Role of Circadian Clock on the Pathogenesis and Lifestyle Management in Non-Alcoholic Fatty Liver Disease
Abstract
1. Introduction
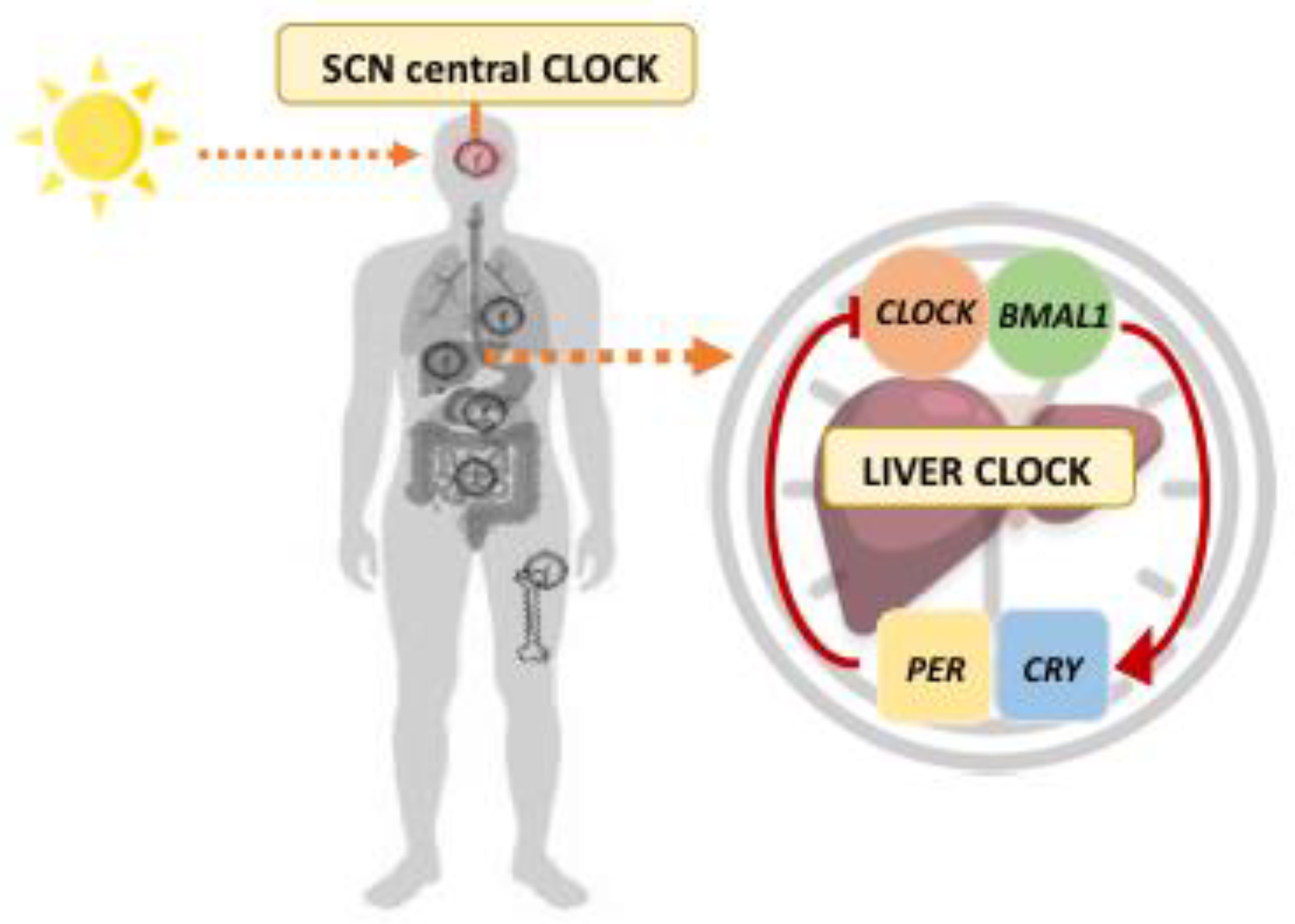
2. Circadian Rhythms in Liver Metabolism
3. Impact of Sleeping Disturbances on NAFLD Risk
3.1. Sleeping Habits and NAFLD
3.2. The Role of Light and Social Jetlag on NAFLD
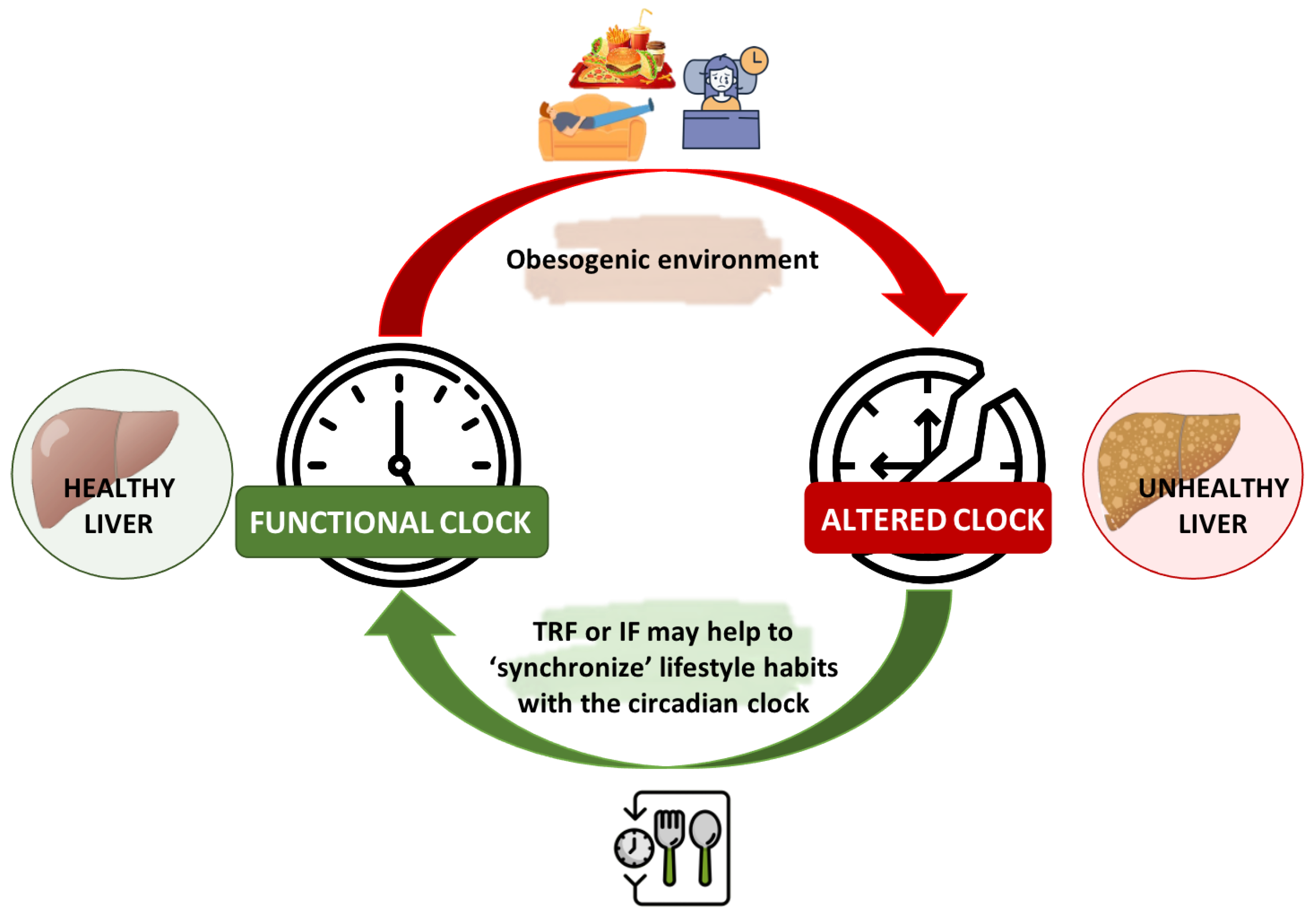
4. Chrononutrition in the Management of NAFLD
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Riazi, K.; Azhari, H.; Charette, J.H.; Underwood, F.E.; King, J.A.; Afshar, E.E.; Swain, M.G.; Congly, S.E.; Kaplan, G.G.; Shaheen, A.A. The prevalence and incidence of NAFLD worldwide: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2022, 7, 851–861. [Google Scholar] [CrossRef] [PubMed]
- Sivell, C. Nonalcoholic Fatty Liver Disease: A Silent Epidemic. Gastroenterol. Nurs. 2019, 42, 428–434. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Scorletti, E.; Mosca, A.; Alisi, A.; Byrne, C.D.; Targher, G. Complications, morbidity and mortality of nonalcoholic fatty liver disease. Metabolism 2020, 111S, 154170. [Google Scholar] [CrossRef] [PubMed]
