Potential Allergenicity Response to Moringa oleifera Leaf Proteins in BALB/c Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Extract Preparation
2.3. Moringa oleifera Leaf Protein Characterization
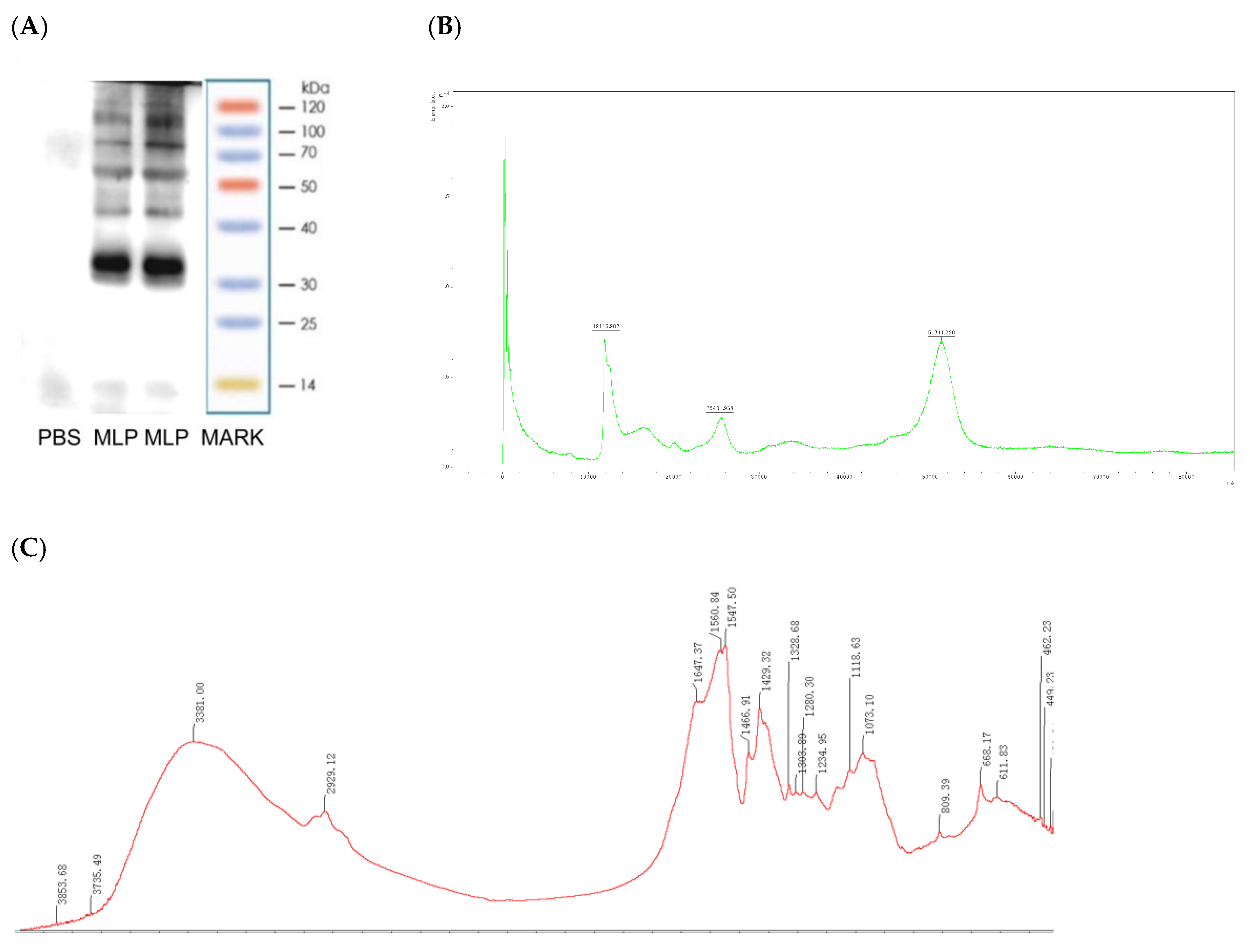
2.3.1. SDS-PAGE
2.3.2. Fourier Transform Infrared (FT-IR) Spectroscopy
2.3.3. Amino Acid Analyze
2.3.4. MALDI−TOF MS Sample Preparation and Data Acquisition
2.4. Sensitization Protocol of BALB/c Mice
2.4.1. Animals
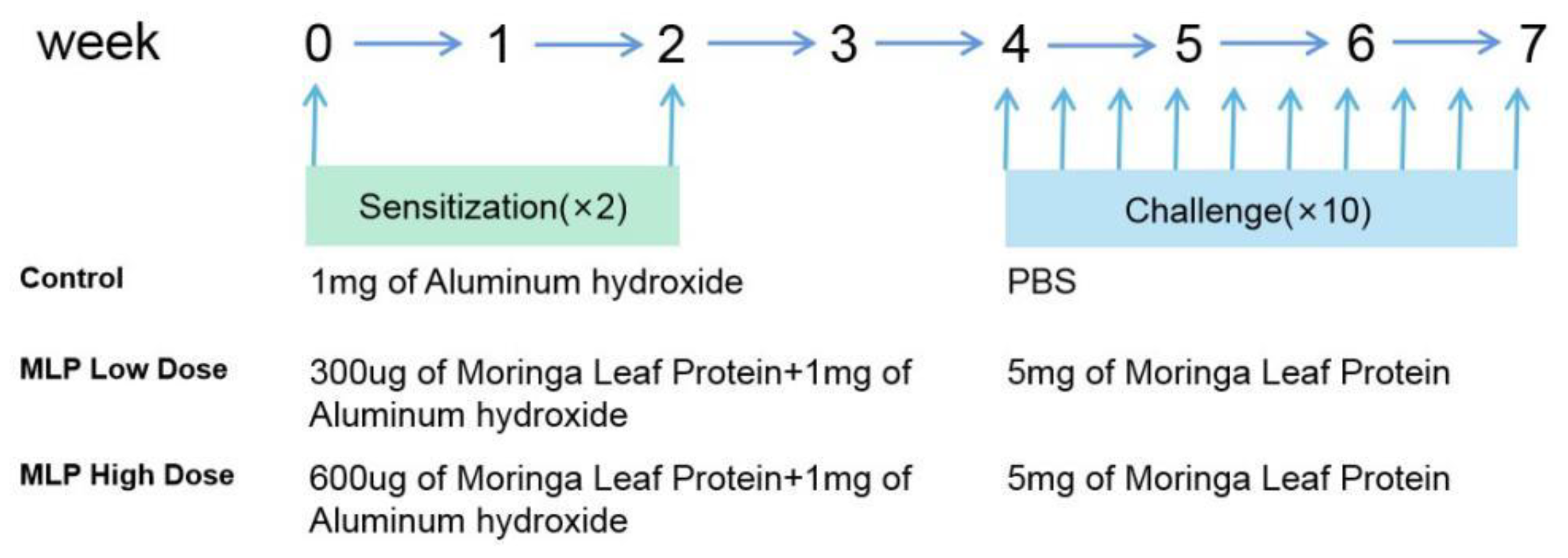
2.4.2. Experimental Design
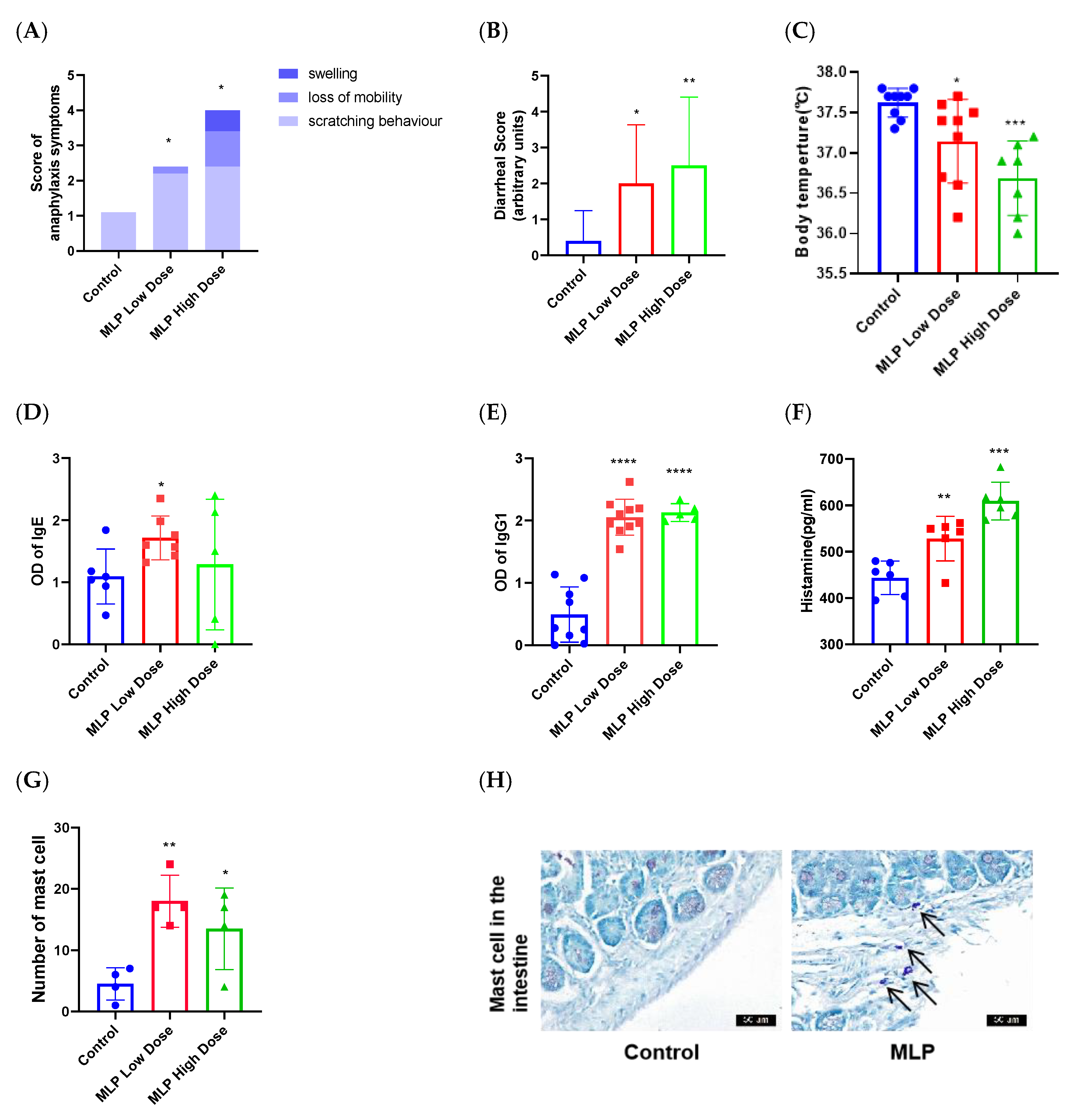
2.4.3. Assessment of the Sensitizing Capacity of MLP
2.4.4. Estimation of Specific IgE and IgG1 Levels
2.4.5. Assessment of Serum Histamine and Cytokine Levels
2.4.6. Cell Culture and Cytokine Evaluation
2.4.7. Histology and Morphometry
2.5. Statistical Analysis
3. Results
3.1. MLP Conforms to the Characteristics of an Allergen
3.2. The Ability of MLP to Induce an Allergic Response in Mice
3.3. Activated T Cells Strongly In Vivo
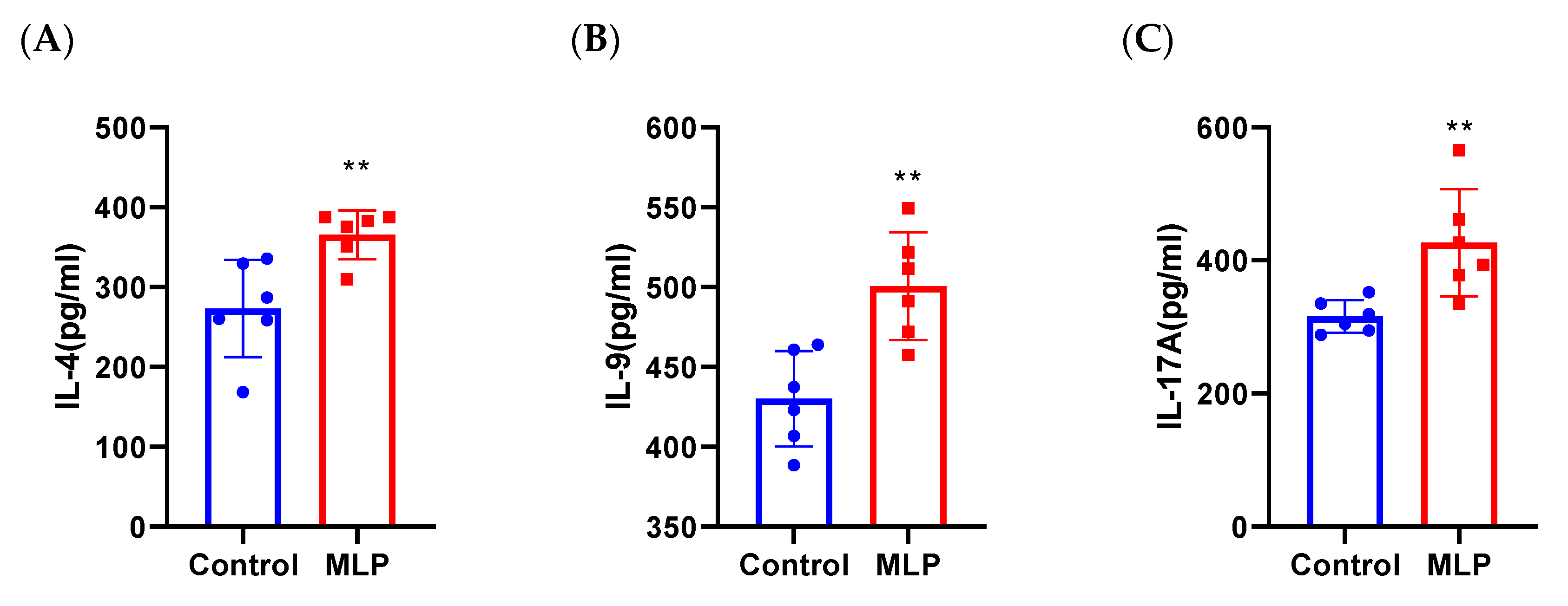
3.3.1. Effects of MLP on Th1 and Th2 Cytokine Secretion
3.3.2. Effects of MLP on Th17 Cytokine Secretion
3.4. Activated T Cells Strongly in Sensitized Mouse Spleen Primary Cells
3.5. Histopathological Changes of Mouse Gut Tissue
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Poussel, M.; Penven, E.; Richard, C.; Jacquenet, S.; Chabot, F.; Paris, C. Occupational asthma to "the miracle tree" (Moringa oleifera): First description. J. Allergy Clin. Immunol. Pract. 2015, 3, 813–814. [Google Scholar] [CrossRef] [PubMed]
- Tarlo, S.M.; Lemiere, C. Occupational asthma. N. Engl. J. Med. 2014, 370, 640–649. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.N.; Singh, B.R.; Singh, R.L.; Prakash, D.; Dhakarey, R.; Upadhyay, G.; Singh, H.B. Oxidative DNA damage protective activity, anti-oxidant and anti-quorum sensing potentials of Moringa oleifera. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2009, 47, 1109–1116.4. [Google Scholar]
- Sreelatha, S.; Jeyachitra, A.; Padma, P.R. Antiproliferation and induction of apoptosis by Moringa oleifera leaf extract on human cancer cells. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2011, 49, 1270–1275. [Google Scholar] [CrossRef]
