Dietary Supplement Intake and Fecundability in a Singapore Preconception Cohort Study
Abstract
1. Introduction
2. Materials and Methods
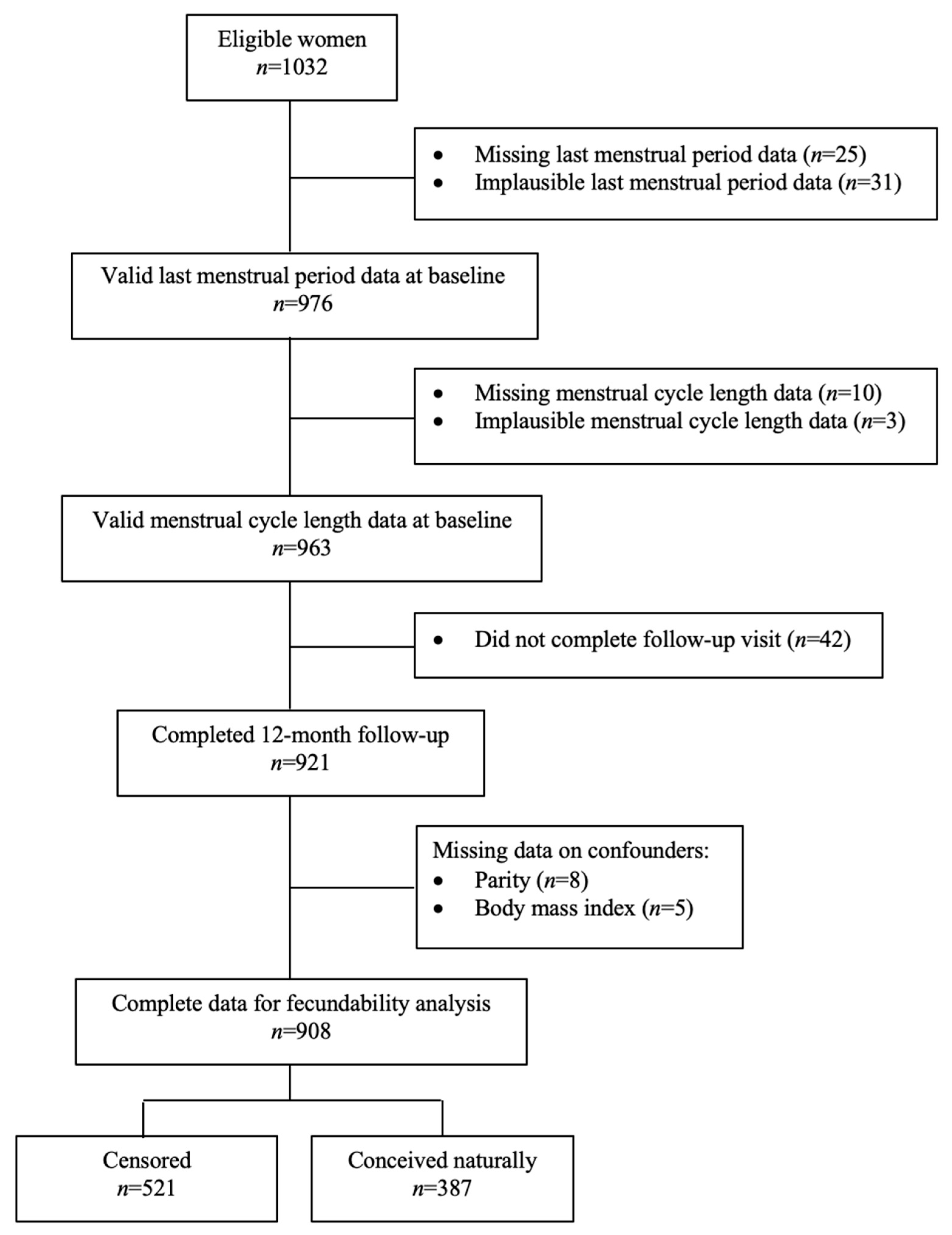
2.1. Study Design and Participants
2.2. Study Procedure
2.3. Supplement Intake
2.4. Assessment of Time to Pregnancy
2.5. Assessment of Confounders
2.6. Statistical Analysis
3. Results
3.1. Participant Characteristics
3.2. Associations between Supplement Intake Status and Fecundability
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gnoth, C.; Godehardt, E.; Frank-Herrmann, P.; Friol, K.; Tigges, J.; Freundl, G. Definition and prevalence of subfertility and infertility. Hum. Reprod. 2005, 20, 1144–1147. [Google Scholar] [CrossRef] [PubMed]
- Fertility Rate, Total (Births Per Woman). Available online: https://data.worldbank.org/indicator/SP.DYN.TFRT.IN?end=2020&start=1960&view=chart (accessed on 7 May 2022).
