1. Introduction
Lactobacilli spp. and
Bifidobacteria spp. are widely used as probiotics in human food supplements [
1,
2]. The identification of specific probiotic strains is important to discriminate their different biological properties [
1,
3,
4]. In fact, the potential health benefits of probiotics, such as the production of anti-microbial metabolites, synthesis of B-group vitamins, anti-obesity, anti-diabetic effect, cholesterol-lowering effect, oxidative stress, and down-regulation, seem to vary from strain to strain [
1,
5,
6,
7].
Despite the large impact of the probiotic industry and the wide use of probiotics as food supplements, the most common assays currently employed for their identification and characterization are based on classical microbiological methods [
3,
8,
9,
10]. In general, these methods are time consuming, operator dependent, and often, the phenotypic identification of strains based on morphology may be due to their misidentifications [
4,
11,
12]. Morphology screening for colony differentiation is difficult to standardize and is often applied to incorrect discrimination of strains [
4,
13].
Recently, for the species-specific identification of strains, genomic-based profiling by real-time quantitative polymerase chain reaction (RT-qPCR) analysis has been proposed as a key metric in the probiotic industrial field to ensure their correct definition [
14,
15,
16]. The RT-qPCR method combines the qualitative identification of strains with the absolute quantification of bacteria obtainable by creating a standard calibration curve of known quantities of starting templates [
16,
17,
18]. The highlight of RT-qPCR allows the detection of several bacterial species simultaneously in food, environmental matrices, cosmetics, feces, and other complex matrices [
4,
5,
16,
17]. Considering its high sensitivity, RT-qPCR enables the quantification of microorganisms with low abundance within a sample without the necessity of a preliminary enrichment step in culture [
16,
18,
19,
20]. The key point in RT-qPCR development is primer design that needs to specifically target the strain of interest [
3,
15,
21]. Furthermore, both the efficiency and the accuracy of the RT-qPCR reaction depend on DNA quality and quantity [
22,
23]. The main problem for reaching a good quality DNA is the removal of PCR-inhibitory elements derived from the food supplement matrix. Different commercial kits are available to obtain an efficient DNA extraction. In our previous work, we have already tested and validated the best method for DNA isolation and purification from probiotics contained in complex matrices [
24].
In pursuit of a rapid, convenient, and cost-effective method for monitoring bacterial viability, costs and time are important aspects to take into consideration. The ideal procedure should present a cost-effective and rapid management to accurately determine the absolute count of viable cells. Culture procedures are considered expensive and time consuming with low statistical accuracy. Costs are associated mainly with selective culture media and broths for bacterial growth. Approximately 4 to 10 plates are needed to obtain at least a duplicate cell count of an unknown sample. Furthermore, large differences among plate counts, derived from inter-operator variability, result in large standard deviations (about 20–30% variability). Using plating techniques, the time to results is often 2–5 days of incubation time, making product release long [
25,
26].
With flow cytometry and molecular biology approaches, the testing time is relatively short—within 2 h for results with the first technology and within 8 h with the second one—with a significant gain in “worker effort” (e.g., man-hour). In fact, man-hour is the basis for measuring the cost of professional people and their contribution to productivity. Furthermore, flow cytometry shows less variability with a mean of 10% standard deviation. Reagents are relatively cheap, considering the number of tests performable per hour (up to 48 in 1 h) [
27,
28,
29,
30].
RT-qPCR is considered expensive because of reagent composition and instruments. The high number of tests enables substantial savings in reagent costs, technical burden, and time to generate laboratory results [
31,
32,
33].
The implementation of new technologies is cost-effective and an effective long-term solution in the management of the high number of samples per day and charged to the operator [
30,
34,
35]. Compared to classical microbiological methods, flow cytometry and RT-qPCR provide the optimal balance of cost in quality control screening scenarios.
The species of both
Lactobacillus and
Bifidobacterium generis are taxonomically very complex for their phenotypical diversity. Nevertheless, 16S rRNA gene sequences are too similar to be easily distinguished. In particular, closely related species within the
L. acidophilus group (
L. acidophilus,
L. gallinarum, and
L. helveticus), the
L. casei group
(L. casei,
L. paracasei, and
L. rhamnosus), the
L. plantarum group (
L. plantarum,
L. paraplantarum, and
L. pentosus), and the
L. sakei group (
L. sakei,
L. curvatus, and
L. graminis) are notoriously difficult to distinguish due to the high morphological homology [
22,
36,
37].
