Association between Dietary Habit and Clinical Parameters in Patients with Chronic Periodontitis Undergoing Supportive Periodontal Therapy
Abstract
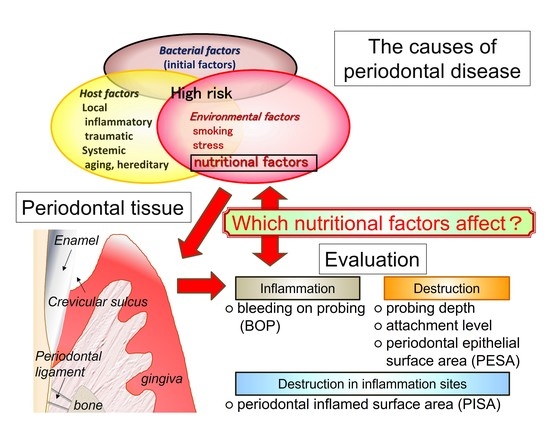
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Periodontal Examinations
2.3. Lifestyle Questionnaire
2.4. Analysis of the Relationship between Clinical Parameters and Environmental and Nutritional Factors
2.5. Quantitative Polymerase Chain Reaction (PCR)
2.6. Statistical Analysis
3. Results
3.1. Profiling for the Periodontal Examinations and Questionnaire
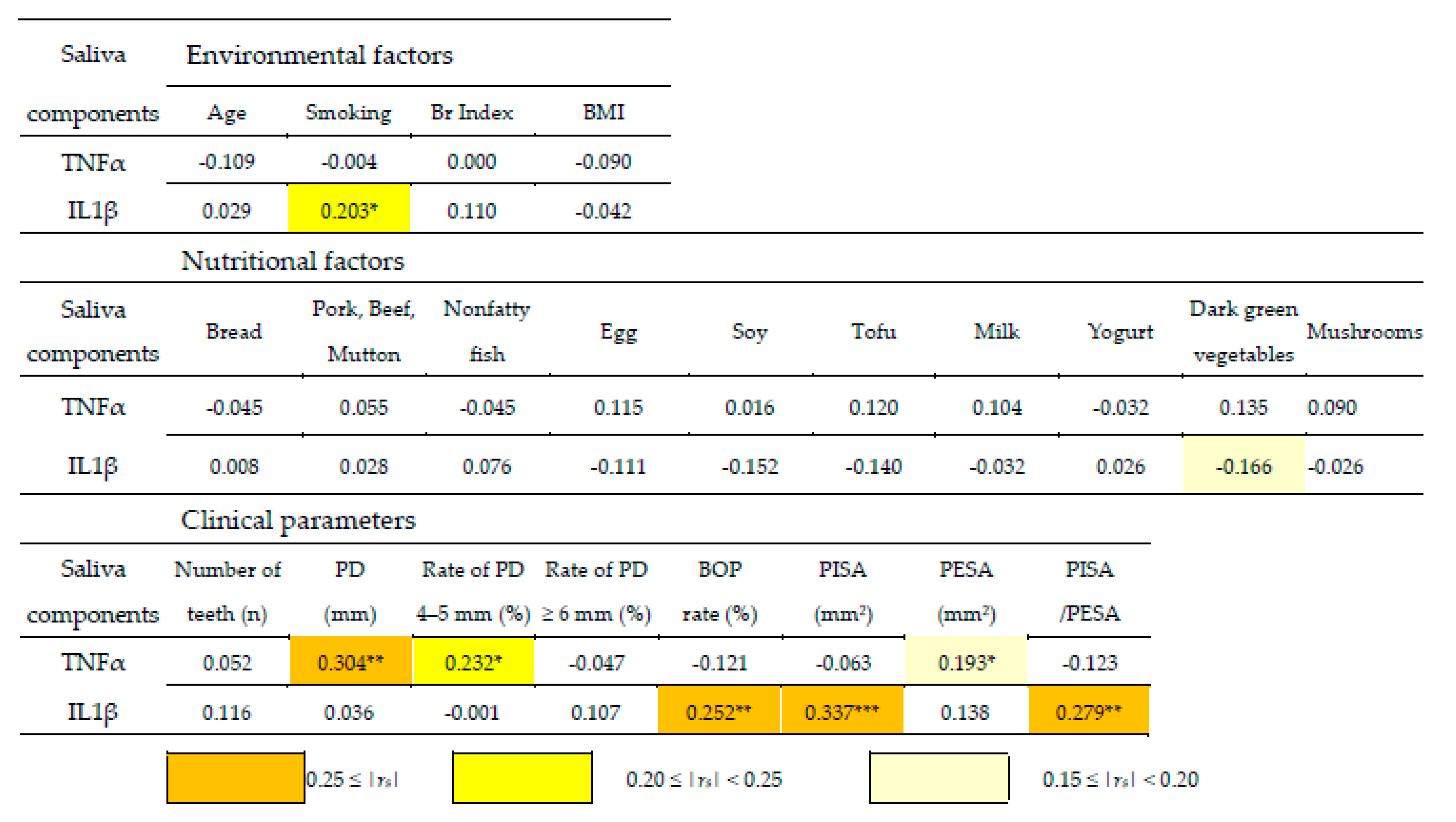
3.2. Correlation Coefficient Matrix between Clinical Parameters and Environmental and Nutritional Factors
3.3. Multiple Linear Regression Analysis
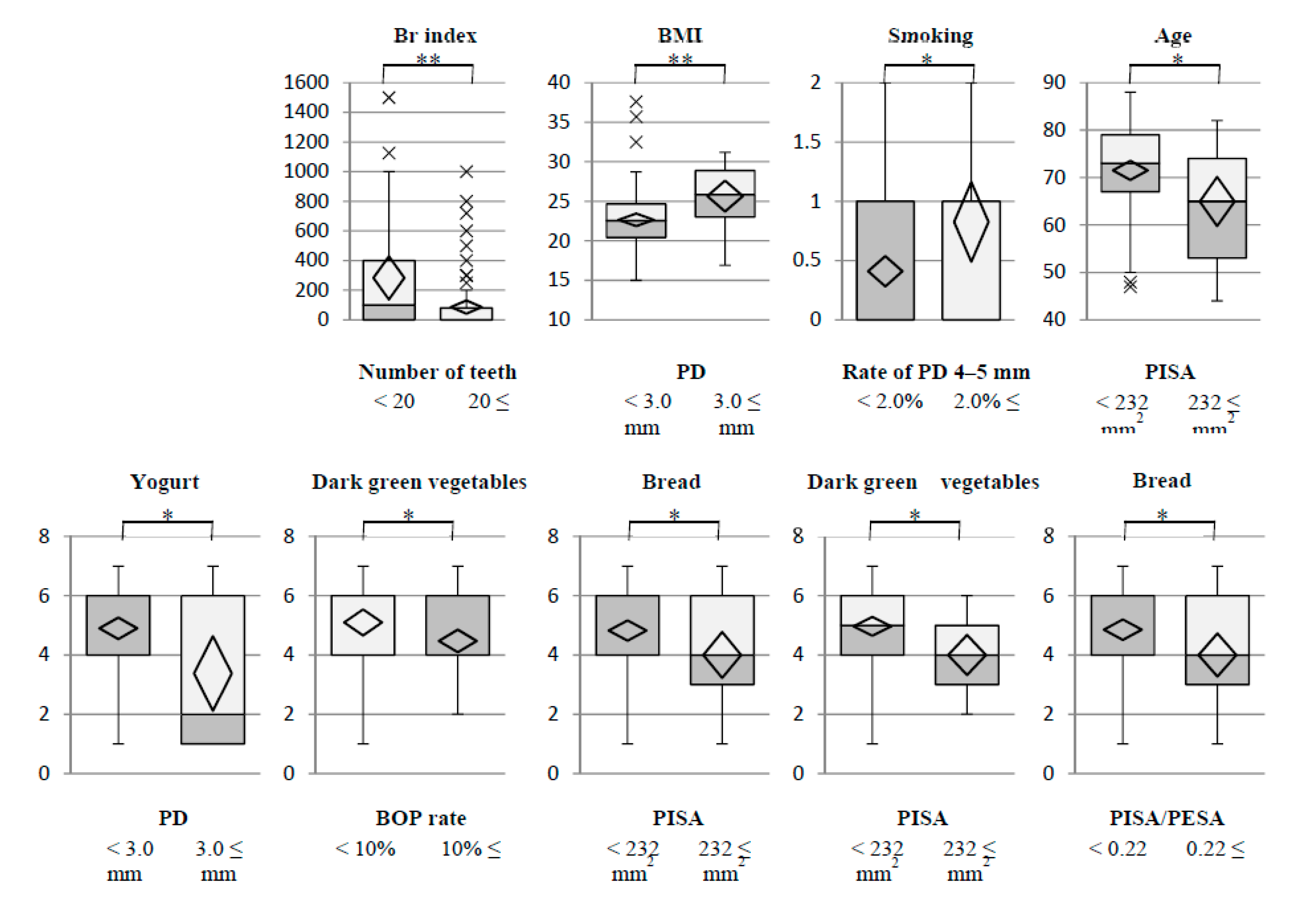
3.4. Stratified Descriptive Statistics between Clinical Parameters and Environmental and Nutritional Factors
3.5. Stratified Descriptive Statistics between Clinical Parameters and Environmental and Nutritional Factors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Orlandi, M.; Muñoz, A.E.; Marletta, D.; Petrie, A.; Suvan, J.; D’Aiuto, F. Impact of the treatment of periodontitis on systemic health and quality of life: A systematic review. J. Clin. Periodontol. 2022, 49 (Suppl. 24), 314–327. [Google Scholar] [CrossRef] [PubMed]
- Desai, R.; Khobaragade, B.; McCracken, G.; Wassall, R.; Taylor, J.J.; Bissett, S.M.; Pumerantz, A.S.; Preshaw, P.M. Impact of diabetes and periodontal status on life quality. BDJ Open 2021, 7, 9. [Google Scholar] [CrossRef] [PubMed]
- Han, P.S.H.; Saub, R.; Baharuddin, N.A.; Sockalingam, S.; Bartold, P.M.; Vaithilingam, R.D. Impact of periodontitis on quality of life among subjects with rheumatoid arthritis: A cross sectional study. BMC Oral Health 2020, 20, 332. [Google Scholar] [CrossRef] [PubMed]
- Gharehghani, M.A.M.; Bayani, A.; Bayat, A.H.; Hemmat, M.; Karimy, M.; Ahounbar, E.; Armoon, B.; Fakhri, Y.; Schroth, R.J. Poor oral health-related quality of life among pregnant women: A systematic review and meta-analysis. Int. J. Dent. Hyg. 2021, 1, 39–49. [Google Scholar] [CrossRef]
- Liang, Y.H.; Chou, C.; Chen, Y.J.; Chou, Y.F.; Lin, C.Y.; Chou, C.; Wang, T.F. Impact of periodontal disease and chewing ability on the quality of life of the elderly in an affluent community. J. Formos. Med. Assoc. 2020, 119, 1693–1701. [Google Scholar] [CrossRef] [PubMed]