- Lonardo, A.; Suzuki, A. Sexual Dimorphism of NAFLD in Adults. Focus on Clinical Aspects and Implications for Practice and Translational Research. J. Clin. Med. 2020, 9, 1278. [Google Scholar] [CrossRef] [PubMed]
- Marchisello, S.; Di Pino, A.; Scicali, R.; Urbano, F.; Piro, S.; Purrello, F.; Rabuazzo, A.M. Pathophysiological, molecular and therapeutic issues of nonalcoholic fatty liver disease: An overview. Int. J. Mol. Sci. 2019, 20, 1948. [Google Scholar] [CrossRef] [PubMed]
- Eslam, M.; Newsome, P.N.; Sarin, S.K.; Anstee, Q.M.; Targher, G.; Romero-Gomez, M.; Zelber-Sagi, S.; Wai-Sun Wong, V.; Dufour, J.F.; Schattenberg, J.M.; et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J. Hepatol. 2020, 73, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Perez-Diaz-Del-Campo, N.; Riezu-Boj, J.I.; Marin-Alejandre, B.A.; Monreal, J.I.; Elorz, M.; Herrero, J.I.; Benito-Boillos, A.; Milagro, F.I.; Bugianesi, E.; Tur, J.A.; et al. A Nutrigenetic Tool for Precision Dietary Management of NAFLD Deeming Insulin Resistance Markers. Panminerva Medica. 2022. Available online: https://doi.org/10.23736/S0031-0808.22.04590-6 (accessed on 11 October 2022).
