Abstract
As the relation between serum non-high-density lipoprotein cholesterol (nHDL) level and renal outcomes has never been investigated in patients with non-dialysis chronic kidney disease (CKD) yet, we here aimed to unveil the association of nHDL with CKD progression. A total of 2152 patients with non-dialysis CKD at stages 1 to 5 from the KNOW-CKD study were categorized into the tertile (i.e., 1st (T1), 2nd (T2), and 3rd (T3) tertiles) by nHDL, and were prospectively analyzed. The primary outcome was the composite renal event, defined as a composite of decline of kidney function or onset of end-stage renal disease. Kaplan–Meier survival curves analysis demonstrated that the cumulative incidence of the composite renal event was significantly increased in T1 and T3, compared to T2 (p = 0.028, by Log-rank test). Cox regression analysis revealed that both T1 (adjusted hazard ratio 1.309, 95% confidence interval 1.074–1.595) and T3 (adjusted hazard ratio 1.272, 95% confidence interval 1.040–1.556) are associated with significantly increased risk of a composite renal event, compared to T2. The restricted cubic spline plot demonstrated a non-linear, U-shaped association between nHDL and the risk of a composite renal event. In conclusion, both low and high serum nHDL levels are associated with increased risk of CKD progression.
1. Introduction
Dyslipidemia is a common complication of chronic kidney disease (CKD) [1,2,3]. The current guidelines on the management of dyslipidemia in CKD recommends the use of statins, which mainly target low-density lipoprotein cholesterol (LDL-C) levels to prevent cardiovascular (CV) events [4]. Yet, among the patients with CKD, abnormalities in triglycerides (TG) and high-density lipoprotein cholesterol (HDL-C) levels are more prominent characteristics [1,2,3], which are known to increase the risk of adverse CV events in the general population [5,6,7] as well as in patients with CKD [8,9]. Thus, it seems obvious that dyslipidemia is a therapeutic target of paramount importance in the management of CKD.
Mounting evidence now suggests that dyslipidemia is not only a complication of CKD, but may also be associated with the development and progression of CKD [10,11,12,13,14], as shown in a number of large-scale, prospective cohort studies. The Physicians’ Health Study demonstrated that a variety of abnormal lipid profiles including high non-high-density lipoprotein cholesterol (nHDL) were closely related to the risk of renal dysfunction in 4483 initially healthy subjects [15]. The Framingham Offspring Study reported that, in the analysis of 2585 participants, the HDL-C level reduced with the risk of incident CKD [16]. By analyzing 12,728 subjects, the Atherosclerosis Risk in Communities Study concluded that high TG and low HDL-C, but not LDL-C, levels are associated with the risk of renal dysfunction [17]. Although numerous lipid indices have been examined so far, there is still no conclusive result that one is superior to the other in the estimation of renal prognosis among the patients with non-dialysis CKD.
The serum nHDL level is equal to total cholesterol minus HDL-C [18], and represents all atherogenic lipoproteins, including intermediate-density lipoprotein, lipoprotein(a), LDL-C, and very low-density lipoprotein remnants [19], indicating that nHDL may be associated with adverse CV outcomes. Indeed, we recently reported that the elevated serum level of nHDL increased the risk of composite CV events among the patients with CKD [20]. To the best of our knowledge, the relation between nHDL and CKD progression, however, has never been investigated yet.
Therefore, we hypothesized that an elevated serum level of nHDL may also be associated with an increased risk of CKD progression. Taking advantage of more than 2000 subjects from the Korean Cohort Study for Outcomes in Patients With Chronic Kidney Disease (KNOW-CKD) cohort, we here aimed to address the association of nHDL with renal outcomes in patients with non-dialysis CKD.
2. Materials and Methods
2.1. Study Design
The KNOW-CKD study is previously described [21]. The study was conducted in accordance with the principles of the Declaration of Helsinki. The Institutional Review Board at each participating center approved the study protocol (Seoul National University Hospital (1104–089-359), Seoul National University Bundang Hospital (B-1106/129–008), Yonsei University Severance Hospital (4–2011-0163), Kangbuk Samsung Medical Center (2011–01-076), Seoul St. Mary’s Hospital (KC11OIMI0441), Gil Hospital (GIRBA2553), Eulji General Hospital (201105–01), Chonnam National University Hospital (CNUH-2011-092), and Busan Paik Hospital (11–091). Patients with non-dialysis CKD at all stages between the ages of 20 and 75 years were enrolled from 2011 through 2016. Informed consent was voluntarily obtained from all the participants. All the participants were under close observation during the follow-up period, and each participating center recorded the study outcomes. Among the participants who are longitudinally followed up (n = 2238), those without the baseline measurement of total cholesterol or HDL-C (n = 74), and those without the information on follow-up duration (n = 8), were excluded (Figure 1). Finally, a total of 2152 patients were included and analyzed. The study observation period ended on 31 March 2021. The median duration of follow-up was 6.940 years.