- Sun, C.; Li, W.; Liu, Y.; Deng, W.; Adu-Frimpong, M.; Zhang, H.; Wang, Q.; Yu, J.; Xu, X. In vitro/in vivo hepatoprotective properties of 1-O-(4-hydroxymethylphenyl)-alpha-L-rhamnopyranoside from Moringa oleifera seeds against carbon tetrachloride-induced hepatic injury. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2019, 131, 110531. [Google Scholar] [CrossRef]
- Islam, Z.; Islam, S.M.R.; Hossen, F.; Mahtab-Ul-Islam, K.; Hasan, M.R.; Karim, R. Moringa oleifera is a Prominent Source of Nutrients with Potential Health Benefits. Int. J. Food Sci. 2021, 2021, 6627265. [Google Scholar] [CrossRef]
- Singh, A.K.; Rana, H.K.; Tshabalala, T.; Kumar, R.; Gupta, A.; Ndhlala, A.R.; Pandey, A.K. Phytochemical, nutraceutical and pharmacological attributes of a functional crop Moringa oleifera Lam: An overview. S. Afr. J. Bot. 2020, 129, 209–220. [Google Scholar] [CrossRef]
- Anwar, F.; Latif, S.; Ashraf, M.; Gilani, A.H. Moringa oleifera: A food plant with multiple medicinal uses. Phytother. Res. PTR 2007, 21, 17–25. [Google Scholar] [CrossRef]
- Wang, J.; Sampson, H.A. Food allergy. J. Clin. Investig. 2011, 121, 827–835. [Google Scholar] [CrossRef]
- Pimentel-Hayashi, J.A.; Del Rio-Navarro, B.E.; Saucedo-Ramirez, O.J. Food allergy, key points for clinical practice. Rev. Alerg. Mex. 2020, 67, 245–267. [Google Scholar]
- Vermeulen, E.M.; Koplin, J.J.; Dharmage, S.C.; Gurrin, L.C.; Peters, R.L.; Mcwilliam, V.; Ponsonby, A.L.; Dwyer, T.; Lowe, A.J.; Tang, M.L.K.; et al. Food Allergy Is an Important Risk Factor for Childhood Asthma, Irrespective of Whether It Resolves. J. Allergy Clin. Immunol. Pract. 2018, 6, 1336–1341.e3. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.F.S.; Luz, L.A.; Argolo, A.C.C.; Teixeira, J.A.; Paiva, P.M.G.; Coelho, L.C.B.B. Isolation of a seed coagulant Moringa oleifera lectin. Process Biochem. 2009, 44, 504–508. [Google Scholar] [CrossRef]
