The Role of Calcium, 25-Hydroxyvitamin D, and Parathyroid Hormone in Irritable Bowel Syndrome: A Bidirectional Two-Sample Mendelian Randomization Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Data Sources and Study Population
2.3. Selection of Instrumental Variables
2.4. Statistical Analyses
3. Results
3.1. Instrumental Variables
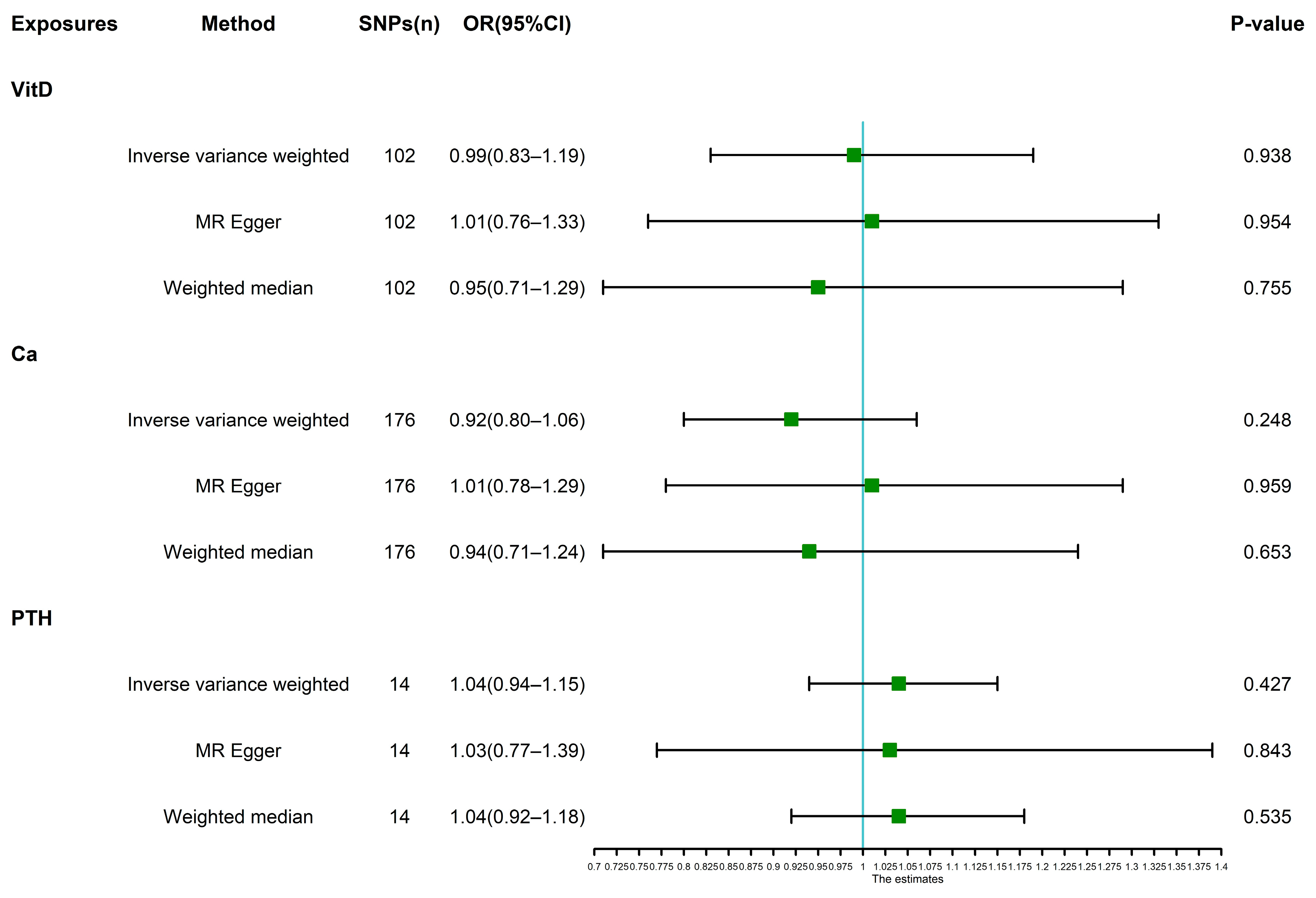
3.2. Main Analyses and Sensitivity Analyses
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| SNP | Eaf | Beta | R2 | F |
|---|---|---|---|---|
| rs10277163 | 0.255 | −0.014 | 0.0001 | 39 |
| rs1038165 | 0.579 | 0.012 | 0.0001 | 32 |
| rs1042034 | 0.792 | −0.015 | 0.0001 | 37 |
| rs10438978 | 0.820 | −0.017 | 0.0001 | 43 |
| rs1047891 | 0.317 | −0.013 | 0.0001 | 39 |
| rs1048328 | 0.080 | 0.031 | 0.0001 | 72 |
| rs10859995 | 0.580 | −0.044 | 0.0009 | 461 |
| rs11023159 | 0.033 | 0.048 | 0.0001 | 73 |
| rs11076175 | 0.176 | 0.023 | 0.0002 | 76 |
| rs111515741 | 0.017 | −0.049 | 0.0001 | 40 |
| rs11207969 | 0.351 | 0.021 | 0.0002 | 99 |
| rs11264361 | 0.251 | 0.017 | 0.0001 | 57 |
| rs11542462 | 0.134 | −0.025 | 0.0001 | 71 |
| rs11600054 | 0.010 | 0.068 | 0.0001 | 46 |
| rs11726886 | 0.291 | −0.054 | 0.0012 | 591 |
| rs117300835 | 0.013 | −0.335 | 0.0029 | 1469 |
| rs11791258 | 0.191 | 0.014 | 0.0001 | 30 |
| rs11867297 | 0.385 | 0.014 | 0.0001 | 43 |
| rs12056768 | 0.584 | −0.023 | 0.0003 | 130 |
| rs12283049 | 0.234 | −0.056 | 0.0011 | 569 |
| rs12324720 | 0.175 | −0.015 | 0.0001 | 32 |
| rs12462826 | 0.370 | −0.013 | 0.0001 | 40 |
| rs12501515 | 0.590 | −0.079 | 0.0030 | 1504 |
| rs12775091 | 0.213 | 0.016 | 0.0001 | 40 |
| rs1321247 | 0.102 | −0.022 | 0.0001 | 45 |
| rs13294734 | 0.466 | 0.013 | 0.0001 | 39 |
| rs138335 | 0.659 | −0.014 | 0.0001 | 42 |
| rs1384687 | 0.132 | −0.017 | 0.0001 | 32 |
| rs142004400 | 0.034 | −0.031 | 0.0001 | 32 |
| rs142158911 | 0.112 | 0.026 | 0.0001 | 68 |
| rs1532085 | 0.617 | 0.025 | 0.0003 | 150 |
| rs1627043 | 0.033 | −0.049 | 0.0002 | 76 |
| rs1684600 | 0.299 | −0.013 | 0.0001 | 33 |
| rs17207784 | 0.324 | −0.013 | 0.0001 | 40 |
| rs17473257 | 0.017 | −0.061 | 0.0001 | 63 |
| rs1800588 | 0.215 | −0.031 | 0.0003 | 156 |
| rs1858889 | 0.503 | 0.013 | 0.0001 | 45 |
| rs1871395 | 0.153 | −0.020 | 0.0001 | 53 |
| rs1949633 | 0.606 | 0.011 | 0.0001 | 31 |
| rs2037511 | 0.166 | 0.018 | 0.0001 | 43 |
| rs2074735 | 0.065 | 0.029 | 0.0001 | 52 |
| rs2229742 | 0.105 | −0.025 | 0.0001 | 58 |
| rs2245133 | 0.164 | −0.021 | 0.0001 | 62 |
| rs2297991 | 0.718 | 0.013 | 0.0001 | 33 |
| rs2494429 | 0.823 | −0.015 | 0.0001 | 32 |
| rs2511279 | 0.960 | 0.098 | 0.0007 | 365 |
| rs2595644 | 0.385 | −0.012 | 0.0001 | 35 |
| rs2710651 | 0.526 | −0.012 | 0.0001 | 33 |
| rs2807834 | 0.685 | −0.015 | 0.0001 | 49 |
| rs28435470 | 0.663 | −0.012 | 0.0001 | 31 |
| rs2847500 | 0.123 | −0.023 | 0.0001 | 55 |
| rs325393 | 0.278 | −0.014 | 0.0001 | 37 |
| rs34186890 | 0.260 | −0.016 | 0.0001 | 47 |
| rs34726834 | 0.252 | 0.014 | 0.0001 | 37 |