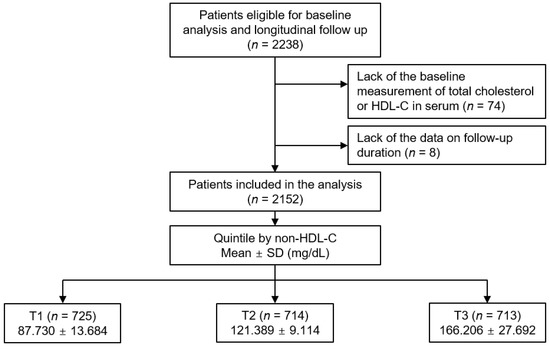
Figure 1.
Study design. Abbreviations: HDL-C, high-density lipoprotein cholesterol; nHDL, non-high-density lipoprotein cholesterol; T1, 1st tertile; T2, 2nd tertile; T3, 3rd tertile; SD, standard deviation.
2.2. Data Collection from Participants
Demographic information included age, gender, Charlson comorbidity index, primary cause of CKD, smoking history, and medication history. Anthropometric measurements included body mass index (BMI) and systolic and diastolic blood pressures (SBP and DBP). After overnight fasting, venous samples were drawn to determine the baseline laboratory measurement, including lipid profiles. The serum nHDL level was defined as total cholesterol minus HDL-C. The estimated glomerular filtration rate (eGFR) was calculated by the Chronic Kidney Disease Epidemiology Collaboration equation using the serum creatinine level [22]. CKD stages were determined by the Kidney Disease Improving Global Outcomes (KDIGO) guidelines [23]. Albuminuria was measured in spot urine samples. Echocardiographic data were collected using complete two-dimensional M-mode and Doppler studies via standard approaches [24]. The cardiologists who are were blinded to the clinical data performed the echocardiography at each participating study site.
2.3. Exposure and Study Outcome
The exposure was nHDL, by which the subjects were categorized into the tertile (i.e., 1st (T1), 2nd (T2), and 3rd (T3) tertiles) (Figure 1). The primary outcome was the composite renal event, defined as a composite of decline of renal function (the first occurrence of > 50% decline of eGFR or doubling of serum creatinine from the baseline) or initiation of renal replacement therapy (RRT) during follow-up periods. The secondary outcome was each of decline of renal function and initiation of RRT. For the accuracy on the clinical outcomes, the participating investigators cross-checked all outcome events.
2.4. Statistical Analysis
In the comparison of the baseline characteristics by nHDL, continuous variates were analyzed by one-way analysis of variance, while categorical variates were analyzed by χ2 test. Cumulative incidences of outcome events were visualized using Kaplan–Meier curves. The participants with any missing data were excluded for further analyses. To evaluate independent associations between nHDL and study outcomes, Cox regression analyses were adopted. Patients lost to follow-up were censored at the date of the last visit. The results of Cox proportional hazard models were presented as hazard ratios (HRs) and 95% confidence intervals (CIs). Restricted cubic spline plots were used to assess the relation between nHDL (as a continuous variable) with the study outcomes. To confirm our findings, we performed sensitivity analyses. First, we exclude the participants with eGFR ≥ 90 mL/min/1.73 m2, because the CKD patients at stage 1 have nearly normal kidney function and were considered not to represent the patients with CKD well. Second, we excluded the subjects with eGFR < 15 mL/min/1.73 m2, because the CKD patients at stage 5, which were relatively scanty, and because the burden of CKD may overly impact the association between serum nHDL and study outcomes. Third, we evaluated the cause-specific HRs of nHDL levels for the study outcomes, in which the death events that occur before the study outcome events were censored at the time of the death event. To examine whether the clinical contexts modify the association of nHDL with the study outcomes, we conducted pre-specified subgroup analyses. The subgroups were defined by age, gender, BMI, eGFR, and spot urine albumin-to-creatinine ratio (ACR). Two-sided p values < 0.05 were considered statistically significant. Statistical analysis was performed using SPSS for Windows version 22.0 (IBM Corp., Armonk, NY, USA) and R (version 4.1.1; R project for Statistical Computing, Vienna, Austria).