- Gonzalez Garza, N.G.; Chuc Koyoc, J.A.; Torres Castillo, J.A.; Garcia Zambrano, E.A.; Betancur Ancona, D.; Chel Guerrero, L.; Sinagawa Garcia, S.R. Biofunctional properties of bioactive peptide fractions from protein isolates of moringa seed (Moringa oleifera). J. Food Sci. Technol. 2017, 54, 4268–4276. [Google Scholar] [CrossRef] [PubMed]
- Arnett, C.; Lange, J.; Boyd, A.; Page, M.; Cropek, D. Expression and secretion of active Moringa oleifera coagulant protein in Bacillus subtilis. Appl. Microbiol. Biotechnol. 2019, 103, 9411–9422. [Google Scholar] [CrossRef] [PubMed]
- Andrade, J.; Pereira, C.G.; Almeida Junior, J.C.d.; Viana, C.C.R.; Neves, L.N.d.O.; Silva, P.H.F.d.; Bell, M.J.V.; Anjos, V.d.C.d. FTIR-ATR determination of protein content to evaluate whey protein concentrate adulteration. LWT 2019, 99, 166–172. [Google Scholar] [CrossRef]
- Ayalew, Y.; Retta, N.; Desse, G.; Mohammed, A.; Mellesse, A. Amino acid profile and protein quality in tuber and leaf of Coccnia abyssinica (Lam.) (Cogn.) accessions of Ethiopia. Food Sci. Nutr. 2017, 5, 722–729. [Google Scholar] [PubMed]
- Fountoulakis, M.; Lahm, H.-W. Hydrolysis and amino acid composition analysis of proteins. J. Chromatogr. A 1998, 826, 109–134. [Google Scholar] [CrossRef]
- Cucu, T.; De Meulenaer, B.; Devreese, B. MALDI based identification of soybean protein markers—Possible analytical targets for allergen detection in processed foods. Peptides 2012, 33, 187–196. [Google Scholar] [CrossRef]
- Brandt, E.B.; Strait, R.T.; Hershko, D.; Wang, Q.; Muntel, E.E.; Scribner, T.A.; Zimmermann, N.; Finkelman, F.D.; Rothenberg, M.E. Mast cells are required for experimental oral allergen-induced diarrhea. J. Clin. Investig. 2003, 112, 1666–1677. [Google Scholar] [CrossRef]
- Marsteller, N.L.; Goodman, R.E.; Andoh-Kumi, K.; Luan, F.; Bogh, K.L.; Baumert, J. Evaluating the potential allergenicity of dietary proteins using model strong to non-allergenic proteins in germ-free mice. Food Chem. Toxicol. 2020, 141, 111398. [Google Scholar] [CrossRef]
