Abstract
Despite the enormous global market of dietary supplements, the impact of dietary supplements on kidney disease is still unclear. Based on the National Health and Nutrition Examination Survey from 2015 to 2017, this study evaluated the association between dietary supplement and chronic kidney disease (CKD) in 13,271 Korean adults. Among the dietary supplements, vitamin and mineral intake was the highest at 61.41%, followed by omega-3 fatty acids at 11.85%, and ginseng at 7.99%. The prevalence of CKD was significantly higher in those who consumed amino acids and proteins, ginseng and red ginseng, and herbal medicine (plant extract)-berries than in those who did not. Conversely, patients who consumed probiotic supplements had a significantly lower prevalence of CKD than those who did not. In the population without CKD risk factors or history of CKD, the prevalence of CKD was high in the group consuming ginseng and red ginseng. After adjusting for covariates, the herbal medicine (plant extract)-berry group showed an independent association with CKD incidence. In conclusion, it is suggested that dietary supplements may affect kidney function. Further large-scale cohort studies are required to elucidate the exact effects of each dietary supplement on CKD.
1. Introduction
Over the past decades, chronic kidney disease (CKD) has affected 10–15% of the population worldwide, and paralleling epidemics of hypertension and diabetes, the number of CKD patients has increased rapidly in recent years, making a significant impact on the global health burden [1,2,3,4]. Owing to the high prevalence, morbidity rates, and medical costs of CKD, prevention and optimal management of the disease is an important public health issue.
However, since there is no effective kidney-targeting drug that can inhibit the progression of CKD other than treatment of underlying diseases such as hypertension, hyperglycemia, and dyslipidemia and monitoring of complications, interest in the effect of nutrition or dietary supplements on kidney disease is increasing. Diets such as protein restriction diet, the Mediterranean diet, and plant-based diets are currently being investigated for their potential roles in delaying CKD progression.
Today, there are thousands of dietary supplements available on the market, including vitamins and minerals, plant ingredients and extracts, proteins and amino acids, omega-3 fatty acids, probiotics, and prebiotics [5,6,7]. Some research has indicated that dietary supplements can compensate for the nutritional deficit derived from unbalanced diets and can assist in prolonging the lifespan and provide some benefits in diseases, although evidence of direct effects is still insufficient [7,8,9].
In the last decade, the prevalence of dietary supplement use has increased dramatically [6,7], and the global market size of dietary supplements was valued at nearly 121 billion USD in 2018 [6]. The use of dietary supplements in modern society is not limited to the middle-aged and elderly, and the interest in dietary supplements is growing among young people as well. This is because most dietary supplements are not classified as drugs by the Food and Drug Administration and are easily obtainable with unrestricted exposure to advertisements. Dietary supplement intake data from the Korean National Health and Nutrition Examination Survey (KNHANES) also showed that approximately 42% of Korean adults aged ≥19 years have taken a dietary supplement at least once in their lifetime [10]. However, the role of dietary supplements is not well understood. Although the beneficial effects of dietary supplements have been shown in some studies, systematic studies on their positive and negative effects have not been reported.
In particular, if there is a problem with the function of the liver or kidney, which is the path through which dietary metabolites are absorbed and excreted, it may affect the dysfunction of these organs, and studies on the effect and safety of dietary supplements are needed. The possibility of drug–supplement interactions should also be considered.
Guidelines for CKD management recommend that patients with CKD should avoid using nutritional protein supplements and herbal remedies and should use them only under the supervision of a physician or pharmacist [11].
Extensive animal and clinical research have been conducted on the role of vitamin/mineral supplements and probiotics/prebiotics in kidney disease [12,13,14]. For example, vitamin D may contribute to improved clinical outcomes of CKD patients [12], and probiotics can lower the levels of inflammatory mediators and have clinical benefits in patients at different stages of CKD [13,14]. However, the association between CKD and numerous dietary supplements (e.g., ginseng and red ginseng, methyl sulfonyl methane, lutein containing supplements, propolis, and milk thistle) has not been well studied. Thus, the present study aimed to characterize the extent of dietary supplement use in a nationally representative sample and to explore the possible consequences of dietary supplements in patients with CKD.
2. Materials and Methods
2.1. Data Source and Study Population
This study used data from the KNHANES conducted from 2015 to 2017, which is a nationwide cross-sectional sample survey conducted by the Korea Centers for Disease Control and Prevention. KNHANES includes an annual survey sample of approximately 10,000 people and consists of biochemical and clinical profiles for diseases, socioeconomic status, anthropometric measures, health-related behaviors, quality of life, health screenings, and nutritional surveys, of which the nutrition survey collects data on dietary supplements through a 24-h recall method [15]. Of the 23,657 survey participants from 2015 to 2017, 20,837 who participated in the dietary supplements survey were selected for this study (Figure 1). In addition, we excluded subjects that aged <19 years (n = 4378), those with missing laboratory data (n = 1586), and those consuming two or more dietary supplements simultaneously (n = 1602). The remaining 13,271 subjects were selected for this study, including 2752 subjects who used dietary supplements and 733 subjects with CKD. We defined CKD as dipstick-positive proteinuria or estimated glomerular filtration rate (eGFR) ≤ 60 mL/min/1.73 m2, calculated using the Chronic Kidney Disease Epidemiology Collaboration equation.
