The Antiplatelet Effect of 4-Methylcatechol in a Real Population Sample and Determination of the Mechanism of Action
Highlights
- The effect of 4 – methylcatechol, a microbiome derived polyphenol/dietary metabolite, on platelet aggregation was investigated using whole human blood and platelet rich plasma.
- 4-methylcatechol is a potent antiplatelet compound that blocks aggregation induced by a wide range of inducers/agonists.
- 4-methylcatechol acts as a disruptor of cyclooxygenase and thromboxane synthase coupling.
- The observed effect of 4-methylcatechol in healthy volunteers may explain, at least in part, the beneficial effects of polyphenol rich diet on cardiovascular health.
- 4-methylcatechol is a strong candidate for a novel antiplatelet compound.
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Methodology
2.2.1. Donor Criteria
2.2.2. Blood Collection
2.2.3. Blood Processing
2.2.4. Biochemical Assessment of Creatinine
2.2.5. Aggregometry—Group Study
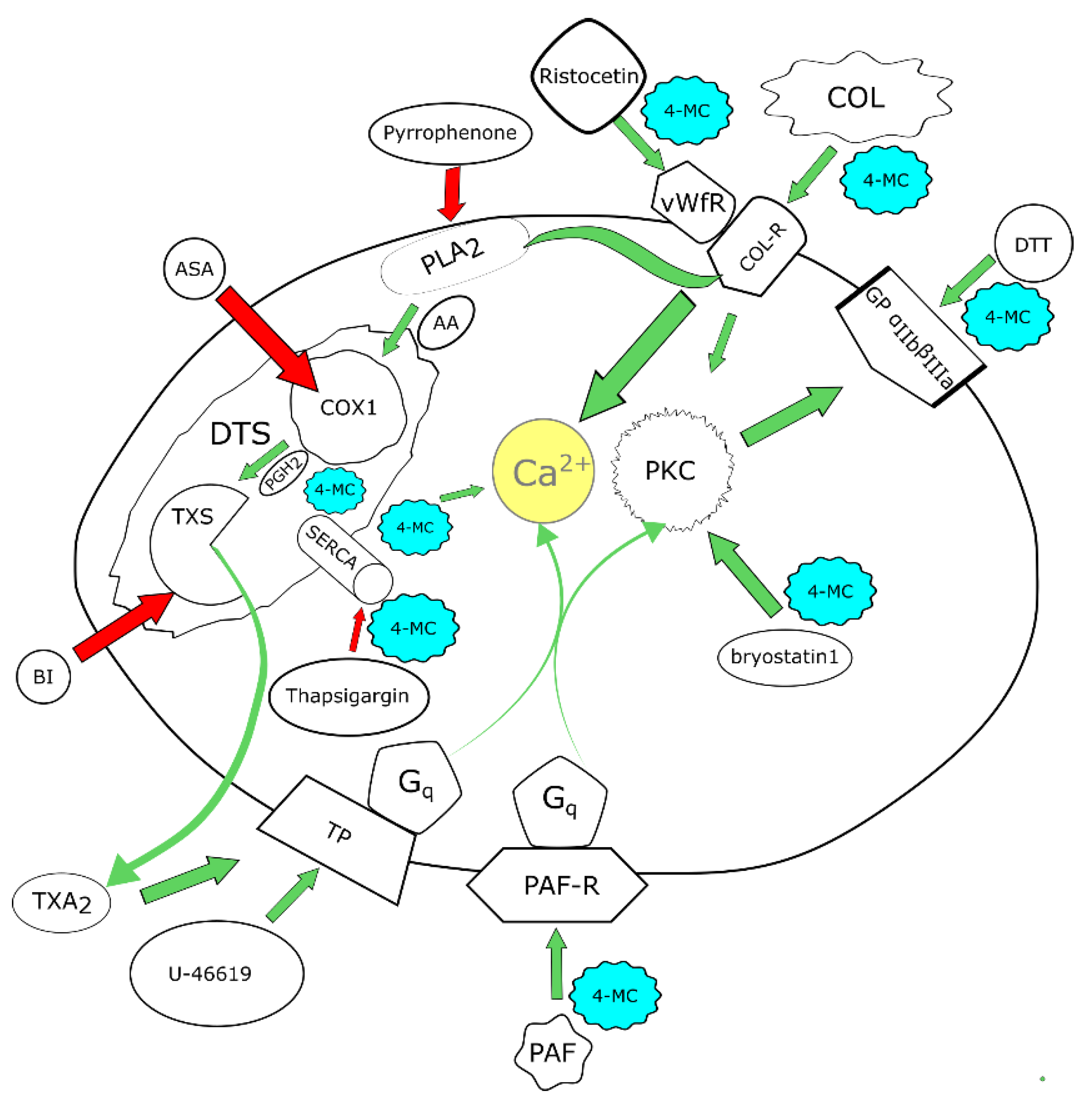
2.2.6. Mechanistic Experiments
Thromboxane Synthase Assay
2.2.7. Statistical Analysis
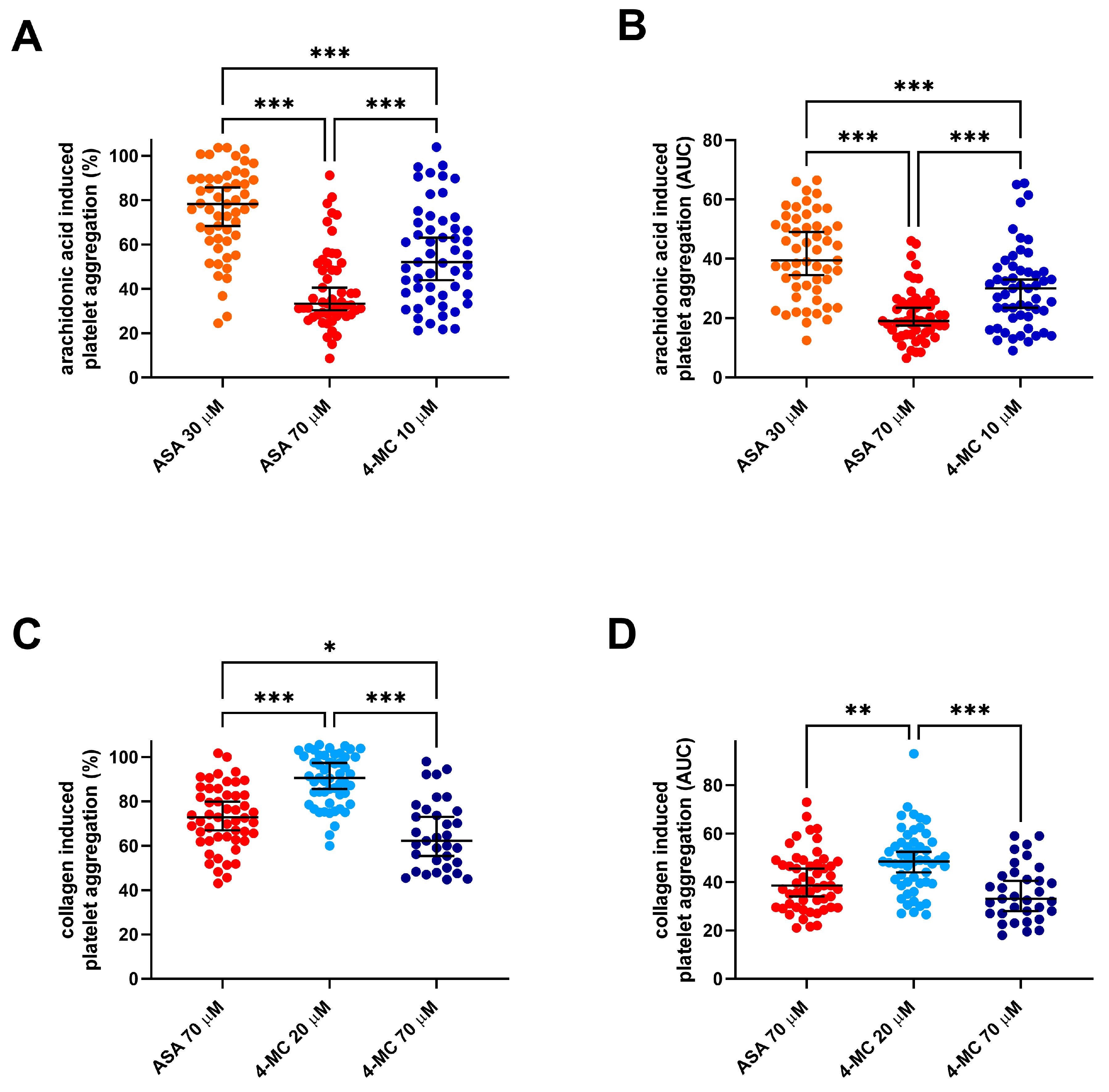
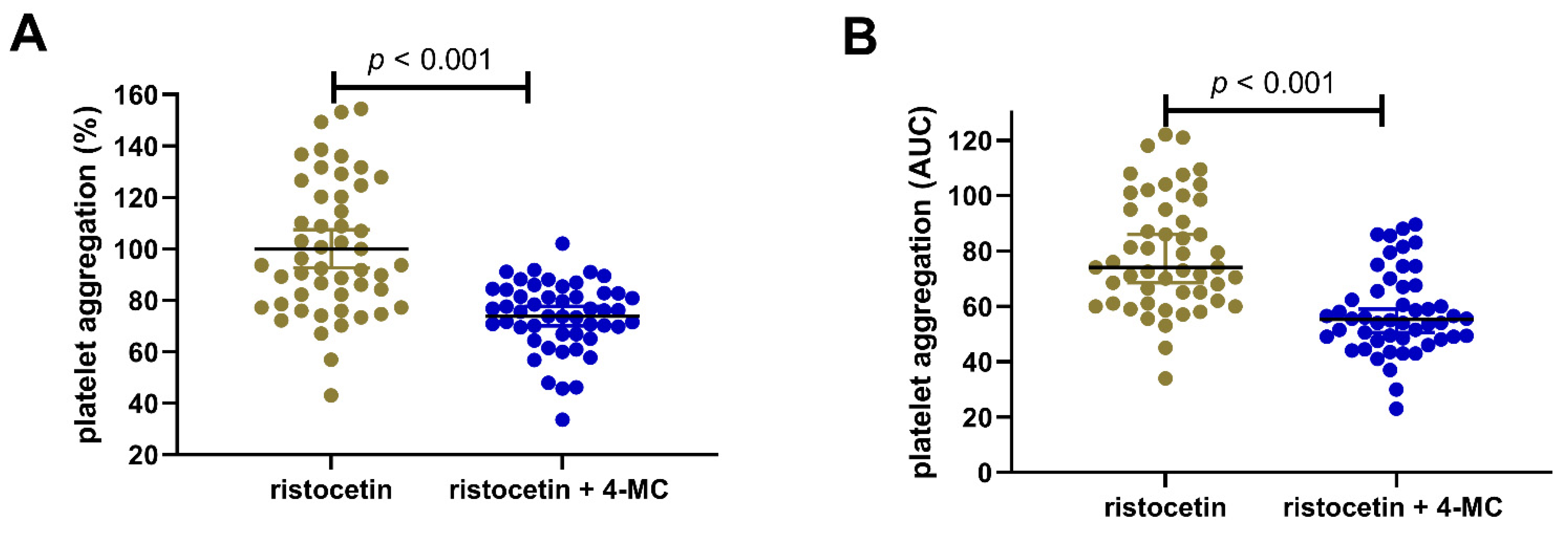
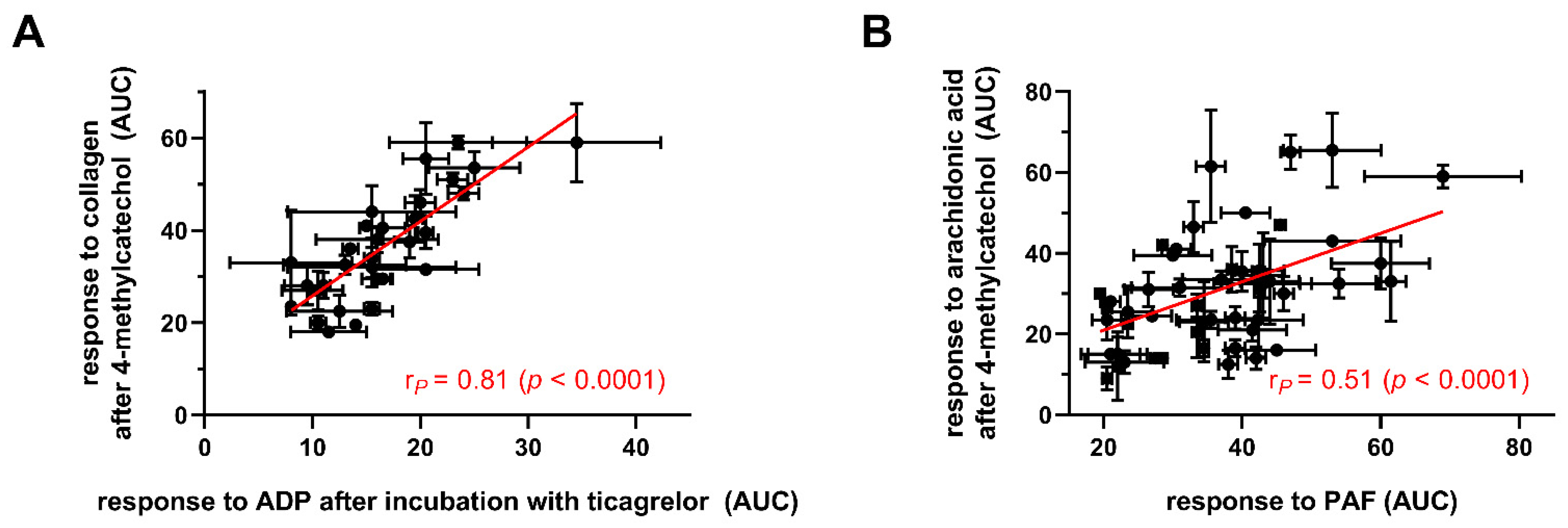
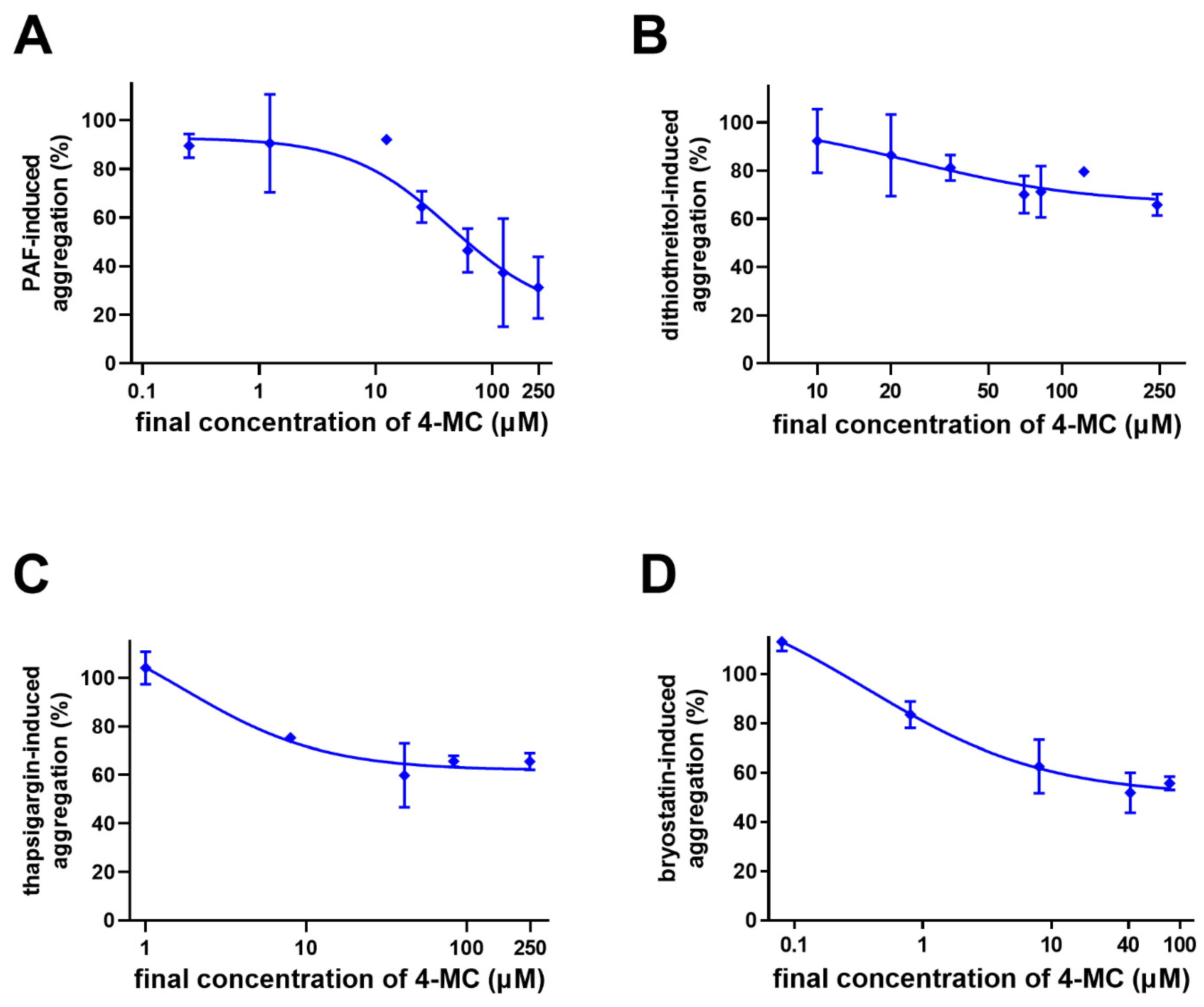
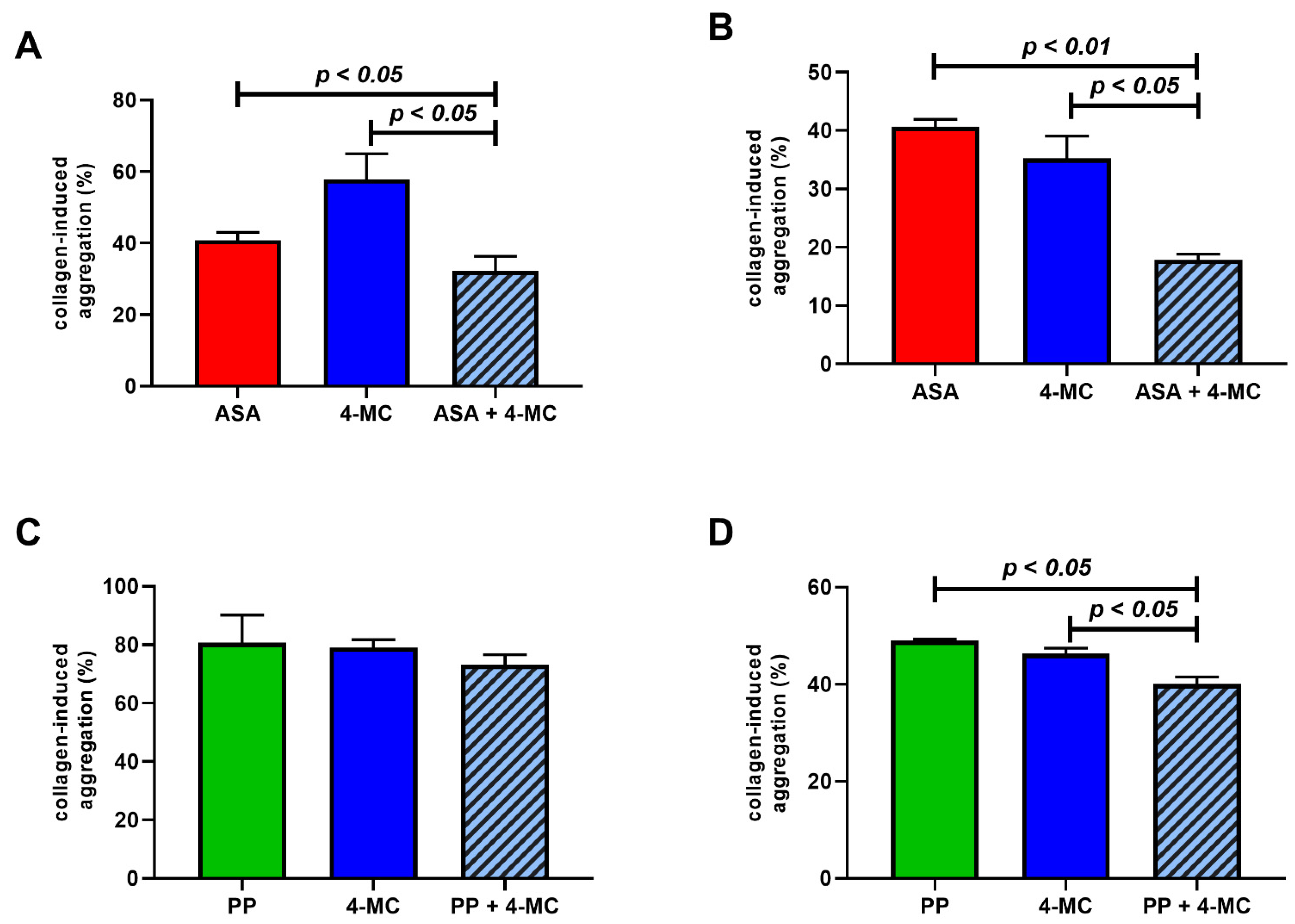
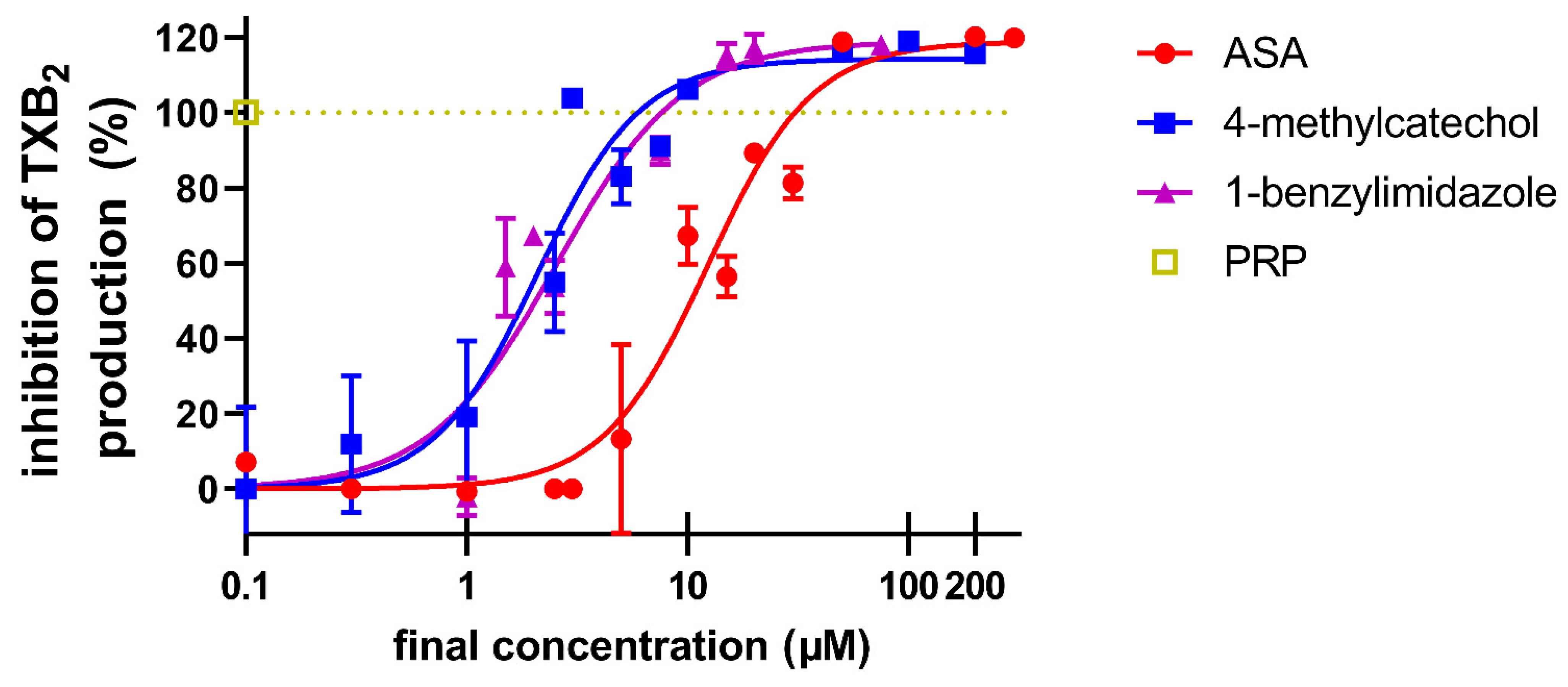
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Gasparyan, A.Y.; Watson, T.; Lip, G.Y. The role of aspirin in cardiovascular prevention: Implications of aspirin resistance. J. Am. Coll. Cardiol. 2008, 51, 1829–1843. [Google Scholar] [CrossRef] [PubMed]
- Giorgi, M.A.; Cohen Arazi, H.; Gonzalez, C.D.; Di Girolamo, G. Beyond efficacy: Pharmacokinetic differences between clopidogrel, prasugrel and ticagrelor. Expert Opin. Pharmacother. 2011, 12, 1285–1295. [Google Scholar] [CrossRef] [PubMed]