To make the assay more robust, the combination with another detection method, such as flow cytometry, has gained prominence in the industry [
19,
38]. The main goal of flow cytometry is the total enumeration of viable, dead, and damaged bacterial cells in a relatively short time [
23,
28,
38,
39]. The assessment of cell viability inherits the advantages of fluorescent dyes. In particular, bacterial viability is differentiated by membrane permeability properties and is based on a dual staining procedure, such as thiazole orange (TO) or SYTO 9 (Thermofisher Scientific, Waltham, MA, USA) and propidium iodide (PI). Both dyes intercalate with nucleic acids. TO/SYTO 9 can cross all bacterial cell membranes, while PI can only enter cells with disrupted membranes allowing differentiation between live and dead cells [
28,
38].
Together, genomics and flow cytometry have revolutionized strain-specific microbial detection, allowing us to specifically quantify viable probiotics and give them a strain genomic identity [
14,
15,
36]. In recent years, the publication by the International Standard in according with ISO 19344 (IDF 232) has suggested a new method for the selective enumeration of active lactic acid bacteria/or total units by flow cytometry to assess the quality of fermented products (ISO 19344 2015) [
13,
24,
40,
41]. High-throughput analysis using a flow-cytometric based-count gave the advantages of a lower variation, a reduction in testing time, the differentiation between active and total cells, and quantification of the fraction of active cells per total cells [
23,
28,
30].
In this study, we describe the development of a combined analytical assay based on flow cytometry and strain-specific RT-qPCRs for the identification and absolute quantification of probiotics in food supplements.
The detection of Bifidobacterium longum, Bifidobacterium animalis spp. lactis, Lactobacillus paracasei, Lactobacillus rhamnosus, Lactobacillus casei, Bifidobacterium breve, Lactobacillus acidophilus, Lactobacillus plantarum, and Lactobacillus helveticus strains have been carried out from two different powder commercial food supplements.
In this work, flow cytometry in combination with RT-qPCR was tested for the first time as a specific quantification and qualification quality control method for Lactobacillus and Bifidobacterium-based products. Primers were tested for specificity with a set of non-target probiotic strains while sensitivity, the limit of detection (LOD), and efficiency were optimized using scalar dilutions. The presence and quantity of probiotics were also determined by plate count enumeration as the reference gold standard method recognized for quality assurance of production. To improve the quality of our study, a multiplex qPCR approach based on TaqMan probes (Thermofisher Scientific, Waltham, MA, USA) was designed. In fact, the simultaneous amplification of more than one target sequence in a single tube allows an even faster screening of different probiotic strains. We performed single reactions per bacterium species to validate the method step by step. In the future, the application of TaqMan probes (Thermofisher Scientific, Waltham, MA, USA) with better specificity will produce faster results in the quality control workflow.
2. Materials and Methods
2.1. Food Supplement Composition
Food supplements employed in this work are 2 anonymized commercial formulations of powder probiotics with different compositions. The probiotic powder composition of food supplement 1 was formulated with 7 species: commercial strain of Bifidobacterium longum, Bifidobacterium animalis spp. lactis, Lactobacillus paracasei, Lactobacillus acidophilus, Lactobacillus plantarum, Bifidobacterium breve, and Lactobacillus helveticus; while the probiotic powder composition of food supplement 2 was composed by 4 strains: Bifidobacterium animalis spp. lactis (≥85.2 × 109 CFU/g), Bifidobacterium breve (≥10.8 × 109 CFU/g), Lactobacillus rhamnosus (≥12 × 109 CFU/g), and Lactobacillus paracasei (≥12 × 109 CFU/g). Food supplement 1 was packaged in 2 different formats, premix and sachets, composed of the same probiotic mix but in different total quantities, ≥285 × 109 CFU/g and 102 × 109 CFU/g, respectively, without any discrimination between strain-specific counts.
Considering that no quantifications for each strain were available, food supplement 1 was employed to set the sensitivity and the specificity of the RT-qPCR reactions and to define the qualitative composition of the probiotic mix; instead, food supplement 2, characterized by declared strain enumeration, was used to optimize quantitative RT-qPCR reactions comparing results.