| rs35270497 | 0.176 | 0.016 | 0.0001 | 35 |
| rs3732220 | 0.085 | −0.048 | 0.0004 | 177 |
| rs3829251 | 0.133 | −0.114 | 0.0030 | 1508 |
| rs4147536 | 0.789 | −0.015 | 0.0001 | 36 |
| rs4348160 | 0.327 | −0.026 | 0.0003 | 146 |
| rs4364259 | 0.199 | 0.017 | 0.0001 | 47 |
| rs4420638 | 0.177 | −0.019 | 0.0001 | 54 |
| rs4580037 | 0.286 | −0.014 | 0.0001 | 37 |
| rs512083 | 0.462 | 0.012 | 0.0001 | 37 |
| rs5770794 | 0.314 | −0.013 | 0.0001 | 38 |
| rs6129648 | 0.380 | 0.014 | 0.0001 | 46 |
| rs61698755 | 0.560 | −0.011 | 0.0001 | 32 |
| rs61747728 | 0.039 | 0.030 | 0.0001 | 34 |
| rs61813875 | 0.025 | 0.082 | 0.0003 | 162 |
| rs61887421 | 0.030 | −0.037 | 0.0001 | 39 |
| rs62007299 | 0.713 | −0.012 | 0.0001 | 31 |
| rs62129966 | 0.161 | 0.061 | 0.0010 | 502 |
| rs635634 | 0.187 | −0.015 | 0.0001 | 34 |
| rs6438900 | 0.256 | 0.015 | 0.0001 | 43 |
| rs6672758 | 0.800 | 0.016 | 0.0001 | 42 |
| rs6834488 | 0.423 | −0.014 | 0.0001 | 51 |
| rs71599974 | 0.148 | 0.026 | 0.0002 | 83 |
| rs727857 | 0.612 | −0.012 | 0.0001 | 34 |
| rs733454 | 0.099 | 0.019 | 0.0001 | 32 |
| rs73413596 | 0.074 | 0.022 | 0.0001 | 34 |
| rs742493 | 0.113 | 0.018 | 0.0001 | 34 |
| rs7528419 | 0.224 | 0.022 | 0.0002 | 80 |
| rs7569755 | 0.289 | 0.014 | 0.0001 | 38 |
| rs7580771 | 0.176 | −0.017 | 0.0001 | 40 |
| rs7712001 | 0.440 | 0.012 | 0.0001 | 35 |
| rs77532868 | 0.052 | 0.026 | 0.0001 | 33 |
| rs77924615 | 0.194 | −0.015 | 0.0001 | 36 |
| rs77960347 | 0.013 | −0.053 | 0.0001 | 34 |
| rs78649910 | 0.106 | −0.019 | 0.0001 | 34 |
| rs8018720 | 0.824 | −0.034 | 0.0003 | 172 |
| rs8107974 | 0.076 | 0.036 | 0.0002 | 89 |
| rs8121940 | 0.198 | −0.044 | 0.0006 | 299 |
| rs9375037 | 0.443 | 0.012 | 0.0001 | 34 |
| rs9409266 | 0.862 | −0.017 | 0.0001 | 33 |
| rs964184 | 0.867 | 0.041 | 0.0004 | 189 |
| rs9847248 | 0.713 | −0.012 | 0.0001 | 31 |
| rs986649 | 0.322 | 0.013 | 0.0001 | 36 |
| rs9946771 | 0.066 | −0.023 | 0.0001 | 34 |
| SNP | Eaf | Beta | R2 | F |
|---|---|---|---|---|
| rs10108887 | 0.388 | 0.014 | 0.0001 | 31 |
| rs1036332 | 0.739 | 0.022 | 0.0002 | 59 |
| rs1061134 | 0.092 | −0.025 | 0.0001 | 33 |
| rs10754439 | 0.417 | 0.014 | 0.0001 | 32 |
| rs10819178 | 0.638 | 0.032 | 0.0005 | 153 |
| rs10858935 | 0.687 | −0.019 | 0.0002 | 51 |
| rs10917386 | 0.690 | 0.020 | 0.0002 | 54 |
| rs10958700 | 0.247 | 0.020 | 0.0002 | 48 |
| rs11078597 | 0.186 | 0.051 | 0.0008 | 249 |
| rs11085015 | 0.800 | −0.018 | 0.0001 | 33 |
| rs112174050 | 0.025 | 0.102 | 0.0005 | 163 |
| rs114949263 | 0.112 | 0.034 | 0.0002 | 72 |
| rs11588907 | 0.342 | −0.016 | 0.0001 | 36 |
| rs11616030 | 0.087 | −0.031 | 0.0002 | 48 |
| rs11621792 | 0.454 | 0.016 | 0.0001 | 39 |
| rs11629876 | 0.331 | −0.018 | 0.0001 | 46 |
| rs11671393 | 0.040 | −0.045 | 0.0002 | 48 |
| rs116769926 | 0.024 | 0.073 | 0.0002 | 76 |
| rs117080418 | 0.011 | −0.099 | 0.0002 | 64 |
| rs117179023 | 0.012 | −0.068 | 0.0001 | 34 |
| rs117213754 | 0.015 | 0.104 | 0.0003 | 100 |
| rs11730491 | 0.168 | 0.021 | 0.0001 | 38 |