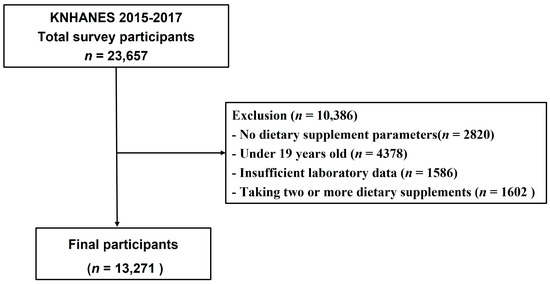
Figure 1.
Flowchart of study population.
2.2. Different Types of Dietary Supplements
Dietary supplement data were assessed by using the following questions: “Did you consume dietary supplements over the past 24 h?” and “What is the brand name and manufacturer name of the dietary supplement that you used over the past 24 h?” According to the Health Fictional Food Code issued by the Ministry of Food and Drug Safety Notification (No. 2020-92) [16], dietary supplements were classified as aloe, amino acids and protein, gamma-linolenic acid, ginseng and red ginseng, glucosamine, herbal medicine and plant extract, lutein containing supplements, methyl sulfonyl methane, milk thistle, chlorella/spirulina, omega-3 fatty acid, probiotics (pre-, post-), propolis, and vitamin and mineral (Figure 2). Owing to the many different types of herbal medicines and plant extract used, we divided herbs into herbal medicine (plant extract) Asian, herbal medicine (plant extract) berry, herbal medicine (plant extract) ginkgo biloba, and herbal medicine (plant extract) others. Although we found 46 different dietary supplements, only the top 17 dietary supplements have been used in this study, and the rest of the dietary supplements were classified into “OTHER category (e.g., honey extract, linolenic acid dietary fiber, hyaluronic acid, placenta and collagen, squalene and alkoxyglycerol, and ursodeoxycholic acid).
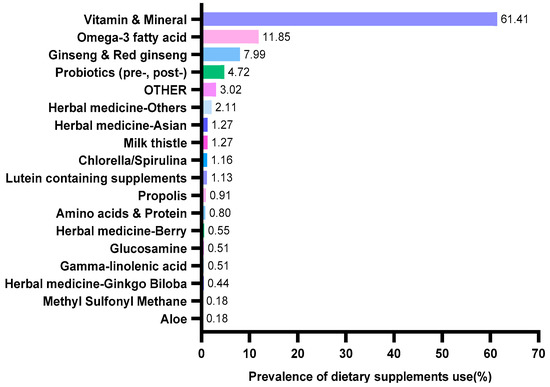
Figure 2.
Prevalence of dietary supplements usage categories by Korean adults.
2.3. Assessment of Covariates
The covariates for this study included sociodemographic variables (age, sex, and education level), body mass index (BMI) (calculated as weight/height2), smoking status, drinking status, physical activity, systolic blood pressure (SBP), diastolic blood pressure (DBP), and medical history (hypertension and diabetes). Serum creatinine (Cr), fasting blood glucose (FBG), and triglyceride (TG) levels were measured.
2.4. Statistical Analyses
All statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA). Differences between continuous variables were analyzed using the independent t-test or Mann–Whitney U test, while categorical variables were examined using the chi-square test. Multivariable logistic regression analysis was used to analyze the association between dietary supplement consumption and CKD. Model 1 is a crude model without adjustment. Model 2 was adjusted for age and gender. Model 3 was adjusted for all variables in Model 2, as well as smoking status (smoker, ex-smoker, and non-smoker), drinking status (never, less than once a month, 1–4 times a month, and ≥5 times a month), education level (less than elementary school graduation, middle School graduation, high School graduation and college graduate or higher), physical activity (≥2 days/week, <2 days/week), SBP, DBP, BMI (<25 kg/m2, ≥25 kg/m2), FBG level, and TG level.
3. Results
3.1. General Characteristics of the Study Subjects
Table 1 shows the characteristics of 13,271 study subjects, including 2752 in the dietary supplement group. The participants in the dietary supplement group were more likely to be women (p < 0.001), older adults (p < 0.001), non-smoker (p < 0.001), and highly educated individuals (p < 0.001). In addition, the DBP (p = 0.048) and serum Cr (p = 0.001) levels were high in the non-dietary supplement group. Among those who taking dietary supplements (Figure 2), most participants consumed vitamin and mineral (61.41%, n = 1690), followed by omega-3 fatty acid (11.85%, n = 326) and ginseng and red ginseng (7.99%, n = 210).