- Infertility. Available online: https://www.who.int/health-topics/infertility#tab=tab_2 (accessed on 7 May 2022).
- Cousineau, T.M.; Domar, A.D. Psychological impact of infertility. Best Pract. Res. Clin. Obstet. Gynaecol. 2007, 21, 293–308. [Google Scholar] [CrossRef]
- Stanhiser, J.; Steiner, A.Z. Psychosocial Aspects of Fertility and Assisted Reproductive Technology. Obstet. Gynecol. Clin. N. Am. 2018, 45, 563–574. [Google Scholar] [CrossRef]
- Tao, P.; Coates, R.; Maycock, B. Investigating marital relationship in infertility: A systematic review of quantitative studies. J. Reprod. Infertil. 2012, 13, 71–80. [Google Scholar] [PubMed]
- Habbema, J.D.; Collins, J.; Leridon, H.; Evers, J.L.; Lunenfeld, B.; te Velde, E.R. Towards less confusing terminology in reproductive medicine: A proposal. Hum. Reprod. 2004, 19, 1497–1501. [Google Scholar] [CrossRef] [PubMed]
- Fan, D.; Liu, L.; Xia, Q.; Wang, W.; Wu, S.; Tian, G.; Liu, Y.; Ni, J.; Wu, S.; Guo, X.; et al. Female alcohol consumption and fecundability: A systematic review and dose-response meta-analysis. Sci. Rep. 2017, 7, 13815. [Google Scholar] [CrossRef] [PubMed]
- Skoracka, K.; Ratajczak, A.E.; Rychter, A.M.; Dobrowolska, A.; Krela-Kaźmierczak, I. Female Fertility and the Nutritional Approach: The Most Essential Aspects. Adv. Nutr. 2021, 12, 2372–2386. [Google Scholar] [CrossRef]
- Taylor, A. ABC of subfertility: Extent of the problem. BMJ 2003, 327, 434–436. [Google Scholar] [CrossRef]
- Choo, X.H.; Ku, C.W.; Cheung, Y.B.; Godfrey, K.M.; Chong, Y.S.; Shek, L.P.; Tan, K.H.; Tan, T.C.; Nadarajah, S.; Yap, F.K.P.; et al. Risk score to stratify miscarriage risk levels in preconception women. Sci. Rep. 2021, 11, 12111. [Google Scholar] [CrossRef]
- Khan, N.N.; Boyle, J.A.; Lang, A.Y.; Harrison, C.L. Preconception Health Attitudes and Behaviours of Women: A Qualitative Investigation. Nutrients 2019, 11, 1490. [Google Scholar] [CrossRef]
- McKenna, E.; Hure, A.; Perkins, A.; Gresham, E. Dietary Supplement Use during Preconception: The Australian Longitudinal Study on Women’s Health. Nutrients 2017, 9, 1119. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, R.; Burt, E.; Gallagher, A.M.; Butler, L.; Venkatakrishnan, R.; Peitsidis, P. Prospective randomized trial of multiple micronutrients in subfertile women undergoing ovulation induction: A pilot study. Reprod. Biomed. Online 2012, 24, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Buhling, K.J.; Grajecki, D. The effect of micronutrient supplements on female fertility. Curr. Opin. Obstet. Gynecol. 2013, 25, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Grajecki, D.; Zyriax, B.C.; Buhling, K.J. The effect of micronutrient supplements on female fertility: A systematic review. Arch. Gynecol. Obstet. 2012, 285, 1463–1471. [Google Scholar] [CrossRef] [PubMed]
- Cederroth, C.R.; Zimmermann, C.; Nef, S. Soy, phytoestrogens and their impact on reproductive health. Mol. Cell. Endocrinol. 2012, 355, 192–200. [Google Scholar] [CrossRef]
- Swanson, D.; Block, R.; Mousa, S.A. Omega-3 fatty acids EPA and DHA: Health benefits throughout life. Adv. Nutr. 2012, 3, 1–7. [Google Scholar] [CrossRef]
- Thaler, C.J. Folate Metabolism and Human Reproduction. Geburtshilfe Frauenheilkd. 2014, 74, 845–851. [Google Scholar] [CrossRef]
- Using Evening Primrose Oil for Fertility. Available online: https://parenting.firstcry.com/articles/evening-primrose-oil-for-fertility-benefits-how-to-use-and-side-effects/ (accessed on 7 May 2022).
