Effect of a Bifidobacterium-Containing Acid-Resistant Microcapsule Formulation on Gut Microbiota: A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Characteristics of the Acid-Resistant Microcapsule
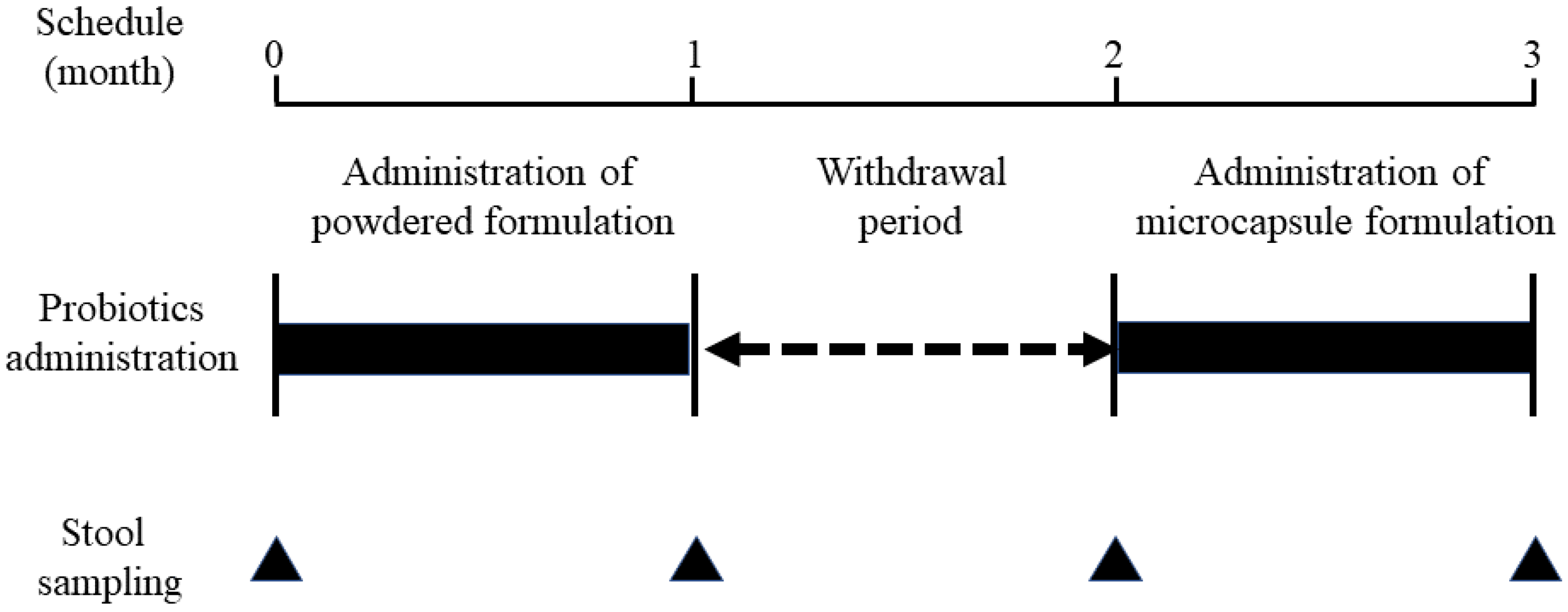
2.3. Evaluation of Gut Microbiota before and after the Oral Administration of Bifidobacteria-Containing Products
2.4. Analysis of Gut Microbiota
2.5. Determination of the 16S rRNA Sequence
2.6. Quantitative Measurement of B. breve in Stools Using PCR
2.7. Statistical Analysis
3. Results
3.1. Characteristics of the Study Participants
3.2. Results of Gut Microbiota Analysis
3.2.1. Taxonomic Composition
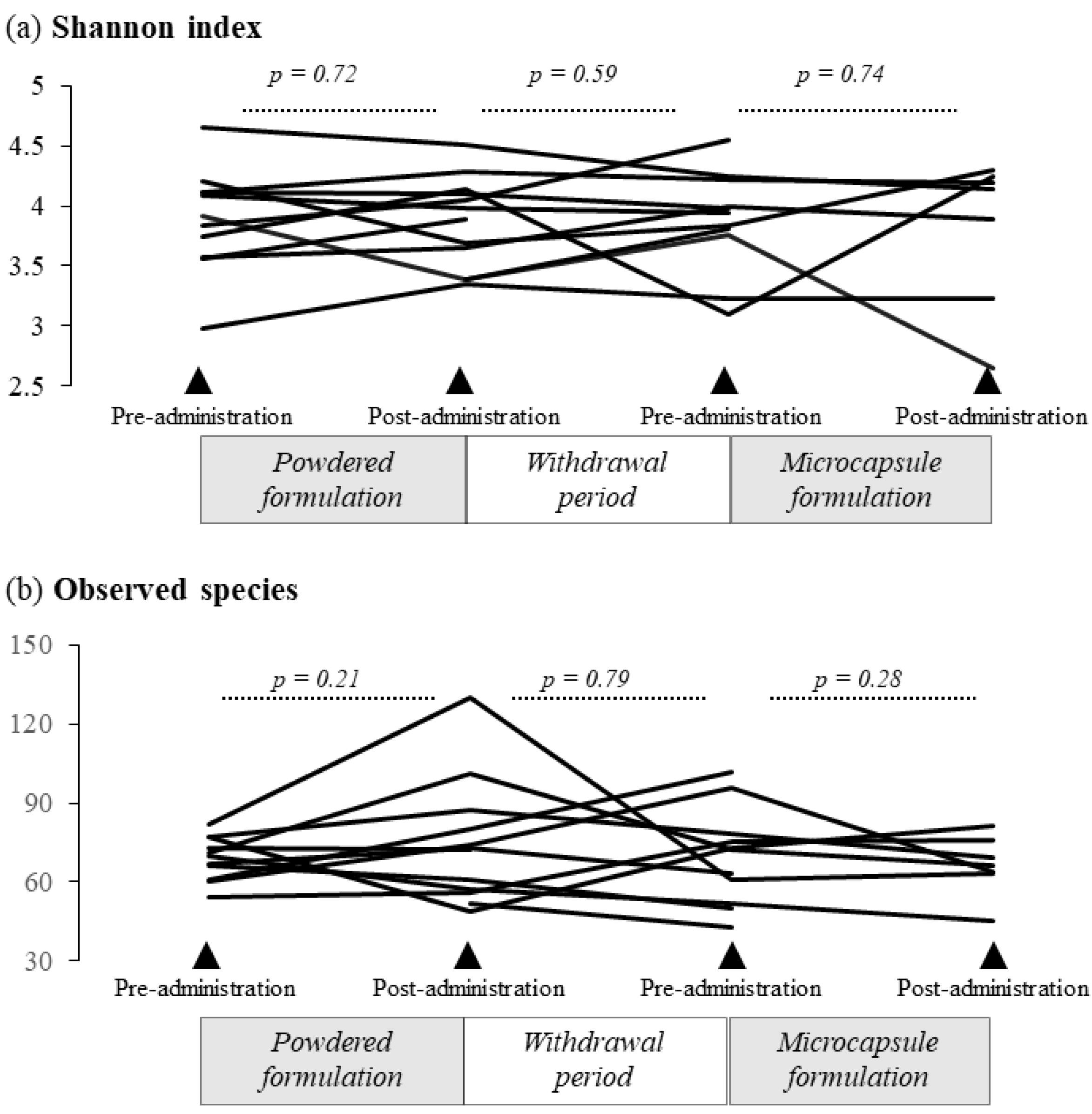
3.2.2. Alpha Diversity
3.2.3. Relative Abundance of B. longum and B. breve in the Gut Microbiota
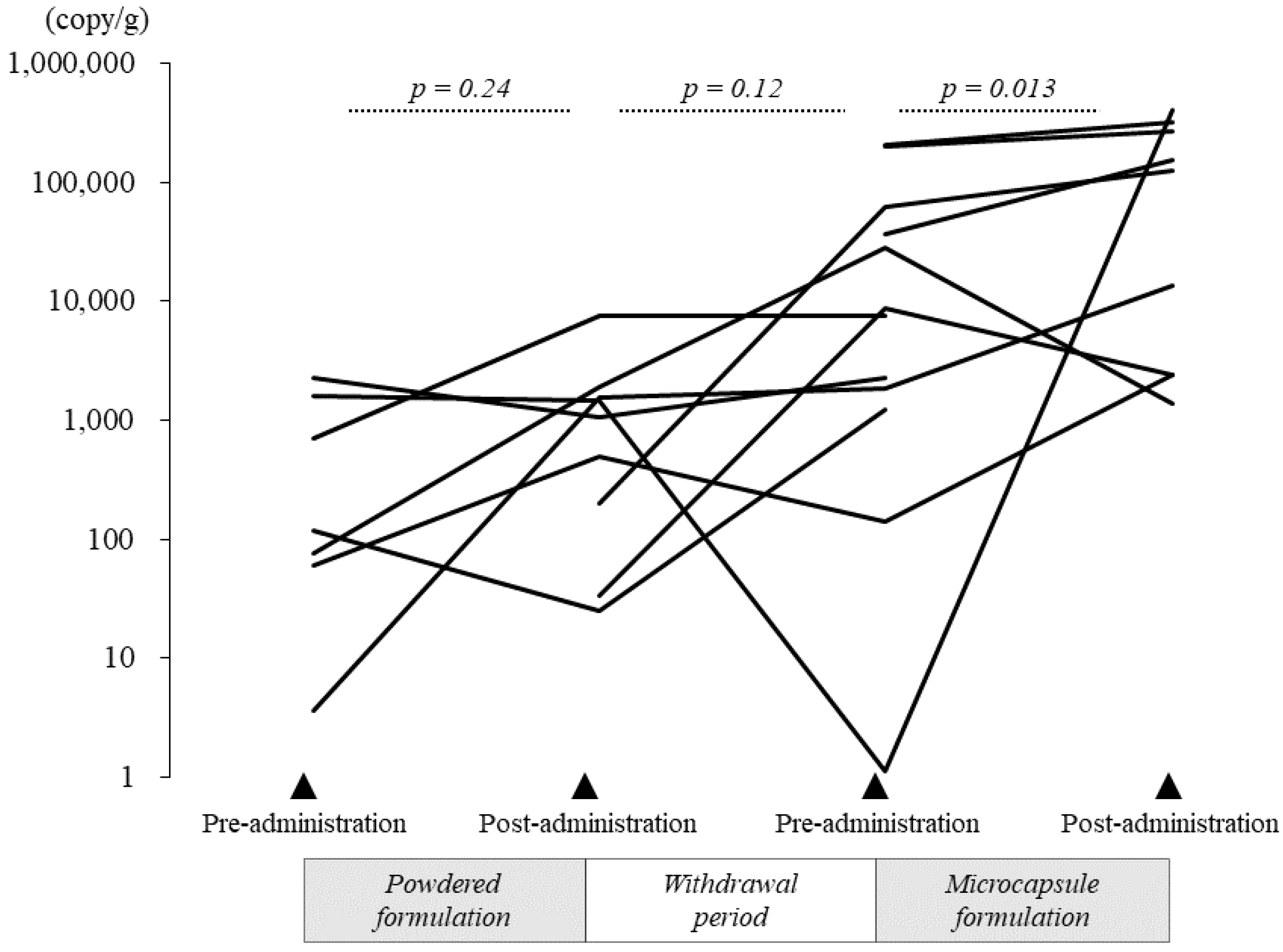
3.2.4. Quantification of B. breve
3.2.5. Comparison of the Colon Delivery Efficiency of the Powder and Microcapsule Formulations of B. breve
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sender, R.; Fuchs, S.; Milo, R. Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLoS Biol. 2016, 14, e1002533. [Google Scholar] [CrossRef]
- Odamaki, T.; Kato, K.; Sugahara, H.; Hashikura, N.; Takahashi, S.; Xiao, J.Z.; Abe, F.; Osawa, R. Age-related changes in gut microbiota composition from newborn to centenarian: A cross-sectional study. BMC Microbiol. 2016, 16, 90. [Google Scholar] [CrossRef] [PubMed]
- Young, R.B.; Marcelino, V.R.; Chonwerawong, M.; Gulliver, E.L.; Forster, S.C. Key Technologies for Progressing Discovery of Microbiome-Based Medicines. Front. Microbiol. 2021, 12, 685935. [Google Scholar] [CrossRef] [PubMed]