Table 1.
General characteristics of participants (n = 13,271).
3.2. Association between Different Dietary Supplements and CKD
Table 2 displays dietary supplements statuses of the participants. Overall, amino acids and protein (p < 0.031), ginseng and red ginseng (p < 0.009), and herbal medicine (plant extract)-berry (p < 0.046) users had a higher prevalence of CKD compared to non-users. However, the CKD prevalence was lower in the group consuming probiotics than in non-user group (p < 0.017). Age is an important confounder; therefore, we redefined the CKD group according to the age-adapted eGFR threshold proposed in a recent study [17], and found that the prevalence of CKD was also high in the group that consumed ginseng and red ginseng (p < 0.03) (Supplementary Table S1). However, the above results cannot rule out the possibility that CKD patients could be overusing dietary supplements. Therefore, to clarify the direct association of dietary supplements with the development of CKD, we further analyzed dietary supplement intake in healthy individuals without a history or risk factors for CKD such as diabetes, hypertension, hyperlipidemia, and cardiovascular diseases. The results showed that the group consuming ginseng and red ginseng had a higher prevalence of CKD compared with the non-users (p < 0.035) (Supplementary Table S2).

Table 2.
Dietary supplements statuses of the participants (n = 13,271).
In subgroup analysis according to sex, we observed that female participants who consumed amino acids and proteins (p < 0.009), ginseng and red ginseng (p < 0.007), and herbal medicine (plant extracts)-berries (p < 0.009) had a higher incidence of CKD than those who did not (Supplementary Table S3).
Logistic regression analysis was used to explore the association between the different dietary supplements and CKD (Table 3). After adjusting for age and sex, we observed that the prevalence of CKD was significantly high only in the herbal medicine (plant extract)-berry group (odds ratio (OR): 4.598, 95% confidence interval (CI): 1.123–18.821). Based on Model 2, we added smoking status, drinking status, education level, activity level, BMI, SBP, DBP, fasting glucose, and triglyceride for adjustment (Model 3). This result was similar to that of Model 2 (OR, 4.809; 95% CI, 1.077–21.473).

Table 3.
Odds ratio (OR) of the incidence of CKD according to take different dietary supplements.
4. Discussion
The present large cross-sectional study assessed the extent of dietary supplement use in Korea and found that participants who consumed some dietary supplements (e.g., acids and protein, ginseng and red ginseng, and herbal medicine-berry) had a higher CKD prevalence than those who did not. In addition, the group consuming probiotics had a lower prevalence of CKD than the group that did not consume probiotics. To our knowledge, this is the first study to examine the association between dietary supplements and CKD in an Asian cohort of over 10,000 participants.
Similar to those previously reported in other studies [5,10,18,19], the most commonly consumed dietary supplements in Korea were vitamin and mineral (61.41%), omega-3 fatty acid (11.85%) and probiotics (pre-, post-) (4.72%). However, the important difference is that Koreans prefer to consume ginseng and red ginseng compared with people in other countries (7.99%). Ginseng is mainly grown in East Asian countries, and Korea is one of the world’s leading producers of ginseng [20,21]. It has been valued for its remarkable therapeutic properties. Increasing evidence has demonstrated that ginsenoside, the main component of ginseng, has antioxidant, anti-apoptotic, and inhibitory effects on inflammatory cytokines. It can also modulate blood pressure and metabolism [21,22]. The association between ginseng and red ginseng consumption and kidney disease is controversial and not fully understood. Karunasagara et al. [23] demonstrated that red ginseng showed renoprotective effects in streptozotocin-induced diabetic rats and suppressed renal inflammation and fibrosis by blocking TGF-β1 activation. Sun et al. [24] revealed that ginsenoside Rb1 can reduce renal apoptosis and alleviate renal dysfunction by activating the Nrf2/ARE signaling pathway and enhancing heme oxygenase expression. However, clinical studies have reported conflicting results. A randomized controlled trial found that long-term ginseng intake did not affect renal function in patients [25]. Additionally, other studies have reported the negative effects of ginseng consumption on the kidneys [26,27,28]. In the present study, we found that participants who consumed ginseng and red ginseng had a high prevalence of CKD. The results were the same even in a healthy population with no history or risk factors for CKD, suggesting that ginseng consumption may have a direct effect on kidney function. Although the mechanism is unknown, it is possible that ginseng has been consumed in excessive quantities, or that interactions between ginseng and drugs have had an effect. A recent study reported that ginseng has a strong interaction effect, especially in patients taking anticoagulants [29]. Considering that dietary supplementation users’ insufficient recognition of the herb-drug interactions, standards and guidelines for the safety of dietary supplements based on information about the medications taken are needed.