- Tantry, U.S.; Bliden, K.P.; Chaudhary, R.; Novakovic, M.; Rout, A.; Gurbel, P.A. Vorapaxar in the treatment of cardiovascular diseases. Future Cardiol. 2020, 16, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Kasmeridis, C.; Apostolakis, S.; Lip, G.Y.H. Aspirin and aspirin resistance in coronary artery disease. Curr. Opin. Pharmacol. 2013, 13, 242–250. [Google Scholar] [CrossRef]
- Mărginean, A.; Bănescu, C.; Scridon, A.; Dobreanu, M. Anti-platelet Therapy Resistance—Concept, Mechanisms and Platelet Function Tests in Intensive Care Facilities. J. Crit. Care Med. 2016, 2, 6–15. [Google Scholar] [CrossRef]
- Lopez, L.R.; Guyer, K.E.; Torre, I.G.; Pitts, K.R.; Matsuura, E.; Ames, P.R. Platelet thromboxane (11-dehydro-Thromboxane B2) and aspirin response in patients with diabetes and coronary artery disease. World J. Diabetes 2014, 5, 115–127. [Google Scholar] [CrossRef]
- Applová, L.; Karlíčková, J.; Warncke, P.; Macáková, K.; Hrubša, M.; Macháček, M.; Tvrdý, V.; Fischer, D.; Mladěnka, P. 4-Methylcatechol, a Flavonoid Metabolite with Potent Antiplatelet Effects. Mol. Nutr. Food Res. 2019, 63, 1900261. [Google Scholar] [CrossRef]
- Fukuhara, K.; Ishikawa, K.; Yasuda, S.; Kishishita, Y.; Kim, H.K.; Kakeda, T.; Yamamoto, M.; Norii, T.; Ishikawa, T. Intracerebroventricular 4-methylcatechol (4-MC) ameliorates chronic pain associated with depression-like behavior via induction of brain-derived neurotrophic factor (BDNF). Cell. Mol. Neurobiol. 2012, 32, 971–977. [Google Scholar] [CrossRef]
- Gezginci-Oktayoglu, S.; Coskun, E.; Ercin, M.; Bolkent, S. 4-Methylcatechol prevents streptozotocin-induced acute kidney injury through modulating NGF/TrkA and ROS-related Akt/GSK3β/β-catenin pathways. Int. Immunopharmacol. 2018, 64, 52–59. [Google Scholar] [CrossRef]
- Hsieh, Y.-L.; Lin, W.-M.; Lue, J.-H.; Chang, M.-F.; Hsieh, S.-T. Effects of 4-Methylcatechol on Skin Reinnervation: Promotion of Cutaneous Nerve Regeneration After Crush Injury. J. Neuropathol. Exp. Neurol. 2009, 68, 1269–1281. [Google Scholar] [CrossRef]