- Saran, A.R.; Dave, S.; Zarrinpar, A. Circadian Rhythms in the Pathogenesis and Treatment of Fatty Liver Disease. Gastroenterology 2020, 158, 1948–1966.e1. [Google Scholar] [CrossRef]
- Morris, C.J.; Purvis, T.E.; Hu, K.; Scheer, F.A.J.L. Circadian misalignment increases cardiovascular disease risk factors in humans. Proc. Natl. Acad. Sci. USA 2016, 113, E1402–E1411. [Google Scholar] [CrossRef]
- Franco, P.; Putois, B.; Guyon, A.; Raoux, A.; Papadopoulou, M.; Guignard-Perret, A.; Bat-Pitault, F.; Hartley, S.; Plancoulaine, S. Sleep during development: Sex and gender differences. Sleep Med. Rev. 2020, 51, 101276. [Google Scholar] [CrossRef]
- Serin, Y.; Acar Tek, N. Effect of Circadian Rhythm on Metabolic Processes and the Regulation of Energy Balance. Ann. Nutr. Metab. 2019, 74, 322–330. [Google Scholar] [CrossRef]
- Adamovich, Y.; Rousso-Noori, L.; Zwighaft, Z.; Neufeld-Cohen, A.; Golik, M.; Kraut-Cohen, J.; Wang, M.; Han, X.; Asher, G. Circadian Clocks and Feeding Time Regulate the Oscillations and Levels of Hepatic Triglycerides. Cell Metab. 2014, 19, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Jacobi, D.; Liu, S.; Burkewitz, K.; Kory, N.; Knudsen, N.H.; Alexander, R.K.; Unluturk, U.; Li, X.; Kong, X.; Hyde, A.L.; et al. Hepatic Bmal1 Regulates Rhythmic Mitochondrial Dynamics and Promotes Metabolic Fitness. Cell Metab. 2015, 22, 709–720. [Google Scholar] [CrossRef] [PubMed]
- Ribas-Latre, A.; Eckel-Mahan, K. Nutrients and the Circadian Clock: A Partnership Controlling Adipose Tissue Function and Health. Nutrients 2022, 14, 2084. [Google Scholar] [CrossRef] [PubMed]
- Charlot, A.; Hutt, F.; Sabatier, E.; Zoll, J. Beneficial Effects of Early Time-Restricted Feeding on Metabolic Diseases: Importance of Aligning Food Habits with the Circadian Clock. Nutrients 2021, 13, 1405. [Google Scholar] [CrossRef] [PubMed]
- Mukherji, A.; Dachraoui, M.; Baumert, T.F. Perturbation of the circadian clock and pathogenesis of NAFLD. Metabolism 2020, 111, 154337. [Google Scholar] [CrossRef]
- Reutrakul, S.; Van Cauter, E. Sleep influences on obesity, insulin resistance, and risk of type 2 diabetes. Metabolism 2018, 84, 56–66. [Google Scholar] [CrossRef]
- Mukherji, A.; Bailey, S.M.; Staels, B.; Baumert, T.F. The Circadian Clock and Liver Function in Health and Disease. J. Hepatol. 2019, 71, 200. [Google Scholar] [CrossRef]
- Breitenbach, T.; Helfrich-Förster, C.; Dandekar, T. An effective model of endogenous clocks and external stimuli determining circadian rhythms. Sci. Rep. 2021, 11, 16165. [Google Scholar] [CrossRef]
- Konopka, R.J.; Benzer, S. Clock mutants of Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 1971, 68, 2112–2116. [Google Scholar] [CrossRef]
- Mure, L.S.; Le, H.D.; Benegiamo, G.; Chang, M.W.; Rios, L.; Jillani, N.; Ngotho, M.; Kariuki, T.; Dkhissi-Benyahya, O.; Cooper, H.M.; et al. Diurnal transcriptome atlas of a primate across major neural and peripheral tissues. Science 2018, 359, eaao0318. [Google Scholar] [CrossRef]
- Crane, B.R.; Young, M.W. Interactive features of proteins composing eukaryotic circadian clocks. Annu. Rev. Biochem. 2014, 83, 191–219. [Google Scholar] [CrossRef] [PubMed]
- Marcheva, B.; Ramsey, K.M.; Buhr, E.D.; Kobayashi, Y.; Su, H.; Ko, C.H.; Ivanova, G.; Omura, C.; Mo, S.; Vitaterna, M.H.; et al. Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes. Nature 2010, 466, 627–631. [Google Scholar] [CrossRef] [PubMed]
- Asher, G.; Sassone-Corsi, P. Time for food: The intimate interplay between nutrition, metabolism, and the circadian clock. Cell 2015, 161, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Lazar, M.A. Transcriptional Control of Circadian Rhythms and Metabolism: A Matter of Time and Space. Endocr. Rev. 2020, 41, 707. [Google Scholar] [CrossRef] [PubMed]
- Astiz, M.; Heyde, I.; Oster, H. Mechanisms of Communication in the Mammalian Circadian Timing System. Int. J. Mol. Sci. 2019, 20, 343. [Google Scholar] [CrossRef] [PubMed]
- Mermet, J.; Yeung, J.; Naef, F. Systems Chronobiology: Global Analysis of Gene Regulation in a 24-Hour Periodic World. Cold Spring Harb. Perspect. Biol. 2017, 9, a028720. [Google Scholar] [CrossRef]
- Ferrell, J.M.; Chiang, J.Y.L. Circadian rhythms in liver metabolism and disease. Acta Pharm. Sin. B 2015, 5, 113. [Google Scholar] [CrossRef]
- Gil-Lozano, M.; Hunter, P.M.; Behan, L.A.; Gladanac, B.; Casper, R.F.; Brubaker, P.L. Short-term sleep deprivation with nocturnal light exposure alters time-dependent glucagon-like peptide-1 and insulin secretion in male volunteers. Am. J. Physiol. Endocrinol. Metab. 2016, 310, E41–E50. [Google Scholar] [CrossRef]
- Martchenko, A.; Oh, R.H.; Wheeler, S.E.; Gurges, P.; Chalmers, J.A.; Brubaker, P.L. Suppression of circadian secretion of glucagon-like peptide-1 by the saturated fatty acid, palmitate. Acta Physiol. 2018, 222, e13007. [Google Scholar] [CrossRef]
- Gnocchi, D.; Bruscalupi, G. Circadian Rhythms and Hormonal Homeostasis: Pathophysiological Implications. Biology 2017, 6, 10. [Google Scholar] [CrossRef]
