Unravelling the Complex Relationship between Diet and Nephrolithiasis: The Role of Nutrigenomics and Nutrigenetics
Abstract
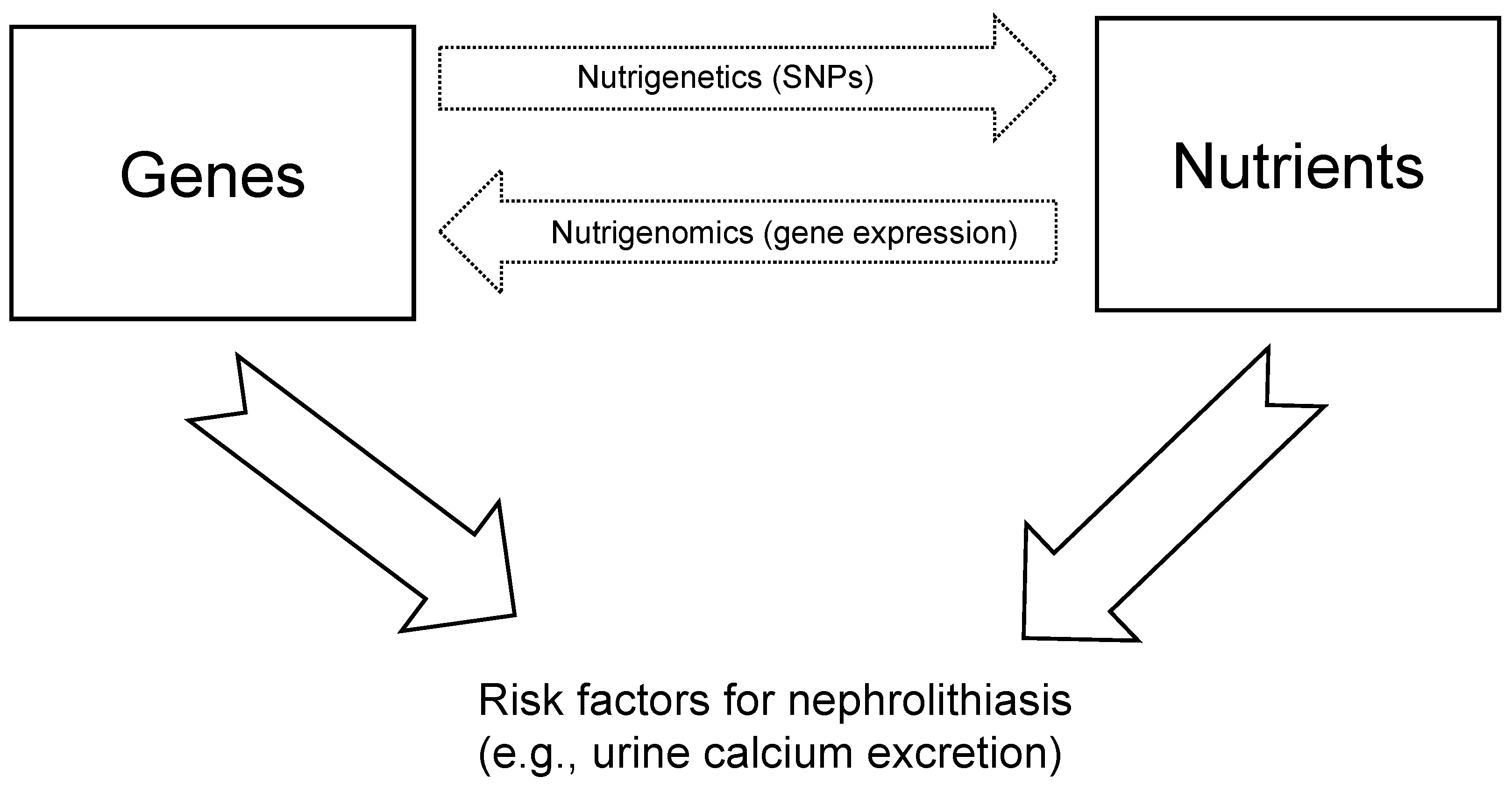
1. Introduction
2. Materials and Methods
- For any given nutrient/dietary pattern, an epigenetic mechanism on either kidney stone formation or risk factors/correlates of nephrolithiasis
- For a nutrient/dietary pattern already known to be associated with kidney stones or risk factors/correlates of nephrolithiasis, an epigenetic mechanism in general (not necessarily related to kidney stone formation)
3. Epigenetics in Nephrolithiasis
4. Nutrigenomics
5. Food Compounds
5.1. Acetic Acid (Vinegar)
5.2. Amino Acids—L-Arginine
5.3. Fructose
5.4. Sodium Chloride
5.5. Vitamin D
5.6. Calcium
5.7. Magnesium
5.8. Phosphate
5.9. Dietary Acid-Base
6. Dietary Patterns
Western Diet
7. Nutrition-Induced Epigenetic Modifications as Determinants of the Multisystemic Nature of Nephrolithiasis and the Possible Role of Fatty Acids
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Abufaraj, M.; Xu, T.; Cao, C.; Waldhoer, T.; Seitz, C.; D’andrea, D.; Siyam, A.; Tarawneh, R.; Fajkovic, H.; Schernhammer, E.; et al. Prevalence and Trends in Kidney Stone Among Adults in the USA: Analyses of National Health and Nutrition Examination Survey 2007–2018 Data. Eur. Urol. Focus 2021, 7, 1468–1475. [Google Scholar] [CrossRef]
- Siener, R.; Herwig, H.; Rüdy, J.; Schaefer, R.M.; Lossin, P.; Hesse, A. Urinary Stone Composition in Germany: Results from 45,783 Stone Analyses. World J. Urol. 2022, 40, 1813–1820. [Google Scholar] [CrossRef] [PubMed]
- Goldfarb, D.S. The Search for Monogenic Causes of Kidney Stones. J. Am. Soc. Nephrol. JASN 2015, 26, 507–510. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ferraro, P.M.; Taylor, E.N.; Gambaro, G.; Curhan, G.C. Dietary and Lifestyle Risk Factors Associated with Incident Kidney Stones in Men and Women. J. Urol. 2017, 198, 858–863. [Google Scholar] [CrossRef]
- Daudon, M.; Jungers, P. Diabetes and Nephrolithiasis. Curr. Diabetes Rep. 2007, 7, 443–448. [Google Scholar] [CrossRef]
- Daudon, M.; Traxer, O.; Conort, P.; Lacour, B.; Jungers, P. Type 2 Diabetes Increases the Risk for Uric Acid Stones. J. Am. Soc. Nephrol. JASN 2006, 17, 2026–2033. [Google Scholar] [CrossRef]
- West, B.; Luke, A.; Durazo-Arvizu, R.A.; Cao, G.; Shoham, D.; Kramer, H. Metabolic Syndrome and Self-Reported History of Kidney Stones: The National Health and Nutrition Examination Survey (NHANES III) 1988-1994. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2008, 51, 741–747. [Google Scholar] [CrossRef]
- Kohjimoto, Y.; Sasaki, Y.; Iguchi, M.; Matsumura, N.; Inagaki, T.; Hara, I. Association of Metabolic Syndrome Traits and Severity of Kidney Stones: Results from a Nationwide Survey on Urolithiasis in Japan. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2013, 61, 923–929. [Google Scholar] [CrossRef]
- Jeong, I.G.; Kang, T.; Bang, J.K.; Park, J.; Kim, W.; Hwang, S.S.; Kim, H.K.; Park, H.K. Association between Metabolic Syndrome and the Presence of Kidney Stones in a Screened Population. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2011, 58, 383–388. [Google Scholar] [CrossRef]
- Ferraro, P.M.; Bargagli, M.; Trinchieri, A.; Gambaro, G. Risk of Kidney Stones: Influence of Dietary Factors, Dietary Patterns, and Vegetarian-Vegan Diets. Nutrients 2020, 12, 779. [Google Scholar] [CrossRef]
- Heilberg, I.P.; Goldfarb, D.S. Optimum Nutrition for Kidney Stone Disease. Adv. Chronic Kidney Dis. 2013, 20, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Brockis, J.G.; Levitt, A.J.; Cruthers, S.M. The Effects of Vegetable and Animal Protein Diets on Calcium, Urate and Oxalate Excretion. Br. J. Urol. 1982, 54, 590–593. [Google Scholar] [CrossRef] [PubMed]
- Gambaro, A.; Lombardi, G.; Caletti, C.; Ribichini, F.L.; Ferraro, P.M.; Gambaro, G. Nephrolithiasis: A Red Flag for Cardiovascular Risk. J. Clin. Med. 2022, 11, 5512. [Google Scholar] [CrossRef] [PubMed]