2.2. Growth Conditions and Plate Count Enumeration
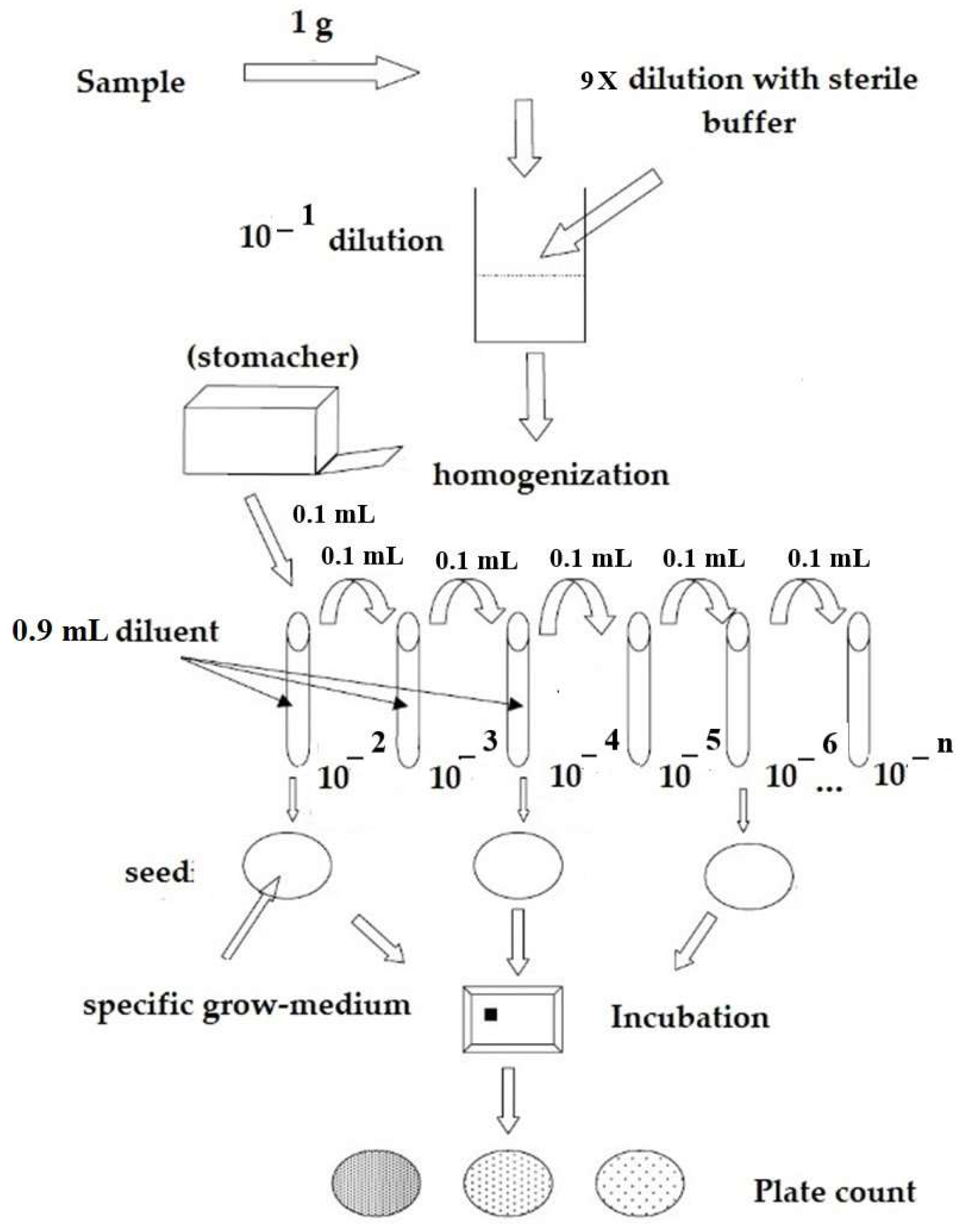
Briefly, 1 g of each lyophilized probiotic was diluted in Maximum Recovery Diluent (MRD-Biolife Italiana, Rome, Italy), according to the Italian National Institute of Health (ISTISAN 08/36) guidelines [
40]. Considering food supplement 1, a premix aliquot and 3 batches of sachets (A, B, and C) were tested, while for food supplement 2, 3 sachets of the same batch were analyzed. Culture of all strains was performed following the ISTISAN 08/36 for
Lactobacillus spp. and ISO 29981:2012 for the
Bifidobacteria spp., which allows discriminating probiotics on the bases of colony’s morphology [
39,
40].