Probiotics, prebiotics, and synbiotics are important dietary supplements whose market has rapidly increased in recent years. Several studies have revealed that a bidirectional relationship exists between the gut microbiota and the kidney, and CKD itself can trigger dysbiosis and an altered intestinal environment. This substantial derangement is mainly caused by the decreased consumption of dietary fibers, frequent use of antibiotics, intestinal wall edema, metabolic acidosis, and uremia [30,31,32]. Recent studies have shown that dysbiosis and leaky gut in CKD are associated with an altered mucosal immune response through activation of intestinal immune cells with inflammatory cytokine production, potentially resulting in systemic inflammation and exacerbated cardiovascular/renal complications [33,34]. Therefore, improving the gut environment is considered one of the interventions to slow down the progression of CKD and prevent CKD-related complications, and probiotic consumption has attracted much attention as an adjuvant therapy to modulate gut dysbiosis. Several animal studies have demonstrated that probiotic supplements improve renal inflammation and fibrosis progression in animals with CKD [35,36]. In addition, some clinical studies have demonstrated that synbiotics have beneficial effects on improving intestinal dysbiosis and reducing serum p-cresyl sulfate levels in pre-dialysis CKD patients, and probiotic supplementation improves glucose homeostasis and systemic inflammation in dialysis patients [37,38,39]. However, owing to the limited number of studies and small sample sizes, they do not provide strong evidence for the efficacy of prebiotics or probiotics in treating CKD patients [39].
In our study, the group that consumed probiotics (pre-and post-) had a lower prevalence of CKD than the group that did not consume probiotics (pre-and post-). Given the abundance of evidence indicating the importance of kidney-gut interactions in patients with kidney disease, clinical studies are needed to demonstrate the effectiveness of microbiome-modifying therapies in large-scale CKD patients.
Modifying protein intake is an important dietary strategy for slowing CKD. Several RCTs have evaluated the effect of dietary protein restriction on renal outcomes, and overall, they suggested the benefit of dietary protein restriction. The 2020 Kidney Disease Outcomes Quality Initiative guidelines recommend dietary protein restriction in patients with metabolically stable pre-dialysis CKD to reduce disease progression or mortality [40].
According to a prospective cohort study based on the Korean Genome and Epidemiology Study conducted between 2001 and 2014, high total protein intake-induced renal hyperfiltration causes fast eGFR decline in healthy adults with normal renal function [41]. A large Italian general-population study also evaluated the effects of protein intake on serum creatinine and eGFR through questionnaire [42]. Results showed an association between protein intake and decreased renal function, and also confirmed the association between higher protein diets and eGFR levels, even excluding participants with known diabetes, hypertension or CKD. Similarly, we observed a higher prevalence of CKD among participants consuming amino acid or protein supplements. According to a recent study, the average dietary protein intake of Koreans is almost twice the estimated average requirement [43]. Therefore, additional protein supplementation can lead to an excessive protein load on the body and renal damage in several ways through increased glomerular pressure and an additional acid load on the kidney [42,44,45]. Therefore, protein supplementation should be prescribed with particular caution in patients with kidney disease, and further evaluation of the effects of prolonged exposure to high protein intake on renal function in healthy subjects is warranted.
Interestingly, we observed that consumption of herbal supplements, including berries, was associated with CKD incidence, even after adjusting for the major risk factors for the development of CKD, such as age, BMI, SBP, DBP, and fasting glucose level; this relationship was prevalent among participants with a history of CKD and CKD risk factors. Berries have conquered the global market as dietary supplements rich in vitamins, dietary prebiotic fibers, and micronutrients (e.g., zinc and iron) [46]. Morsy. et al. [47] showed that prophylactic administration of açaí berry extract in an ischemia-reperfusion animal model can improve renal function parameters and suppress the expression of renal proinflammatory cytokines and endothelin-1. Nair et al. [48] found that blueberries can protect rats with metabolic syndrome from chronic kidney injury by inhibiting Toll-like receptor 4 and attenuating mitogen-activated protein kinase activity. This renoprotective effect is attributed to the high content of flavonoids, polyphenols, and other bioactive compounds with powerful antioxidant and anti-inflammatory properties [46,49]. However, high concentrations of flavonoids in berries, especially anthocyanins, have been reported to have a cyclooxygenase (COX) inhibitory action similar to non-steroidal anti-inflammatory drugs (NSAIDs) [26,50]. NSAID-induced COX inhibition is known to be associated with CKD progression; therefore, we do not exclude the possibility that chronic use of berries may produce a similar clinical phenotype [51,52]. Another noteworthy relationship between berry consumption and kidney disease is that some berries contain a significant amount of potassium, such as 100 g of blackcurrants contain 322 mg of potassium [53], and hyperkalemia may be associated with the risk of arrhythmia or worsening of heart failure and CKD [54,55]. However, in this study, further analysis such as potassium levels or possible toxicants in berry consumers could not be performed and berries were not analyzed by type. Considering that some types of berries have a positive effect on kidney health, additional research is needed to analyze changes in kidney function after consumption of different berry types.