- Melton, L.J.; Crowson, C.S.; Khosla, S.; Wilson, D.M.; O’Fallon, W.M. Fracture Risk among Patients with Urolithiasis: A Population-Based Cohort Study. Kidney Int. 1998, 53, 459–464. [Google Scholar] [CrossRef]
- Lombardi, G.; Ferraro, P.M.; Calvaruso, L.; Naticchia, A.; D’Alonzo, S.; Gambaro, G. Sodium Fluctuations and Mortality in a General Hospitalized Population. Kidney Blood Press. Res. 2019, 44, 604–614. [Google Scholar] [CrossRef]
- Taylor, E.N.; Feskanich, D.; Paik, J.M.; Curhan, G.C. Nephrolithiasis and Risk of Incident Bone Fracture. J. Urol. 2016, 195, 1482–1486. [Google Scholar] [CrossRef]
- Ferraro, P.M.; Taylor, E.N.; Eisner, B.H.; Gambaro, G.; Rimm, E.B.; Mukamal, K.J.; Curhan, G.C. History of Kidney Stones and the Risk of Coronary Heart Disease. JAMA 2013, 310, 408–415. [Google Scholar] [CrossRef]
- Oton-Gonzalez, L.; Mazziotta, C.; Iaquinta, M.R.; Mazzoni, E.; Nocini, R.; Trevisiol, L.; D’Agostino, A.; Tognon, M.; Rotondo, J.C.; Martini, F. Genetics and Epigenetics of Bone Remodeling and Metabolic Bone Diseases. Int. J. Mol. Sci. 2022, 23, 1500. [Google Scholar] [CrossRef]
- Hunter, D.J. Gene-Environment Interactions in Human Diseases. Nat. Rev. Genet. 2005, 6, 287–298. [Google Scholar] [CrossRef]
- Sharp, G.C.; Relton, C.L. Epigenetics and Noncommunicable Diseases. Epigenomics 2017, 9, 789–791. [Google Scholar] [CrossRef]
- Choi, S.-W.; Friso, S. Epigenetics: A New Bridge between Nutrition and Health. Adv. Nutr. 2010, 1, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.-W.; Claycombe, K.J.; Martinez, J.A.; Friso, S.; Schalinske, K.L. Nutritional Epigenomics: A Portal to Disease Prevention. Adv. Nutr. 2013, 4, 530–532. [Google Scholar] [CrossRef] [PubMed]
- Samblas, M.; Milagro, F.I.; Martínez, A. DNA Methylation Markers in Obesity, Metabolic Syndrome, and Weight Loss. Epigenetics 2019, 14, 421–444. [Google Scholar] [CrossRef] [PubMed]
- Friso, S.; Carvajal, C.A.; Fardella, C.E.; Olivieri, O. Epigenetics and Arterial Hypertension: The Challenge of Emerging Evidence. Transl. Res. J. Lab. Clin. Med. 2015, 165, 154–165. [Google Scholar] [CrossRef] [PubMed]
- Brenna, I.; Dogliotti, E.; Terranegra, A.; Raspini, B.; Soldati, L. Nephrolithiasis: Nutrition as Cause or Therapeutic Tool. J. Transl. Med. 2013, 11, 178. [Google Scholar] [CrossRef]
- Khatami, F.; Gorji, A.; Khoshchehreh, M.; Mashhadi, R.; Pishkuhi, M.A.; Khajavi, A.; Shabestari, A.N.; Aghamir, S.M.K. The Correlation between Promoter Hypermethylation of VDR, CLDN, and CasR Genes and Recurrent Stone Formation. BMC Med. Genom. 2022, 15, 109. [Google Scholar] [CrossRef]
- Guo, S.; Chia, W.; Wang, H.; Bushinsky, D.A.; Zhong, B.; Favus, M.J. Vitamin D Receptor (VDR) Contributes to the Development of Hypercalciuria by Sensitizing VDR Target Genes to Vitamin D in a Genetic Hypercalciuric Stone-Forming (GHS) Rat Model. Genes Dis. 2022, 9, 797–806. [Google Scholar] [CrossRef]
- Scheinman, S.J.; Cox, J.P.; Lloyd, S.E.; Pearce, S.H.; Salenger, P.V.; Hoopes, R.R.; Bushinsky, D.A.; Wrong, O.; Asplin, J.R.; Langman, C.B.; et al. Isolated Hypercalciuria with Mutation in CLCN5: Relevance to Idiopathic Hypercalciuria. Kidney Int. 2000, 57, 232–239. [Google Scholar] [CrossRef][Green Version]
- Gillion, V.; Devuyst, O. Genetic Variation in Claudin-2, Hypercalciuria, and Kidney Stones. Kidney Int. 2020, 98, 1076–1078. [Google Scholar] [CrossRef]
- Curry, J.N.; Saurette, M.; Askari, M.; Pei, L.; Filla, M.B.; Beggs, M.R.; Rowe, P.S.; Fields, T.; Sommer, A.J.; Tanikawa, C.; et al. Claudin-2 Deficiency Associates with Hypercalciuria in Mice and Human Kidney Stone Disease. J. Clin. Investig. 2020, 130, 1948–1960. [Google Scholar] [CrossRef]
- Toka, H.R.; Genovese, G.; Mount, D.B.; Pollak, M.R.; Curhan, G.C. Frequency of Rare Allelic Variation in Candidate Genes among Individuals with Low and High Urinary Calcium Excretion. PLoS ONE 2013, 8, e71885. [Google Scholar] [CrossRef] [PubMed]