3. Results
3.1. Baseline Characteristics
To describe the baseline characteristics, the participants were categorized into the tertile by nHDL level (Table 1). The follow-up duration was not significantly different among the three groups. The mean age was highest and lowest in T1 and T3, respectively. The proportion of male gender was not significantly different among the three groups. The proportion with age-adjusted Charlson comorbidity index ≥ 4 was relatively higher in T1. The distribution of the primary cause of CKD, smoking history, and medication history were not significantly different among the three groups. BMI, waist circumference, and SBP and DBP were lowest and highest in T1 and T3, respectively. Hemoglobin, total cholesterol, LDL-C, TG and fasting glucose were also lowest and highest in T1 and T3, respectively. The serum albumin level was highest in T2, while the serum HDL-C level was highest in T1. Kidney function was best preserved in T2, as the spot urine ACR and serum creatinine level were lowest in T2. The echocardiographic indices, except for interventricular wall thickness, were not significantly different among the three groups (Supplementary Materials Table S1). The interventricular wall thickness was significantly increased in T3.

Table 1.
Baseline characteristics of study participants by nHDL level.
3.2. Association of nHDL with Renal Outcomes in Patients with Non-Dialysis CKD
To visualize the cumulative incidence of the study outcomes by nHDL levels, Kaplan–Meier survival curves were analyzed. The cumulative incidence of a composite renal event was significantly higher in T1 and T3, compared to T2 (p = 0.028 by Log-rank test, Figure 2), whereas that of decline of kidney function event (Supplementary Materials Figure S1) or onset of end-stage renal disease (ESRD) event (Supplementary Materials Figure S2) was not significantly different among the groups. To demonstrate an independent association between nHDL and CKD progression, Cox proportional hazard regression models were adopted, where both T1 (adjusted HR (aHR) 1.309, 95% CIs 1.074–1.595) and T3 (aHR 1.272, 95% CIs 1.040–1.556) are associated with significantly increased risk of a composite renal event, compared to T2 (Table 2). The risk of the secondary outcomes was not significantly different among the groups (Table 3). To assess the relation between nHDL (as a continuous variable) with the study outcomes, restricted cubic spline plots were used, which demonstrated a U-shaped association between nHDL and the risk of a composite renal event (Figure 3), whereas the relation of nHDL with the risk of decline of kidney function event (Supplementary Materials Figure S3) or onset of ESRD event (Supplementary Materials Figure S4) was rather linear, but substantially blunted.
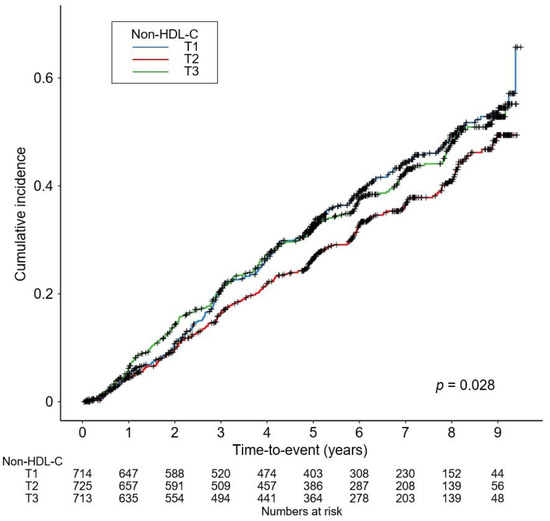
Figure 2.
Kaplan–Meier survival curve for cumulative incidence of composite renal event by nHDL. p value by Log-rank test. Abbreviations: nHDL, non-high-density lipoprotein cholesterol; T1, 1st tertile; T2, 2nd tertile; T3, 3rd tertile.

Table 2.
HRs for the primary outcome by nHDL level.

Table 3.
HRs for the secondary outcomes by nHDL level.
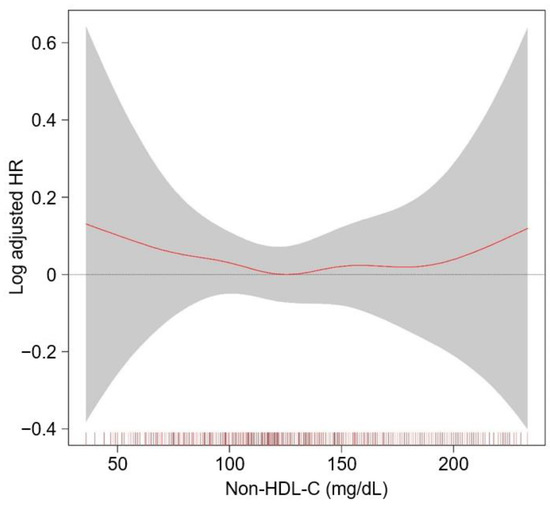
Figure 3.