- Ishikawa, K.; Yasuda, S.; Fukuhara, K.; Iwanaga, Y.; Ida, Y.; Ishikawa, J.; Yamagata, H.; Ono, M.; Kakeda, T.; Ishikawa, T. 4-Methylcatechol prevents derangements of brain-derived neurotrophic factor and TrkB-related signaling in anterior cingulate cortex in chronic pain with depression-like behavior. Neuroreport 2014, 25, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.E.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (poly)phenolics in human health: Structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid. Redox Signal. 2013, 18, 1818–1892. [Google Scholar] [CrossRef]
- Mursu, J.; Voutilainen, S.; Nurmi, T.; Tuomainen, T.P.; Kurl, S.; Salonen, J.T. Flavonoid intake and the risk of ischaemic stroke and CVD mortality in middle-aged Finnish men: The Kuopio Ischaemic Heart Disease Risk Factor Study. Br. J. Nutr. 2008, 100, 890–895. [Google Scholar] [CrossRef] [PubMed]
- Feliciano, R.P.; Boeres, A.; Massacessi, L.; Istas, G.; Ventura, M.R.; Nunes Dos Santos, C.; Heiss, C.; Rodriguez-Mateos, A. Identification and quantification of novel cranberry-derived plasma and urinary (poly)phenols. Arch. Biochem. Biophys. 2016, 599, 31–41. [Google Scholar] [CrossRef]
- Nieman, D.C.; Gillitt, N.D.; Knab, A.M.; Shanely, R.A.; Pappan, K.L.; Jin, F.; Lila, M.A. Influence of a polyphenol-enriched protein powder on exercise-induced inflammation and oxidative stress in athletes: A randomized trial using a metabolomics approach. PLoS ONE 2013, 8, e72215. [Google Scholar] [CrossRef] [PubMed]
- Stalmach, A.; Edwards, C.A.; Wightman, J.D.; Crozier, A. Colonic catabolism of dietary phenolic and polyphenolic compounds from Concord grape juice. Food Funct. 2013, 4, 52–62. [Google Scholar] [CrossRef]
- Pan, P.; Skaer, C.W.; Stirdivant, S.M.; Young, M.R.; Stoner, G.D.; Lechner, J.F.; Huang, Y.W.; Wang, L.S. Beneficial Regulation of Metabolic Profiles by Black Raspberries in Human Colorectal Cancer Patients. Cancer Prev. Res. 2015, 8, 743–750. [Google Scholar] [CrossRef]