- Yu, A.S.L. Claudins and the Kidney. J. Am. Soc. Nephrol. JASN 2015, 26, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Milagro, F.I.; Mansego, M.L.; de Miguel, C.; Martínez, J.A. Dietary Factors, Epigenetic Modifications and Obesity Outcomes: Progresses and Perspectives. Mol. Aspects Med. 2013, 34, 782–812. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Liu, Y.; Lan, Y.; Li, X.; Luo, L.; Duan, X.; Lei, M.; Liu, G.; Yang, Z.; Mai, X.; et al. Dietary Vinegar Prevents Kidney Stone Recurrence via Epigenetic Regulations. eBioMedicine 2019, 45, 231–250. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Jin, X.; Ma, Y.; Sun, Q.; Li, H.; Wang, K. Vinegar Reduced Renal Calcium Oxalate Stones by Regulating Acetate Metabolism in Gut Microbiota and Crystal Adhesion in Rats. Int. Urol. Nephrol. 2022, 54, 2485–2495. [Google Scholar] [CrossRef] [PubMed]
- Kandhare, A.D.; Patil, M.V.K.; Bodhankar, S.L. L-Arginine Attenuates the Ethylene Glycol Induced Urolithiasis in Ininephrectomized Hypertensive Rats: Role of KIM-1, NGAL, and NOs. Ren. Fail. 2015, 37, 709–721. [Google Scholar] [CrossRef]
- Taylor, E.N.; Curhan, G.C. Fructose Consumption and the Risk of Kidney Stones. Kidney Int. 2008, 73, 207–212. [Google Scholar] [CrossRef]
- Osis, G.; Webster, K.L.; Harris, A.N.; Lee, H.-W.; Chen, C.; Fang, L.; Romero, M.F.; Khattri, R.B.; Merritt, M.E.; Verlander, J.W.; et al. Regulation of Renal NaDC1 Expression and Citrate Excretion by NBCe1-A. Am. J. Physiol. Renal Physiol. 2019, 317, F489–F501. [Google Scholar] [CrossRef]
- Tavasoli, S.; Alebouyeh, M.; Naji, M.; Shakiba Majd, G.; Shabani Nashtaei, M.; Broumandnia, N.; Basiri, A. Association of Intestinal Oxalate-Degrading Bacteria with Recurrent Calcium Kidney Stone Formation and Hyperoxaluria: A Case-Control Study. BJU Int. 2020, 125, 133–143. [Google Scholar] [CrossRef]
- Stern, J.M.; Moazami, S.; Qiu, Y.; Kurland, I.; Chen, Z.; Agalliu, I.; Burk, R.; Davies, K.P. Evidence for a Distinct Gut Microbiome in Kidney Stone Formers Compared to Non-Stone Formers. Urolithiasis 2016, 44, 399–407. [Google Scholar] [CrossRef]
- Tang, R.; Jiang, Y.; Tan, A.; Ye, J.; Xian, X.; Xie, Y.; Wang, Q.; Yao, Z.; Mo, Z. 16S RRNA Gene Sequencing Reveals Altered Composition of Gut Microbiota in Individuals with Kidney Stones. Urolithiasis 2018, 46, 503–514. [Google Scholar] [CrossRef]
- Ticinesi, A.; Milani, C.; Guerra, A.; Allegri, F.; Lauretani, F.; Nouvenne, A.; Mancabelli, L.; Lugli, G.A.; Turroni, F.; Duranti, S.; et al. Understanding the Gut-Kidney Axis in Nephrolithiasis: An Analysis of the Gut Microbiota Composition and Functionality of Stone Formers. Gut 2018, 67, 2097–2106. [Google Scholar] [CrossRef] [PubMed]
- Zampini, A.; Nguyen, A.H.; Rose, E.; Monga, M.; Miller, A.W. Defining Dysbiosis in Patients with Urolithiasis. Sci. Rep. 2019, 9, 5425. [Google Scholar] [CrossRef]
- Peerapen, P.; Thongboonkerd, V. Effects of Calcium Oxalate Monohydrate Crystals on Expression and Function of Tight Junction of Renal Tubular Epithelial Cells. Lab. Investig. J. Tech. Methods Pathol. 2011, 91, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Verkoelen, C.F. Crystal Retention in Renal Stone Disease: A Crucial Role for the Glycosaminoglycan Hyaluronan? J. Am. Soc. Nephrol. JASN 2006, 17, 1673–1687. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Hu, Y.; Guo, D.; Zhang, A.; Gu, Y.; Zhang, S.; Zhao, C.; Gong, P.; Shen, X.; Li, Y.; et al. DNA Methyltransferase 3A Isoform b Contributes to Repressing E-Cadherin through Cooperation of DNA Methylation and H3K27/H3K9 Methylation in EMT-Related Metastasis of Gastric Cancer. Oncogene 2018, 37, 4358–4371. [Google Scholar] [CrossRef] [PubMed]
- Nowak, E.; Bednarek, I. Aspects of the Epigenetic Regulation of EMT Related to Cancer Metastasis. Cells 2021, 10, 3435. [Google Scholar] [CrossRef]
- Pragasam, V.; Kalaiselvi, P.; Sumitra, K.; Srinivasan, S.; Varalakshmi, P. Counteraction of Oxalate Induced Nitrosative Stress by Supplementation of L-Arginine, a Potent Antilithic Agent. Clin. Chim. Acta Int. J. Clin. Chem. 2005, 354, 159–166. [Google Scholar] [CrossRef]