- Enhance Overall Fertility with Evening Primrose. Available online: https://natural-fertility-info.com/fertility-evening-primrose-oil.html (accessed on 7 May 2022).
- Supplements to Help Boost Fertility. Available online: https://www.craigranchobgyn.com/2019/03/30/supplements-to-help-boost-fertility/ (accessed on 7 May 2022).
- Jacobsen, B.K.; Jaceldo-Siegl, K.; Knutsen, S.F.; Fan, J.; Oda, K.; Fraser, G.E. Soy isoflavone intake and the likelihood of ever becoming a mother: The Adventist Health Study-2. Int. J. Womens Health 2014, 6, 377–384. [Google Scholar] [CrossRef]
- Wesselink, A.K.; Hatch, E.E.; Mikkelsen, E.M.; Trolle, E.; Willis, S.K.; McCann, S.E.; Valsta, L.; Lundqvist, A.; Tucker, K.L.; Rothman, K.J.; et al. Dietary phytoestrogen intakes of adult women are not strongly related to fecundability in 2 preconception cohort studies. J. Nutr. 2020, 150, 1240–1251. [Google Scholar] [CrossRef]
- Pilz, S.; Zittermann, A.; Obeid, R.; Hahn, A.; Pludowski, P.; Trummer, C.; Lerchbaum, E.; Pérez-López, F.R.; Karras, S.N.; März, W. The Role of Vitamin D in Fertility and during Pregnancy and Lactation: A Review of Clinical Data. Int. J. Environ. Res. Public Health 2018, 15, 2241. [Google Scholar] [CrossRef] [PubMed]
- Cueto, H.T.; Riis, A.H.; Hatch, E.E.; Wise, L.A.; Rothman, K.J.; Sørensen, H.T.; Mikkelsen, E.M. Folic acid supplementation and fecundability: A Danish prospective cohort study. Eur. J. Clin. Nutr. 2016, 70, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Hahn, K.A.; Wesselink, A.K.; Wise, L.A.; Mikkelsen, E.M.; Cueto, H.T.; Tucker, K.L.; Vinceti, M.; Rothman, K.J.; Sorensen, H.T.; Hatch, E.E. Iron Consumption Is Not Consistently Associated with Fecundability among North American and Danish Pregnancy Planners. J. Nutr. 2019, 149, 1585–1595. [Google Scholar] [CrossRef] [PubMed]
- Wise, L.A.; Wesselink, A.K.; Tucker, K.L.; Saklani, S.; Mikkelsen, E.M.; Cueto, H.; Riis, A.H.; Trolle, E.; McKinnon, C.J.; Hahn, K.A.; et al. Dietary Fat Intake and Fecundability in 2 Preconception Cohort Studies. Am. J. Epidemiol. 2018, 187, 60–74. [Google Scholar] [CrossRef] [PubMed]
- Loo, E.X.L.; Soh, S.E.; Loy, S.L.; Ng, S.; Tint, M.T.; Chan, S.Y.; Huang, J.Y.; Yap, F.; Tan, K.H.; Chern, B.S.M.; et al. Cohort profile: Singapore Preconception Study of Long-Term Maternal and Child Outcomes (S-PRESTO). Eur. J. Epidemiol. 2021, 36, 129–142. [Google Scholar] [CrossRef] [PubMed]
- Collins, G.G.; Rossi, B.V. The impact of lifestyle modifications, diet, and vitamin supplementation on natural fertility. Fertil. Res. Pract. 2015, 1, 11. [Google Scholar] [CrossRef]
- Gaskins, A.J.; Chiu, Y.H.; Williams, P.L.; Ford, J.B.; Toth, T.L.; Hauser, R.; Chavarro, J.E. Association between serum folate and vitamin B-12 and outcomes of assisted reproductive technologies. Am. J. Clin. Nutr. 2015, 102, 943–950. [Google Scholar] [CrossRef]