Restricted cubic spline of nHDL on composite renal event. Adjusted HR of nHDL as a continuous variable for a composite CV event is depicted. The model was adjusted for age and sex, age-adjusted Charlson comorbidity index, primary cause of CKD, smoking history, medication (angiotensin-converting enzyme inhibitors/angiotensin receptor blockers, diuretic use, number of antihypertensive drugs, statins), body mass index, waist circumference, systolic blood pressure, hemoglobin, albumin, fasting glucose, hs-CRP, CKD stage, and spot urine ACR. Abbreviations: CI, confidence interval; HR, hazard ratio; nHDL, non-high-density lipoprotein cholesterol; CV, cardiovascular; CKD, chronic kidney disease; hs-CRP, high-sensitivity C-reactive protein; ACR, albumin-to-creatinine ratio.
3.3. Sensitivity Analyses
First, after excluding the subjects with eGFR ≥ 90 mL/min/1.73 m2, Cox regression analysis revealed that the risk of primary outcome was still significantly increased both in T1 (aHR 1.290, 95% CIs 1.054–1.579) and T3 (aHR 1.296, 95% CIs 1.055–1.596), compared to T2 (Supplementary Materials Table S2). After excluding the subjects with eGFR ≥ 90 mL/min/1.73 m2, the risk of decline of renal function event was not significantly different among the groups, while the risk of initiation of an RRT event was significantly increased in T3, compared to T2 (aHR 1.282, 95% CIs 1.014–1.619). Second, even after excluding the subjects with eGFR < 15 mL/min/1.73 m2, the risk of primary outcome was significantly increased both in T1 (aHR 1.369, 95% CIs 1.110–1.688) and T3 (aHR 1.278, 95% CIs 1.031–1.584), compared to T2 (Supplementary Materials Table S3). After excluding the subjects with eGFR < 15 mL/min/1.73 m2, the risk of decline of kidney function event was not significantly different among the groups either, whereas the risk of initiation of an RRT event was significantly increased in T3, compared to T2 (aHR 1.332, 95% CIs 1.032–1.719). Finally, in the competing risks analysis to estimate cause-specific HRs, the risk of primary outcome was robustly and significantly higher in T1 (aHR 1.309, 95% CIs 1.051–1.630) and T3 (aHR 1.273, 95% CIs 1.013–1.598), compared to T2, although the risk of the secondary outcomes was not significantly different among the groups (Table 4).

Table 4.
Cause-specific HRs for the study outcomes by nHDL level.
3.4. Subgroup Analyses
Subgroup analyses revealed that the association of nHDL with the risk of a composite renal event is altered by gender, BMI, and eGFR (Table 5). More specifically, the risk of a composite renal event was increased only in T3 and T1, compared to that in T2, among the male and female subjects, respectively (p for interaction = 0.007). The risk of a composite renal event in T1 and T3 tended to be decreased in the subjects with BMI < 23 kg/m2 and eGFR ≥ 45 mL/min/1.73 m2, compared to that in T2, whereas the risk in T1 and T3 was significantly increased in the subjects with BMI ≥ 23 kg/m2 (p for interaction = 0.035) and eGFR < 45 mL/min/1.73 m2 (p for interaction = 0.011).

Table 5.
HRs for the primary outcome by nHDL level in various subgroups.
4. Discussion
In the present study, we found that, contrary to our initial hypothesis, both low and high nHDL are associated with increased risk of CKD progression, demonstrating a non-linear, U-shaped association. We believe that our finding is robust, as the similar results were found in a series of sensitivity analyses, including the analysis of cause-specific hazard models. Moreover, we proved that, in the subgroup analyses, several clinical conditions, such as gender, BMI, and eGFR, alter the association of nHDL with the risk of CKD progression.
It is readily expected that high nHDL increases the risk of CKD progression, which is in agreement with the observation from healthy subjects, where high nHDL significantly increases the risk of incident renal dysfunction [15]. In fact, it has long been suggested that dyslipidemia may promote the development and progression of CKD [25]. Supporting the observations from clinical research, dietary lipid loading aggravates the glomerular lesions in a rodent model of experimental CKD [26]. It has been also reported that elevated levels of very low-density lipoprotein and intermediate-density lipoprotein, which are known to be highly atherogenic, induce proteinuria and glomerulosclerosis in a certain strain of female rats [27]. Therefore, our finding that high nHDL increases the risk of CKD progression seems reasonable.
In contrast, the other finding of the present study that even low nHDL increases the risk of CKD progression is beyond our initial expectation. Based on the definition, either low total cholesterol or a high serum HDL-C level may lead to low nHDL. A low total cholesterol level is closely related to malnutrition and inflammation among the patients with ESRD, leading to mortality [28]. A recent study also reported that those with a high risk of malnutrition were associated with poor baseline kidney function and an increased risk of CKD progression, especially among the elderly [29], suggesting that the association of low nHDL with increased risk of CKD progression in the current study could be attributed to a low total cholesterol level related to the underlying malnutrition-inflammation process.