- Yu, H.-R.; Tsai, C.-C.; Chang, L.-S.; Huang, H.-C.; Cheng, H.-H.; Wang, J.-Y.; Sheen, J.-M.; Kuo, H.-C.; Hsieh, K.-S.; Huang, Y.-H.; et al. L-Arginine-Dependent Epigenetic Regulation of Interleukin-10, but Not Transforming Growth Factor-β, Production by Neonatal Regulatory T Lymphocytes. Front. Immunol. 2017, 8, 487. [Google Scholar] [CrossRef]
- Hsu, S.-C.; Chen, C.-L.; Cheng, M.-L.; Chu, C.-Y.; Changou, C.A.; Yu, Y.-L.; Yeh, S.-D.; Kuo, T.-C.; Kuo, C.-C.; Chuu, C.-P.; et al. Arginine Starvation Elicits Chromatin Leakage and CGAS-STING Activation via Epigenetic Silencing of Metabolic and DNA-Repair Genes. Theranostics 2021, 11, 7527–7545. [Google Scholar] [CrossRef]
- Ferraro, P.M.; Taylor, E.N.; Gambaro, G.; Curhan, G.C. Soda and Other Beverages and the Risk of Kidney Stones. Clin. J. Am. Soc. Nephrol. CJASN 2013, 8, 1389–1395. [Google Scholar] [CrossRef] [PubMed]
- Koh, E.T.; Min, K.W. Fructose Precipitates Calcium Phosphate in the Kidneys of Female Rats Fed Magnesium-Deficient Diets. Magnes. Res. 1991, 4, 171–176. [Google Scholar] [PubMed]
- Koh, E.T.; Reiser, S.; Fields, M. Dietary Fructose as Compared to Glucose and Starch Increases the Calcium Content of Kidney of Magnesium-Deficient Rats. J. Nutr. 1989, 119, 1173–1178. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.U.; Dumoulin, G.; Henriet, M.T.; Regnard, J. Increase in Urinary Calcium and Oxalate after Fructose Infusion. Horm. Metab. Res. Horm. Stoffwechs. Horm. Metab. 1995, 27, 155–158. [Google Scholar] [CrossRef] [PubMed]
- Milne, D.B.; Nielsen, F.H. The Interaction between Dietary Fructose and Magnesium Adversely Affects Macromineral Homeostasis in Men. J. Am. Coll. Nutr. 2000, 19, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Abate, N.; Chandalia, M.; Cabo-Chan, A.V.; Moe, O.W.; Sakhaee, K. The Metabolic Syndrome and Uric Acid Nephrolithiasis: Novel Features of Renal Manifestation of Insulin Resistance. Kidney Int. 2004, 65, 386–392. [Google Scholar] [CrossRef]
- Kageyama, I.; Yamada, H.; Munetsuna, E.; Yamazaki, M.; Ando, Y.; Mizuno, G.; Fujii, R.; Nouchi, Y.; Wakasugi, T.; Sakakibara, T.; et al. Differential Effects of Excess High-Fructose Corn Syrup on the DNA Methylation of Hippocampal Neurotrophic Factor in Childhood and Adolescence. PLoS ONE 2022, 17, e0270144. [Google Scholar] [CrossRef]
- Assante, G.; Chandrasekaran, S.; Ng, S.; Tourna, A.; Chung, C.H.; Isse, K.A.; Banks, J.L.; Soffientini, U.; Filippi, C.; Dhawan, A.; et al. Acetyl-CoA Metabolism Drives Epigenome Change and Contributes to Carcinogenesis Risk in Fatty Liver Disease. Genome Med. 2022, 14, 67. [Google Scholar] [CrossRef]
- Luo, Y.; Zhang, Z.; Xiang, L.; Zhou, B.; Wang, X.; Lin, Y.; Ding, X.; Liu, F.; Lu, Y.; Peng, Y. Analysis of N6-Methyladenosine Methylation Modification in Fructose-Induced Non-Alcoholic Fatty Liver Disease. Front. Endocrinol. 2021, 12, 780617. [Google Scholar] [CrossRef]
- Jegatheesan, P.; de Bandt, J.-P. Fructose and NAFLD: The Multifaceted Aspects of Fructose Metabolism. Nutrients 2017, 9, 230. [Google Scholar] [CrossRef]
- Lee, J.; Kim, Y.; Friso, S.; Choi, S.-W. Epigenetics in Non-Alcoholic Fatty Liver Disease. Mol. Aspects Med. 2017, 54, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Bae, H.; Song, W.-S.; Jang, C. Dietary Fructose and Fructose-Induced Pathologies. Annu. Rev. Nutr. 2022, 42, 45–66. [Google Scholar] [CrossRef] [PubMed]
- Grillo, A.; Salvi, L.; Coruzzi, P.; Salvi, P.; Parati, G. Sodium Intake and Hypertension. Nutrients 2019, 11, 1970. [Google Scholar] [CrossRef] [PubMed]
- Cappuccio, F.P. Cardiovascular and Other Effects of Salt Consumption. Kidney Int. Suppl. 2013, 3, 312–315. [Google Scholar] [CrossRef] [PubMed]