| rs11743466 | 0.364 | 0.018 | 0.0001 | 45 |
| rs117896857 | 0.027 | −0.055 | 0.0002 | 51 |
| rs11792928 | 0.295 | −0.017 | 0.0001 | 36 |
| rs12132412 | 0.388 | 0.028 | 0.0004 | 121 |
| rs12135382 | 0.584 | 0.022 | 0.0002 | 76 |
| rs12147703 | 0.895 | −0.028 | 0.0001 | 47 |
| rs12339541 | 0.064 | −0.061 | 0.0004 | 141 |
| rs12378991 | 0.080 | −0.038 | 0.0002 | 67 |
| rs12583851 | 0.751 | −0.023 | 0.0002 | 60 |
| rs12613807 | 0.444 | 0.016 | 0.0001 | 40 |
| rs12675477 | 0.274 | 0.017 | 0.0001 | 37 |
| rs12793731 | 0.511 | 0.015 | 0.0001 | 34 |
| rs12918968 | 0.439 | −0.032 | 0.0005 | 157 |
| rs12922549 | 0.237 | −0.022 | 0.0002 | 55 |
| rs12933858 | 0.493 | 0.019 | 0.0002 | 56 |
| rs12982234 | 0.040 | −0.058 | 0.0003 | 82 |
| rs12998379 | 0.194 | −0.024 | 0.0002 | 58 |
| rs13073106 | 0.641 | 0.038 | 0.0007 | 205 |
| rs13389219 | 0.394 | −0.016 | 0.0001 | 40 |
| rs1354034 | 0.601 | −0.018 | 0.0002 | 49 |
| rs1374161 | 0.491 | −0.023 | 0.0003 | 80 |
| rs138789759 | 0.075 | 0.047 | 0.0003 | 96 |
| rs147233090 | 0.024 | 0.103 | 0.0005 | 157 |
| rs1476698 | 0.369 | −0.021 | 0.0002 | 62 |
| rs1497826 | 0.373 | 0.024 | 0.0003 | 86 |
| rs149807892 | 0.016 | 0.066 | 0.0001 | 43 |
| rs1500187 | 0.456 | −0.014 | 0.0001 | 29 |
| rs164751 | 0.410 | −0.019 | 0.0002 | 54 |
| rs165316 | 0.198 | −0.018 | 0.0001 | 31 |
| rs1672991 | 0.934 | 0.075 | 0.0007 | 221 |
| rs17132144 | 0.093 | −0.026 | 0.0001 | 35 |
| rs17164683 | 0.270 | −0.021 | 0.0002 | 55 |
| rs17580 | 0.049 | 0.047 | 0.0002 | 64 |
| rs1763519 | 0.607 | −0.032 | 0.0005 | 149 |
| rs17774672 | 0.158 | −0.027 | 0.0002 | 62 |
| rs17884869 | 0.025 | −0.111 | 0.0006 | 190 |
| rs1801282 | 0.120 | −0.037 | 0.0003 | 92 |
| rs1858800 | 0.345 | 0.031 | 0.0004 | 134 |
| rs2001884 | 0.507 | −0.017 | 0.0001 | 46 |
| rs2004315 | 0.625 | 0.033 | 0.0005 | 160 |
| rs2241699 | 0.277 | −0.026 | 0.0003 | 84 |
| rs2243010 | 0.206 | −0.018 | 0.0001 | 32 |
| rs2249825 | 0.269 | −0.016 | 0.0001 | 33 |
| rs2274224 | 0.432 | −0.017 | 0.0001 | 47 |
| rs2309233 | 0.731 | 0.021 | 0.0002 | 56 |
| rs2327774 | 0.378 | −0.022 | 0.0002 | 71 |
| rs2335534 | 0.179 | −0.038 | 0.0004 | 136 |
| rs2343592 | 0.267 | −0.023 | 0.0002 | 67 |
| rs2370218 | 0.766 | −0.022 | 0.0002 | 53 |
| rs2419886 | 0.257 | −0.020 | 0.0001 | 47 |
| rs255755 | 0.270 | 0.015 | 0.0001 | 29 |
| rs2647242 | 0.798 | −0.020 | 0.0001 | 40 |
| rs2762938 | 0.587 | 0.016 | 0.0001 | 37 |
| rs28520334 | 0.119 | 0.022 | 0.0001 | 31 |
| rs28929474 | 0.020 | 0.123 | 0.0006 | 189 |
| rs2971855 | 0.302 | 0.019 | 0.0002 | 49 |
| rs3011642 | 0.243 | 0.020 | 0.0001 | 45 |
| rs3026445 | 0.367 | −0.018 | 0.0001 | 45 |
| rs302650 | 0.432 | −0.019 | 0.0002 | 58 |
| rs3091842 | 0.044 | 0.094 | 0.0007 | 235 |
| rs3133548 | 0.142 | 0.020 | 0.0001 | 31 |
| rs34042070 | 0.186 | 0.021 | 0.0001 | 42 |
| rs34066945 | 0.358 | −0.026 | 0.0003 | 97 |
| rs34290411 | 0.286 | 0.018 | 0.0001 | 41 |
| rs34395935 | 0.152 | 0.059 | 0.0009 | 285 |
| rs35118755 | 0.149 | 0.023 | 0.0001 | 43 |