- Ronnenberg, A.G.; Venners, S.A.; Xu, X.; Chen, C.; Wang, L.; Guang, W.; Huang, A.; Wang, X. Preconception B-vitamin and homocysteine status, conception, and early pregnancy loss. Am. J. Epidemiol. 2007, 166, 304–312. [Google Scholar] [CrossRef]
- Ruder, E.H.; Hartman, T.J.; Reindollar, R.H.; Goldman, M.B. Female dietary antioxidant intake and time to pregnancy among couples treated for unexplained infertility. Fertil. Steril. 2014, 101, 759–766. [Google Scholar] [CrossRef][Green Version]
- Midttun, Ø.; Hustad, S.; Ueland, P.M. Quantitative profiling of biomarkers related to B-vitamin status, tryptophan metabolism and inflammation in human plasma by liquid chromatography/tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2009, 23, 1371–1379. [Google Scholar] [CrossRef] [PubMed]
- Midttun, Ø.; Ueland, P.M. Determination of vitamins A, D and E in a small volume of human plasma by a high-throughput method based on liquid chromatography/tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2011, 25, 1942–1948. [Google Scholar] [CrossRef]
- Loy, S.L.; Ku, C.W.; Lai, A.E.Q.; Choo, X.H.; Ho, A.H.M.; Cheung, Y.B.; Godfrey, K.M.; Chong, Y.S.; Gluckman, P.D.; Shek, L.P.; et al. Plasma glycemic measures and fecundability in a Singapore preconception cohort study. Fertil. Steril. 2021, 115, 138–147. [Google Scholar] [CrossRef]
- McKinnon, C.J.; Hatch, E.E.; Rothman, K.J.; Mikkelsen, E.M.; Wesselink, A.K.; Hahn, K.A.; Wise, L.A. Body mass index, physical activity and fecundability in a North American preconception cohort study. Fertil. Steril. 2016, 106, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Schrager, N.L.; Wesselink, A.K.; Wang, T.R.; Hatch, E.E.; Rothman, K.J.; Mikkelsen, E.M.; Boynton-Jarrett, R.D.; Wise, L.A. Association of income and education with fecundability in a North American preconception cohort. Ann. Epidemiol. 2020, 50, 41–47.e41. [Google Scholar] [CrossRef]
- Wesselink, A.K.; Hatch, E.E.; Rothman, K.J.; Mikkelsen, E.M.; Aschengrau, A.; Wise, L.A. Prospective study of cigarette smoking and fecundability. Hum. Reprod. 2019, 34, 558–567. [Google Scholar] [CrossRef]
- Wesselink, A.K.; Rothman, K.J.; Hatch, E.E.; Mikkelsen, E.M.; Sørensen, H.T.; Wise, L.A. Age and fecundability in a North American preconception cohort study. Am. J. Obstet. Gynecol. 2017, 217, 667.e661–667.e668. [Google Scholar] [CrossRef] [PubMed]
- Consultation, W.E. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004, 363, 157–163. [Google Scholar] [CrossRef]
- Lim, S.X.; Loy, S.L.; Colega, M.T.; Lai, J.S.; Godfrey, K.M.; Lee, Y.S.; Tan, K.H.; Yap, F.; Shek, L.P.; Chong, Y.S.; et al. Prepregnancy adherence to plant-based diet indices and exploratory dietary patterns in relation to fecundability. Am. J. Clin. Nutr. 2022, 115, 559–569. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, A.J. Fertility and Pregnancy: An Epidemiologic Perspective; Oxford University Press: Oxford, UK, 2010. [Google Scholar]