Meanwhile, low nHDL could result from high serum HDL-C, which is true in the current study (Table 1). It should be noted that, although HDL-C has long been believed as a “good cholesterol” that exerts an anti-inflammatory effect, recent studies report that its anti-inflammatory activity is decreased in patients with CKD [30,31]. Moreover, even the pro-inflammatory role of HDL-C under uremic conditions has been reported [32,33]. Importantly, a study that investigated the association of serum HDL-C level and the risk of CKD progression reported that, not only low, but also high serum HDL-C level increases the risk of CKD progression [14]. Thus, the association between low nHDL and the risk of CKD progression shown in the present study could be directly explained by an elevated serum HDL-C level in patients with CKD.
A previous study reported that high nHDL increases the risk of incident CKD among the healthy male subjects [15], where no significant impact of low nHDL was reported. This may seem somewhat contradictory to our findings, though one should be reminded that the study population is entirely different between the two studies. As our study enrolled only CKD patients at stage 1 to pre-dialysis 5, the biological interpretation of low serum total cholesterol or a high serum HDL-C level should be substantially modified. Indeed, in the subgroup analyses (Table 5), we found that the association of nHDL and the risk of a composite renal event is significantly more prominent in the subjects with eGFR < 45 mL/min/1.73 m2, compared to those with eGFR ≥ 45 mL/min/1.73 m2.
The use of statins primarily targets a lower serum LDL-C level, though the optimal range of the goal has not been established, due to the lack of firm evidence supporting the benefits of statin therapy on CV outcome in patients with CKD [4,34]. It is of note that the current KDIGO clinical practice guideline does not state the role of LDL-C as well as nHDL in term of renal outcomes, despite accumulating evidence suggesting the detrimental role of dyslipidemia in CKD progression. In this regard, we propose that further studies should determine the optimal target range of nHDL level in patients with CKD.
We acknowledge a number of limitations in the current study. First, we are not able to definitively define the casual relation of nHDL with the risk of CKD progression, due to the observational nature of the present study. Second, the variables measured once at the baseline were included for the regression analyses. Third, as the cohort enrolled only Koreans resident in South Korea, the extrapolation of the data from the current study to the other populations requires a precaution.
5. Conclusions
In conclusion, we report that both low and high nHDL are associated with increased risk of CKD progression. Further studies are warranted to determine the optimal target range of nHDL level in patients with CKD.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/nu14214704/s1, Figure S1: Kaplan–Meier survival curve for cumulative incidence of decline of kidney function by nHDL; Figure S2: Kaplan–Meier survival curve for cumulative incidence of onset of ESRD by nHDL; Figure S3: Restricted cubic spline of nHDL on decline of kidney function; Figure S4: Restricted cubic spline of nHDL on onset of ESRD; Table S1: Summary of echocardiographic findings of study participants by nHDL; Table S2: HRs for the primary and secondary outcomes by nHDL level after excluding the subjects at CKD stage 1; Table S3: HRs for the primary and secondary outcomes by nHDL level after excluding the subjects at CKD stage 5.
Author Contributions
Conceptualization, S.H.S.; methodology, S.H.S., T.R.O., H.S.C. and C.S.K.; formal analysis, S.H.S.; resources, E.H.B., K.-H.O., Y.Y.H. and S.S.; data curation, S.H.S.; writing—original draft preparation, S.H.S.; writing—review and editing, S.H.S., S.K.M. and S.W.K.; supervision, S.K.M. and S.W.K.; funding acquisition, S.H.S., K.-H.O. and S.W.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Research Program funded by the Korea Disease Control and Prevention Agency (2011E3300300, 2012E3301100, 2013E3301600, 2013E3301601, 2013E3301602, 2016E3300200, 2016E3300201, 2016E3300202, 2019E320100, 2019E320101, 2019E320102, and 2022-11-007), by the National Research Foundation of Korea (NRF) funded by the Korea government (MSIT) (NRF-2020R1F1A1074001, NRF-2019R1A2C2086276), and by a grant (BCRI22042 and BCRI22079) from the Chonnam National University Hospital Biomedical Research Institute.
Institutional Review Board Statement
The study protocol was approved by the Institutional Review Board at each participating clinical center (Seoul National University Hospital (1104–089-359, 25 May 2011), Seoul National University Bundang Hospital (B-1106/129–008, 24 August 2011), Yonsei University Severance Hospital (4–2011-0163, 2 June 2011), Kangbuk Samsung Medical Center (2011–01-076, 16 June 2012), Seoul St. Mary’s Hospital (KC11OIMI0441, 30 June 2011), Gil Hospital (GIRBA2553, 8 August 2011), Eulji General Hospital (201105–01, 10 June 2011), Chonnam National University Hospital (CNUH-2011-092, 5 July 2011), and Busan Paik Hospital (11–091, 26 July2011)).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Not applicable.