- Elkholy, R.; Balaha, M.; El-Anwar, N.; Kandeel, S.; Hedya, S.; Abd-El Rahman, M.N. Fisetin and telmisartan each alone or in low-dose combination alleviate OVA-induced food allergy in mice. Pharmacol. Rep. PR 2019, 71, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Xuanyi Meng, Y.; Xuefang, W.; Gao, J.; Xie, Y.; Zhao, X.; Yuan, J.; Yang, H.; Zeng, Z.; Li, X.; Chen, H. Dietary Linolenic Acid Increases Sensitizing and Eliciting Capacities of Cow’s Milk Whey Proteins in BALB/c Mice. Nutrients 2022, 14, 16. [Google Scholar]
- Arae, K.; Oboki, K.; Ohno, T.; Hirata, M.; Nakae, S.; Taguchi, H.; Saito, H.; Nakajima, T. Cimetidine enhances antigen-specific IgE and Th2 cytokine production. Allergol. Int. 2011, 60, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Gowthaman, U.; Chen, J.S.; Zhang, B.; Flynn, W.F.; Lu, Y.; Song, W.; Joseph, J.; Gertie, J.A.; Xu, L.; Collet, M.A.; et al. Identification of a T follicular helper cell subset that drives anaphylactic IgE. Science 2019, 365, eaaw6433. [Google Scholar] [CrossRef]
- Aalberse, R.C. Structural biology of allergens. J. Allergy Clin. Immunol. 2000, 106, 228–238. [Google Scholar] [CrossRef]
- Huby, R.D.J.; Dearman, R.J.; Kimber, I. Why are some proteins allergens. Soc. Toxicol. 2000, 55, 235–246. [Google Scholar] [CrossRef]
- Lin, H.; Zheng, L.; Li, Z.; Haider, S. Identification of the major allergen in greasy-back shrimp (Metapenaeus ensis) by MALDI-TOF-MS. J. Ocean. Univ. China 2010, 9, 178–184. [Google Scholar] [CrossRef]
- Guler, G.; Vorob’ev, M.M.; Vogel, V.; Mantele, W. Proteolytically-induced changes of secondary structural protein conformation of bovine serum albumin monitored by Fourier transform infrared (FT-IR) and UV-circular dichroism spectroscopy. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2016, 161, 8–18. [Google Scholar] [CrossRef]
- Kumar, D.; Kumar, S.; Verma, A.K.; Sharma, A.; Tripathi, A.; Chaudhari, B.P.; Kant, S.; Das, M.; Jain, S.K.; Dwivedi, P.D. Hypersensitivity linked to exposure of broad bean protein(s) in allergic patients and BALB/c mice. Nutrition 2014, 30, 903–914. [Google Scholar] [CrossRef]
- Tang, X.; Meng, X.; Wang, H.; Wang, T.; Li, Q.; Jiang, S. Egg allergy was alleviated after baking and frying cooking by weakening Jagged2-Notch induced Th2 immunity in a mice model. Eur. Food Res. Technol. 2022, 248, 917–927. [Google Scholar] [CrossRef]