| rs35590487 | 0.241 | −0.021 | 0.0002 | 53 |
| rs35751693 | 0.039 | 0.043 | 0.0001 | 45 |
| rs35852840 | 0.059 | 0.029 | 0.0001 | 29 |
| rs36086195 | 0.580 | 0.020 | 0.0002 | 63 |
| rs36104352 | 0.121 | 0.028 | 0.0002 | 52 |
| rs3748861 | 0.202 | −0.017 | 0.0001 | 31 |
| rs3795243 | 0.126 | 0.022 | 0.0001 | 32 |
| rs3822858 | 0.407 | −0.016 | 0.0001 | 40 |
| rs3931841 | 0.681 | −0.026 | 0.0003 | 94 |
| rs4082330 | 0.814 | 0.021 | 0.0001 | 41 |
| rs41278174 | 0.027 | 0.050 | 0.0001 | 42 |
| rs41393948 | 0.113 | −0.022 | 0.0001 | 29 |
| rs4239142 | 0.745 | −0.017 | 0.0001 | 35 |
| rs4320103 | 0.039 | 0.045 | 0.0002 | 49 |
| rs4324076 | 0.530 | −0.018 | 0.0002 | 53 |
| rs4633480 | 0.556 | 0.020 | 0.0002 | 62 |
| rs4721467 | 0.738 | −0.021 | 0.0002 | 54 |
| rs4744854 | 0.629 | −0.033 | 0.0005 | 156 |
| rs4758621 | 0.308 | −0.026 | 0.0003 | 94 |
| rs4790310 | 0.575 | −0.020 | 0.0002 | 59 |
| rs4841132 | 0.908 | 0.057 | 0.0005 | 171 |
| rs4938642 | 0.074 | 0.034 | 0.0002 | 49 |
| rs4976647 | 0.332 | 0.018 | 0.0001 | 47 |
| rs498490 | 0.164 | −0.031 | 0.0003 | 82 |
| rs55772024 | 0.242 | −0.017 | 0.0001 | 32 |
| rs56406311 | 0.386 | 0.018 | 0.0002 | 47 |
| rs567743 | 0.708 | 0.015 | 0.0001 | 29 |
| rs5751350 | 0.330 | 0.016 | 0.0001 | 37 |
| rs5760495 | 0.354 | 0.017 | 0.0001 | 41 |
| rs5786388 | 0.581 | 0.022 | 0.0002 | 71 |
| rs58579887 | 0.404 | 0.016 | 0.0001 | 40 |
| rs59821684 | 0.029 | 0.054 | 0.0002 | 52 |
| rs6127099 | 0.278 | −0.053 | 0.0011 | 358 |
| rs62134679 | 0.148 | 0.022 | 0.0001 | 39 |
| rs62211622 | 0.184 | −0.018 | 0.0001 | 32 |
| rs62309863 | 0.586 | −0.014 | 0.0001 | 32 |
| rs62472728 | 0.060 | 0.032 | 0.0001 | 36 |
| rs634916 | 0.511 | −0.014 | 0.0001 | 31 |
| rs648514 | 0.467 | −0.014 | 0.0001 | 30 |
| rs6580981 | 0.458 | −0.018 | 0.0002 | 52 |
| rs66920316 | 0.196 | −0.021 | 0.0001 | 45 |
| rs6741561 | 0.393 | −0.038 | 0.0007 | 222 |
| rs6771438 | 0.116 | −0.027 | 0.0002 | 48 |
| rs6841429 | 0.166 | −0.040 | 0.0004 | 137 |
| rs6909201 | 0.517 | −0.042 | 0.0009 | 281 |
| rs710217 | 0.485 | 0.024 | 0.0003 | 94 |
| rs71565393 | 0.177 | 0.018 | 0.0001 | 30 |
| rs7221118 | 0.215 | −0.020 | 0.0001 | 41 |
| rs722298 | 0.438 | 0.015 | 0.0001 | 34 |
| rs72660383 | 0.064 | −0.028 | 0.0001 | 30 |
| rs72697816 | 0.163 | 0.020 | 0.0001 | 33 |
| rs73001065 | 0.071 | 0.036 | 0.0002 | 53 |
| rs73186030 | 0.128 | 0.193 | 0.0083 | 2637 |
| rs73186098 | 0.015 | −0.091 | 0.0002 | 78 |
| rs73536752 | 0.043 | −0.033 | 0.0001 | 29 |
| rs7370877 | 0.420 | −0.017 | 0.0001 | 44 |
| rs7402977 | 0.267 | −0.016 | 0.0001 | 30 |
| rs74230087 | 0.078 | −0.055 | 0.0004 | 134 |
| rs7533348 | 0.352 | −0.021 | 0.0002 | 61 |
| rs7546838 | 0.651 | 0.020 | 0.0002 | 55 |
| rs7559013 | 0.130 | 0.025 | 0.0001 | 46 |
| rs75702986 | 0.188 | 0.037 | 0.0004 | 134 |
| rs7587636 | 0.529 | −0.016 | 0.0001 | 38 |
| rs75895430 | 0.033 | 0.080 | 0.0004 | 126 |
| rs7592216 | 0.869 | −0.020 | 0.0001 | 30 |
| rs7599 | 0.634 | −0.019 | 0.0002 | 55 |
| rs7688574 | 0.369 | 0.015 | 0.0001 | 32 |