- McHill, A.W.; Phillips, A.J.K.; Czeisler, C.A.; Keating, L.; Yee, K.; Barger, L.K.; Garaulet, M.; Scheer, F.A.J.L.; Klerman, E.B. Later circadian timing of food intake is associated with increased body fat. Am. J. Clin. Nutr. 2017, 106, 1213–1219. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.; Chen, J.; Wang, J.; Yao, J.; Huang, Y.; Zhang, G.; Bao, Z. Circadian Clock Genes in the Metabolism of Non-alcoholic Fatty Liver Disease. Front. Physiol. 2019, 10, 423. [Google Scholar] [CrossRef] [PubMed]
- Salgado-Delgado, R.; Ángeles-Castellanos, M.; Buijs, M.R.; Escobar, C. Internal desynchronization in a model of night-work by forced activity in rats. Neuroscience 2008, 154, 922–931. [Google Scholar] [CrossRef] [PubMed]
- Zarrinpar, A.; Chaix, A.; Panda, S. Daily Eating Patterns and Their Impact on Health and Disease. Trends Endocrinol. Metab. 2016, 27, 69. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, A.; Debelius, J.; Brenner, D.A.; Karin, M.; Loomba, R.; Schnabl, B.; Knight, R. The gut-liver axis and the intersection with the microbiome. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 397–411. [Google Scholar] [CrossRef] [PubMed]
- Frazier, K.; Chang, E.B. Intersection of the Gut Microbiome and Circadian Rhythms in Metabolism. Trends Endocrinol. Metab. 2020, 31, 25–36. [Google Scholar] [CrossRef]
- Leone, V.; Gibbons, S.M.; Martinez, K.; Hutchison, A.L.; Huang, E.Y.; Cham, C.M.; Pierre, J.F.; Heneghan, A.F.; Nadimpalli, A.; Hubert, N.; et al. Effects of Diurnal Variation of Gut Microbes and High-Fat Feeding on Host Circadian Clock Function and Metabolism. Cell Host Microbe 2015, 17, 681–689. [Google Scholar] [CrossRef]
- Liang, X.; Bushman, F.D.; FitzGerald, G.A. Rhythmicity of the intestinal microbiota is regulated by gender and the host circadian clock. Proc. Natl. Acad. Sci. USA 2015, 112, 10479–10484. [Google Scholar] [CrossRef]
- Pearson, J.A.; Voisey, A.C.; Boest-Bjerg, K.; Wong, F.S.; Wen, L. Circadian Rhythm Modulation of Microbes During Health and Infection. Front. Microbiol. 2021, 12, 2468. [Google Scholar] [CrossRef]
- Marjot, T.; Ray, D.W.; Williams, F.R.; Tomlinson, J.W.; Armstrong, M.J. Sleep and liver disease: A bidirectional relationship. Lancet Gastroenterol. Hepatol. 2021, 6, 850–863. [Google Scholar] [CrossRef]
- Wijarnpreecha, K.; Thongprayoon, C.; Panjawatanan, P.; Ungprasert, P. Short sleep duration and risk of nonalcoholic fatty liver disease: A systematic review and meta-analysis. J. Gastroenterol. Hepatol. 2016, 31, 1802–1807. [Google Scholar] [CrossRef] [PubMed]
- Bernsmeier, C.; Weisskopf, D.M.; Pflueger, M.O.; Mosimann, J.; Campana, B.; Terracciano, L.; Beglinger, C.; Heim, M.H.; Cajochen, C. Sleep Disruption and Daytime Sleepiness Correlating with Disease Severity and Insulin Resistance in Non-Alcoholic Fatty Liver Disease: A Comparison with Healthy Controls. PLoS ONE 2015, 10, e0143293. [Google Scholar] [CrossRef] [PubMed]
- Al-Jahdali, H.; Al Enezi, A.; Anwar, A.E.; Al-Harbi, A.; Baharoon, S.; Aljumah, A.; Shimemeri, A.; Abdullah, K. Prevalence of Insomnia and Sleep Patterns among Liver Cirrhosis. J. Circadian Rhythm. 2014, 12, 2. [Google Scholar] [CrossRef]
- Imaizumi, H.; Takahashi, A.; Tanji, N.; Abe, K.; Sato, Y.; Anzai, Y.; Watanabe, H.; Ohira, H. The Association between Sleep Duration and Non-Alcoholic Fatty Liver Disease among Japanese Men and Women. Obes. Facts 2015, 8, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Shen, N.; Wang, P.; Yan, W. Sleep Duration and the Risk of Fatty Liver Disease: A Systematic Review and Meta-analysis. Sci. Rep. 2016, 6, 31956. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, K.; Leproult, R.; L’Hermite-Balériaux, M.; Copinschi, G.; Penev, P.D.; Van Cauter, E. Leptin levels are dependent on sleep duration: Relationships with sympathovagal balance, carbohydrate regulation, cortisol, and thyrotropin. J. Clin. Endocrinol. Metab. 2004, 89, 5762–5771. [Google Scholar] [CrossRef]
- Nesto, R.W.; Mackie, K. Endocannabinoid system and its implications for obesity and cardiometabolic risk. Eur. Heart J. Suppl. 2008, 10, B34–B41. [Google Scholar] [CrossRef]
- Richey, J.M.; Woolcott, O. Re-visiting the Endocannabinoid System and Its Therapeutic Potential in Obesity and Associated Diseases. Curr. Diab. Rep. 2017, 17, 99. [Google Scholar] [CrossRef]
- VanItallie, T.B. Sleep and energy balance: Interactive homeostatic systems. Metabolism 2006, 55, S30–S35. [Google Scholar] [CrossRef]
- Spaeth, A.M.; Dinges, D.F.; Goel, N. Effects of Experimental Sleep Restriction on Weight Gain, Caloric Intake, and Meal Timing in Healthy Adults. Sleep 2013, 36, 981–990. [Google Scholar] [CrossRef]