- Stahl, S.S. Host resistance and periodontal disease. J. Dent. Res. 1970, 49, 248–255. [Google Scholar] [CrossRef]
- Mergenhagen, S.E.; Tempel, T.R.; Snyderman, R. Immunologic reactions and periodontal inflammation. J. Dent. Res. 1970, 49, 256–261. [Google Scholar] [CrossRef]
- Woods, J.R., Jr.; Dandavino, A.; Murayama, K.; Brinkman, C.R., 3rd; Assali, N.S. Autonomic control of cardiovascular functions during neonatal development and in adult sheep. Circ. Res. 1977, 40, 401–407. [Google Scholar] [CrossRef]
- Alberti, K.; Zimmet, P.; Shaw, J. Metabolic syndrome—A new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet. Med. 2006, 23, 469–480. [Google Scholar] [CrossRef]
- Tonetti, M.S.; Greenwell, H.; Kornman, K.S. Staging and grading of periodontitis: Framework and proposal of a new classification and case definition. J. Periodontol. 2018, 89 (Suppl. 1), S159–S172. [Google Scholar] [CrossRef]
- Groeger, S.; Jarzina, F.; Windhorst, A.; Meyle, J. Influence of retinoic acid on human gingival epithelial barriers. J. Periodontal. Res. 2016, 51, 748–757. [Google Scholar] [CrossRef] [PubMed]
- Nanayakkara, V.; Ekanayake, L.; Silva, R. Dietary intake of calcium, vitamins A and E and bleeding on probing in Sri Lankan preschoolers. Community Dent. Health 2014, 31, 153–157. [Google Scholar] [PubMed]
- Saito, T.; Shimazaki, Y.; Sakamoto, M. Obesity and periodontitis. N. Engl. J. Med. 1998, 339, 482–483. [Google Scholar] [CrossRef] [PubMed]
- Lang, N.P.; Tonetti, M.S. Periodontal risk assessment (PRA) for patients in supportive periodontal therapy (SPT). Oral Health Prev. Dent. 2003, 1, 7–16. [Google Scholar] [PubMed]
- Li, W.; Song, J.; Chen, Z. The association between dietary vitamin C intake and periodontitis: Result from the NHANES (2009–2014). BMC Oral Health 2022, 22, 390. [Google Scholar] [CrossRef]
- Naruishi, K. Carotenoids and Periodontal Infection. Nutrients 2020, 12, 269. [Google Scholar] [CrossRef]
- Nesse, W.; Abbas, F.; van der Ploeg, I.; Spijkervet, F.K.; Dijkstra, P.U.; Vissink, A. Periodontal inflamed surface area: Quantifying inflammatory burden. J. Clin. Periodontol. 2008, 35, 668–673. [Google Scholar] [CrossRef]