| rs7730344 | 0.293 | 0.017 | 0.0001 | 40 |
| rs77542162 | 0.023 | −0.089 | 0.0004 | 113 |
| rs777588 | 0.578 | −0.029 | 0.0004 | 126 |
| rs778368 | 0.585 | −0.014 | 0.0001 | 30 |
| rs7786368 | 0.416 | −0.024 | 0.0003 | 90 |
| rs7864156 | 0.390 | 0.019 | 0.0002 | 55 |
| rs7913072 | 0.859 | −0.021 | 0.0001 | 33 |
| rs7924737 | 0.349 | −0.015 | 0.0001 | 34 |
| rs7940215 | 0.434 | 0.014 | 0.0001 | 30 |
| rs79501693 | 0.021 | −0.050 | 0.0001 | 32 |
| rs8041057 | 0.714 | −0.015 | 0.0001 | 30 |
| rs835664 | 0.456 | −0.017 | 0.0001 | 46 |
| rs838717 | 0.566 | −0.046 | 0.0010 | 327 |
| rs883951 | 0.261 | 0.023 | 0.0002 | 66 |
| rs928760 | 0.303 | −0.018 | 0.0001 | 45 |
| rs9388399 | 0.312 | −0.024 | 0.0003 | 81 |
| rs9399697 | 0.464 | 0.014 | 0.0001 | 32 |
| rs9419741 | 0.479 | 0.015 | 0.0001 | 36 |
| rs945890 | 0.714 | −0.017 | 0.0001 | 37 |
| rs949300 | 0.380 | 0.015 | 0.0001 | 35 |
| rs9530 | 0.549 | −0.031 | 0.0005 | 149 |
| rs9532958 | 0.855 | 0.035 | 0.0003 | 99 |
| rs9611396 | 0.655 | −0.014 | 0.0001 | 29 |
| rs9895661 | 0.831 | −0.028 | 0.0002 | 69 |
| SNP | Eaf | Beta | R2 | F |
|---|---|---|---|---|
| rs10886704 | 0.425 | 0.121 | 0.007 | 24 |
| rs10902764 | 0.576 | 0.131 | 0.008 | 28 |
| rs150350229 | 0.026 | −0.388 | 0.008 | 25 |
| rs16895559 | 0.043 | 0.286 | 0.007 | 22 |
| rs17267730 | 0.045 | 0.292 | 0.007 | 24 |
| rs2585442 | 0.249 | −0.158 | 0.009 | 31 |
| rs34421071 | 0.171 | −0.155 | 0.007 | 23 |
| rs464202 | 0.888 | −0.181 | 0.007 | 22 |
| rs62533188 | 0.106 | −0.191 | 0.007 | 23 |
| rs678360 | 0.962 | −0.346 | 0.009 | 29 |
| rs73556612 | 0.035 | 0.328 | 0.007 | 24 |
| rs7514637 | 0.788 | −0.148 | 0.007 | 24 |
| rs753409 | 0.201 | 0.151 | 0.007 | 24 |
| rs9316680 | 0.384 | −0.137 | 0.009 | 30 |
| rs950455 | 0.317 | 0.129 | 0.007 | 24 |
| SNP | Eaf | Beta | R2 | F |
|---|---|---|---|---|
| rs10275986 | 0.316 | −0.113 | 0.005 | 1028 |
| rs10985554 | 0.394 | 0.103 | 0.005 | 947 |
| rs11952072 | 0.113 | 0.157 | 0.005 | 932 |
| rs12570677 | 0.112 | −0.158 | 0.005 | 935 |
| rs12956689 | 0.400 | −0.102 | 0.005 | 930 |
| rs147367149 | 0.010 | −0.521 | 0.006 | 1042 |
| rs177503 | 0.358 | −0.105 | 0.005 | 951 |
| rs45506200 | 0.015 | 0.416 | 0.005 | 957 |
| rs735820 | 0.001 | 2.070 | 0.009 | 1623 |
| rs763614 | 0.329 | 0.107 | 0.005 | 948 |
References
- Ford, A.C.; Sperber, A.D.; Corsetti, M.; Camilleri, M. Irritable bowel syndrome. Lancet 2020, 396, 1675–1688. [Google Scholar] [CrossRef]
- Camilleri, M. Diagnosis and Treatment of Irritable Bowel Syndrome: A Review. JAMA 2021, 325, 865–877. [Google Scholar] [CrossRef]
- Black, C.; Ford, A. Global burden of irritable bowel syndrome: Trends, predictions and risk factors. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 473–486. [Google Scholar] [CrossRef]
- Camilleri, M.; Zhernakova, A.; Bozzarelli, I.; D’Amato, M. Genetics of irritable bowel syndrome: Shifting gear via biobank-scale studies. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 689–702. [Google Scholar] [CrossRef]