- Bosy-Westphal, A.; Hinrichs, S.; Jauch-Chara, K.; Hitze, B.; Later, W.; Wilms, B.; Settler, U.; Peters, A.; Kiosz, D.; Müller, M.J. Influence of partial sleep deprivation on energy balance and insulin sensitivity in healthy women. Obes. Facts 2008, 1, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Calvin, A.D.; Carter, R.E.; Adachi, T.; MacEdo, P.G.; Albuquerque, F.N.; Van Der Walt, C.; Bukartyk, J.; Davison, D.E.; Levine, J.A.; Somers, V.K. Effects of experimental sleep restriction on caloric intake and activity energy expenditure. Chest 2013, 144, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Capers, P.L.; Fobian, A.D.; Kaiser, K.A.; Borah, R.; Allison, D.B. A systematic review and meta-analysis of randomized controlled trials of the impact of sleep duration on adiposity and components of energy balance. Obes. Rev. 2015, 16, 771–782. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.W.; Yun, K.E.; Jung, H.S.; Chang, Y.; Choi, E.S.; Kwon, M.J.; Lee, E.H.; Woo, E.J.; Kim, N.H.; Shin, H.; et al. Sleep duration and quality in relation to non-alcoholic fatty liver disease in middle-aged workers and their spouses. J. Hepatol. 2013, 59, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Trovato, F.M.; Martines, G.F.; Brischetto, D.; Catalano, D.; Musumeci, G.; Trovato, G.M. Fatty liver disease and lifestyle in youngsters: Diet, food intake frequency, exercise, sleep shortage and fashion. Liver Int. 2016, 36, 427–433. [Google Scholar] [CrossRef]
- Spiegel, K.; Leproult, R.; Van Cauter, E. Impact of sleep debt on metabolic and endocrine function. Lancet 1999, 354, 1435–1439. [Google Scholar] [CrossRef]
- Leproult, R.; Van Cauter, E. Role of sleep and sleep loss in hormonal release and metabolism. Endocr. Dev. 2010, 17, 11–21. [Google Scholar] [CrossRef]
- Katsagoni, C.N.; Georgoulis, M.; Papatheodoridis, G.V.; Fragopoulou, E.; Ioannidou, P.; Papageorgiou, M.; Alexopoulou, A.; Papadopoulos, N.; Deutsch, M.; Kontogianni, M.D. Associations Between Lifestyle Characteristics and the Presence of Nonalcoholic Fatty Liver Disease: A Case-Control Study. Metab. Syndr. Relat. Disord. 2017, 15, 72–79. [Google Scholar] [CrossRef]
- Tasali, E.; Chapotot, F.; Wroblewski, K.; Schoeller, D. The effects of extended bedtimes on sleep duration and food desire in overweight young adults: A home-based intervention. Appetite 2014, 80, 220–224. [Google Scholar] [CrossRef]
- Miyake, T.; Kumagi, T.; Furukawa, S.; Hirooka, M.; Kawasaki, K.; Koizumi, M.; Todo, Y.; Yamamoto, S.; Tokumoto, Y.; Ikeda, Y.; et al. Short sleep duration reduces the risk of nonalcoholic fatty liver disease onset in men: A community-based longitudinal cohort study. J. Gastroenterol. 2015, 50, 583–589. [Google Scholar] [CrossRef]
- Liu, C.; Zhong, R.; Lou, J.; Pan, A.; Tang, Y.; Chang, J.; Ke, J.; Li, J.; Yuan, J.; Wang, Y.; et al. Nighttime sleep duration and risk of nonalcoholic fatty liver disease: The Dongfeng-Tongji prospective study. Ann. Med. 2016, 48, 468–476. [Google Scholar] [CrossRef] [PubMed]
- Robles, M.S.; Cox, J.; Mann, M. In-vivo quantitative proteomics reveals a key contribution of post-transcriptional mechanisms to the circadian regulation of liver metabolism. PLoS Genet. 2014, 10, e1004047. [Google Scholar] [CrossRef] [PubMed]
- Salgado-Delgado, R.C.; Saderi, N.; Basualdo, M.d.C.; Guerrero-Vargas, N.N.; Escobar, C.; Buijs, R.M. Shift Work or Food Intake during the Rest Phase Promotes Metabolic Disruption and Desynchrony of Liver Genes in Male Rats. PLoS ONE 2013, 8, e60052. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, Y.; Wang, Z.; Wang, H.; Xue, C.; Li, Q.; Guan, W.; Yuan, J. Rotating night shift work and non-alcoholic fatty liver disease among steelworkers in China: A cross-sectional survey. Occup. Environ. Med. 2020, 77, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.C.; Chen, P.C. Persistent rotating shift work exposure is a tough second hit contributing to abnormal liver function among on-site workers having sonographic fatty liver. Asia Pac. J. Public Health 2015, 27, NP1765–NP1774. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhang, L.; Wu, S.; Li, W.; Sun, M.; Feng, W.; Ding, D.; Wong, S.Y.; Zhu, P.; Evans, G.J.; et al. Night shift work and abnormal liver function: Is non-alcohol fatty liver a necessary mediator? Occup. Environ. Med. 2019, 76, 83–89. [Google Scholar] [CrossRef]
- Balakrishnan, M.; El-Serag, H.B.; Kanwal, F.; Thrift, A.P. Shiftwork Is Not Associated with Increased Risk of NAFLD: Findings from the National Health and Nutrition Examination Survey. Dig. Dis. Sci. 2017, 62, 526–533. [Google Scholar] [CrossRef]
- Zubidat, A.E.; Haim, A. Artificial light-at-night-a novel lifestyle risk factor for metabolic disorder and cancer morbidity. J. Basic Clin. Physiol. Pharmacol. 2017, 28, 295–313. [Google Scholar] [CrossRef]
- Lunn, R.M.; Blask, D.E.; Coogan, A.N.; Figueiro, M.G.; Gorman, M.R.; Hall, J.E.; Hansen, J.; Nelson, R.J.; Panda, S.; Smolensky, M.H.; et al. Health consequences of electric lighting practices in the modern world: A report on the National Toxicology Program’s workshop on shift work at night, artificial light at night, and circadian disruption. Sci. Total Environ. 2017, 607–608, 1073–1084. [Google Scholar] [CrossRef]
- Rijo-Ferreira, F.; Takahashi, J.S. Genomics of circadian rhythms in health and disease. Genome Med. 2019, 11, 82. [Google Scholar] [CrossRef]
- Artificial Light during Sleep Linked to Obesity|National Institutes of Health (NIH). Available online: https://www.nih.gov/news-events/nih-research-matters/artificial-light-during-sleep-linked-obesity (accessed on 18 October 2022).