- El, O.N.; Pichot, H.; Soulier-Peigue, D.; Hennequin, M.; Tubert-Jeannin, S. Validation of the child oral health impact profile (COHIP) french questionnaire among 12 years-old children in New Caledonia. Health Qual Life Outcomes 2015, 13, 176. [Google Scholar]
- Zhu, H.; Zhou, H.; Qin, Q.; Zhang, W. Association between Smoking and Sugar-Sweetened Beverage Consumption, Tooth Brushing among Adolescents in China. Children 2022, 9, 1008. [Google Scholar] [CrossRef]
- Chmielik, L.P.; Mielnik-Niedzielska, G.; Kasprzyk, A.; Stankiewicz, T.; Niedzielski, A. Health-Related Quality of Life Assessed in Children with Chronic Rhinitis and Sinusitis. Children 2021, 8, 1133. [Google Scholar] [CrossRef]
- Lang, N.P.; Adler, R.; Joss, A.; Nyman, S. Absence of bleeding on probing. An indicator of periodontal stability. J. Clin. Periodontol. 1990, 17, 714–721. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, T.J.; Drake, R.B.; Naylor, J.E. The plaque control record. J. Periodontol. 1972, 43, 38. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, Y.; Matsuda, H.; Itoh, S.; Iwai, Y.; Takai, H.; Mezawa, M.; Yoshino, S.; Ogata, Y. Impact of adjunctive procedures on recombinant human fibroblast growth factor-2-mediated periodontal regeneration therapy: A retrospective study. J. Periodontol. 2021, 92, 983–994. [Google Scholar] [CrossRef] [PubMed]
- Eichner, K. Renewed examination of the group classification of partially edentulous arches by Eichner and application advice for studies on morbidity statistics. Stomatologie 1990, 40, 321–325. [Google Scholar]
- Faizuddin, M.; Bharathi, S.H.; Rohini, N.V. Estimation of interleukin1beta levels in the gingival crevicular fluid in health and in infammatory periodontal disease. J. Periodontal. Res. 2003, 38, 111–114. [Google Scholar] [CrossRef]
- Ulker, A.E.; Tulunoglu, O.; Ozmeric, N.; Can, M.; Demirtas, S. The evaluation of cystatin C, IL-1beta, and TNF-alpha levels in total saliva and gingival crevicular fluid from 11- to 16-year-old children. J. Periodontol. 2008, 79, 854–860. [Google Scholar] [CrossRef]
- Nakayama, Y.; Inoue, E.; Kato, A.; Iwai, Y.; Takai-Yamazaki, M.; Tsuruya, Y.; Yamaguchi, A.; Noda, K.; Nomoto, T.; Ganss, B.; et al. Follicular dendritic cell-secreted protein gene expression is upregulated and spread in nifedipine-induced gingival overgrowth. Odontology 2020, 108, 532–544. [Google Scholar] [CrossRef]