For plate seeding, samples were serially diluted, and the 10 × 10
−9/10
−10 dilutions of food supplement 1 were inoculated on Petri plates containing de Man, Rogosa, Sharpe agar (MRS—Biolife Italiana, Rome, Italy), added with 0.05% L-cystein hydrochloric acid (HCl) (Sigma Aldrich, Milwaukee, WI, USA) while for the food supplement 2 the 10 × 10
−8/10
−9/10
−10 dilutions were inoculated on Petri plates containing de Man, Rogosa, Sharpe agar (MRS—Biolife Italiana, Rome, Italy), added with 0.05% L-cystein HCl (Sigma Aldrich, Milwaukee, WI, USA) and TOS-Propionate Agar base (TOS—Merck Millipore, Burlington, MA, USA), with MUP Selective Supplement, as appropriate, all in duplicates (
Figure 1). Plates were incubated at 37 ± 2 °C for 48–72 h in anaerobiosis. Grown colonies have been counted in duplicate on two consecutive dilutions (two plates for 10 × 10
−9 dilution and two plates for 10 × 10
−10 dilution), where the expected number of colonies was between 3 and 300. The proportionality was verified with the calculation of the Kp (coverage factor for measurement uncertainty, as described by ISO 13005 [
39,
40]) and the G2 (chi square test).
Plate counts were performed, ensuring proportionality between the different dilutions tested. Results were expressed as colony-forming units per gram (CFU/g), in scientific notation, with 1 or 2 decimal numbers, considering the weighted average value.
2.3. Flow-Cytometry
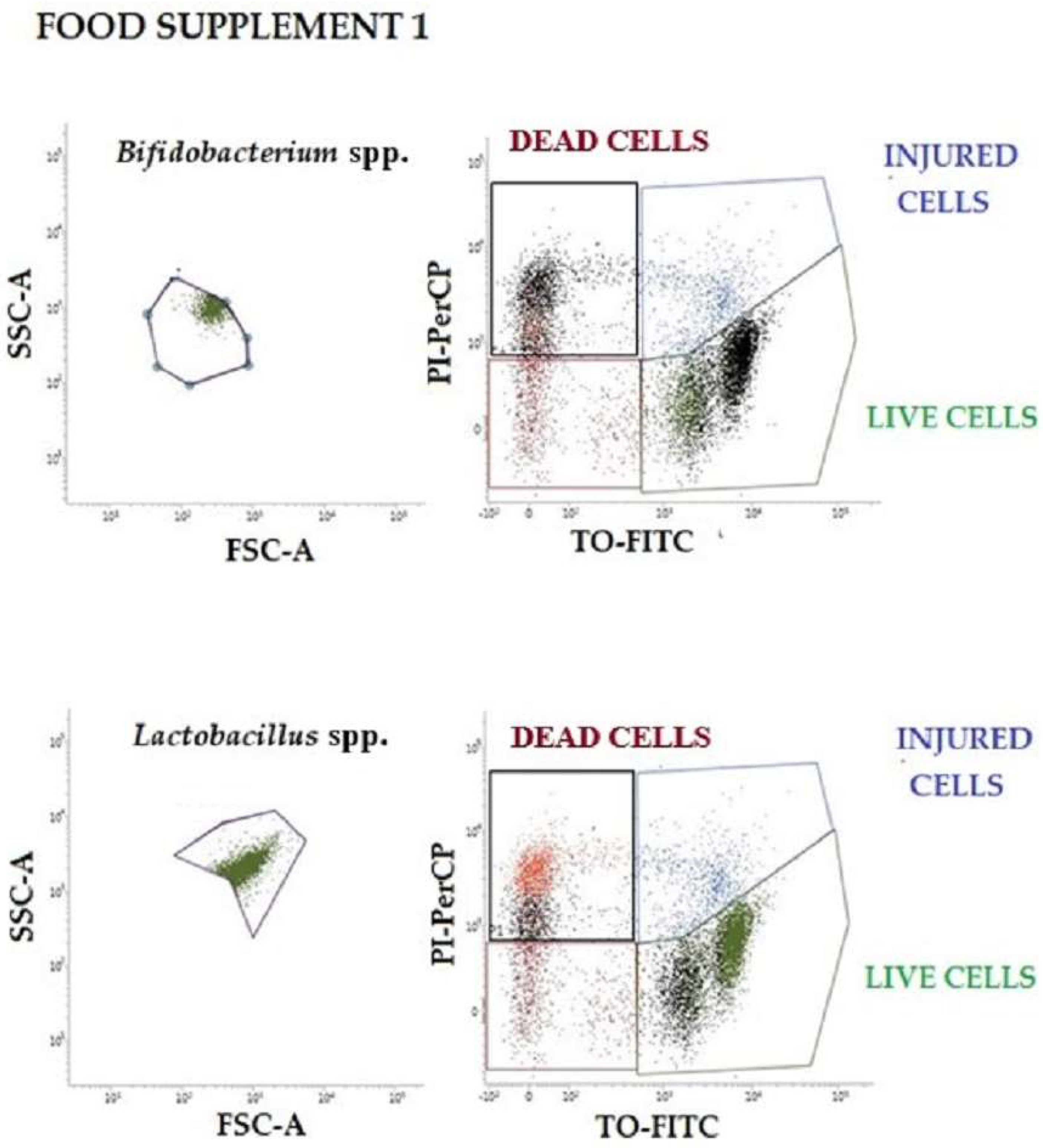
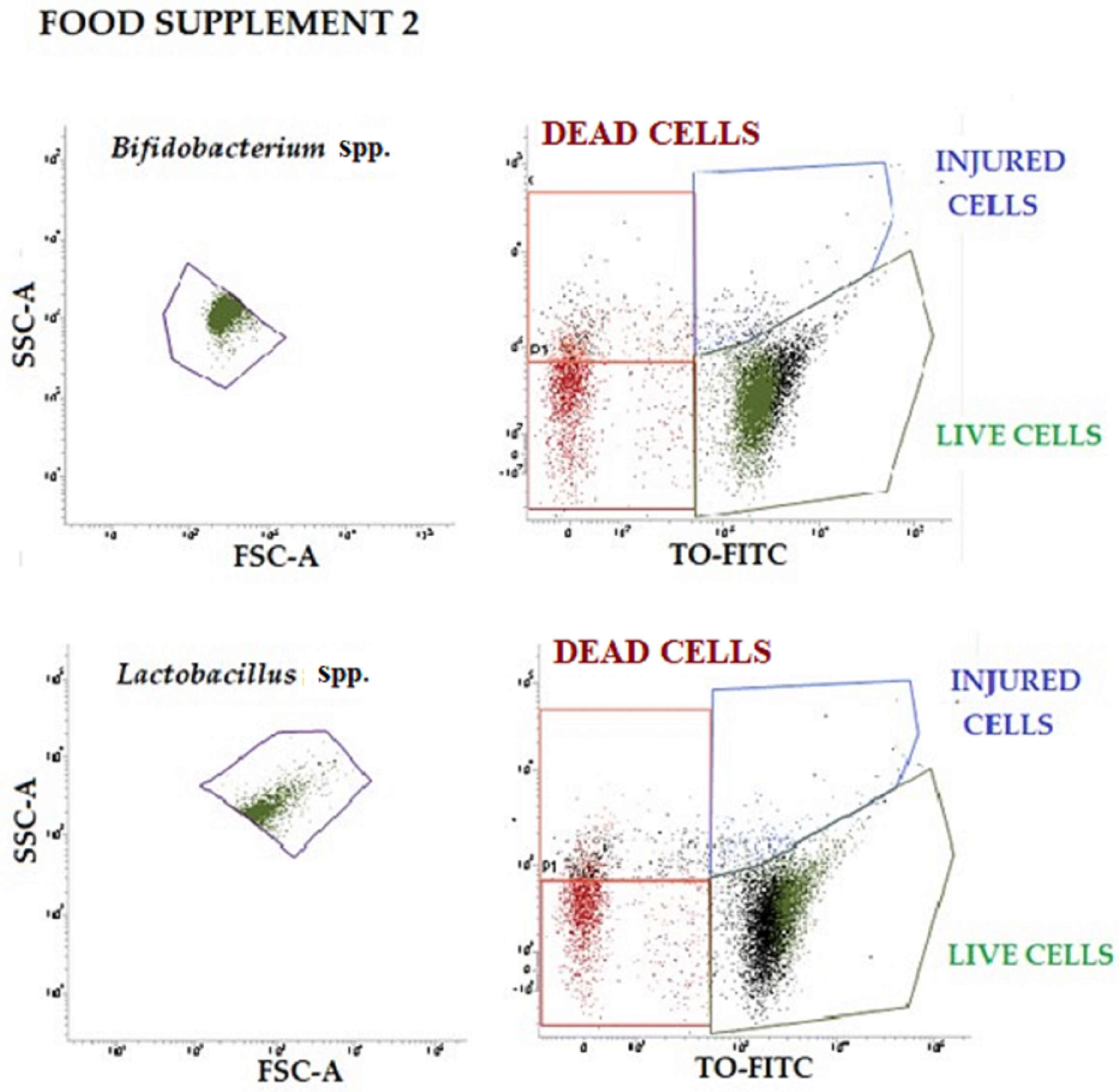