- Krcmova, L.; Solichova, D.; Melichar, B.; Kasparova, M.; Plisek, J.; Sobotka, L.; Solich, P. Determination of neopterin, kynurenine, tryptophan and creatinine in human serum by high throuput HPLC. Talanta 2011, 85, 1466–1471. [Google Scholar] [CrossRef]
- Chemical, C. Thromboxane B2 ELISA Kit (Item No. 501020). Available online: https://www.caymanchem.com/product/501020 (accessed on 8 January 2022).
- Mladěnka, P.; Zatloukalová, L.; Filipský, T.; Hrdina, R. Cardiovascular effects of flavonoids are not caused only by direct antioxidant activity. Free Radic. Biol. Med. 2010, 49, 963–975. [Google Scholar] [CrossRef]
- Rothwell, J.A.; Urpi-Sarda, M.; Boto-Ordoñez, M.; Llorach, R.; Farran-Codina, A.; Barupal, D.K.; Neveu, V.; Manach, C.; Andres-Lacueva, C.; Scalbert, A. Systematic analysis of the polyphenol metabolome using the Phenol-Explorer database. Mol. Nutr. Food Res. 2016, 60, 203–211. [Google Scholar] [CrossRef]
- Pourová, J.; Najmanová, I.; Vopršalová, M.; Migkos, T.; Pilařová, V.; Applová, L.; Nováková, L.; Mladěnka, P. Two flavonoid metabolites, 3,4-dihydroxyphenylacetic acid and 4-methylcatechol, relax arteries ex vivo and decrease blood pressure in vivo. Vasc. Pharmacol. 2018, 111, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Kamato, D.; Thach, L.; Bernard, R.; Chan, V.; Zheng, W.; Kaur, H.; Brimble, M.; Osman, N.; Little, P.J. Structure, Function, Pharmacology, and Therapeutic Potential of the G Protein, Gα/q,11. Front. Cardiovasc. Med. 2015, 2, 14. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, N.; Itoh, H. Functions and Regulatory Mechanisms of Gq-Signaling Pathways. Neurosignals 2009, 17, 42–54. [Google Scholar] [CrossRef] [PubMed]
- Grabarek, J.; Ware, J.A. Protein kinase C activation without membrane contact in platelets stimulated by bryostatin. J. Biol. Chem. 1993, 268, 5543–5549. [Google Scholar] [CrossRef]
- Carr, M.E., Jr.; Carr, S.L.; Grant, S. A sensitive platelet activation-based functional assay for the antileukemic agent bryostatin 1. Anticancer. Drugs 1995, 6, 384–391. [Google Scholar] [CrossRef]