This study has several important advantages. Despite the steady growth of the global supplement market over the past decade, there is a dearth of information regarding the association between dietary supplements and kidney disease. This study did not simply examine the association between dietary supplements and CKD but explored as many as 17 different dietary supplements. In addition, we analyzed the general Korean population of more than 10,000 individuals using KNHANES data that are representative of the entire population. Third, to reduce the effects of the interaction between different dietary supplements, we excluded participants who consumed two or more dietary supplements from the study design. However, this study also has some limitations. First, because this was a cross-sectional study, the results cannot prove a causal relationship between CKD and dietary supplements. Secondly, since these data are only for Korean adults, the results may not be extended to other ethnic groups, and future studies in other countries and races should be conducted.
5. Conclusions
Our study was a large cross-sectional study that examined the association between dietary supplementation and CKD in >10,000 individuals. The study found that high incidence of CKD in the groups consuming dietary supplements, indicating that not all dietary supplements were safe for kidneys. Due to the growth of the dietary supplement market, we require special attention to the potential risk of dietary supplements to the kidney health. Furthermore, future large cohort studies are needed to provide knowledge about the precise role of each dietary supplement in CKD progression and to establish safety recommendations and guidelines for dietary supplements.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu15040822/s1, Supplementary Table S1. General participant characteristics Supplementary Table S2. General characteristics of participants with and without a history of CKD or risk factors for CKD. Supplementary Table S3. General characteristics of participants according to sex.
Author Contributions
Conceptualization, M.-G.K.; data analysis and interpretation, Y.F., H.L., S.S. and M.-G.K.; writing—original draft preparation, Y.F.; critical revision of the article, S.O., S.-K.J., W.C. and M.-G.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was conducted in accordance with the guidelines of the Declaration of Helsinki, and the study protocol for the survey was approved by the Institutional Review Board of the Korea Centers for Disease Control and Prevention (IRB No. 2018-01-03-P-A).
Informed Consent Statement
Written informed consent was obtained from each participant in the KNHANES at the time of enrollment.
Data Availability Statement
The Korea National Health and Nutrition Examination Survey (KNHANES) data are publicly available on the official KNHANES website (https://knhanes.kdca.go.kr, accessed on 19 November 2022).
Acknowledgments
We are grateful to the KNHANES participants, and we thank the field staff for data collection.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Levin, A.; Tonelli, M.; Bonventre, J.; Coresh, J.; Donner, J.A.; Fogo, A.B.; Fox, C.S.; Gansevoort, R.T.; Heerspink, H.J.L.; Jardine, M.; et al. Global kidney health 2017 and beyond: A roadmap for closing gaps in care, research, and policy. Lancet 2017, 390, 1888–1917. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.H.; Tsai, C.C.; Liu, Y.H.; Wu, P.Y.; Huang, J.C.; Chung, T.L.; Su, H.M.; Chen, S.C. Sex Difference in the Associations among Hyperuricemia with New-Onset Chronic Kidney Disease in a Large Taiwanese Population Follow-Up Study. Nutrients 2022, 14, 3832. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Grams, M.E. Epidemiology research to foster improvement in chronic kidney disease care. Kidney Int. 2020, 97, 477–486. [Google Scholar] [CrossRef]
- Lin, P.C.; Chou, C.L.; Ou, S.H.; Fang, T.C.; Chen, J.S. Systematic Review of Nutrition Supplements in Chronic Kidney Diseases: A GRADE Approach. Nutrients 2021, 13, 469. [Google Scholar] [CrossRef]