- Abbring, S.; Kusche, D.; Roos, T.C.; Diks, M.A.P.; Hols, G.; Garssen, J.; Baars, T.; van Esch, B. Milk processing increases the allergenicity of cow’s milk-Preclinical evidence supported by a human proof-of-concept provocation pilot. Clin. Exp. Allergy J. Br. Soc. Allergy Clin. Immunol. 2019, 49, 1013–1025. [Google Scholar] [CrossRef] [PubMed]
- Krempski, J.W.; Lama, J.K.; Iijima, K.; Kobayashi, T.; Matsunaga, M.; Kita, H. A mouse model of the LEAP study reveals a role for CTLA-4 in preventing peanut allergy induced by environmental peanut exposure. J. Allergy Clin. Immunol. 2022, 150, 425–439.e3. [Google Scholar] [CrossRef] [PubMed]
- Berin, M.C.; Sampson, H.A. Food allergy: An enigmatic epidemic. Trends Immunol. 2013, 34, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Freeland, D.M.H.; Nadeau, K.C. Food allergy: Immune mechanisms, diagnosis and immunotherapy. Nat. Rev. Immunol. 2016, 16, 751–765. [Google Scholar] [CrossRef]
- Gocki, J.; Bartuzi, Z. Role of immunoglobulin G antibodies in diagnosis of food allergy. Postep. Dermatol. Alergol. 2016, 33, 253–256. [Google Scholar] [CrossRef]
- Dinarvand, N.; Azizi, R. Histamine and Food Allergy. Int. J. Adv. Biol. Biomed. Res. 2020, 8, 100–111. [Google Scholar]
- De Benedetto, A.; Yoshida, T.; Fridy, S.; Park, J.E.; Kuo, I.H.; Beck, L.A. Histamine and Skin Barrier: Are Histamine Antagonists Useful for the Prevention or Treatment of Atopic Dermatitis? J. Clin. Med. 2015, 4, 741–755. [Google Scholar] [CrossRef]
- Dwyer, D.F.; Barrett, N.A.; Austen, K.F.; The Immunological Genome Project Consortium. Expression profiling of constitutive mast cells reveals a unique identity within the immune system. Nat. Immunol. 2016, 17, 878–887. [Google Scholar] [CrossRef]
- Maggi, E. The TH1/TH2 paradigm in allergy. Immunotechnology 1998, 3, 233–244. [Google Scholar] [CrossRef]
- Rotmagnani, S. The Th1/Th2 paradigm and allergic disorders. Allergy 2010, 53, 12–15. [Google Scholar] [CrossRef]