- Authi, K.S.; Bokkala, S.; Patel, Y.; Kakkar, V.V.; Munkonge, F. Ca2+ release from platelet intracellular stores by thapsigargin and 2,5-di-(t-butyl)-1,4-benzohydroquinone: Relationship to Ca2+ pools and relevance in platelet activation. Biochem. J. 1993, 294 Pt 1, 119–126. [Google Scholar] [CrossRef]
- Thastrup, O.; Linnebjerg, H.; Bjerrum, P.J.; Knudsen, J.B.; Christensen, S.B. The inflammatory and tumor-promoting sesquiterpene lactone, thapsigargin, activates platelets by selective mobilization of calcium as shown by protein phosphorylations. Biochim. Biophys. Acta 1987, 927, 65–73. [Google Scholar] [CrossRef]
- Shibata, K.; Kitayama, S.; Morita, K.; Shirakawa, M.; Okamoto, H.; Dohi, T. Regulation by protein kinase C of platelet-activating factor- and thapsigargin-induced calcium entry in rabbit neutrophils. Jpn. J. Pharmacol. 1994, 66, 273–276. [Google Scholar] [CrossRef]
- Margaritis, A.; Priora, R.; Frosali, S.; Di Giuseppe, D.; Summa, D.; Coppo, L.; Di Stefano, A.; Di Simplicio, P. The role of protein sulfhydryl groups and protein disulfides of the platelet surface in aggregation processes involving thiol exchange reactions. Pharmacol. Res. 2011, 63, 77–84. [Google Scholar] [CrossRef]
- Yan, B.; Smith, J.W. Mechanism of integrin activation by disulfide bond reduction. Biochemistry 2001, 40, 8861–8867. [Google Scholar] [CrossRef]
- Ono, T.; Yamada, K.; Chikazawa, Y.; Ueno, M.; Nakamoto, S.; Okuno, T.; Seno, K. Characterization of a novel inhibitor of cytosolic phospholipase A2alpha, pyrrophenone. Biochem. J. 2002, 363, 727–735. [Google Scholar] [CrossRef] [PubMed]
- Prévost, N.; Mitsios, J.V.; Kato, H.; Burke, J.E.; Dennis, E.A.; Shimizu, T.; Shattil, S.J. Group IVA cytosolic phospholipase A2 (cPLA2alpha) and integrin alphaIIbbeta3 reinforce each other’s functions during alphaIIbbeta3 signaling in platelets. Blood 2009, 113, 447–457. [Google Scholar] [CrossRef] [PubMed]