Two-color staining using the BD Cell Viability Kit (BD Biosciences, San Jose, CA, USA) was performed on all probiotic suspensions of food supplements 1 and 2 to distinguish between live and dead cells by flow cytometry. First, the appropriate dilutions of samples were incubated for 30 min in the dark with 42 µmol/L of TO and then an additional 30 min with 4.3 mmol/L of PI. Properly conjugated isotype-matched dyes and unstained bacterial samples were used as negative controls (the same bacterial cells incubated without any dye). Stained samples were analyzed employing a FACS Verse (BD Biosciences, San Jose, CA, USA) flow cytometer. A total of 10,000 events were acquired per sample using an automatic compensation tool [
41]. Data were analyzed with the FACSuite™ (BD Biosciences, San Jose, CA, USA) software, in the “Acquisition-to-Analysis” mode. A forward scatter (FSC) vs. side scatter (SSC) plot with a physical gate was designed to identify the bacterial population of interest. Then, another specific region in the fluorescence parameter 1 (FL1 (TO)) vs. fluorescence parameter 3 (FL3 (PI)) plot was gated to display the live/dead stain results. To determine the absolute count, expressed as UFC/g, the following equation was used:
(
n = number of events per µL;
d = dilution factor corresponding to the dilution).
2.4. DNA Extraction from Powder Probiotics
Bacterial DNA was extracted starting from 1 mL of the 10−1 dilution of 6 samples of the premix and from 6 sachets belonging to 3 batches (two per each batch A, B, and C) for food supplement 1 and from 3 sachets belonging to one batch for the food supplement 2. DNA was isolated using the Power Soil Kit (Qiagen GmbH, Hilden, Germany), according to the manufacturer’s instructions using a Qiacube Connect extractor (Qiagen GmbH, Hilden, Germany). Prior to starting with the automated kit protocol, samples were incubated with Lysozyme (50 mg/mL) for 45 min at 37 °C and then homogenized for 10 s at 1000 g using Precellys® SK38 bead beating tubes (Peqlab Biotechnology GmbH, Erlangen, Germany) and the Precellys® 24-Dual homogenizer (Peqlab Biotechnology GmbH, Erlangen, Germany). The volume of extraction and the elution solution was chosen according to the manufacturer’s instruction. Isolated DNA was visualized by agarose gel electrophoresis, and concentration was determined by NanoDrop ND-1000 spectrophotometer (Peqlab Biotechnology GmbH, Erlangen, Germany).
2.5. Strain-Specific Primer Design and qPCR Assay
Strain-specific PCR primers were designed or modified from literature and checked for sequence similarity by the primer BLAST tool of the National Center of Biotechnology Information (NCBI) [
32,
33,
34,
35,
36]. A set of primers for each species was tested for sensitivity, specificity, and efficiency using an annealing temperature gradient-qPCR. The following conditions were applied: initial denaturation at 95 °C for 3 min followed by 40 cycles at 95 °C for 30 s, annealing at 58 °C–63 °C for 15 s, elongation at 72 °C for 30 s, and a final step at 72 °C for 5 min. To ensure that the correct PCR product was amplified, a melting curve analysis was added at the end of the PCR program using the following gradient thermal protocol: 65 °C–95 °C with a delta temperature of 0.5 °C every 5 s. Amplification was carried out in a 15 µL final volume containing 1 × qPCR ssoAdvance SYBR Master Mix (Biorad, Hercules, CA, USA), 500 nM of each primer, and 2 µL target DNA using a QIA-quant 96 instrument. The best-performing primers employed on food supplement 1 for the sole qualitative discrimination are listed in
Table 1.