- Cetin, I.; Berti, C.; Calabrese, S. Role of micronutrients in the periconceptional period. Hum. Reprod. Update 2010, 16, 80–95. [Google Scholar] [CrossRef] [PubMed]
- Kilicdag, E.B.; Bagis, T.; Tarim, E.; Aslan, E.; Erkanli, S.; Simsek, E.; Haydardedeoglu, B.; Kuscu, E. Administration of B-group vitamins reduces circulating homocysteine in polycystic ovarian syndrome patients treated with metformin: A randomized trial. Hum. Reprod. 2005, 20, 1521–1528. [Google Scholar] [CrossRef] [PubMed]
- Lonn, E.; Yusuf, S.; Arnold, M.J.; Sheridan, P.; Pogue, J.; Micks, M.; McQueen, M.J.; Probstfield, J.; Fodor, G.; Held, C.; et al. Homocysteine lowering with folic acid and B vitamins in vascular disease. N. Engl. J. Med. 2006, 354, 1567–1577. [Google Scholar] [CrossRef]
- Di Simone, N.; Maggiano, N.; Caliandro, D.; Riccardi, P.; Evangelista, A.; Carducci, B.; Caruso, A. Homocysteine induces trophoblast cell death with apoptotic features. Biol. Reprod. 2003, 69, 1129–1134. [Google Scholar] [CrossRef] [PubMed]
- Mills, J.L.; Buck Louis, G.M.; Kannan, K.; Weck, J.; Wan, Y.; Maisog, J.; Giannakou, A.; Wu, Q.; Sundaram, R. Delayed conception in women with low-urinary iodine concentrations: A population-based prospective cohort study. Hum. Reprod. 2018, 33, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Mathews, D.M.; Johnson, N.P.; Sim, R.G.; O’Sullivan, S.; Peart, J.M.; Hofman, P.L. Iodine and fertility: Do we know enough? Hum. Reprod. 2021, 36, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Clarke, M.W.; Burnett, J.R.; Croft, K.D. Vitamin E in human health and disease. Crit. Rev. Clin. Lab. Sci. 2008, 45, 417–450. [Google Scholar] [CrossRef]
- Heaney, R.P. Vitamin D in health and disease. Clin. J. Am. Soc. Nephrol. 2008, 3, 1535–1541. [Google Scholar] [CrossRef]
- Bayles, B.; Usatine, R. Evening primrose oil. Am. Fam. Physician 2009, 80, 1405–1408. [Google Scholar]
- Kazemi, F.; Masoumi, S.Z.; Shayan, A.; Oshvandi, K. The Effect of Evening Primrose Oil Capsule on Hot Flashes and Night Sweats in Postmenopausal Women: A Single-Blind Randomized Controlled Trial. J. Menopausal. Med. 2021, 27, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Křížová, L.; Dadáková, K.; Kašparovská, J.; Kašparovský, T. Isoflavones. Molecules 2019, 24, 1076. [Google Scholar] [CrossRef] [PubMed]
- Evening Primrose Oil. Available online: https://www.nccih.nih.gov/health/evening-primrose-oil#:~:text=Today%2C%20evening%20primrose%20oil%20dietary,are%20applied%20to%20the%20skin (accessed on 7 May 2022).
- Cervical Mucus Changes During Ovulation. Available online: https://www.fairhavenhealth.com/cervical-mucus (accessed on 7 May 2022).
- Female Fertility Supplements: An Evidence-Based Guide. Available online: https://extendfertility.com/female-fertility-supplements/ (accessed on 7 May 2022).