- Rybnikova, N.A.; Haim, A.; Portnov, B.A. Does artificial light-at-night exposure contribute to the worldwide obesity pandemic? Int. J. Obes. 2016, 40, 815–823. [Google Scholar] [CrossRef]
- Fonken, L.K.; Nelson, R.J. The effects of light at night on circadian clocks and metabolism. Endocr. Rev. 2014, 35, 648–670. [Google Scholar] [CrossRef] [PubMed]
- Fonken, L.K.; Workman, J.L.; Walton, J.C.; Weil, Z.M.; Morris, J.S.; Haim, A.; Nelson, R.J. Light at night increases body mass by shifting the time of food intake. Proc. Natl. Acad. Sci. USA 2010, 107, 18664. [Google Scholar] [CrossRef] [PubMed]
- Rybnikova, N.; Portnov, B.A. Population-level study links short-wavelength nighttime illumination with breast cancer incidence in a major metropolitan area. Chronobiol. Int. 2018, 35, 1198–1208. [Google Scholar] [CrossRef] [PubMed]
- Kloog, I.; Haim, A.; Stevens, R.G.; Barchana, M.; Portnov, B.A. Light at Night Co-distributes with Incident Breast but not Lung Cancer in the Female Population of Israel. Chronobiol. Int. 2009, 25, 65–81. [Google Scholar] [CrossRef]
- Kim, K.Y.; Lee, E.; Kim, Y.J.; Kim, J. The association between artificial light at night and prostate cancer in Gwangju City and South Jeolla Province of South Korea. Chronobiol. Int. 2016, 34, 203–211. [Google Scholar] [CrossRef]
- Muscogiuri, G.; Poggiogalle, E.; Barrea, L.; Tarsitano, M.G.; Garifalos, F.; Liccardi, A.; Pugliese, G.; Savastano, S.; Colao, A.; Colao, A.; et al. Exposure to artificial light at night: A common link for obesity and cancer? Eur. J. Cancer 2022, 173, 263–275. [Google Scholar] [CrossRef]
- Jones, R.R. Exposure to artificial light at night and risk of cancer: Where do we go from here? Br. J. Cancer 2021, 124, 1467–1468. [Google Scholar] [CrossRef]
- Lai, K.Y.; Sarkar, C.; Ni, M.Y.; Cheung, L.W.T.; Gallacher, J.; Webster, C. Exposure to light at night (LAN) and risk of breast cancer: A systematic review and meta-analysis. Sci. Total Environ. 2021, 762, 143159. [Google Scholar] [CrossRef]
- Stevens, R.G.; Hansen, J.; Costa, G.; Haus, E.; Kauppinen, T.; Aronson, K.J.; Castaño-Vinyals, G.; Davis, S.; Frings-Dresen, M.H.W.; Fritschi, L.; et al. Considerations of circadian impact for defining “shift work” in cancer studies: IARC Working Group Report. Occup. Environ. Med. 2011, 68, 154–162. [Google Scholar] [CrossRef]
- Wittmann, M.; Dinich, J.; Merrow, M.; Roenneberg, T. Social jetlag: Misalignment of biological and social time. Chronobiol. Int. 2006, 23, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Roenneberg, T.; Allebrandt, K.V.; Merrow, M.; Vetter, C. Social Jetlag and Obesity. Curr. Biol. 2012, 22, 939–943. [Google Scholar] [CrossRef] [PubMed]
- Caliandro, R.; Streng, A.A.; van Kerkhof, L.W.M.; van der Horst, G.T.J.; Chaves, I. Social Jetlag and Related Risks for Human Health: A Timely Review. Nutrients 2021, 13, 4543. [Google Scholar] [CrossRef] [PubMed]
- McMahon, D.M.; Burch, J.B.; Youngstedt, S.D.; Wirth, M.D.; Hardin, J.W.; Hurley, T.G.; Blair, S.N.; Hand, G.A.; Shook, R.P.; Drenowatz, C.; et al. Relationships between chronotype, social jetlag, sleep, obesity and blood pressure in healthy young adults. Chronobiol. Int. 2019, 36, 493–509. [Google Scholar] [CrossRef]
- Kantermann, T.; Duboutay, F.; Haubruge, D.; Kerkhofs, M.; Schmidt-Trucksäss, A.; Skene, D.J. Atherosclerotic risk and social jetlag in rotating shift-workers: First evidence from a pilot study. Work 2013, 46, 273–282. [Google Scholar] [CrossRef]
- Stoner, L.; Beets, M.W.; Brazendale, K.; Moore, J.B.; Weaver, R.G. Social Jetlag Is Associated With Adiposity in Children. Glob. Pediatr. Health 2018, 5, 2333794X18816921. [Google Scholar] [CrossRef]
- Parsons, M.J.; Moffitt, T.E.; Gregory, A.M.; Goldman-Mellor, S.; Nolan, P.M.; Poulton, R.; Caspi, A. Social jetlag, obesity and metabolic disorder: Investigation in a cohort study. Int. J. Obes. 2015, 39, 842–848. [Google Scholar] [CrossRef]
- de Zwart, B.J.; Beulens, J.W.J.; Elders, P.; Rutters, F. Pilot data on the association between social jetlag and obesity-related characteristics in Dutch adolescents over one year. Sleep Med. 2018, 47, 32–35. [Google Scholar] [CrossRef]
- Bodur, M.; Baspinar, B.; Özçelik, A.Ö. A cross-sectional evaluation of the relationship between social jetlag and diet quality. Chronobiol. Int. 2021, 38, 1557–1568. [Google Scholar] [CrossRef]
- Rutters, F.; Lemmens, S.G.; Adam, T.C.; Bremmer, M.A.; Elders, P.J.; Nijpels, G.; Dekker, J.M. Is social jetlag associated with an adverse endocrine, behavioral, and cardiovascular risk profile? J. Biol. Rhythm. 2014, 29, 377–383. [Google Scholar] [CrossRef]