- Inoue, Y.; Hatanaka, K.; Amamoto, T.; Hirata, T.; Minabe, M.; Yamamoto, T.; Naito, T.; Yamamoto, M.; Sato, S.; Ishihata, H.; et al. Reference values of periodontal inflamed surface area as a clinical index determined by a multicenter retrospective observational study. J. Jpn. Soc. Periodontol. 2019, 61, 159–167. [Google Scholar] [CrossRef]
- Asgari, M.M.; Brasky, T.M.; White, E. Association of vitamin A and carotenoid intake with melanoma risk in a large prospective cohort. J. Investig. Dermatol. 2012, 132, 1573–1582. [Google Scholar] [CrossRef]
- Li, X.Y.; Wen, M.Z.; Liu, H.; Shen, Y.C.; Su, L.X.; Yang, X.T. Dietary magnesium intake is protective in patients with periodontitis. Front. Nutr. 2022, 9, 976518. [Google Scholar] [CrossRef]
- Rivera-Hidalgo, F. Smoking and periodontal disease. Periodontol. 2000 2003, 32, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Johnson, G.K.; Hill, M. Cigarette smoking and the periodontal patient. J. Periodontol. 2004, 75, 196–209. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, K.; Saito, I.; Maruyama, K.; Eguchi, E.; Mori, H.; Tanno, S.; Sakurai, S.; Kishida, T.; Nishida, W.; Osawa, H.; et al. Associations of serum β-carotene and retinol concentrations with insulin resistance: The Toon Health Study. Nutrition 2015, 7-8, 975–980. [Google Scholar] [CrossRef] [PubMed]
- Amengual, J.; Gouranton, E.; van Helden, Y.G.; Hessel, S.; Ribot, J.; Kramer, E.; Kiec-Wilk, B.; Razny, U.; Lietz, G.; Wyss, A.; et al. Beta-carotene reduces body adiposity of mice via BCMO1. PLoS ONE 2011, 6, e20644. [Google Scholar] [CrossRef]
- Ebersole, J.L.; Lambert, J.; Bush, H.; Huja, P.E.; Basu, A. Serum Nutrient Levels and Aging Effects on Periodontitis. Nutrients 2018, 10, 1986. [Google Scholar] [CrossRef]
- Belludi, S.A.; Verma, S.; Banthia, R.; Bhusari, P.; Parwani, S.; Kedia, S.; Saiprasad, S.V. Effect of lycopene in the treatment of periodontal disease: A clinical study. J. Contemp. Dent. Pract. 2013, 14, 1054–1059. [Google Scholar] [CrossRef]
- Arora, N.; Avula, H.; Avula, J.K. The adjunctive use of systemic antioxidant therapy (lycopene) in nonsurgical treatment of chronic periodontitis: A short-term evaluation. Quintessence Int. 2013, 44, 395–405. [Google Scholar] [PubMed]
- Chandra, R.V.; Sandhya, Y.P.; Nagarajan, S.; Reddy, B.H.; Naveen, A.; Murthy, K.R. Efficacy of lycopene as a locally delivered gel in the treatment of chronic periodontitis: Smokers vs nonsmokers. Quintessence Int. 2012, 43, 401–411. [Google Scholar]