Primer sequences used for the absolute quantification of species in food supplement 2 are reported in
Table 2.
For each amplification reaction, the melting temperature (Tm) and cycle-threshold (Ct) values were computed. For the absolute quantification of DNA copies, a standard curve composed of points, representative of different concentrations, was built. The sensitivity of RT-qPCR was determined by the lowest concentration tested (10 × 10−11), while the specificity and the threshold signal were defined using a no-probiotic DNA or other probiotic DNAs, and a no template control as references.
2.6. Optimization of RT-qPCR
For the experimental set-up, optimization of assay parameters, such as primer concentration, annealing temperature, and amplification efficiency conditions, was performed. The specificity of primers was studied with other 3 distinct bacteria strains (no-probiotic DNA) and with the DNA of the other probiotic strains compounding food supplement mixture 1 and 2. For specificity testing, DNA concentrations in all samples were normalized to 10 ng/µL. The quantitative PCR experiment was conducted in triplicate using the protocol described previously in
Section 2.6. Furthermore, sensitivity tests on serially diluted DNA concentrations were performed. The DNA-based sensitivity was tested by 6 tenfold serial dilutions starting from DNA extracted from 10 × 10
−5 to 10 × 10
−11. All tests were carried out in triplicate using the quantitative PCR protocol described above. The reaction efficiency was estimated by building reference standard curves obtained between the Ct values and logarithmic DNA dilutions. The linear regression was calculated using the QIA-quant software (Qiagen, Hilden, Germany). The slope and R squared values were computed using the same software as well.
2.7. Statistical Analyses
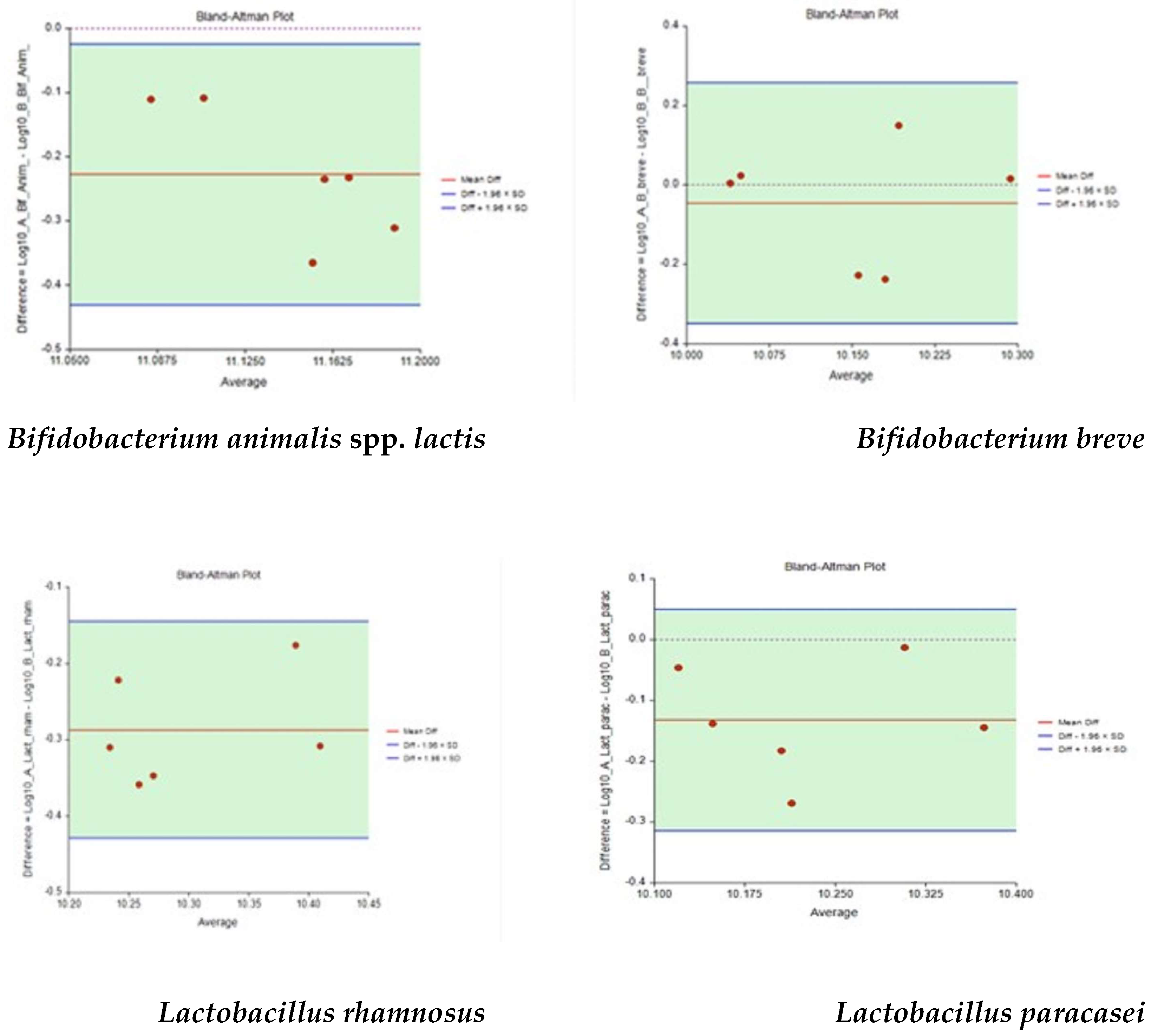
Data were described using exponential values and the statistical treatment has been performed on the values transformed in Log10. The standard curve was built using the QIA-quant 96 software (Qiagen, Hilden, Germany). Statistics were performed by NCSS 2020 v 20.0.2 software (NCSS Statistical Software, East Kaysville, UT, USA) for the Bland–Altman plot to test the agreement between methods and Minitab 19 software (GMSL, Milan, Italy) for the One-way ANOVA analysis.
4. Discussion
The establishment of new quality control methods to enumerate raw materials in food supplements can help in overcoming some of the current limitations of the microbiological-based probiotic industry [
9,
13,
24]. The described method based on RT-qPCR and flow cytometry could contribute significantly to improving the current methods available [