- Ronis, M.J.J.; Pedersen, K.B.; Watt, J. Adverse Effects of Nutraceuticals and Dietary Supplements. Annu. Rev. Pharmacol. Toxicol. 2018, 58, 583–601. [Google Scholar] [CrossRef] [PubMed]
- Tan, E.C.K.; Eshetie, T.C.; Gray, S.L.; Marcum, Z.A. Dietary Supplement Use in Middle-aged and Older Adults. J. Nutr. Health Aging 2022, 26, 133–138. [Google Scholar] [CrossRef]
- Wierzejska, R.E. Dietary Supplements-For Whom? The Current State of Knowledge about the Health Effects of Selected Supplement Use. Int. J. Environ. Res. Public Health 2021, 18, 8897. [Google Scholar] [CrossRef]
- Crawford, C.; Brown, L.L.; Costello, R.B.; Deuster, P.A. Select Dietary Supplement Ingredients for Preserving and Protecting the Immune System in Healthy Individuals: A Systematic Review. Nutrients 2022, 14, 4604. [Google Scholar] [CrossRef]
- Kim, M.; Lee, Y.; Park, K. Vitamin and Mineral Supplement Use among Korean Adults: Baseline Data from the Trace Element Study of Korean Adults in Yeungnam Area. Nutrients 2018, 10, 50. [Google Scholar] [CrossRef]
- Choi, J. The Association between Health Conditions, Consciousness, Involvement, and Knowledge and Dietary Supplement Intake among University Students in South Korea. Int. J. Environ. Res. Public Health 2019, 16, 4028. [Google Scholar] [CrossRef]
- Rovin, B.H.; Adler, S.G.; Barratt, J.; Bridoux, F.; Burdge, K.A.; Chan, T.M.; Cook, H.T.; Fervenza, F.C.; Gibson, K.L.; Glassock, R.J.; et al. Executive summary of the KDIGO 2021 Guideline for the Management of Glomerular Diseases. Kidney Int. 2021, 100, 753–779. [Google Scholar] [CrossRef] [PubMed]
- Junarta, J.; Jha, V.; Banerjee, D. Insight into the impact of vitamin D on cardiovascular outcomes in chronic kidney disease. Nephrology 2019, 24, 781–790. [Google Scholar] [CrossRef] [PubMed]
- Tian, N.; Li, L.; Ng, J.K.; Li, P.K. The Potential Benefits and Controversies of Probiotics Use in Patients at Different Stages of Chronic Kidney Disease. Nutrients 2022, 14, 4044. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.J.; Guo, J.; Wang, Q.; Wang, L.; Wang, Y.; Zhang, F.; Huang, W.J.; Zhang, W.; Liu, W.J.; Wang, Y. Probiotics, prebiotics, and synbiotics for the improvement of metabolic profiles in patients with chronic kidney disease: A systematic review and meta-analysis of randomized controlled trials. Crit. Rev. Food Sci. Nutr. 2021, 61, 577–598. [Google Scholar] [CrossRef]
- The Homepage of Korea National Health and Nutrition Examination Survey. Available online: https://kosis.kr/index/index.do (accessed on 15 May 2022).
- Health Functional Food Code. Available online: https://www.mfds.go.kr/index.do (accessed on 1 May 2022).
- Delanaye, P.; Jager, K.J.; Bökenkamp, A.; Christensson, A.; Dubourg, L.; Eriksen, B.O.; Gaillard, F.; Gambaro, G.; van der Giet, M.; Glassock, R.J.; et al. CKD: A Call for an Age-Adapted Definition. J. Am. Soc. Nephrol. JASN 2019, 30, 1785–1805. [Google Scholar] [CrossRef]
- Alsaleem, S.A.; Asiri, M.M.; Alsaleem, M.A.; AlShahrani, A.N.; Alamer, K.A.; Mahfouz, A.A. Dietary Supplement Use among Primary Health Care Attendants in Abha City, Southwestern Saudi Arabia. Nutrients 2021, 13, 2968. [Google Scholar] [CrossRef]
- Brown, A.C. An overview of herb and dietary supplement efficacy, safety and government regulations in the United States with suggested improvements. Part 1 of 5 series. Food Chem. Toxicol. 2017, 107, 449–471. [Google Scholar] [CrossRef]
- Xu, W.; Choi, H.K.; Huang, L. State of Panax ginseng Research: A Global Analysis. Molecules 2017, 22, 1518. [Google Scholar] [CrossRef]
- Zhang, H.; Abid, S.; Ahn, J.C.; Mathiyalagan, R.; Kim, Y.J.; Yang, D.C.; Wang, Y. Characteristics of Panax ginseng Cultivars in Korea and China. Molecules 2020, 25, 2635. [Google Scholar] [CrossRef]
- Park, S.H.; Chung, S.; Chung, M.Y.; Choi, H.K.; Hwang, J.T.; Park, J.H. Effects of Panax ginseng on hyperglycemia, hypertension, and hyperlipidemia: A systematic review and meta-analysis. J. Ginseng Res. 2022, 46, 188–205. [Google Scholar] [CrossRef]