- Kose, O.; Arabaci, T.; Yemenoglu, H.; Kara, A.; Ozkanlar, S.; Kayis, S.; Duymus, Z.Y. Influences of Fucoxanthin on Alveolar Bone Resorption in Induced Periodontitis in Rat Molars. Mar. Drugs 2016, 14, 70. [Google Scholar] [CrossRef]
- Balci, Y.H.; Lektemur, A.A.; Gevrek, F.; Toker, H. Investigation of the effect of astaxanthin on alveolar bone loss in expemental periodontitis. J. Periodontal. Res. 2018, 53, 131–138. [Google Scholar] [CrossRef]
- Wang, L.; Wang, J.; Jin, Y.; Gao, H.; Lin, X. Oral administration of all-trans retinoic acid suppresses experimental periodontitis by modulating the Th17/Treg imbalance. J. Periodontol. 2014, 85, 740–750. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, R.; Hashizume-Takizawa, T.; Kurita-Ochiai, T. Lactic acid bacteria prevent both periodontitis and atherosclerosis exacerbated by periodontitis in spontaneously hyperlipidemic mice. J. Periodontal. Res. 2021, 56, 753–760. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, R.; Kobayashi, T.; Sakai, F.; Hosoya, T.; Yamamoto, M.; Kurita-Ochiai, T. Oral administration of Lactobacillus gasseri SBT2055 is effective in preventing Porphyromonas gingivalis-accelerated periodontal disease. Sci. Rep. 2017, 7, 545. [Google Scholar] [CrossRef] [PubMed]

| Environmental Factors | ||||||||
|---|---|---|---|---|---|---|---|---|
| Multiple Regression Analysis 1 | ||||||||
| Clinical Parameters | Correlation Coefficient |rs| > 0.15 | * p < 0.05 ** p < 0.01 | Standardized Partial Regression Coefficient | Collinearity Statistics | R R2 Regression Variation | DW | MAE | |
| Tolerance | VIF | |||||||
| Number of teeth | •Age •Smoking •Br index | •Age ** •Smoking •Br index * •Constant term ** | −0.2811 −0.2045 −0.2354 - | 0.9764 0.6567 0.6586 | 1.0241 1.5228 1.5185 | 0.4800 0.2304 p < 0.001 | 2.035 | 3.9 |
| PD (mm) | •Smoking •BMI | •Smoking * •BMI * •Constant term ** | -0.1960 -0.2177 - | 0.9879 0.9879 | 1.0123 1.0123 | 0.3085 0.0952 p < 0.01 | 1.243 | 0.3 |
| Rate of PD of 4–5 mm (%) | •Smoking •BMI | •Smoking * •BMI * •Constant term ** | 0.2196 -0.2156 - | 0.9879 0.9879 | 1.0123 1.0123 | 0.3242 0.1051 p < 0.01 | 1.360 | 3.2 |
| Rate of PD ≥6 mm (%) | •BMI | •BMI •Constant term | 0.1835 - | 1.0000 | 1.0000 | 0.1835 0.0337 p = 0.06 | 1.644 | 1.5 |