- Ticinesi, A.; Nouvenne, A.; Maalouf, N.M.; Borghi, L.; Meschi, T. Salt and Nephrolithiasis. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc.-Eur. Ren. Assoc. 2016, 31, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Hsi, R.S.; Spieker, A.J.; Stoller, M.L.; Jacobs, D.R.; Reiner, A.P.; McClelland, R.L.; Kahn, A.J.; Chi, T.; Szklo, M.; Sorensen, M.D. Coronary Artery Calcium Score and Association with Recurrent Nephrolithiasis: The Multi-Ethnic Study of Atherosclerosis. J. Urol. 2016, 195, 971–976. [Google Scholar] [CrossRef] [PubMed]
- Blackwood, A.M.; Sagnella, G.A.; Cook, D.G.; Cappuccio, F.P. Urinary Calcium Excretion, Sodium Intake and Blood Pressure in a Multi-Ethnic Population: Results of the Wandsworth Heart and Stroke Study. J. Hum. Hypertens. 2001, 15, 229–237. [Google Scholar] [CrossRef]
- Massey, L.K.; Whiting, S.J. Dietary Salt, Urinary Calcium, and Kidney Stone Risk. Nutr. Rev. 1995, 53, 131–139. [Google Scholar] [CrossRef]
- Lin, P.-H.; Ginty, F.; Appel, L.J.; Aickin, M.; Bohannon, A.; Garnero, P.; Barclay, D.; Svetkey, L.P. The DASH Diet and Sodium Reduction Improve Markers of Bone Turnover and Calcium Metabolism in Adults. J. Nutr. 2003, 133, 3130–3136. [Google Scholar] [CrossRef]
- Sorensen, M.D.; Kahn, A.J.; Reiner, A.P.; Tseng, T.Y.; Shikany, J.M.; Wallace, R.B.; Chi, T.; Wactawski-Wende, J.; Jackson, R.D.; O’Sullivan, M.J.; et al. Impact of Nutritional Factors on Incident Kidney Stone Formation: A Report from the WHI OS. J. Urol. 2012, 187, 1645–1649. [Google Scholar] [CrossRef]
- Jaeger, P.; Robertson, W.G. Role of Dietary Intake and Intestinal Absorption of Oxalate in Calcium Stone Formation. Nephron Physiol. 2004, 98, p64–p71. [Google Scholar] [CrossRef] [PubMed]
- Yatabe, M.S.; Yatabe, J.; Takano, K.; Murakami, Y.; Sakuta, R.; Abe, S.; Sanada, H.; Kimura, J.; Watanabe, T. Effects of a High-Sodium Diet on Renal Tubule Ca2+ Transporter and Claudin Expression in Wistar-Kyoto Rats. BMC Nephrol. 2012, 13, 160. [Google Scholar] [CrossRef] [PubMed]
- Amara, V.R.; Surapaneni, S.K.; Tikoo, K. Dysregulation of MicroRNAs and Renin-Angiotensin System in High Salt Diet-Induced Cardiac Dysfunction in Uninephrectomized Rats. PLoS ONE 2017, 12, e0180490. [Google Scholar] [CrossRef] [PubMed]
- Carlberg, C. Nutrigenomics of Vitamin, D. Nutrients 2019, 11, 676. [Google Scholar] [CrossRef] [PubMed]
- Bocheva, G.; Boyadjieva, N. Epigenetic Regulation of Fetal Bone Development and Placental Transfer of Nutrients: Progress for Osteoporosis. Interdiscip. Toxicol. 2011, 4, 167–172. [Google Scholar] [CrossRef]
- Haroun, N.; Bennour, I.; Seipelt, E.; Astier, J.; Couturier, C.; Mounien, L.; Landrier, J.-F. Maternal Vitamin D Deficiency in Mice Increases White Adipose Tissue Inflammation in Offspring. Cells 2022, 11, 2024. [Google Scholar] [CrossRef]
- Di Costanzo, M.; de Paulis, N.; Capra, M.E.; Biasucci, G. Nutrition during Pregnancy and Lactation: Epigenetic Effects on Infants’ Immune System in Food Allergy. Nutrients 2022, 14, 1766. [Google Scholar] [CrossRef]
- Koemel, N.A.; Skilton, M.R. Epigenetic Aging in Early Life: Role of Maternal and Early Childhood Nutrition. Curr. Nutr. Rep. 2022, 11, 318–328. [Google Scholar] [CrossRef]
- Krstic, N.; Bishop, N.; Curtis, B.; Cooper, C.; Harvey, N.; Lilycrop, K.; Murray, R.; Owen, R.; Reilly, G.; Skerry, T.; et al. Early Life Vitamin D Depletion and Mechanical Loading Determine Methylation Changes in the RUNX2, RXRA, and Osterix Promoters in Mice. Genes Nutr. 2022, 17, 7. [Google Scholar] [CrossRef]
- Snegarova, V.; Naydenova, D. Vitamin D: A Review of Its Effects on Epigenetics and Gene Regulation. Folia Med. 2020, 62, 662–667. [Google Scholar] [CrossRef]
- Borghi, L.; Schianchi, T.; Meschi, T.; Guerra, A.; Allegri, F.; Maggiore, U.; Novarini, A. Comparison of Two Diets for the Prevention of Recurrent Stones in Idiopathic Hypercalciuria. N. Engl. J. Med. 2002, 346, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Curhan, G.C.; Willett, W.C.; Rimm, E.B.; Stampfer, M.J. A Prospective Study of Dietary Calcium and Other Nutrients and the Risk of Symptomatic Kidney Stones. N. Engl. J. Med. 1993, 328, 833–838. [Google Scholar] [CrossRef] [PubMed]