- Eigenmann, P.A.; Frossard, C.P. The T lymphocyte in food-allergy disorders. Curr. Opin. Allergy Clin. Immunol. 2003, 3, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Tomičić, S.; Fälth-Magnusson, K.; Böttcher, M.F. Dysregulated Th1 and Th2 responses in food-allergic children--does elimination diet contribute to the dysregulation? Pediatr. Allergy Immunol. 2010, 21, 649–655. [Google Scholar] [CrossRef]
- Cosmi, L.; Liotta, F.; Maggi, E.; Romagnani, S.; Annunziato, F. Th17 cells: New players in asthma pathogenesis. Allergy 2011, 66, 989–998. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Lee, J.B.; Liu, B.; Ohta, S.; Wang, P.Y.; Kartashov, A.V.; Mugge, L.; Abonia, J.P.; Barski, A.; Izuhara, K.; et al. Induction of Interleukin-9-Producing Mucosal Mast Cells Promotes Susceptibility to IgE-Mediated Experimental Food Allergy. Immunity 2015, 43, 788–802. [Google Scholar] [CrossRef]
- Shroba, J.; Das, R.; Bilaver, L.; Vincent, E.; Brown, E.; Polk, B.; Ramos, A.; Russell, A.F.; Bird, J.A.; Ciaccio, C.E.; et al. Food Insecurity in the Food Allergic Population: A Work Group Report of the AAAAI Adverse Reactions to Foods Committee. J. Allergy Clin. Immunol. Pract. 2022, 10, 81–90. [Google Scholar] [CrossRef]
- Tokuhara, D.; Kurashima, Y.; Kamioka, M.; Nakayama, T.; Ernst, P.; Kiyono, H. A comprehensive understanding of the gut mucosal immune system in allergic inflammation. Allergol. Int. 2019, 68, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhong, J.; Meng, X.; Gao, J.; Li, H.; Sun, J.; Li, X.; Chen, H. The gut microbiome-immune axis as a target for nutrition-mediated modulation of food allergy. Trends Food Sci. Technol. 2021, 114, 116–132. [Google Scholar] [CrossRef]
- Drønen, E.K.; Namork, E.; Dirven, H.; Hylland, K.; Nygaard, U.C. Does altered gut barrier function facilitate food allergy development? Toxicol. Lett. 2018, 295, S178. [Google Scholar] [CrossRef]
- Chen, C.; Lianhua, L.; Nana, S.; Yongning, L.; Xudong, J. Development of a BALB/c mouse model for food allergy: Comparison of allergy-related responses to peanut agglutinin, beta-lactoglobulin and potato acid phosphatase. Toxicol. Res. 2017, 6, 251–261. [Google Scholar] [CrossRef]