- Yatsunenko, T.; Rey, F.E.; Manary, M.J.; Trehan, I.; Dominguez-Bello, M.G.; Contreras, M.; Magris, M.; Hidalgo, G.; Baldassano, R.N.; Anokhin, A.P.; et al. Human gut microbiome viewed across age and geography. Nature 2012, 486, 222–227. [Google Scholar] [CrossRef]
- Akagawa, S.; Akagawa, Y.; Yamanouchi, S.; Kimata, T.; Tsuji, S.; Kaneko, K. Development of the gut microbiota and dysbiosis in children. Biosci. Microbiota Food Health 2021, 40, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Malard, F.; Dore, J.; Gaugler, B.; Mohty, M. Introduction to host microbiome symbiosis in health and disease. Mucosal. Immunol. 2021, 14, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Schwarzenberg, S.J.; Georgieff, M.K.; Committee On, N. Advocacy for Improving Nutrition in the First 1000 Days to Support Childhood Development and Adult Health. Pediatrics 2018, 141, e20173716. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Marras, L.; Caputo, M.; Bisicchia, S.; Soato, M.; Bertolino, G.; Vaccaro, S.; Inturri, R. The Role of Bifidobacteria in Predictive and Preventive Medicine: A Focus on Eczema and Hypercholesterolemia. Microorganisms 2021, 9, 836. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo-Cantabrana, C.; Delgado, S.; Ruiz, L.; Ruas-Madiedo, P.; Sanchez, B.; Margolles, A. Bifidobacteria and Their Health-Promoting Effects. Microbiol. Spectr. 2017, 5, 73–98. [Google Scholar] [CrossRef]
- Wong, C.B.; Sugahara, H.; Odamaki, T.; Xiao, J.Z. Different physiological properties of human-residential and non-human-residential bifidobacteria in human health. Benef. Microbes 2018, 9, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Inoue, Y.; Iwabuchi, N.; Xiao, J.Z.; Yaeshima, T.; Iwatsuki, K. Suppressive effects of bifidobacterium breve strain M-16V on T-helper type 2 immune responses in a murine model. Biol. Pharm. Bull. 2009, 32, 760–763. [Google Scholar] [CrossRef]