- Nouvenne, A.; Meschi, T.; Guerra, A.; Allegri, F.; Prati, B.; Borghi, L. Dietary Treatment of Nephrolithiasis. Clin. Cases Miner. Bone Metab. Off. J. Ital. Soc. Osteoporos. Miner. Metab. Skelet. Dis. 2008, 5, 135–141. [Google Scholar]
- Cross, H.S.; Hulla, W.; Tong, W.M.; Peterlik, M. Growth Regulation of Human Colon Adenocarcinoma-Derived Cells by Calcium, Vitamin D and Epidermal Growth Factor. J. Nutr. 1995, 125, 2004S–2008S. [Google Scholar] [CrossRef]
- Chang, X.; Li, P.; Yan, K.; Lu, Y.; Tang, T.; Fan, X.; Fan, C.; Zhan, D.; Qi, K. Maternal Dietary Calcium Status during Pregnancy and Lactation Affects Brain DHA Accretion through Modifying DNA Methylation of Fatty Acid Desaturases in the Mouse Offspring. Nutr. Res. 2019, 65, 29–42. [Google Scholar] [CrossRef]
- Chaplin, A.; Palou, A.; Serra, F. Methylation Analysis in Fatty-Acid-Related Genes Reveals Their Plasticity Associated with Conjugated Linoleic Acid and Calcium Supplementation in Adult Mice. Eur. J. Nutr. 2017, 56, 879–891. [Google Scholar] [CrossRef]
- Takaya, J.; Yamanouchi, S.; Tanabe, Y.; Kaneko, K. A Calcium-Deficient Diet in Rat Dams during Gestation Decreases HOMA-Β% in 3 Generations of Offspring. J. Nutr. Nutr. 2016, 9, 276–286. [Google Scholar] [CrossRef]
- Li, H.; Klett, D.E.; Littleton, R.; Elder, J.S.; Sammon, J.D. Role of Insulin Resistance in Uric Acid Nephrolithiasis. World J. Nephrol. 2014, 3, 237–242. [Google Scholar] [CrossRef]
- Houillier, P. Mechanisms and Regulation of Renal Magnesium Transport. Annu. Rev. Physiol. 2014, 76, 411–430. [Google Scholar] [CrossRef]
- Johansson, G.; Backman, U.; Danielson, B.G.; Fellström, B.; Ljunghall, S.; Wikström, B. Effects of Magnesium Hydroxide in Renal Stone Disease. J. Am. Coll. Nutr. 1982, 1, 179–185. [Google Scholar] [CrossRef]
- Liebman, M.; Costa, G. Effects of Calcium and Magnesium on Urinary Oxalate Excretion after Oxalate Loads. J. Urol. 2000, 163, 1565–1569. [Google Scholar] [CrossRef] [PubMed]
- Lindberg, J.; Harvey, J.; Pak, C.Y. Effect of Magnesium Citrate and Magnesium Oxide on the Crystallization of Calcium Salts in Urine: Changes Produced by Food-Magnesium Interaction. J. Urol. 1990, 143, 248–251. [Google Scholar] [CrossRef] [PubMed]
- Massey, L. Magnesium Therapy for Nephrolithiasis. Magnes. Res. 2005, 18, 123–126. [Google Scholar] [PubMed]
- Amenyah, S.D.; Ward, M.; Strain, J.J.; McNulty, H.; Hughes, C.F.; Dollin, C.; Walsh, C.P.; Lees-Murdock, D.J. Nutritional Epigenomics and Age-Related Disease. Curr. Dev. Nutr. 2020, 4, nzaa097. [Google Scholar] [CrossRef] [PubMed]
- Rosanoff, A.; Weaver, C.M.; Rude, R.K. Suboptimal Magnesium Status in the United States: Are the Health Consequences Underestimated? Nutr. Rev. 2012, 70, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.-S.; Liu, D.-X.; Fan, Y.-H.; Wu, J.-K. LncRNA GAS5 Promotes Epilepsy Progression through the Epigenetic Repression of MiR-219, in Turn Affecting CaMKIIγ/NMDAR Pathway. J. Neurogenet. 2022, 36, 32–42. [Google Scholar] [CrossRef]
- Manghat, P.; Sodi, R.; Swaminathan, R. Phosphate Homeostasis and Disorders. Ann. Clin. Biochem. 2014, 51, 631–656. [Google Scholar] [CrossRef]
- Ha, Y.-S.; Tchey, D.-U.; Kang, H.W.; Kim, Y.-J.; Yun, S.-J.; Lee, S.-C.; Kim, W.-J. Phosphaturia as a Promising Predictor of Recurrent Stone Formation in Patients with Urolithiasis. Korean J. Urol. 2010, 51, 54–59. [Google Scholar] [CrossRef]
- Prié, D.; Ravery, V.; Boccon-Gibod, L.; Friedlander, G. Frequency of Renal Phosphate Leak among Patients with Calcium Nephrolithiasis. Kidney Int. 2001, 60, 272–276. [Google Scholar] [CrossRef]
- Dhayat, N.A.; Lüthi, D.; Schneider, L.; Mattmann, C.; Vogt, B.; Fuster, D.G. Distinct Phenotype of Kidney Stone Formers with Renal Phosphate Leak. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc.-Eur. Ren. Assoc. 2019, 34, 129–137. [Google Scholar] [CrossRef]