| Rate of BOP (%) | N/A | N/A | - | - | - | - | - | - |
| PISA (mm2) | •Age •Br index | •Age * •Br index •Constant term ** | −0.1959 −0.1576 - | 0.9963 0.9963 | 1.0037 1.0037 | 0.2588 0.0670 p < 0.05 | 1.907 | 91.2 |
| PESA (mm2) | •Age •Smoking •Br index | •Age * •Br index ** •Constant term ** | −0.2290 −0.2831 - | 0.9963 0.9963 | 1.0037 1.0037 | 0.3748 0.1450 p < 0.001 | 2.281 | 237.1 |
| PISA/PESA | N/A | N/A | - | - | - | - | - | - |
| Environmental Factors | ||||||||
|---|---|---|---|---|---|---|---|---|
| Multiple Regression Analysis 1 | ||||||||
| Clinical Parameters | Correlation Coefficient |rs| > 0.15 | * p < 0.05 ** p < 0.01 | Standardized Partial Regression Coefficient | Collinearity Statistics | R R2 Regression Variation | DW | MAE | |
| Tolerance | VIF | |||||||
| Number of teeth | •Pork, beef, mutton | •Pork, beef, mutton •Constant term ** | 0.1806 - | 1.0000 | 1.0000 | 0.1806 0.0326 p = 0.06 | 1.976 | 4.6 |
| PD (mm) | •Bread soy •Nonfatty fish •Yogurt •Dark green vegetables | •Bread •Nonfatty fish * •Yogurt * •Constant term ** | −0.1740 −0.1847 −0.2288 - | 0.9889 0.9902 0.9986 | 1.0113 1.0099 1.0014 | 0.3544 0.1256 p < 0.01 | 1.364 | 0.3 |
| Rate of PD of 4–5 mm (%) | •Bread •Pork, beef, mutton •Nonfatty fish •Yogurt | •Pork, beef, mutton •Nonfatty fish * •Yogurt ** •Constant term ** | −0.1342 −0.1919 −0.2457 - | 0.9361 0.9445 0.9904 | 1.0683 1.0588 1.0097 | 0.3647 0.1330 p < 0.01 | 1.453 | 3.4 |
| Rate of PD ≥6 mm (%) | •Pork, beef, mutton •Egg soy •Tofu •Dark green vegetables •Mushrooms | •Tofu •Dark green vegetables •Constant term ** | −0.1620 −0.1600 - | 0.8528 0.8528 | 1.1726 1.1726 | 0.2678 0.0717 p < 0.05 | 1.782 | 1.5 |
| BOP rate (%) | •Bread soy •Milk yogurt •Dark green vegetables •Mushrooms | •Bread •Milk ** •Dark green vegetables * •Constant term ** | −0.1670 −0.2413 −0.1672 - | 0.9939 0.9877 0.9935 | 1.0061 1.0124 1.0066 | 0.3572 0.1276 p < 0.05 | 1.627 | 6.5 |
| PISA (mm2) | •Bread •Soy tofu •Milk •Dark green vegetables •Mushrooms | •Bread * •Milk •Dark green vegetables * •Constant term ** | −0.1950 −0.1620 −0.2110 - | 0.9939 0.9877 0.9935 | 1.0061 1.0124 1.0066 | 0.3470 0.1204 p < 0.01 | 2.020 | 87.9 |
| PESA (mm2) | •Pork, beef, mutton | •Pork, beef, mutton •Constant term ** | 0.1550 - | 1.0000 | 1.0000 | 0.1550 0.0240 p = 0.11 | 2.141 | 255.4 |