- Zuidmeer, L.; Goldhahn, K.; Rona, R.J.; Gislason, D.; Madsen, C.; Summers, C.; Sodergren, E.; Dahlstrom, J.; Lindner, T.; Sigurdardottir, S.T.; et al. The prevalence of plant food allergies: A systematic review. J. Allergy Clin. Immunol. 2008, 121, 1210–1218.e1214. [Google Scholar] [CrossRef]
- Zhang, W.; Zhu, Q.; Zhang, T.; Cai, Q.; Chen, Q. Thermal processing effects on peanut allergen Ara h 2 allergenicity in mice and its antigenic epitope structure. Food Chem. 2016, 212, 657–662. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, A.; Mills, E.N.C.; Koning, F.; Moreno, F.J. Allergenicity Assessment of Novel Food Proteins: What Should Be Improved? Trends Biotechnol. 2021, 39, 4–8. [Google Scholar] [CrossRef] [PubMed]
- Oyoshi, M.K.; Oettgen, H.C.; Chatila, T.A.; Geha, R.S.; Bryce, P.J. Food allergy: Insights into etiology 2008, prevention, and treatment provided by murine models. J. Allergy Clin. Immunol. 2014, 133, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Tong, P.; Chen, S.; Gao, J.; Li, X.; Wu, Z.; Yang, A.; Yuan, J.; Chen, H. Caffeic acid-assisted cross-linking catalyzed by polyphenol oxidase decreases the allergenicity of ovalbumin in a Balb/c mouse model. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2018, 111, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Li, X.; Gao, J.; Chen, H. Characterization of the potential allergenicity of irradiated bovine alpha-lactalbumin in a BALB/c mouse model. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2016, 97, 402–410. [Google Scholar] [CrossRef]
- Asarea, G.A.; Gyan, B.; Bugyei, K.; Adjei, S.; Mahama, R.; Addo, P.; Otu-Nyarko, L.; Wiredu, E.K.; Nyarko, A. Toxicity potentials of the nutraceutical Moringa oleifera at supra-supplementation levels. J. Ethnopharmacol. 2012, 39, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Dearman, R.J.; Skinner, R.A.; Herouet, C.; Labay, K.; Debruyne, E.; Kimber, I. Induction of IgE antibody responses by protein allergens: Inter-laboratory comparisons. Food Chem. Toxicol. 2003, 41, 1509–1516. [Google Scholar] [CrossRef]
- Paul, W.E.; Zhu, J. How are T(H)2-type immune responses initiated and amplified? Nature reviews. Immunology 2010, 10, 225–235. [Google Scholar]