- Curlin, M.; Bursac, D. Cervical mucus: From biochemical structure to clinical implications. Front. Biosci. (Schol. Ed.) 2013, 5, 507–515. [Google Scholar] [CrossRef]
- Jefferson, W.N.; Williams, C.J. Circulating levels of genistein in the neonate, apart from dose and route, predict future adverse female reproductive outcomes. Reprod. Toxicol. 2011, 31, 272–279. [Google Scholar] [CrossRef]
- Vinther, S.; Christensen, M.; Christensen, H. The importance of convenience for patient adherence to drug treatments–an overview of secondary Literature. Clin. Ther. 2015, 37, e95–e96. [Google Scholar] [CrossRef]
- Chen, F.; Du, M.; Blumberg, J.B.; Ho Chui, K.K.; Ruan, M.; Rogers, G.; Shan, Z.; Zeng, L.; Zhang, F.F. Association Among Dietary Supplement Use, Nutrient Intake, and Mortality Among U.S. Adults: A Cohort Study. Ann. Intern. Med. 2019, 170, 604–613. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Hong, X.; Zhang, H.; Dai, Q.; Huang, K.; Zhang, X.; Liu, Y.; Wu, J.; Wang, Q.; Shen, H.; et al. Pre-pregnancy maternal fasting plasma glucose levels in relation to time to pregnancy among the couples attempting first pregnancy. Hum. Reprod. 2019, 34, 1325–1333. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Total (n = 908) | No Supplements (n = 295) | Any Supplement (n = 613) | p a |
|---|---|---|---|---|
| Age | 0.021 | |||
| <35 years | 774 (85.2) | 263 (89.2) | 511 (83.4) | |
| ≥35 years | 134 (14.8) | 32 (10.8) | 102 (16.6) | |
| Ethnicity | <0.001 | |||
| Chinese | 654 (72.0) | 171 (58.0) | 483 (78.8) | |
| Malay | 143 (15.7) | 75 (25.4) | 68 (11.1) | |
| Indian | 82 (9.0) | 38 (12.9) | 44 (7.2) | |
| Mix | 29 (3.2) | 11 (3.7) | 18 (2.9) | |
| Parity | <0.001 | |||
| 0 | 596 (65.6) | 162 (54.9) | 434 (70.8) | |
| ≥1 | 312 (34.4) | 133 (45.1) | 179 (29.2) | |
| Highest education | <0.001 | |||
| Below tertiary | 337 (37.1) | 143 (48.5) | 194 (31.6) | |
| Tertiary and above | 571 (62.9) | 152 (51.5) | 419 (68.4) | |
| Body mass index b | <0.001 | |||
| Underweight <18.5 kg/m2 | 76 (8.4) | 21 (7.1) | 55 (9.0) | |
| Normal 18.5–22.9 kg/m2 | 420 (46.3) | 112 (38.0) | 308 (50.2) | |
| Overweight 23–27.4 kg/m2 | 238 (26.2) | 86 (29.2) | 152 (24.8) | |
| Obese ≥27.5 kg/m2 | 174 (19.2) | 76 (25.8) | 98 (16.0) | |
| Cycle regularity | 0.053 | |||
| Regular | 597 (65.7) | 181 (61.4) | 416 (67.9) | |
| Irregular | 311 (34.3) | 114 (38.6) | 197 (32.1) | |
| Cycle length, days | 29.5 (29.0–32.5) | 29.5 (29.0–32.5) | 29.5 (29.0–32.5) | 0.678 |
| Smoking exposure | <0.001 | |||
| No | 698 (76.9) | 206 (69.8) | 492 (80.3) | |