- Gerrard, J.M.; White, J.G.; Peterson, D.A. The platelet dense tubular system: Its relationship to prostaglandin synthesis and calcium flux. Thromb. Haemost. 1978, 40, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Hrubša, M.; Alva, R.; Parvin, M.S.; Macáková, K.; Karlíčková, J.; Fadraersada, J.; Konečný, L.; Moravcová, M.; Carazo, A.; Mladěnka, P. Comparison of Antiplatelet Effects of Phenol Derivatives in Humans. Biomolecules 2022, 12, 117. [Google Scholar] [CrossRef]
- Nitta, A.; Ito, M.; Fukumitsu, H.; Ohmiya, M.; Ito, H.; Sometani, A.; Nomoto, H.; Furukawa, Y.; Furukawa, S. 4-Methylcatechol Increases Brain-Derived Neurotrophic Factor Content and mRNA Expression in Cultured Brain Cells and in Rat Brain In Vivo. J. Pharmacol. Exp. Ther. 1999, 291, 1276. [Google Scholar]
- Glässer, G.; Graefe, E.U.; Struck, F.; Veit, M.; Gebhardt, R. Comparison of antioxidative capacities and inhibitory effects on cholesterol biosynthesis of quercetin and potential metabolites. Phytomedicine 2002, 9, 33–40. [Google Scholar] [CrossRef]
- Morita, K.; Arimochi, H.; Ohnishi, Y. In vitro cytotoxicity of 4-methylcatechol in murine tumor cells: Induction of apoptotic cell death by extracellular pro-oxidant action. J. Pharmacol. Exp. Ther. 2003, 306, 317–323. [Google Scholar] [CrossRef]
- Furukawa, Y.; Urano, T.; Minamimura, M.; Nakajima, M.; Okuyama, S.; Furukawa, S. 4-Methylcatechol-induced heme oxygenase-1 exerts a protective effect against oxidative stress in cultured neural stem/progenitor cells via PI3 kinase/Akt pathway. Biomed. Res. 2010, 31, 45–52. [Google Scholar] [CrossRef]
- Payton, F.; Bose, R.; Alworth, W.L.; Kumar, A.P.; Ghosh, R. 4-Methylcatechol-induced oxidative stress induces intrinsic apoptotic pathway in metastatic melanoma cells. Biochem. Pharmacol. 2011, 81, 1211–1218. [Google Scholar] [CrossRef]
- Senger, D.R.; Hoang, M.V.; Kim, K.H.; Li, C.; Cao, S. Anti-inflammatory activity of Barleria lupulina: Identification of active compounds that activate the Nrf2 cell defense pathway, organize cortical actin, reduce stress fibers, and improve cell junctions in microvascular endothelial cells. J. Ethnopharmacol. 2016, 193, 397–407. [Google Scholar] [CrossRef]