Acknowledgments
KNOW-CKD: Study Group Clinical Centers. Seoul National University, Curie Ahn, Kook-Hwan Oh, Dong Wan Chae, Ho Jun Chin, Hayne Cho Park, Seungmi Lee, Hyun Hwa Jang and Hyun Jin Cho. Yonsei University, Severance Hospital, Kyu Hun Choi, Seung Hyeok Han, Tae Hyun Yoo and Mi Hyun Yu. Kangbuk Samsung Medical Center, Kyubeck Lee and Sooyeon Jin. The Catholic University of Korea, Seoul St. Mary’s Hospital, Yong-Soo Kim and Sol Ji Kim. Gachon University, Gil Hospital, Wookyung Chung, Youkyoung Jang and Ji Hye Park. Eulji University, Eulji General Hospital. Young-Hwan Hwang, Su-Ah Sung and Jeong Ok So. Chonnam University, Soo Wan Kim, MD, and Ji Seon Lee. Inje University, Pusan Paik Hospital, Yeong Hoon Kim, Sun Woo Kang and Yun Jin Kim. Epidemiology and Biostatistics Department of Preventive Medicine, Seoul National University College of Medicine, Byung-Joo Park, Sue Kyung Park and Juyeon Lee. Coordinating Center. Medical Research Collaborating Center, Seoul National University Hospital and Seoul National University College of Medicine, Joongyub Lee, Dayeon Nam, Soohee Kang and Heejung Ahn. Central Laboratory, Donghee Seo, Lab Genomics, Korea and Dae Yeon Cho, Lab Genomics, Korea. Biobank. Korea Biobank, Korea Centers for Disease Control and Prevention, Osong, Korea. Korea Centers for Disease Control and Prevention, Dukhyoung Lee, Hy-ekyung Park (Project Officer), Eunkyeong Jung (Project Officer), Suyeon Jeong, Eunmi Ahn and Sil-Hea Sung.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tsuruya, K.; Yoshida, H.; Nagata, M.; Kitazono, T.; Iseki, K.; Iseki, C.; Fujimoto, S.; Konta, T.; Moriyama, T.; Yamagata, K.; et al. Impact of the Triglycerides to High-Density Lipoprotein Cholesterol Ratio on the Incidence and Progression of CKD: A Longitudinal Study in a Large Japanese Population. Am. J. Kidney Dis. 2015, 66, 972–983. [Google Scholar] [CrossRef]
- Kochan, Z.; Szupryczynska, N.; Malgorzewicz, S.; Karbowska, J. Dietary Lipids and Dyslipidemia in Chronic Kidney Disease. Nutrients 2021, 13, 3138. [Google Scholar] [CrossRef]
- Tsuruya, K.; Yoshida, H.; Nagata, M.; Kitazono, T.; Iseki, K.; Iseki, C.; Fujimoto, S.; Konta, T.; Moriyama, T.; Yamagata, K.; et al. Association of Hypertriglyceridemia With the Incidence and Progression of Chronic Kidney Disease and Modification of the Association by Daily Alcohol Consumption. J. Ren. Nutr. Off. J. Counc. Ren. Nutr. Natl. Kidney Found. 2017, 27, 381–394. [Google Scholar] [CrossRef]
- Wanner, C.; Tonelli, M. KDIGO Clinical Practice Guideline for Lipid Management in CKD: Summary of recommendation statements and clinical approach to the patient. Kidney Int. 2014, 85, 1303–1309. [Google Scholar] [CrossRef]
- Toth, P.P.; Philip, S.; Hull, M.; Granowitz, C. Association of Elevated Triglycerides With Increased Cardiovascular Risk and Direct Costs in Statin-Treated Patients. Mayo Clin. Proc. 2019, 94, 1670–1680. [Google Scholar] [CrossRef]
- Nelson, A.J.; Navar, A.M.; Mulder, H.; Wojdyla, D.; Philip, S.; Granowitz, C.; Peterson, E.D.; Pagidipati, N.J. Association Between Triglycerides and Residual Cardiovascular Risk in Patients With Type 2 Diabetes Mellitus and Established Cardiovascular Disease (From the Bypass Angioplasty Revascularization Investigation 2 Diabetes [BARI 2D] Trial). Am. J. Cardiol. 2020, 132, 36–43. [Google Scholar] [CrossRef]
- Li, Y.H.; Tseng, W.K.; Yin, W.H.; Lin, F.J.; Wu, Y.W.; Hsieh, I.C.; Lin, T.H.; Sheu, W.H.; Yeh, H.I.; Chen, J.W.; et al. Prognostic effect of high-density lipoprotein cholesterol level in patients with atherosclerotic cardiovascular disease under statin treatment. Sci. Rep. 2020, 10, 21835. [Google Scholar] [CrossRef]