- Karunasagara, S.; Hong, G.L.; Park, S.R.; Lee, N.H.; Jung, D.Y.; Kim, T.W.; Jung, J.Y. Korean red ginseng attenuates hyperglycemia-induced renal inflammation and fibrosis via accelerated autophagy and protects against diabetic kidney disease. J. Ethnopharmacol. 2020, 254, 112693. [Google Scholar] [CrossRef]
- Sun, Q.; Meng, Q.T.; Jiang, Y.; Liu, H.M.; Lei, S.Q.; Su, W.T.; Duan, W.N.; Wu, Y.; Xia, Z.Y.; Xia, Z.Y. Protective effect of ginsenoside Rb1 against intestinal ischemia-reperfusion induced acute renal injury in mice. PLoS ONE 2013, 8, e80859. [Google Scholar] [CrossRef] [PubMed]
- Stavro, P.M.; Woo, M.; Leiter, L.A.; Heim, T.F.; Sievenpiper, J.L.; Vuksan, V. Long-term intake of North American ginseng has no effect on 24-hour blood pressure and renal function. Hypertension 2006, 47, 791–796. [Google Scholar] [CrossRef] [PubMed]
- Grubbs, V.; Plantinga, L.C.; Tuot, D.S.; Hedgeman, E.; Saran, R.; Saydah, S.; Rolka, D.; Powe, N.R. Americans’ use of dietary supplements that are potentially harmful in CKD. Am. J. Kidney Dis. 2013, 61, 739–747. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Yoon, J.H.; Kim, S.S.; Ma, S.K.; Kim, S.W.; Bae, E.H. Panax Ginseng Induces Toxic Hepatitis and Acute Kidney Injury. Chonnam Med. J. 2017, 53, 168–169. [Google Scholar] [CrossRef]
- Paik, D.J.; Lee, C.H. Review of cases of patient risk associated with ginseng abuse and misuse. J. Ginseng Res. 2015, 39, 89–93. [Google Scholar] [CrossRef]
- Mohammadi, S.; Asghari, G.; Emami-Naini, A.; Mansourian, M.; Badri, S. Herbal Supplement Use and Herb-drug Interactions among Patients with Kidney Disease. J. Res. Pharm. Pract. 2020, 9, 61–67. [Google Scholar] [CrossRef]
- Mahmoodpoor, F.; Rahbar Saadat, Y.; Barzegari, A.; Ardalan, M.; Zununi Vahed, S. The impact of gut microbiota on kidney function and pathogenesis. Biomed. Pharmacother. Biomed. Pharmacother. 2017, 93, 412–419. [Google Scholar] [CrossRef]
- Feng, Z.; Wang, T.; Dong, S.; Jiang, H.; Zhang, J.; Raza, H.K.; Lei, G. Association between gut dysbiosis and chronic kidney disease: A narrative review of the literature. J. Int. Med. Res. 2021, 49, 3000605211053276. [Google Scholar] [CrossRef]
- Kim, M.G.; Yang, J.; Jo, S.K. Intestinal microbiota and kidney diseases. Kidney Res. Clin. Pract. 2021, 40, 335–343. [Google Scholar] [CrossRef]
- Yang, J.; Lim, S.Y.; Ko, Y.S.; Lee, H.Y.; Oh, S.W.; Kim, M.G.; Cho, W.Y.; Jo, S.K. Intestinal barrier disruption and dysregulated mucosal immunity contribute to kidney fibrosis in chronic kidney disease. Nephrol Dial Transpl. 2019, 34, 419–428. [Google Scholar] [CrossRef]
- Ramezani, A.; Raj, D.S. The gut microbiome, kidney disease, and targeted interventions. J. Am. Soc. Nephrol. 2014, 25, 657–670. [Google Scholar] [CrossRef]
- Wang, I.K.; Yen, T.H.; Hsieh, P.S.; Ho, H.H.; Kuo, Y.W.; Huang, Y.Y.; Kuo, Y.L.; Li, C.Y.; Lin, H.C.; Wang, J.Y. Effect of a Probiotic Combination in an Experimental Mouse Model and Clinical Patients with Chronic Kidney Disease: A Pilot Study. Front. Nutr. 2021, 8, 661794. [Google Scholar] [CrossRef]
- Zhu, H.; Cao, C.; Wu, Z.; Zhang, H.; Sun, Z.; Wang, M.; Xu, H.; Zhao, Z.; Wang, Y.; Pei, G.; et al. The probiotic L. casei Zhang slows the progression of acute and chronic kidney disease. Cell Metab. 2021, 33, 1926–1942. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.; Johnson, D.W.; Morrison, M.; Pascoe, E.M.; Coombes, J.S.; Forbes, J.M.; Szeto, C.C.; McWhinney, B.C.; Ungerer, J.P.; Campbell, K.L. Synbiotics Easing Renal Failure by Improving Gut Microbiology (SYNERGY): A Randomized Trial. Clin. J. Am. Soc. Nephrol. CJASN 2016, 11, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Soleimani, A.; Zarrati Mojarrad, M.; Bahmani, F.; Taghizadeh, M.; Ramezani, M.; Tajabadi-Ebrahimi, M.; Jafari, P.; Esmaillzadeh, A.; Asemi, Z. Probiotic supplementation in diabetic hemodialysis patients has beneficial metabolic effects. Kidney Int. 2017, 91, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.; Yang, J.; Ji, G.E.; Park, M.S.; Seong, Y.; Oh, S.W.; Kim, M.G.; Cho, W.Y.; Jo, S.K. The effect of probiotic supplementation on systemic inflammation in dialysis patients. Kidney Res. Clin. Pract. 2022, 41, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Ikizler, T.A.; Burrowes, J.D.; Byham-Gray, L.D.; Campbell, K.L.; Carrero, J.J.; Chan, W.; Fouque, D.; Friedman, A.N.; Ghaddar, S.; Goldstein-Fuchs, D.J.; et al. KDOQI Clinical Practice Guideline for Nutrition in CKD: 2020 Update. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2020, 76, S1–S107. [Google Scholar] [CrossRef]