| Yes | 210 (23.1) | 89 (30.2) | 121 (19.7) | |
| Alcohol intake | <0.001 | |||
| No | 479 (52.8) | 186 (63.1) | 293 (47.8) | |
| Yes | 429 (47.2) | 109 (36.9) | 320 (52.2) | |
| Unhealthful plant-based diet index | 0.093 | |||
| Tertile 1 ≤41 | 331 (36.5) | 95 (32.2) | 236 (38.5) | |
| Tertile 2 >41 to ≤47 | 290 (31.9) | 94 (31.9) | 196 (32.0) | |
| Tertile 3 >47 | 287 (31.6) | 106 (35.9) | 181 (29.5) | |
| Total daily energy intake, kcal/d | 1945 (1567–2385) | 2020 (1572–2502) | 1918 (1553–2317) | 0.032 |
| Attempt time to conceive at study entry, cycles | 1.0 (0–7.0) | 0 (0–6.0) | 2.0 (0–8.0) | 0.002 |
| Unadjusted | Model 1 a | Model 2 b | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Type of Supplements | n | Pregnancies | Cycles | FR | 95% CI | FR | 95% CI | FR | 95% CI |
| Supplement intake status | |||||||||
| No supplements | 295 | 114 | 698 | 1.00 | (Ref.) | 1.00 | (Ref.) | 1.00 | (Ref.) |
| Any supplement | 613 | 273 | 1303 | 1.31 | 1.05, 1.62 | 1.34 | 1.06, 1.68 | 1.30 | 1.03, 1.63 |
| Folic acid | |||||||||
| Non-user | 441 | 182 | 1077 | 1.00 | (Ref.) | 1.00 | (Ref.) | 1.00 | (Ref.) |
| User | 467 | 205 | 924 | 1.22 | 1.00, 1.49 | 1.27 | 1.03, 1.56 | 1.26 | 1.03, 1.56 |
| Folic acid type | |||||||||
| Non-user | 441 | 182 | 1077 | 1.00 | (Ref.) | 1.00 | (Ref.) | 1.00 | (Ref.) |
| Single vitamin | 235 | 96 | 422 | 1.18 | 0.92, 1.51 | 1.25 | 0.97, 1.61 | 1.25 | 0.97, 1.62 |
| Multivitamin | 168 | 79 | 365 | 1.23 | 0.95, 1.61 | 1.25 | 0.95, 1.64 | 1.23 | 0.94, 1.62 |
| Single vitamin and multivitamin | 64 | 30 | 137 | 1.34 | 0.91, 1.98 | 1.38 | 0.93, 2.05 | 1.39 | 0.94, 2.07 |
| Fish oil | |||||||||
| Non-user | 723 | 308 | 1611 | 1.00 | (Ref.) | 1.00 | (Ref.) | 1.00 | (Ref.) |
| User | 185 | 79 | 390 | 0.99 | 0.78, 1.27 | 0.99 | 0.77, 1.27 | 0.98 | 0.76, 1.26 |
| Evening primrose oil | |||||||||
| Non-user | 865 | 375 | 1943 | 1.00 | (Ref.) | 1.00 | (Ref.) | 1.00 | (Ref.) |
| User | 43 | 12 | 58 | 0.59 | 0.33, 1.04 | 0.55 | 0.31, 0.98 | 0.56 | 0.31, 0.99 |
| Iron | |||||||||
| Non-user | 688 | 284 | 1552 | 1.00 | (Ref.) | 1.00 | (Ref.) | 1.00 | (Ref.) |
| User | 220 | 103 | 449 | 1.20 | 0.96, 1.50 | 1.20 | 0.95, 1.51 | 1.19 | 0.94, 1.49 |
| Zinc | |||||||||
| Non-user | 702 | 293 | 1569 | 1.00 | (Ref.) | 1.00 | (Ref.) | 1.00 | (Ref.) |
| User | 206 | 94 | 432 | 1.16 | 0.92, 1.46 | 1.15 | 0.91, 1.47 | 1.13 | 0.89, 1.43 |
| Selenium | |||||||||
| Non-user | 737 | 308 | 1634 | 1.00 | (Ref.) | 1.00 | (Ref.) | 1.00 | (Ref.) |