- Khairallah, P.; Isakova, T.; Asplin, J.; Hamm, L.; Dobre, M.; Rahman, M.; Sharma, K.; Leonard, M.; Miller, E.; Jaar, B.; et al. Acid Load and Phosphorus Homeostasis in CKD. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2017, 70, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, T.J.; de Araújo, C.C.; da Lima, A.C.S.; Matida, L.M.; Griebeler, A.F.M.; Coelho, A.S.G.; Gontijo, A.P.M.; Cominetti, C.; Vêncio, E.F.; Horst, M.A. Dietary Intake Is Associated with MiR-31 and MiR-375 Expression in Patients with Head and Neck Squamous Cell Carcinoma. Nutr. Cancer 2022, 74, 2049–2058. [Google Scholar] [CrossRef] [PubMed]
- Frick, K.K.; Bushinsky, D.A. Metabolic Acidosis Stimulates RANKL RNA Expression in Bone through a Cyclo-Oxygenase-Dependent Mechanism. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2003, 18, 1317–1325. [Google Scholar] [CrossRef] [PubMed]
- Espino, L.; Suarez, M.L.; Santamarina, G.; Goicoa, A.; Fidalgo, L.E. Effects of Dietary Cation-Anion Difference on Blood Cortisol and ACTH Levels in Reproducing Ewes. J. Vet. Med. A Physiol. Pathol. Clin. Med. 2005, 52, 8–12. [Google Scholar] [CrossRef]
- Lim, S.Y.; Chan, Y.M.; Ramachandran, V.; Shariff, Z.M.; Chin, Y.S.; Arumugam, M. Dietary Acid Load and Its Interaction with IGF1 (Rs35767 and Rs7136446) and IL6 (Rs1800796) Polymorphisms on Metabolic Traits among Postmenopausal Women. Nutrients 2021, 13, 2161. [Google Scholar] [CrossRef]
- Turney, B.W.; Appleby, P.N.; Reynard, J.M.; Noble, J.G.; Key, T.J.; Allen, N.E. Diet and Risk of Kidney Stones in the Oxford Cohort of the European Prospective Investigation into Cancer and Nutrition (EPIC). Eur. J. Epidemiol. 2014, 29, 363–369. [Google Scholar] [CrossRef]
- Leone, A.; Fernández-Montero, A.; de la Fuente-Arrillaga, C.; Martínez-González, M.Á.; Bertoli, S.; Battezzati, A.; Bes-Rastrollo, M. Adherence to the Mediterranean Dietary Pattern and Incidence of Nephrolithiasis in the Seguimiento Universidad de Navarra Follow-up (SUN) Cohort. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2017, 70, 778–786. [Google Scholar] [CrossRef]
- Taylor, E.N.; Fung, T.T.; Curhan, G.C. DASH-Style Diet Associates with Reduced Risk for Kidney Stones. J. Am. Soc. Nephrol. JASN 2009, 20, 2253–2259. [Google Scholar] [CrossRef]
- Rodriguez, A.; Curhan, G.C.; Gambaro, G.; Taylor, E.N.; Ferraro, P.M. Mediterranean Diet Adherence and Risk of Incident Kidney Stones. Am. J. Clin. Nutr. 2020, 111, 1100–1106. [Google Scholar] [CrossRef]
- Ferraro, P.M.; Mandel, E.I.; Curhan, G.C.; Gambaro, G.; Taylor, E.N. Dietary Protein and Potassium, Diet-Dependent Net Acid Load, and Risk of Incident Kidney Stones. Clin. J. Am. Soc. Nephrol. CJASN 2016, 11, 1834–1844. [Google Scholar] [CrossRef]
- Frassetto, L.A.; Nash, E.; Morris, R.C.; Sebastian, A. Comparative Effects of Potassium Chloride and Bicarbonate on Thiazide-Induced Reduction in Urinary Calcium Excretion. Kidney Int. 2000, 58, 748–752. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.W.; Park, J.T.; Yoo, T.-H.; Lee, J.; Chung, W.; Lee, K.-B.; Chae, D.-W.; Ahn, C.; Kang, S.-W.; Choi, K.H.; et al. Urinary Potassium Excretion and Progression of CKD. Clin. J. Am. Soc. Nephrol. 2019, 14, 330–340. [Google Scholar] [CrossRef] [PubMed]
- Kieneker, L.M.; Gansevoort, R.T.; Mukamal, K.J.; de Boer, R.A.; Navis, G.; Bakker, S.J.L.; Joosten, M.M. Urinary Potassium Excretion and Risk of Developing Hypertension. Hypertension 2014, 64, 769–776. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.-W.; Tammen, S.A.; Liu, Z.; Friso, S. A Lifelong Exposure to a Western-Style Diet, but Not Aging, Alters Global DNA Methylation in Mouse Colon. Nutr. Res. Pract. 2015, 9, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo, A.G.; Carreira, M.C.; Boughanem, H.; Moreno-Navarrete, J.M.; Nicoletti, C.F.; Oliver, P.; de Luis, D.; Nonino, C.B.; Portillo, M.P.; Martinez-Olmos, M.A.; et al. Adipose Tissue and Blood Leukocytes ACE2 DNA Methylation in Obesity and after Weight Loss. Eur. J. Clin. Investig. 2022, 52, e13685. [Google Scholar] [CrossRef]