- Jhee, J.H.; Kee, Y.K.; Park, S.; Kim, H.; Park, J.T.; Han, S.H.; Kang, S.W.; Yoo, T.H. High-protein diet with renal hyperfiltration is associated with rapid decline rate of renal function: A community-based prospective cohort study. Nephrol. Dial. Transplant. 2020, 35, 98–106. [Google Scholar] [CrossRef]
- Vukovic, V.; Hantikainen, E.; Raftopoulou, A.; Gögele, M.; Rainer, J.; Domingues, F.S.; Pramstaller, P.P.; Garcia-Larsen, V.; Pattaro, C. Association of dietary proteins with serum creatinine and estimated glomerular filtration rate in a general population sample: The CHRIS study. J. Nephrol. 2022, 36, 103–114. [Google Scholar] [CrossRef]
- Kim, E.; Chung, S.; Hwang, J.-T.; Park, Y.J. 2020 Korean Dietary Reference Intakes for Protein: Estimation of protein requirements and the status of dietary protein intake in the Korean population. J. Nutr. Health 2022, 55, 10–20. [Google Scholar] [CrossRef]
- Ko, G.J.; Obi, Y.; Tortorici, A.R.; Kalantar-Zadeh, K. Dietary protein intake and chronic kidney disease. Curr. Opin. Clin. Nutr. Metab. Care 2017, 20, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Oba, R.; Kanzaki, G.; Sasaki, T.; Okabayashi, Y.; Haruhara, K.; Koike, K.; Kobayashi, A.; Yamamoto, I.; Tsuboi, N.; Yokoo, T. Dietary Protein Intake and Single-Nephron Glomerular Filtration Rate. Nutrients 2020, 12, 2549. [Google Scholar] [CrossRef]
- Nunes, S.; Vieira, P.; Gomes, P.; Viana, S.D.; Reis, F. Blueberry as an Attractive Functional Fruit to Prevent (Pre)Diabetes Progression. Antioxidants 2021, 10, 1162. [Google Scholar] [CrossRef] [PubMed]
- El Morsy, E.M.; Ahmed, M.A.; Ahmed, A.A. Attenuation of renal ischemia/reperfusion injury by açaí extract preconditioning in a rat model. Life Sci. 2015, 123, 35–42. [Google Scholar] [CrossRef]
- Nair, A.R.; Elks, C.M.; Vila, J.; Del Piero, F.; Paulsen, D.B.; Francis, J. A blueberry-enriched diet improves renal function and reduces oxidative stress in metabolic syndrome animals: Potential mechanism of TLR4-MAPK signaling pathway. PLoS ONE 2014, 9, e111976. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Sun, Z.; Zeng, Y.; Luo, M.; Yang, J. Molecular Mechanism and Health Role of Functional Ingredients in Blueberry for Chronic Disease in Human Beings. Int. J. Mol. Sci. 2018, 19, 2785. [Google Scholar] [CrossRef] [PubMed]
- Belmonte-Herrera, B.H.; Domínguez-Avila, J.A.; Wall-Medrano, A.; Ayala-Zavala, J.F.; Preciado-Saldaña, A.M.; Salazar-López, N.J.; López-Martínez, L.X.; Yahia, E.M.; Robles-Sánchez, R.M.; González-Aguilar, G.A. Lesser-Consumed Tropical Fruits and Their by-Products: Phytochemical Content and Their Antioxidant and Anti-Inflammatory Potential. Nutrients 2022, 14, 3663. [Google Scholar] [CrossRef]
- Wan, E.Y.F.; Yu, E.Y.T.; Chan, L.; Mok, A.H.Y.; Wang, Y.; Chan, E.W.Y.; Wong, I.C.K.; Lam, C.L.K. Comparative Risks of Nonsteroidal Anti-Inflammatory Drugs on CKD. Clin. J. Am. Soc. Nephrol. CJASN 2021, 16, 898–907. [Google Scholar] [CrossRef]
- Gooch, K.; Culleton, B.F.; Manns, B.J.; Zhang, J.; Alfonso, H.; Tonelli, M.; Frank, C.; Klarenbach, S.; Hemmelgarn, B.R. NSAID use and progression of chronic kidney disease. Am. J. Med. 2007, 120, 280.e281–e287. [Google Scholar] [CrossRef]
- Are Berries High in Potassium? Available online: https://tastylicious.com/berries-potassium/ (accessed on 17 December 2022).
- Kovesdy, C.P.; Matsushita, K.; Sang, Y.; Brunskill, N.J.; Carrero, J.J.; Chodick, G.; Hasegawa, T.; Heerspink, H.L.; Hirayama, A.; Landman, G.W.D.; et al. Serum potassium and adverse outcomes across the range of kidney function: A CKD Prognosis Consortium meta-analysis. Eur. Heart J. 2018, 39, 1535–1542. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S.; Inaba, M. Potassium Metabolism and Management in Patients with CKD. Nutrients 2021, 13, 1751. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).