- Fukuda, S.; Toh, H.; Hase, K.; Oshima, K.; Nakanishi, Y.; Yoshimura, K.; Tobe, T.; Clarke, J.M.; Topping, D.L.; Suzuki, T.; et al. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature 2011, 469, 543–547. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.C.; Su, B.H.; Chen, A.C.; Lin, T.W.; Tsai, C.H.; Yeh, T.F.; Oh, W. Oral probiotics reduce the incidence and severity of necrotizing enterocolitis in very low birth weight infants. Pediatrics 2005, 115, 1–4. [Google Scholar] [CrossRef]
- Lin, H.C.; Hsu, C.H.; Chen, H.L.; Chung, M.Y.; Hsu, J.F.; Lien, R.I.; Tsao, L.Y.; Chen, C.H.; Su, B.H. Oral probiotics prevent necrotizing enterocolitis in very low birth weight preterm infants: A multicenter, randomized, controlled trial. Pediatrics 2008, 122, 693–700. [Google Scholar] [CrossRef]
- Sistek, D.; Kelly, R.; Wickens, K.; Stanley, T.; Fitzharris, P.; Crane, J. Is the effect of probiotics on atopic dermatitis confined to food sensitized children? Clin. Exp. Allergy 2006, 36, 629–633. [Google Scholar] [CrossRef] [PubMed]
- Gerasimov, S.V.; Vasjuta, V.V.; Myhovych, O.O.; Bondarchuk, L.I. Probiotic supplement reduces atopic dermatitis in preschool children: A randomized, double-blind, placebo-controlled, clinical trial. Am. J. Clin. Dermatol. 2010, 11, 351–361. [Google Scholar] [CrossRef]