| User | 171 | 79 | 367 | 1.17 | 0.92, 1.50 | 1.18 | 0.92, 1.52 | 1.17 | 0.91, 1.51 |
| Iodine | |||||||||
| Non-user | 749 | 309 | 1641 | 1.00 | (Ref.) | 1.00 | (Ref.) | 1.00 | (Ref.) |
| User | 159 | 78 | 360 | 1.27 | 0.99, 1.63 | 1.30 | 1.01, 1.67 | 1.28 | 1.00, 1.65 |
| Vitamin B6 | |||||||||
| Non-user | 676 | 278 | 1487 | 1.00 | (Ref.) | 1.00 | (Ref.) | 1.00 | (Ref.) |
| User | 232 | 109 | 514 | 1.19 | 0.96, 1.49 | 1.18 | 0.94, 1.48 | 1.17 | 0.93, 1.47 |
| Vitamin B12 | |||||||||
| Non-user | 673 | 276 | 1491 | 1.00 | (Ref.) | 1.00 | (Ref.) | 1.00 | (Ref.) |
| User | 235 | 111 | 510 | 1.21 | 0.97, 1.51 | 1.20 | 0.96, 1.50 | 1.19 | 0.95, 1.49 |
| Vitamin C | |||||||||
| Non-user | 615 | 245 | 1337 | 1.00 | (Ref.) | 1.00 | (Ref.) | 1.00 | (Ref.) |
| User | 293 | 142 | 664 | 1.27 | 1.03, 1.56 | 1.22 | 0.98, 1.51 | 1.19 | 0.96, 1.47 |
| Vitamin D | |||||||||
| Non-user | 687 | 285 | 1499 | 1.00 | (Ref.) | 1.00 | (Ref.) | 1.00 | (Ref.) |
| User | 221 | 102 | 502 | 1.14 | 0.91, 1.43 | 1.18 | 0.94, 1.48 | 1.15 | 0.91, 1.45 |
| Vitamin E | |||||||||
| Non-user | 704 | 297 | 1591 | 1.00 | (Ref.) | 1.00 | (Ref.) | 1.00 | (Ref.) |
| User | 204 | 90 | 410 | 1.08 | 0.85, 1.37 | 1.11 | 0.88, 1.42 | 1.09 | 0.86, 1.39 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ku, C.W.; Ku, C.O.; Tay, L.P.C.; Xing, H.K.; Cheung, Y.B.; Godfrey, K.M.; Colega, M.T.; Teo, C.; Tan, K.M.L.; Chong, Y.-S.; et al. Dietary Supplement Intake and Fecundability in a Singapore Preconception Cohort Study. Nutrients 2022, 14, 5110. https://doi.org/10.3390/nu14235110
Ku CW, Ku CO, Tay LPC, Xing HK, Cheung YB, Godfrey KM, Colega MT, Teo C, Tan KML, Chong Y-S, et al. Dietary Supplement Intake and Fecundability in a Singapore Preconception Cohort Study. Nutrients. 2022; 14(23):5110. https://doi.org/10.3390/nu14235110
Chicago/Turabian StyleKu, Chee Wai, Chee Onn Ku, Liza Pui Chin Tay, Hui Kun Xing, Yin Bun Cheung, Keith M. Godfrey, Marjorelee T. Colega, Cherlyen Teo, Karen Mei Ling Tan, Yap-Seng Chong, and et al. 2022. "Dietary Supplement Intake and Fecundability in a Singapore Preconception Cohort Study" Nutrients 14, no. 23: 5110. https://doi.org/10.3390/nu14235110
APA StyleKu, C. W., Ku, C. O., Tay, L. P. C., Xing, H. K., Cheung, Y. B., Godfrey, K. M., Colega, M. T., Teo, C., Tan, K. M. L., Chong, Y.-S., Shek, L. P.-C., Tan, K. H., Chan, S.-Y., Lim, S. X., Chong, M. F.-F., Yap, F., Chan, J. K. Y., & Loy, S. L. (2022). Dietary Supplement Intake and Fecundability in a Singapore Preconception Cohort Study. Nutrients, 14(23), 5110. https://doi.org/10.3390/nu14235110