| PISA/PESA | •Bread •Egg soy •Milk yogurt •Dark green vegetables •Mushrooms | •Bread * •Milk * •Dark green vegetables •Constant term ** | −0.2061 −0.2305 −0.0061 - | 0.9939 0.9877 0.9935 | 1.0061 1.0124 1.0066 | 0.3720 0.1384 p < 0.01 | 1.701 | 0.074 |
| Environmental Factors | ||||||||
|---|---|---|---|---|---|---|---|---|
| Clinical Parameters | Multiple Regression Analysis Explanatory Variables | Stratified Descriptive Statistics | Testing of Differences of Population Mean | |||||
| Stratified Standard of Response Variables | Numbers | Mean ± SD of Explanatory Variables | Correlation Ratio (η2) * p < 0.05 ** p < 0.01 | Hypothesis Testing for the Homogeneity of Variances | Methods | p Value Statistical Power | ||
| Number of teeth |
| <20 20≤ | 31 75 | 72.7 ± 7.52 69.2 ± 10.83 | 0.0249 | p < 0.05 | t-test | p = 0.10 0.3650 |
| 282.71 ± 396.86 85.800 ± 194.24 | 0.1016 ** | p < 0.001 | t-test | p < 0.001 ** 0.9248 | |||
| PD (mm) |
| <3.0 3.0≤ | 16 90 | 0.456 ± 0.621 0.750 ± 0.775 | 0.0265 | p = 0.21 | Welch’s t-test | p = 0.17 0.2769 |
| 22.7 ± 3.81 25.7 ± 3.68 | 0.0759 ** | p = 0.94 | Welch’s t-test | p < 0.01 * 0.8150 | |||
| Rate of PD of 4–5 mm (%) |
| <2.0 2.0≤ | 41 65 | 0.369 ± 0.575 0.707 ± 0.716 | 0.0646 ** | p = 0.12 | Welch’s t-test | p < 0.05 * 0.7109 |
| 22.8 ± 4.016 23.6 ± 3.767 | 0.0091 | p = 0.67 | Welch’s t-test | p = 0.32 0.1660 | |||
| PISA (mm2) |
| <232 232≤ | 85 21 | 71.5 ± 9.35 65.0 ± 11.4 | 0.0681 ** | p = 0.22 | Welch’s t-test | p < 0.05 * 0.6550 |
| 153.99 ± 299.1 100.48 ± 200.8 | 0.0058 | p < 0.05 | t-test | p = 0.33 0.1606 | |||
| PESA (mm2) |
| <1026 1026≤ | 75 31 | 71.2 ± 9.77 67.9 ± 10.6 | 0.0220 | p = 0.58 | Welch’s t-test | p = 0.14 0.3065 |
| 163.52 ± 301.8 94.68 ± 225.40 | 0.0124 | p = 0.08 | Welch’s t-test | p = 0.20 0.2464 | |||
| Nutritional Factors | ||||||||
|---|---|---|---|---|---|---|---|---|
| Clinical parameters | Multiple Regression Analysis Explanatory Variables | Stratified Descriptive Statistics | Testing of Differences of Population Mean | |||||
| Stratified Standard of Response Variables | Numbers | Mean ± SD of Explanatory Variables | Correlation Ratio (η2) * p < 0.05 ** p < 0.01 | Hypothesis Testing for the Homogeneity of Variances | Methods | p Value Statistical Power | ||
| PD (mm) |