- Liu, Y.L.; Liu, J.S. Irritable bowel syndrome in China: A review on the epidemiology, diagnosis, and management. Chin. Med. J. 2021, 134, 1396–1401. [Google Scholar] [CrossRef]
- Böhn, L.; Störsrud, S.; Törnblom, H.; Bengtsson, U.; Simrén, M. Self-reported food-related gastrointestinal symptoms in IBS are common and associated with more severe symptoms and reduced quality of life. Am. J. Gastroenterol. 2013, 108, 634–641. [Google Scholar] [CrossRef]
- Bek, S.; Teo, Y.N.; Tan, X.H.; Fan, K.H.R.; Siah, K.T.H. Association between irritable bowel syndrome and micronutrients: A systematic review. J. Gastroenterol. Hepatol. 2022, 37, 1485–1497. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.; Rossi, M.; Bajka, B.; Whelan, K. Dietary fibre in gastrointestinal health and disease. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 101–116. [Google Scholar] [CrossRef]
- Müller, M.; Canfora, E.E.; Blaak, E.E. Gastrointestinal Transit Time, Glucose Homeostasis and Metabolic Health: Modulation by Dietary Fibers. Nutrients 2018, 10, 275. [Google Scholar] [CrossRef]
- Bijkerk, C.J.; de Wit, N.J.; Muris, J.W.; Whorwell, P.J.; Knottnerus, J.A.; Hoes, A.W. Soluble or insoluble fibre in irritable bowel syndrome in primary care? Randomised placebo controlled trial. BMJ 2009, 339, b3154. [Google Scholar] [CrossRef]
- Black, C.J.; Staudacher, H.M.; Ford, A.C. Efficacy of a low FODMAP diet in irritable bowel syndrome: Systematic review and network meta-analysis. Gut 2022, 71, 1117–1126. [Google Scholar] [CrossRef] [PubMed]
- Matikainen, N.; Pekkarinen, T.; Ryhänen, E.M.; Schalin-Jäntti, C. Physiology of Calcium Homeostasis: An Overview. Endocrinol. Metab. Clin. N. Am. 2021, 50, 575–590. [Google Scholar] [CrossRef] [PubMed]
- Glabska, D.; Kolota, A.; Lachowicz, K.; Skolmowska, D.; Stachon, M.; Guzek, D. Vitamin D Supplementation and Mental Health in Inflammatory Bowel Diseases and Irritable Bowel Syndrome Patients: A Systematic Review. Nutrients 2021, 13, 3662. [Google Scholar] [CrossRef] [PubMed]
- Jalili, M.; Vahedi, H.; Poustchi, H.; Hekmatdoost, A. Effects of Vitamin D Supplementation in Patients with Irritable Bowel Syndrome: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Int. J. Prev. Med. 2019, 10, 16. [Google Scholar] [CrossRef]
- Williams, C.E.; Williams, E.A.; Corfe, B.M. Vitamin D supplementation in people with IBS has no effect on symptom severity and quality of life: Results of a randomised controlled trial. Eur. J. Nutr. 2022, 61, 299–308. [Google Scholar] [CrossRef]
- Abboud, M.; Haidar, S.; Mahboub, N.; Papandreou, D.; Al Anouti, F.; Rizk, R. Association between Serum Vitamin D and Irritable Bowel Syndrome Symptoms in a Sample of Adults. Nutrients 2022, 14, 4157. [Google Scholar] [CrossRef]
- Emdin, C.A.; Khera, A.V.; Kathiresan, S. Mendelian Randomization. JAMA 2017, 318, 1925–1926. [Google Scholar] [CrossRef]