- Sonmez, A.; Yilmaz, M.I.; Saglam, M.; Unal, H.U.; Gok, M.; Cetinkaya, H.; Karaman, M.; Haymana, C.; Eyileten, T.; Oguz, Y.; et al. The role of plasma triglyceride/high-density lipoprotein cholesterol ratio to predict cardiovascular outcomes in chronic kidney disease. Lipids Health Dis. 2015, 14, 29. [Google Scholar] [CrossRef]
- Kim, J.Y.; Park, J.T.; Kim, H.W.; Chang, T.I.; Kang, E.W.; Ahn, C.; Oh, K.H.; Lee, J.; Chung, W.; Kim, Y.S.; et al. Inflammation Alters Relationship Between High-Density Lipoprotein Cholesterol and Cardiovascular Risk in Patients With Chronic Kidney Disease: Results From KNOW-CKD. J. Am. Heart Assoc. 2021, 10, e021731. [Google Scholar] [CrossRef]
- Kang, H.T.; Kim, J.K.; Kim, J.Y.; Linton, J.A.; Yoon, J.H.; Koh, S.B. Independent association of TG/HDL-C with urinary albumin excretion in normotensive subjects in a rural Korean population. Clin. Chim. Acta Int. J. Clin. Chem. 2012, 413, 319–324. [Google Scholar] [CrossRef]
- Kang, H.T.; Shim, J.Y.; Lee, Y.J.; Lee, J.E.; Linton, J.A.; Kim, J.K.; Lee, H.R. Association between the ratio of triglycerides to high-density lipoprotein cholesterol and chronic kidney disease in Korean adults: The 2005 Korean National Health and Nutrition Examination Survey. Kidney Blood Press. Res. 2011, 34, 173–179. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kang, H.T.; Lee, H.R.; Lee, Y.J.; Shim, J.Y. Comparison of lipid-related ratios for prediction of chronic kidney disease stage 3 or more in Korean adults. J. Korean Med. Sci. 2012, 27, 1524–1529. [Google Scholar] [CrossRef]
- Tsuruya, K.; Yoshida, H.; Nagata, M.; Kitazono, T.; Hirakata, H.; Iseki, K.; Moriyama, T.; Yamagata, K.; Yoshida, H.; Fujimoto, S.; et al. Association of the triglycerides to high-density lipoprotein cholesterol ratio with the risk of chronic kidney disease: Analysis in a large Japanese population. Atherosclerosis 2014, 233, 260–267. [Google Scholar] [CrossRef]
- Nam, K.H.; Chang, T.I.; Joo, Y.S.; Kim, J.; Lee, S.; Lee, C.; Yun, H.R.; Park, J.T.; Yoo, T.H.; Sung, S.A.; et al. Association Between Serum High-Density Lipoprotein Cholesterol Levels and Progression of Chronic Kidney Disease: Results From the KNOW-CKD. J. Am. Heart Assoc. 2019, 8, e011162. [Google Scholar] [CrossRef]
- Schaeffner, E.S.; Kurth, T.; Curhan, G.C.; Glynn, R.J.; Rexrode, K.M.; Baigent, C.; Buring, J.E.; Gaziano, J.M. Cholesterol and the risk of renal dysfunction in apparently healthy men. J. Am. Soc. Nephrol. 2003, 14, 2084–2091. [Google Scholar] [CrossRef]
- Muntner, P.; Coresh, J.; Smith, J.C.; Eckfeldt, J.; Klag, M.J. Plasma lipids and risk of developing renal dysfunction: The atherosclerosis risk in communities study. Kidney Int. 2000, 58, 293–301. [Google Scholar] [CrossRef]
- Fox, C.S.; Larson, M.G.; Leip, E.P.; Culleton, B.; Wilson, P.W.; Levy, D. Predictors of new-onset kidney disease in a community-based population. JAMA 2004, 291, 844–850. [Google Scholar] [CrossRef]
- Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP). Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA 2001, 285, 2486–2497. [Google Scholar] [CrossRef]
- Su, X.; Kong, Y.; Peng, D. Evidence for changing lipid management strategy to focus on non-high density lipoprotein cholesterol. Lipids Health Dis. 2019, 18, 134. [Google Scholar] [CrossRef]
- Suh, S.H.; Oh, T.R.; Choi, H.S.; Kim, C.S.; Bae, E.H.; Ma, S.K.; Oh, K.-H.; Han, S.H.; Kim, S.W. Non-High-Density Lipoprotein Cholesterol and Cardiovascular Outcomes in Chronic Kidney Disease: Results from KNOW-CKD Study. Nutrients 2022, 14, 3792. [Google Scholar] [CrossRef]