- Koopman, A.D.M.; Rauh, S.P.; Van ’T Riet, E.; Groeneveld, L.; Van Der Heijden, A.A.; Elders, P.J.; Dekker, J.M.; Nijpels, G.; Beulens, J.W.; Rutters, F. The Association between Social Jetlag, the Metabolic Syndrome, and Type 2 Diabetes Mellitus in the General Population: The New Hoorn Study. J. Biol. Rhythm. 2017, 32, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Mota, M.C.; Silva, C.M.; Balieiro, L.C.T.; Fahmy, W.M.; Marqueze, E.C.; Moreno, C.R.d.C.; Crispim, C.A. Social Jetlag Is Associated With Impaired Metabolic Control During a 1-Year Follow-Up. Front. Physiol. 2021, 12, 1346. [Google Scholar] [CrossRef] [PubMed]
- Wong, P.M.; Hasler, B.P.; Kamarck, T.W.; Muldoon, M.F.; Manuck, S.B. Social Jetlag, Chronotype, and Cardiometabolic Risk. J. Clin. Endocrinol. Metab. 2015, 100, 4612–4620. [Google Scholar] [CrossRef]
- Levandovski, R.; Dantas, G.; Fernandes, L.C.; Caumo, W.; Torres, I.; Roenneberg, T.; Hidalgo, M.P.L.; Allebrandt, K.V. Depression scores associate with chronotype and social jetlag in a rural population. Chronobiol. Int. 2011, 28, 771–778. [Google Scholar] [CrossRef]
- Dhurandhar, N.V.; Binks, M. When to eat! Am. J. Clin. Nutr. 2017, 106, 1171–1172. [Google Scholar] [CrossRef][Green Version]
- Erren, T.C.; Reiter, R.J. Defining chronodisruption. J. Pineal Res. 2009, 46, 245–247. [Google Scholar] [CrossRef] [PubMed]
- Neuhouser, M.L. The importance of healthy dietary patterns in chronic disease prevention. Nutr. Res. 2019, 70, 3–6. [Google Scholar] [CrossRef]
- Pot, G.K. Sleep and dietary habits in the urban environment: The role of chrono-nutrition. Proc. Nutr. Soc. 2018, 77, 189–198. [Google Scholar] [CrossRef]
- Yin, C.; Li, Z.; Xiang, Y.; Peng, H.; Yang, P.; Yuan, S.; Zhang, X.; Wu, Y.; Huang, M.; Li, J. Effect of Intermittent Fasting on Non-Alcoholic Fatty Liver Disease: Systematic Review and Meta-Analysis. Front. Nutr. 2021, 8, 709683. [Google Scholar] [CrossRef]
- McHill, A.W.; Melanson, E.L.; Higgins, J.; Connick, E.; Moehlman, T.M.; Stothard, E.R.; Wright, K.P. Impact of circadian misalignment on energy metabolism during simulated nightshift work. Proc. Natl. Acad. Sci. USA 2014, 111, 17302–17307. [Google Scholar] [CrossRef]
- Von Behren, J.; Hurley, S.; Goldberg, D.; Clague DeHart, J.; Wang, S.S.; Reynolds, P. Chronotype and Risk of Post-Menopausal Endometrial Cancer in the California Teachers Study. Chronobiol. Int. 2021, 38, 1151. [Google Scholar] [CrossRef] [PubMed]
- Baehr, E.K.; Revelle, W.; Eastman, C.I. Individual differences in the phase and amplitude of the human circadian temperature rhythm: With an emphasis on morningness-eveningness. J. Sleep Res. 2000, 9, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Roenneberg, T.; Kuehnle, T.; Juda, M.; Kantermann, T.; Allebrandt, K.; Gordijn, M.; Merrow, M. Epidemiology of the human circadian clock. Sleep Med. Rev. 2007, 11, 429–438. [Google Scholar] [CrossRef]
- Vetrani, C.; Barrea, L.; Verde, L.; Sarno, G.; Docimo, A.; de Alteriis, G.; Savastano, S.; Colao, A.; Muscogiuri, G. Evening chronotype is associated with severe NAFLD in obesity. Int. J. Obes. 2022, 46, 1638–1643. [Google Scholar] [CrossRef]
- Bazzani, A.; Marantonio, S.; Andreozzi, G.; Lorenzoni, V.; Bruno, S.; Cruz-Sanabria, F.; d’Ascanio, P.; Turchetti, G.; Faraguna, U. Late chronotypes, late mealtimes. Chrononutrition and sleep habits during the COVID-19 lockdown in Italy. Appetite 2022, 172, 105951. [Google Scholar] [CrossRef]
- Patterson, F.; Malone, S.K.; Lozano, A.; Grandner, M.A.; Hanlon, A.L. Smoking, Screen-Based Sedentary Behavior, and Diet Associated with Habitual Sleep Duration and Chronotype: Data from the UK Biobank. Ann. Behav. Med. 2016, 50, 715–726. [Google Scholar] [CrossRef] [PubMed]
- Reutrakul, S.; Hood, M.M.; Crowley, S.J.; Morgan, M.K.; Teodori, M.; Knutson, K.L. The relationship between breakfast skipping, chronotype, and glycemic control in type 2 diabetes. Chronobiol. Int. 2014, 31, 64–71. [Google Scholar] [CrossRef]
- Xie, J.; Huang, H.; Chen, Y.; Xu, L.; Xu, C. Skipping breakfast is associated with an increased long-term cardiovascular mortality in metabolic dysfunction-associated fatty liver disease (MAFLD) but not MAFLD-free individuals. Aliment. Pharmacol. Ther. 2022, 55, 212–224. [Google Scholar] [CrossRef]
- Romero-Gómez, M.; Zelber-Sagi, S.; Trenell, M. Treatment of NAFLD with diet, physical activity and exercise. J. Hepatol. 2017, 67, 829–846. [Google Scholar] [CrossRef]
- Basolo, A.; Bechi Genzano, S.; Piaggi, P.; Krakoff, J.; Santini, F. Energy Balance and Control of Body Weight: Possible Effects of Meal Timing and Circadian Rhythm Dysregulation. Nutrients 2021, 13, 3276. [Google Scholar] [CrossRef]
- Longo, V.D.; Panda, S. Fasting, Circadian Rhythms, and Time-Restricted Feeding in Healthy Lifespan. Cell Metab. 2016, 23, 1048–1059. [Google Scholar] [CrossRef] [PubMed]
- Patikorn, C.; Roubal, K.; Veettil, S.K.; Chandran, V.; Pham, T.; Lee, Y.Y.; Giovannucci, E.L.; Varady, K.A.; Chaiyakunapruk, N. Intermittent Fasting and Obesity-Related Health Outcomes: An Umbrella Review of Meta-analyses of Randomized Clinical Trials. JAMA Netw. Open 2021, 4, e2139558. [Google Scholar] [CrossRef] [PubMed]
