | 16.2 (4.5) | 16.8 (4.1) | 21.8 (9.3) | 23.0 (10.2) * |
| ROO (n = 12) | EVOO (n = 13) | ||||
|---|---|---|---|---|---|
| Mean a (SD) | p | Mean a (SD) | p | 95% CI for Difference of Differences | |
| MMSE | 1.00 (2.73) | 0.23 | −0.23 (1.54) | 0.60 | (−0.583, 3.044) |
| CDR | −0.291 (0.257) | 0.0024 | −0.154 (0.240) | 0.039 | (−0.344, 0.068) |
| WMS-IV | |||||
| Overall score | −0.323 (5.068) | 0.83 | 2.034 (4.347) | 0.05 | (−5.897, 1.182) |
| LM-I | −3.036 (20.9) | 0.63 | 6.615 (14.9) | 0.13 | (−24.573, 5.272) |
| LM-II | −4.786 (29.17) | 0.58 | 13.451 (20.77) | 0.038 | (−39.056, 2.582) |
| LM-IIR | 4.375 (9.80) | 0.15 | 2.244 (18.57) | 0.67 | (−10.321, 14.591) |
| VR I | 3.333 (5.10) | 0.04 | 2.308 (4.35) | 0.08 | (−2.887, 4.938) |
| VR II | −1.167 (4.43) | 0.38 | −0.385 (2.43) | 0.58 | (−3.706, 2.142) |
| VR IIR | 0.0 (1.13) | 1.00 | 1.462 (1.81) | 0.013 | (−2.721, −0.202) |
| ROO (n = 12) | EVOO (n = 13) | ||||
|---|---|---|---|---|---|
| Mean a (SD) | p | Mean a (SD) | p | 95% CI for Differences of Differences | |
| Aβ40 | −8.835 (59.6) | 0.63 | −1.150 (56.8) | 0.94 | (−57.038, 41.669) |
| Aβ42 | −1.29 (1.85) | 0.07 | −0.901 (2.15) | 0.158 | (−2.235, 1.456) |
| Tau | 0.098 (0.42) | 0.46 | −0.180 (0.69) | 0.368 | (−0.220, 0.770) |
| p-Tau 181 | −0.344 (0.66) | 0.12 | −0.698 (1.18) | 0.05 | (−0.476, 1.184) |
| Aβ42/Aβ40 ratio | −0.0034 (0.004) | 0.041 | −0.0036 (0.004) | 0.007 | (−0.003, 0.004) |
| p-Tau/Tau ratio | −0.347 (0.251) | 0.001 | −0.286 (0.446) | 0.039 | (−0.376, 0.253) |
| NFL | 0.553 (2.25) | 0.43 | 1.206 (1.91) | 0.042 | (−2.414, 1.109) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaddoumi, A.; Denney, T.S., Jr.; Deshpande, G.; Robinson, J.L.; Beyers, R.J.; Redden, D.T.; Praticò, D.; Kyriakides, T.C.; Lu, B.; Kirby, A.N.; et al. Extra-Virgin Olive Oil Enhances the Blood–Brain Barrier Function in Mild Cognitive Impairment: A Randomized Controlled Trial. Nutrients 2022, 14, 5102. https://doi.org/10.3390/nu14235102
Kaddoumi A, Denney TS Jr., Deshpande G, Robinson JL, Beyers RJ, Redden DT, Praticò D, Kyriakides TC, Lu B, Kirby AN, et al. Extra-Virgin Olive Oil Enhances the Blood–Brain Barrier Function in Mild Cognitive Impairment: A Randomized Controlled Trial. Nutrients. 2022; 14(23):5102. https://doi.org/10.3390/nu14235102
Chicago/Turabian StyleKaddoumi, Amal, Thomas S. Denney, Jr., Gopikrishna Deshpande, Jennifer L. Robinson, Ronald J. Beyers, David T. Redden, Domenico Praticò, Tassos C. Kyriakides, Bonian Lu, Anna N. Kirby, and et al. 2022. "Extra-Virgin Olive Oil Enhances the Blood–Brain Barrier Function in Mild Cognitive Impairment: A Randomized Controlled Trial" Nutrients 14, no. 23: 5102. https://doi.org/10.3390/nu14235102
APA StyleKaddoumi, A., Denney, T. S., Jr., Deshpande, G., Robinson, J. L., Beyers, R. J., Redden, D. T., Praticò, D., Kyriakides, T. C., Lu, B., Kirby, A. N., Beck, D. T., & Merner, N. D. (2022). Extra-Virgin Olive Oil Enhances the Blood–Brain Barrier Function in Mild Cognitive Impairment: A Randomized Controlled Trial. Nutrients, 14(23), 5102. https://doi.org/10.3390/nu14235102