16,
18,
38,
39,
40]. In routine laboratories, culture methods are considered the gold standard for quality control assurance, but it is a time-consuming, operator-dependent, and cost-effective test. RT-qPCR is considerably faster and has lower variability. Another key advantage of qPCR is the detection of a strain-specific DNA target, which can be easily developed for any species based on the unique genetic identity [
14,
22,
37]. Current culture methods rely on growth using general and selective media, which only detect probiotics that can create a colony [
9,
42,
43,
44].
Concerning costs, the implementation of new technologies, such as flow cytometry and RT-qPCR, is a long-term, cost-effective solution in the management of a high number of samples per day compared to probiotic cultures [
23,
30,
34]. These methods provide the optimal balance of costs between reagents and man-hours, representing a suitable solution in routine testing.
The importance of identifying specific probiotic strains is the peculiar ability of each to produce health benefits in a human environment [
1,
5,
44]. This is an important issue with classical microbiology methods. The inability to identify specific strains from one another can be overcome by genetic tests [
14,
22]. RT-qPCR can be used to distinguish and quantify very similar strains of probiotics using their variability in gene sequences, and this technology can be used in combination with flow cytometry to also define probiotics’ vitality [
18,
23,
30,
38].
As demonstrated in our work, RT-qPCR can be used as a qualitative test (e.g., in food supplement 1) and as a quantitative test (food supplement 2). The results obtained from the quantification of food supplement 2 have demonstrated that RT-qPCR can be used to absolute the enumerate probiotic cells at the strain level for the dietary supplement industry. This technology is more sensitive for strain-specific detection with less operator variability than the current plate count enumeration. In our case, the quantification by RT-qPCR overestimates the number of probiotics declared by the producer and count by culture method, probably due to its higher sensitivity in also identifying single cells and for the amplification of genetic targets as well as dead and damaged cells.
New experiments may need to be implemented in order to create a unique molecular assay able to simultaneously detect strains and cell viability using specific DNA markers. To now, a combined approach based on flow cytometry and RT-qPCR could represent an optimal way to perform quality control assessments for product release and overcome classical microbiological methods.