- Revez, J.A.; Lin, T.; Qiao, Z.; Xue, A.; Holtz, Y.; Zhu, Z.; Zeng, J.; Wang, H.; Sidorenko, J.; Kemper, K.E.; et al. Genome-wide association study identifies 143 loci associated with 25 hydroxyvitamin D concentration. Nat. Commun. 2020, 11, 1647. [Google Scholar] [CrossRef]
- Sun, B.B.; Maranville, J.C.; Peters, J.E.; Stacey, D.; Staley, J.R.; Blackshaw, J.; Burgess, S.; Jiang, T.; Paige, E.; Surendran, P.; et al. Genomic atlas of the human plasma proteome. Nature 2018, 558, 73–79. [Google Scholar] [CrossRef]
- Kurki, M.I.; Karjalainen, J.; Palta, P.; Sipilä, T.P.; Kristiansson, K.; Donner, K.; Reeve, M.P.; Laivuori, H.; Aavikko, M.; Kaunisto, M.A.; et al. FinnGen: Unique genetic insights from combining isolated population and national health register data. medRxiv 2022. [Google Scholar]
- Kamat, M.A.; Blackshaw, J.A.; Young, R.; Surendran, P.; Burgess, S.; Danesh, J.; Butterworth, A.S.; Staley, J.R. PhenoScanner V2: An expanded tool for searching human genotype-phenotype associations. Bioinformatics 2019, 35, 4851–4853. [Google Scholar] [CrossRef] [PubMed]
- Reding, K.W.; Cain, K.C.; Jarrett, M.E.; Eugenio, M.D.; Heitkemper, M.M. Relationship between patterns of alcohol consumption and gastrointestinal symptoms among patients with irritable bowel syndrome. Am. J. Gastroenterol. 2013, 108, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Talley, N.J.; Powell, N.; Walker, M.M.; Jones, M.P.; Ronkainen, J.; Forsberg, A.; Kjellstrom, L.; Hellstrom, P.M.; Aro, P.; Wallner, B.; et al. Role of smoking in functional dyspepsia and irritable bowel syndrome: Three random population-based studies. Aliment Pharm. Ther. 2021, 54, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Creed, F. Review article: The incidence and risk factors for irritable bowel syndrome in population-based studies. Aliment Pharm. Ther. 2019, 50, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Palmer, T.M.; Lawlor, D.A.; Harbord, R.M.; Sheehan, N.A.; Tobias, J.H.; Timpson, N.J.; Davey Smith, G.; Sterne, J.A. Using multiple genetic variants as instrumental variables for modifiable risk factors. Stat. Methods Med. Res. 2012, 21, 223–242. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Ye, D.; Yang, H.; Song, J.; Sun, X.; Mao, Y.; He, Z. Two-Sample Mendelian Randomization Analysis Investigates Causal Associations Between Gut Microbial Genera and Inflammatory Bowel Disease, and Specificity Causal Associations in Ulcerative Colitis or Crohn’s Disease. Front. Immunol. 2022, 13, 921546. [Google Scholar] [CrossRef]
- Pham, V.T.; Fehlbaum, S.; Seifert, N.; Richard, N.; Bruins, M.J.; Sybesma, W.; Rehman, A.; Steinert, R.E. Effects of colon-targeted vitamins on the composition and metabolic activity of the human gut microbiome—A pilot study. Gut Microbes 2021, 13, 1–20. [Google Scholar] [CrossRef]
- Peacock, M. Calcium metabolism in health and disease. Clin. J. Am. Soc. Nephrol. 2010, 5 (Suppl. S1), S23–S30. [Google Scholar] [CrossRef]
- Na, S.Y.; Kim, K.B.; Lim, Y.J.; Song, H.J. Vitamin D and Colorectal Cancer: Current Perspectives and Future Directions. J. Cancer Prev. 2022, 27, 147–156. [Google Scholar] [CrossRef]