- van der Aa, L.B.; Heymans, H.S.; van Aalderen, W.M.; Sillevis Smitt, J.H.; Knol, J.; Ben Amor, K.; Goossens, D.A.; Sprikkelman, A.B.; Synbad Study, G. Effect of a new synbiotic mixture on atopic dermatitis in infants: A randomized-controlled trial. Clin. Exp. Allergy 2010, 40, 795–804. [Google Scholar] [CrossRef] [PubMed]
- Yesilova, Y.; Calka, O.; Akdeniz, N.; Berktas, M. Effect of probiotics on the treatment of children with atopic dermatitis. Ann. Dermatol. 2012, 24, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Dianawati, D.; Mishra, V.; Shah, N.P. Viability, Acid and Bile Tolerance of Spray Dried Probiotic Bacteria and Some Commercial Probiotic Supplement Products Kept at Room Temperature. J. Food Sci. 2016, 81, M1472–M1479. [Google Scholar] [CrossRef]
- Kohno, M.; Tanaka-Azuma, Y.; Kamaguchi, R.; Tagawa, D. Application of enteric seamless capsules containing Bifidobacterium longum to functional foods. Folia. Pharmacol. Jpn. (Nihon Yakurigaku Zasshi) 2016, 148, 310–314. (In Japanese) [Google Scholar] [CrossRef] [PubMed][Green Version]
- Junick, J.; Blaut, M. Quantification of human fecal bifidobacterium species by use of quantitative real-time PCR analysis targeting the groEL gene. Appl. Environ. Microbiol. 2012, 78, 2613–2622. [Google Scholar] [CrossRef] [PubMed]
- Lewis, S.J.; Heaton, K.W. Stool form scale as a useful guide to intestinal transit time. Scand. J. Gastroenterol. 1997, 32, 920–924. [Google Scholar] [CrossRef] [PubMed]
- Venema, K.; Verhoeven, J.; Beckman, C.; Keller, D. Survival of a probiotic-containing product using capsule-within-capsule technology in an in vitro model of the stomach and small intestine (TIM-1). Benef. Microbes 2020, 11, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.B.; Iwabuchi, N.; Xiao, J.Z. Exploring the Science behind Bifidobacterium breve M-16V in Infant Health. Nutrients 2019, 11, 1724. [Google Scholar] [CrossRef]