- Crujeiras, A.B.; Izquierdo, A.G.; Primo, D.; Milagro, F.I.; Sajoux, I.; Jácome, A.; Fernandez-Quintela, A.; Portillo, M.P.; Martínez, J.A.; Martinez-Olmos, M.A.; et al. Epigenetic Landscape in Blood Leukocytes Following Ketosis and Weight Loss Induced by a Very Low Calorie Ketogenic Diet (VLCKD) in Patients with Obesity. Clin. Nutr. Edinb. Scotl. 2021, 40, 3959–3972. [Google Scholar] [CrossRef]
- Gluckman, P.D.; Hanson, M.A.; Cooper, C.; Thornburg, K.L. Effect of in Utero and Early-Life Conditions on Adult Health and Disease. N. Engl. J. Med. 2008, 359, 61–73. [Google Scholar] [CrossRef]
- Ma, Y.; Ordovas, J.M. The Integration of Epigenetics and Genetics in Nutrition Research for CVD Risk Factors. Proc. Nutr. Soc. 2017, 76, 333–346. [Google Scholar] [CrossRef]
- Baggio, B.; Gambaro, G.; Zambon, S.; Marchini, F.; Bassi, A.; Bordin, L.; Clari, G.; Manzato, E. Anomalous Phospholipid N-6 Polyunsaturated Fatty Acid Composition in Idiopathic Calcium Nephrolithiasis. J. Am. Soc. Nephrol. JASN 1996, 7, 613–620. [Google Scholar] [CrossRef]
- Brenner, R. R Factors Influencing Fatty Acid Chain Elongation and Desaturation. The Role of Fats in Human Nutrition (1989), 2nd ed.; Academic Press: London, UK, 1989; Volume 1989, pp. 45–79. [Google Scholar]
- Gambaro, G.; Bordoni, A.; Hrelia, S.; Bordin, L.; Biagi, P.; Semplicini, A.; Clari, G.; Manzato, E.; Baggio, B. Dietary Manipulation of Delta-6-Desaturase Modifies Phospholipid Arachidonic Acid Levels and the Urinary Excretion of Calcium and Oxalate in the Rat: Insight in Calcium Lithogenesis. J. Lab. Clin. Med. 2000, 135, 89–95. [Google Scholar] [CrossRef]
- Baggio, B.; Budakovic, A.; Nassuato, M.A.; Vezzoli, G.; Manzato, E.; Luisetto, G.; Zaninotto, M. Plasma Phospholipid Arachidonic Acid Content and Calcium Metabolism in Idiopathic Calcium Nephrolithiasis. Kidney Int. 2000, 58, 1278–1284. [Google Scholar] [CrossRef] [PubMed]
- Baggio, B.; Budakovic, A.; Priante, G.; Gambaro, G.; Manzato, E.; Khan, S. Dietary Fatty Acid Supplementation Modulates the Urinary Excretion of Calcium and Oxalate in the Rat. Insight into Calcium Lithogenesis. Nephron 2002, 91, 486–491. [Google Scholar] [CrossRef] [PubMed]
| Food | Epigenetic Mechanism | Phenotype |
|---|---|---|
| Acetic acid (vinegar) [34] | ncRNA-mediated gene silencing (miRNA) by promoting expression of microRNAs -130a-3p, -148b-3p, and -374b-5p by increasing H3K9, H3K27 acetylation at their promoter regions | Reduced urinary calcium Increased urinary citrate |
| Acetic acid (vinegar) [35] | unknown | Reduced urinary oxalate Reduced CaOx crystals adhesion Reduced plasma acetate -> increased occludins in tight junctions -> reduced CaOx stone formation |
| L-arginine [36] | unknown | Reduced urinary calcium Increased urinary citrate |
| Fructose [37] | unknown | Increased urinary calcium Increased urinary oxalate |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Ambrosio, V.; Ferraro, P.M.; Lombardi, G.; Friso, S.; Gambaro, G. Unravelling the Complex Relationship between Diet and Nephrolithiasis: The Role of Nutrigenomics and Nutrigenetics. Nutrients 2022, 14, 4961. https://doi.org/10.3390/nu14234961
D’Ambrosio V, Ferraro PM, Lombardi G, Friso S, Gambaro G. Unravelling the Complex Relationship between Diet and Nephrolithiasis: The Role of Nutrigenomics and Nutrigenetics. Nutrients. 2022; 14(23):4961. https://doi.org/10.3390/nu14234961
Chicago/Turabian StyleD’Ambrosio, Viola, Pietro Manuel Ferraro, Gianmarco Lombardi, Simonetta Friso, and Giovanni Gambaro. 2022. "Unravelling the Complex Relationship between Diet and Nephrolithiasis: The Role of Nutrigenomics and Nutrigenetics" Nutrients 14, no. 23: 4961. https://doi.org/10.3390/nu14234961
APA StyleD’Ambrosio, V., Ferraro, P. M., Lombardi, G., Friso, S., & Gambaro, G. (2022). Unravelling the Complex Relationship between Diet and Nephrolithiasis: The Role of Nutrigenomics and Nutrigenetics. Nutrients, 14(23), 4961. https://doi.org/10.3390/nu14234961