- Oh, K.H.; Park, S.K.; Park, H.C.; Chin, H.J.; Chae, D.W.; Choi, K.H.; Han, S.H.; Yoo, T.H.; Lee, K.; Kim, Y.S.; et al. KNOW-CKD (KoreaN cohort study for Outcome in patients With Chronic Kidney Disease): Design and methods. BMC Nephrol. 2014, 15, 80. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., 3rd; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Eckardt, K.U.; Tsukamoto, Y.; Levin, A.; Coresh, J.; Rossert, J.; De Zeeuw, D.; Hostetter, T.H.; Lameire, N.; Eknoyan, G. Definition and classification of chronic kidney disease: A position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2005, 67, 2089–2100. [Google Scholar] [CrossRef]
- Lang, R.M.; Bierig, M.; Devereux, R.B.; Flachskampf, F.A.; Foster, E.; Pellikka, P.A.; Picard, M.H.; Roman, M.J.; Seward, J.; Shanewise, J.S.; et al. Recommendations for chamber quantification: A report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J. Am. Soc. Echocardiogr. 2005, 18, 1440–1463. [Google Scholar] [CrossRef] [PubMed]
- Moorhead, J.F.; Chan, M.K.; El-Nahas, M.; Varghese, Z. Lipid nephrotoxicity in chronic progressive glomerular and tubulo-interstitial disease. Lancet 1982, 2, 1309–1311. [Google Scholar] [CrossRef]
- Gröne, H.J.; Walli, A.; Gröne, E.; Niedmann, P.; Thiery, J.; Seidel, D.; Helmchen, U. Induction of glomerulosclerosis by dietary lipids. A functional and morphologic study in the rat. Lab. Investig. A J. Tech. Methods Pathol. 1989, 60, 433–446. [Google Scholar]
- Joles, J.A.; van Goor, H.; van der Horst, M.L.; van Tol, A.; Elema, J.D.; Koomans, H.A. High lipid levels in very low density lipoprotein and intermediate density lipoprotein may cause proteinuria and glomerulosclerosis in aging female analbuminemic rats. Lab. Investig. A J. Tech. Methods Pathol. 1995, 73, 912–921. [Google Scholar]
- Liu, Y.; Coresh, J.; Eustace, J.A.; Longenecker, J.C.; Jaar, B.; Fink, N.E.; Tracy, R.P.; Powe, N.R.; Klag, M.J. Association between cholesterol level and mortality in dialysis patients: Role of inflammation and malnutrition. JAMA 2004, 291, 451–459. [Google Scholar] [CrossRef]
- Lu, Y.; Nyunt, M.S.Z.; Gao, Q.; Gwee, X.; Chua, D.Q.; Yap, K.B.; Pan, F.; Ng, T.P. Malnutrition Risk and Kidney Function and Decline in Community-Dwelling Older Adults. J. Ren. Nutr. 2022, 32, 560–568. [Google Scholar] [CrossRef]
- Gluba-Brzozka, A.; Franczyk, B.; Rysz, J. Cholesterol Disturbances and the Role of Proper Nutrition in CKD Patients. Nutrients 2019, 11, 2820. [Google Scholar] [CrossRef]
- Vaziri, N.D.; Navab, M.; Fogelman, A.M. HDL metabolism and activity in chronic kidney disease. Nat. Rev. Nephrol. 2010, 6, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Yancey, P.G.; Ikizler, T.A.; Jerome, W.G.; Kaseda, R.; Cox, B.; Bian, A.; Shintani, A.; Fogo, A.B.; Linton, M.F.; et al. Dysfunctional high-density lipoprotein in patients on chronic hemodialysis. J. Am. Coll. Cardiol. 2012, 60, 2372–2379. [Google Scholar] [CrossRef] [PubMed]
- Moradi, H.; Vaziri, N.D.; Kashyap, M.L.; Said, H.M.; Kalantar-Zadeh, K. Role of HDL dysfunction in end-stage renal disease: A double-edged sword. J. Ren. Nutr. 2013, 23, 203–206. [Google Scholar] [CrossRef] [PubMed]
- Baigent, C.; Landray, M.J.; Reith, C.; Emberson, J.; Wheeler, D.C.; Tomson, C.; Wanner, C.; Krane, V.; Cass, A.; Craig, J.; et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): A randomised placebo-controlled trial. Lancet 2011, 377, 2181–2192. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).