- Toscano, M.; De Vecchi, E.; Gabrieli, A.; Zuccotti, G.V.; Drago, L. Probiotic characteristics and in vitro compatibility of a combination of Bifidobacterium breve M-16 V, Bifidobacterium longum subsp. infantis M-63 and Bifidobacterium longum subsp. longum BB536. Ann. Microbiol. 2014, 65, 1079–1086. [Google Scholar] [CrossRef]
- Xiao, J.Z.; Takahashi, S.; Odamaki, T.; Yaeshima, T.; Iwatsuki, K. Antibiotic susceptibility of bifidobacterial strains distributed in the Japanese market. Biosci. Biotechnol. Biochem. 2010, 74, 336–342. [Google Scholar] [CrossRef]
- Abe, F.; Muto, M.; Yaeshima, T.; Iwatsuki, K.; Aihara, H.; Ohashi, Y.; Fujisawa, T. Safety evaluation of probiotic bifidobacteria by analysis of mucin degradation activity and translocation ability. Anaerobe 2010, 16, 131–136. [Google Scholar] [CrossRef]
- Wong, C.B.; Odamaki, T.; Xiao, J.-z. Beneficial effects of Bifidobacterium longum subsp. longum BB536 on human health: Modulation of gut microbiome as the principal action. J. Funct. Foods 2019, 54, 506–519. [Google Scholar] [CrossRef]
- Sjoberg, F.; Nookaew, I.; Yazdanshenas, S.; Gio-Batta, M.; Adlerberth, I.; Wold, A.E. Are all faecal bacteria detected with equal efficiency? A study using next-generation sequencing and quantitative culture of infants’ faecal samples. J. Microbiol. Methods 2020, 177, 106018. [Google Scholar] [CrossRef]
- Saxelin, M.; Lassig, A.; Karjalainen, H.; Tynkkynen, S.; Surakka, A.; Vapaatalo, H.; Jarvenpaa, S.; Korpela, R.; Mutanen, M.; Hatakka, K. Persistence of probiotic strains in the gastrointestinal tract when administered as capsules, yoghurt, or cheese. Int. J. Food Microbiol. 2010, 144, 293–300. [Google Scholar] [CrossRef] [PubMed]