- Lavallee, C.M.; Bruno, A.; Ma, C.; Raman, M. The Role of Intermittent Fasting in the Management of Nonalcoholic Fatty Liver Disease: A Narrative Review. Nutrients 2022, 14, 4655. [Google Scholar] [CrossRef] [PubMed]
- Pickel, L.; Sung, H.K. Feeding Rhythms and the Circadian Regulation of Metabolism. Front. Nutr. 2020, 7, 39. [Google Scholar] [CrossRef] [PubMed]
- Moore, M.P.; Cunningham, R.P.; Dashek, R.J.; Mucinski, J.M.; Rector, R.S. A fad too far? Dietary strategies for the prevention and treatment of NAFLD. Obesity 2020, 28, 1843. [Google Scholar] [CrossRef]
- Park, S.; Zhang, T.; Wu, X.; Yi Qiu, J. Ketone production by ketogenic diet and by intermittent fasting has different effects on the gut microbiota and disease progression in an Alzheimer’s disease rat model. J. Clin. Biochem. Nutr. 2020, 67, 188. [Google Scholar] [CrossRef]
- Cho, Y.; Hong, N.; Kim, K.W.; Cho, S.J.; Lee, M.; Lee, Y.H.; Lee, Y.H.; Kang, E.S.; Cha, B.S.; Lee, B.W. The Effectiveness of Intermittent Fasting to Reduce Body Mass Index and Glucose Metabolism: A Systematic Review and Meta-Analysis. J. Clin. Med. 2019, 8, 1645. [Google Scholar] [CrossRef]
- Harris, L.; Hamilton, S.; Azevedo, L.B.; Olajide, J.; De Brún, C.; Waller, G.; Whittaker, V.; Sharp, T.; Lean, M.; Hankey, C.; et al. Intermittent fasting interventions for treatment of overweight and obesity in adults: A systematic review and meta-analysis. JBI Database Syst. Rev. Implement. Rep. 2018, 16, 507–547. [Google Scholar] [CrossRef]
- Jamshed, H.; Beyl, R.A.; Manna, D.L.D.; Yang, E.S.; Ravussin, E.; Peterson, C.M. Early Time-Restricted Feeding Improves 24-Hour Glucose Levels and Affects Markers of the Circadian Clock, Aging, and Autophagy in Humans. Nutrients 2019, 11, 1234. [Google Scholar] [CrossRef]
- Peters, B.; Koppold-Liebscher, D.A.; Schuppelius, B.; Steckhan, N.; Pfeiffer, A.F.H.; Kramer, A.; Michalsen, A.; Pivovarova-Ramich, O. Effects of Early vs. Late Time-Restricted Eating on Cardiometabolic Health, Inflammation, and Sleep in Overweight and Obese Women: A Study Protocol for the ChronoFast Trial. Front. Nutr. 2021, 8, 897. [Google Scholar] [CrossRef]
- Cienfuegos, S.; Gabel, K.; Kalam, F.; Ezpeleta, M.; Wiseman, E.; Pavlou, V.; Lin, S.; Oliveira, M.L.; Varady, K.A. Effects of 4- and 6-h Time-Restricted Feeding on Weight and Cardiometabolic Health: A Randomized Controlled Trial in Adults with Obesity. Cell Metab. 2020, 32, 366–378.e3. [Google Scholar] [CrossRef] [PubMed]
- Chaix, A.; Zarrinpar, A.; Miu, P.; Panda, S. Time-restricted feeding is a preventative and therapeutic intervention against diverse nutritional challenges. Cell Metab. 2014, 20, 991–1005. [Google Scholar] [CrossRef] [PubMed]
- Varkaneh, H.K.; Salehisahlabadi, A.; Tinsley, G.M.; Santos, H.O.; Hekmatdoost, A. Effects of Time-Restricted Feeding (16/8) Combined with a Low-Sugar Diet on the Management of Nonalcoholic Fatty Liver Disease: A Randomized Controlled Trial. Nutrition 2022, 105, 111847. [Google Scholar] [CrossRef] [PubMed]
- Hutchison, A.T.; Regmi, P.; Manoogian, E.N.C.; Fleischer, J.G.; Wittert, G.A.; Panda, S.; Heilbronn, L.K. Time-Restricted Feeding Improves Glucose Tolerance in Men at Risk for Type 2 Diabetes: A Randomized Crossover Trial. Obesity 2019, 27, 724–732. [Google Scholar] [CrossRef]
- Mindikoglu, A.L.; Opekun, A.R.; Gagan, S.K.; Devaraj, S. Impact of Time-Restricted Feeding and Dawn-to-Sunset Fasting on Circadian Rhythm, Obesity, Metabolic Syndrome, and Nonalcoholic Fatty Liver Disease. Gastroenterol. Res. Pract. 2017, 2017, 3932491. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perez-Diaz-del-Campo, N.; Castelnuovo, G.; Caviglia, G.P.; Armandi, A.; Rosso, C.; Bugianesi, E. Role of Circadian Clock on the Pathogenesis and Lifestyle Management in Non-Alcoholic Fatty Liver Disease. Nutrients 2022, 14, 5053. https://doi.org/10.3390/nu14235053
Perez-Diaz-del-Campo N, Castelnuovo G, Caviglia GP, Armandi A, Rosso C, Bugianesi E. Role of Circadian Clock on the Pathogenesis and Lifestyle Management in Non-Alcoholic Fatty Liver Disease. Nutrients. 2022; 14(23):5053. https://doi.org/10.3390/nu14235053
Chicago/Turabian StylePerez-Diaz-del-Campo, Nuria, Gabriele Castelnuovo, Gian Paolo Caviglia, Angelo Armandi, Chiara Rosso, and Elisabetta Bugianesi. 2022. "Role of Circadian Clock on the Pathogenesis and Lifestyle Management in Non-Alcoholic Fatty Liver Disease" Nutrients 14, no. 23: 5053. https://doi.org/10.3390/nu14235053
APA StylePerez-Diaz-del-Campo, N., Castelnuovo, G., Caviglia, G. P., Armandi, A., Rosso, C., & Bugianesi, E. (2022). Role of Circadian Clock on the Pathogenesis and Lifestyle Management in Non-Alcoholic Fatty Liver Disease. Nutrients, 14(23), 5053. https://doi.org/10.3390/nu14235053