| <3.0 3.0≤ | 31 75 | 3.26 ± 1.11 2.69 ± 1.14 | 0.0330 | p = 0.81 | Welch’s t-test | p = 0.08 0.4207 |
| 4.90 ± 1.74 3.38 ± 2.36 | 0.0824 ** | p = 0.09 | Welch’s t-test | p < 0.05 * 0.6454 | |||
| Rate of PD of 4–5 mm (%) |
| <2.0 2.0≤ | 16 90 | 3.19 ± 1.07 3.15 ± 1.22 | 0.0003 | p = 0.37 | Welch’s t-test | p = 0.87 0.0530 |
| 4.94 ± 1.66 4.24 ± 2.21 | 0.0316 | p = 0.94 | t-test | p = 0.07 0.4468 | |||
| BOP rate (%) |
| <10.0 10.0≤ | 51 55 | 4.67 ± 2.02 4.01 ±2.09 | 0.0211 | p = 0.81 | Welch’s t-test | p = 0.13 0.3309 |
| 5.10 ± 1.57 4.47 ± 1.41 | 0.0430 * | p = 0.46 | Welch’s t-test | p < 0.05 * 0.5687 | |||
| PISA (mm2) |
| <232 232≤ | 85 21 | 4.82 ± 1.59 4.00 ± 1.70 | 0.0405 * | p = 0.64 | Welch’s t-test | p < 0.05 * 0.5465 |
| 4.97 ± 1.47 4.00 ± 1.48 | 0.0651 ** | p = 0.89 | Welch’s t-test | p < 0.05 * 0.7355 | |||
| PISA/PESA |
| <0.22 0.22≤ | 82 24 | 4.85 ± 1.57 4.00 ± 1.72 | 0.0480 * | p = 0.55 | Welch’s t-test | p < 0.05 * 0.5636 |
| 4.45 ± 2.03 4.00 ± 2.21 | 0.0084 | p = 0.56 | Welch’s t-test | p = 0.38 0.1408 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tabe, S.; Nakayama, Y.; Kobayashi, R.; Oyama, K.; Kitano, D.; Ogihara, J.; Senpuku, H.; Ogata, Y. Association between Dietary Habit and Clinical Parameters in Patients with Chronic Periodontitis Undergoing Supportive Periodontal Therapy. Nutrients 2022, 14, 4993. https://doi.org/10.3390/nu14234993
Tabe S, Nakayama Y, Kobayashi R, Oyama K, Kitano D, Ogihara J, Senpuku H, Ogata Y. Association between Dietary Habit and Clinical Parameters in Patients with Chronic Periodontitis Undergoing Supportive Periodontal Therapy. Nutrients. 2022; 14(23):4993. https://doi.org/10.3390/nu14234993
Chicago/Turabian StyleTabe, Shinichi, Yohei Nakayama, Ryoki Kobayashi, Kstsunori Oyama, Daisuke Kitano, Jun Ogihara, Hidenobu Senpuku, and Yorimasa Ogata. 2022. "Association between Dietary Habit and Clinical Parameters in Patients with Chronic Periodontitis Undergoing Supportive Periodontal Therapy" Nutrients 14, no. 23: 4993. https://doi.org/10.3390/nu14234993
APA StyleTabe, S., Nakayama, Y., Kobayashi, R., Oyama, K., Kitano, D., Ogihara, J., Senpuku, H., & Ogata, Y. (2022). Association between Dietary Habit and Clinical Parameters in Patients with Chronic Periodontitis Undergoing Supportive Periodontal Therapy. Nutrients, 14(23), 4993. https://doi.org/10.3390/nu14234993