- Huang, H.; Lu, L.; Chen, Y.; Zeng, Y.; Xu, C. The efficacy of vitamin D supplementation for irritable bowel syndrome: A systematic review with meta-analysis. Nutr. J. 2022, 21, 24. [Google Scholar] [CrossRef]
- Abuelazm, M.; Muhammad, S.; Gamal, M.; Labieb, F.; Amin, M.A.; Abdelazeem, B.; Brasic, J.R. The Effect of Vitamin D Supplementation on the Severity of Symptoms and the Quality of Life in Irritable Bowel Syndrome Patients: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2022, 14, 2618. [Google Scholar] [CrossRef] [PubMed]
- Wongtrakul, W.; Charoenngam, N.; Ungprasert, P. The association between irritable bowel syndrome and osteoporosis: A systematic review and meta-analysis. Osteoporos. Int. 2020, 31, 1049–1057. [Google Scholar] [CrossRef] [PubMed]
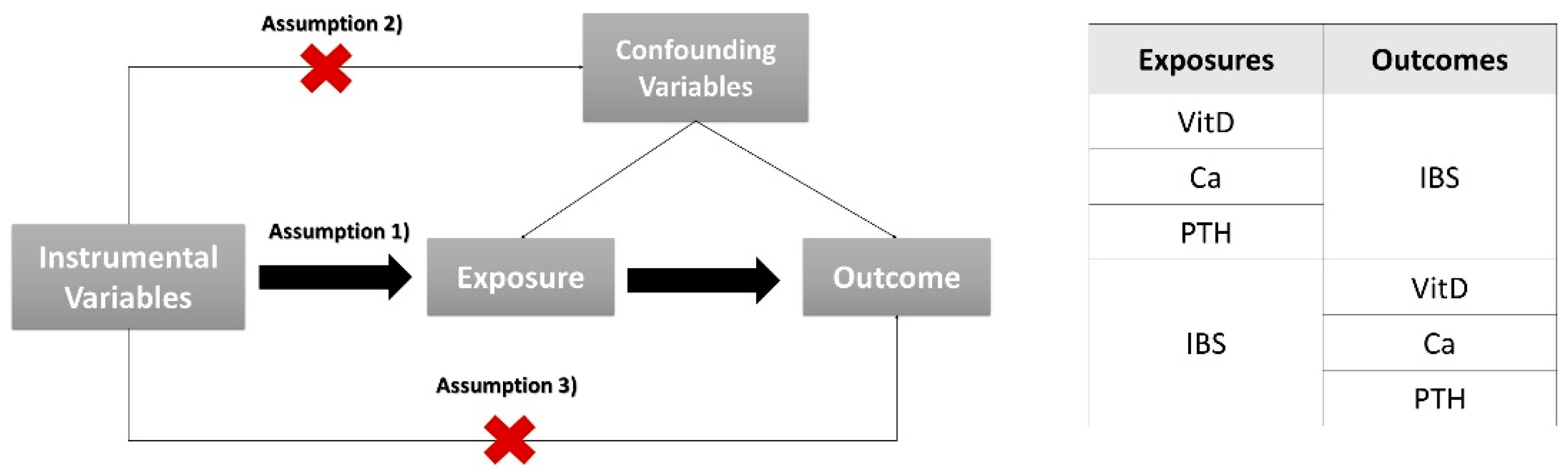

| Traits | GWAS ID | Author | PMID | Ancestor | Sample Size |
|---|---|---|---|---|---|
| VitD | ebi-a-GCST90000618 | Revez et al. | 32242144 | European | 496,946 |
| Ca | ukb-d-30680_irnt | Neale lab | NA | European | 315,153 |
| PTH | prot-a-2431 | Sun et al. | 29875488 | European | 3301 |
| IBS | finn-b-K11_IBS | NA | NA | European | 187,028 |
| Exposure-Outcome | MR-PRESSO | IVW Estimates | MR-Egger Pleiotropy Test | ||
|---|---|---|---|---|---|
| Global p-Value | Cochran’s Q | p-Value | MR-Egger Intercept | p-Value | |
| VitD-IBS | 0.384 | 102.68 | 0.435 | −0.001 | 0.886 |
| Ca-IBS | 0.670 | 166.66 | 0.662 | −0.003 | 0.417 |
| PTH-IBS | 0.555 | 12.79 | 0.464 | 0.002 | 0.949 |
| IBS-VitD | 0.645 | 5.329 | 0.620 | −0.002 | 0.452 |
| IBS-Ca | 0.617 | 6.515 | 0.687 | 0.002 | 0.361 |
| IBS-PTH | 0.480 | 8.100 | 0.424 | −0.009 | 0.732 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, N.; Xie, J.; Wang, Z.; Shu, Q.; Shi, H.; Wang, J.; Liu, N.; Xu, F.; Wu, J. The Role of Calcium, 25-Hydroxyvitamin D, and Parathyroid Hormone in Irritable Bowel Syndrome: A Bidirectional Two-Sample Mendelian Randomization Study. Nutrients 2022, 14, 5109. https://doi.org/10.3390/nu14235109
Xie N, Xie J, Wang Z, Shu Q, Shi H, Wang J, Liu N, Xu F, Wu J. The Role of Calcium, 25-Hydroxyvitamin D, and Parathyroid Hormone in Irritable Bowel Syndrome: A Bidirectional Two-Sample Mendelian Randomization Study. Nutrients. 2022; 14(23):5109. https://doi.org/10.3390/nu14235109
Chicago/Turabian StyleXie, Ning, Jiale Xie, Ziwei Wang, Qiuai Shu, Haitao Shi, Jinhai Wang, Na Liu, Feng Xu, and Jian Wu. 2022. "The Role of Calcium, 25-Hydroxyvitamin D, and Parathyroid Hormone in Irritable Bowel Syndrome: A Bidirectional Two-Sample Mendelian Randomization Study" Nutrients 14, no. 23: 5109. https://doi.org/10.3390/nu14235109
APA StyleXie, N., Xie, J., Wang, Z., Shu, Q., Shi, H., Wang, J., Liu, N., Xu, F., & Wu, J. (2022). The Role of Calcium, 25-Hydroxyvitamin D, and Parathyroid Hormone in Irritable Bowel Syndrome: A Bidirectional Two-Sample Mendelian Randomization Study. Nutrients, 14(23), 5109. https://doi.org/10.3390/nu14235109