- Li, C.J.; Jiang, Y.W.; Chen, S.X.; Li, H.J.; Chen, L.; Liu, Y.T.; Gao, S.; Zhao, Y.; Zhu, X.L.; Wang, H.T.; et al. 4-Methylcatechol inhibits cell growth and testosterone production in TM3 Leydig cells by reducing mitochondrial activity. Andrologia 2017, 49, e12581. [Google Scholar] [CrossRef] [PubMed]
- Karatug Kacar, A.; Gezginci-Oktayoglu, S.; Bolkent, S. 4-Methylcatechol stimulates apoptosis and reduces insulin secretion by decreasing betacellulin and inhibin beta-A in INS-1 beta-cells. Hum. Exp. Toxicol. 2018, 37, 1123–1130. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.; Zhou, Q.; Lin, J.; Chi, L. Aspirin resistance in Chinese stroke patients increased the rate of recurrent stroke and other vascular events. Int J. Stroke 2013, 8, 535–539. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.D.; Park, C.-Y.; Ahn, K.J.; Cho, J.H.; Choi, K.M.; Kang, J.G.; Kim, J.H.; Lee, K.Y.; Lee, B.W.; Mok, J.O.; et al. Non-HDL cholesterol is an independent risk factor for aspirin resistance in obese patients with type 2 diabetes. Atherosclerosis 2014, 234, 146–151. [Google Scholar] [CrossRef] [PubMed]
| Tested Compound | Final Concentration | Inducer | Final Concentration 1 |
|---|---|---|---|
| 4-MC | 240 µM | ristocetin | 400 µM |
| 70 µM | collagen | 1 µg/mL | |
| 20 µM | |||
| 10 µM | AA | 200 µM | |
| ASA | 70 µM | collagen | 1 µg/mL |
| AA | 200 µM | ||
| 30 µM | AA | 200 µM | |
| ticagrelor | 0.5 µM | ADP | 5 µM |
| vorapaxar | 1 and 5 µM | TRAP | 10 µM |
| U-46619 | 80 nM | ||
| PAF | 20 nM |
| 4-MC 10 μM + AA 200 μM | 4-MC 20 μM + Collagen 1 μg/mL | 4-MC 70 µM + Collagen 1 µg/mL | 4-MC 240 µM + Ristocetin | |
|---|---|---|---|---|
| AA 60 µM | 0.67 *** | 0.39 ** | n.s. | 0.26 (p = 0.07) |
| AA 200 µM | 0.36 ** | 0.78 *** | 0.47 ** | 0.60 *** |
| collagen 0.16 µg/mL | 0.55 *** | 0.61 *** | 0.63 *** | 0.29 * |
| collagen 1 µg/mL | 0.29 * | 0.87 *** | 0.60 *** | 0.55 *** |