| Powder Formulation | Microcapsule Formulation | p-Value | |
|---|---|---|---|
| Adherence (%) | 100 (92.9–100) | 100 (96.6–100) | 0.49 |
| Frequency of defecation per week (pre/post) | 5.0 (4.5–6.0)/5.75 (5.0–6.0) | 5.75 (5.0–6.0)/ 3.25 (2.75–5.75) | 0.35/0.18 ‡ |
| BSFS score † (pre/post) | 4.0 (3.0–4.0)/4.0 (3.0–4.0) | 4.0 (3.0–4.0)/4.0 (4.0–4.0) | 0.56/0.32 ‡ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Minami, M.; Tsuji, S.; Akagawa, S.; Akagawa, Y.; Yoshimoto, Y.; Kawakami, H.; Kohno, M.; Kaneko, K. Effect of a Bifidobacterium-Containing Acid-Resistant Microcapsule Formulation on Gut Microbiota: A Pilot Study. Nutrients 2022, 14, 4829. https://doi.org/10.3390/nu14224829
Minami M, Tsuji S, Akagawa S, Akagawa Y, Yoshimoto Y, Kawakami H, Kohno M, Kaneko K. Effect of a Bifidobacterium-Containing Acid-Resistant Microcapsule Formulation on Gut Microbiota: A Pilot Study. Nutrients. 2022; 14(22):4829. https://doi.org/10.3390/nu14224829
Chicago/Turabian StyleMinami, Miki, Shoji Tsuji, Shohei Akagawa, Yuko Akagawa, Yuki Yoshimoto, Hirosato Kawakami, Mamiko Kohno, and Kazunari Kaneko. 2022. "Effect of a Bifidobacterium-Containing Acid-Resistant Microcapsule Formulation on Gut Microbiota: A Pilot Study" Nutrients 14, no. 22: 4829. https://doi.org/10.3390/nu14224829
APA StyleMinami, M., Tsuji, S., Akagawa, S., Akagawa, Y., Yoshimoto, Y., Kawakami, H., Kohno, M., & Kaneko, K. (2022). Effect of a Bifidobacterium-Containing Acid-Resistant Microcapsule Formulation on Gut Microbiota: A Pilot Study. Nutrients, 14(22), 4829. https://doi.org/10.3390/nu14224829