| ADP | 0.54 *** | 0.63 *** | 0.55 ** | 0.58 *** |
| ristocetin | 0.57 *** | 0.65 *** | 0.34 (p = 0.06) | 0.71 *** |
| TRAP | 0.30 * | 0.65 *** | n.s. | 0.49 *** |
| U-44619 | 0.43 ** | 0.60 *** | n.s. | 0.45 ** |
| PAF | 0.51 *** | 0.51 *** | n.s. | 0.52 *** |
| ASA 30 μM + AA 200 μM | 0.69 *** | 0.50 *** | 0.35 * | 0.29 * |
| ASA 70 μM + AA 200 μM | 0.60 *** | 0.28 * | n.s. | n.s. |
| ASA 70 μM + collagen 1 μg/mL | 0.32 * | 0.81 *** | 0.69 *** | 0.47 *** |
| ticagrelor + ADP | 0.36 * | 0.59 *** | 0.81 *** | 0.36 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hrubša, M.; Konečný, L.; Paclíková, M.; Parvin, M.S.; Skořepa, P.; Musil, F.; Karlíčková, J.; Javorská, L.; Matoušová, K.; Krčmová, L.K.; et al. The Antiplatelet Effect of 4-Methylcatechol in a Real Population Sample and Determination of the Mechanism of Action. Nutrients 2022, 14, 4798. https://doi.org/10.3390/nu14224798
Hrubša M, Konečný L, Paclíková M, Parvin MS, Skořepa P, Musil F, Karlíčková J, Javorská L, Matoušová K, Krčmová LK, et al. The Antiplatelet Effect of 4-Methylcatechol in a Real Population Sample and Determination of the Mechanism of Action. Nutrients. 2022; 14(22):4798. https://doi.org/10.3390/nu14224798
Chicago/Turabian StyleHrubša, Marcel, Lukáš Konečný, Markéta Paclíková, Mst Shamima Parvin, Pavel Skořepa, František Musil, Jana Karlíčková, Lenka Javorská, Kateřina Matoušová, Lenka Kujovská Krčmová, and et al. 2022. "The Antiplatelet Effect of 4-Methylcatechol in a Real Population Sample and Determination of the Mechanism of Action" Nutrients 14, no. 22: 4798. https://doi.org/10.3390/nu14224798
APA StyleHrubša, M., Konečný, L., Paclíková, M., Parvin, M. S., Skořepa, P., Musil, F., Karlíčková, J., Javorská, L., Matoušová, K., Krčmová, L. K., Carazo, A., Šmahelová, A., Blaha, V., & Mladěnka, P. (2022). The Antiplatelet Effect of 4-Methylcatechol in a Real Population Sample and Determination of the Mechanism of Action. Nutrients, 14(22), 4798. https://doi.org/10.3390/nu14224798