- Yu, Q.; Sharma, A.; Oh, S.Y.; Moon, H.-G.; Hossain, M.Z.; Salay, T.M.; Leeds, K.E.; Du, H.; Wu, B.; Waterman, M.L.; et al. T cell factor 1 initiates the T helper type 2 fate by inducing the transcription factor GATA-3 and repressing interferon-γ. Nat. Immunol. 2009, 10, 992–999. [Google Scholar] [CrossRef]
- Burton, O.T.; Darling, A.R.; Zhou, J.S.; Noval-Rivas, M.; Jones, T.G.; Gurish, M.F.; Chatila, T.A.; Oettgen, H.C. Direct effects of IL-4 on mast cells drive their intestinal expansion and increase susceptibility to anaphylaxis in a murine model of food allergy. Mucosal Immunol. 2013, 6, 740–750. [Google Scholar] [CrossRef]
- Akbari, O.; Stock, P.; Meyer, E.; Kronenberg, M.; Sidobre, S.; Nakayama, T.; Taniguchi, M.; Grusby, M.J.; DeKruyff, R.H.; Umetsu, D.T. Essential role of NKT cells producing IL-4 and IL-13 in the development of allergen-induced airway hyperreactivity. Nat. Med. 2003, 9, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Minton, K. What’ drives’IL-4 versus IL-13 signalling? Nat. Rev. Immunol. 2008, 8, 167. [Google Scholar] [CrossRef]
- Brandt, E.B.; Munitz, A.; Orekov, T.; Mingler, M.K.; McBride, M.; Finkelman, F.D.; Rothenberg, M.E. Targeting IL-4/IL-13 signaling to alleviate oral allergen-induced diarrhea. J. Allergy Clin. Immunol. 2009, 123, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Brough, H.A.; Cousins, D.J.; Munteanu, A.; Wong, Y.F.; Sudra, A.; Makinson, K.; Stephens, A.C.; Arno, M.; Ciortuz, L.; Lack, G.; et al. IL-9 is a key component of memory TH cell peanut-specific responses from children with peanut allergy. J. Allergy Clin. Immunol. 2014, 134, 1329–1338.e1310. [Google Scholar] [CrossRef]
- Wang, Y.-H. Novel IL-9-Producing Mucosal Mast Cells Promote IgE-Mediated Food Allergy. J. Allergy Clin. Immunol. 2016, 137, AB1. [Google Scholar] [CrossRef]
- Forbes, E.E.; Groschwitz, K.; Abonia, J.P.; Brandt, E.B.; Cohen, E.; Blanchard, C.; Ahrens, R.; Seidu, L.; McKenzie, A.; Strait, R.; et al. IL-9- and mast cell-mediated intestinal permeability predisposes to oral antigen hypersensitivity. J. Exp. Med. 2008, 205, 897–913. [Google Scholar] [CrossRef] [PubMed]
- Bankova, L.G.; Dwyer, D.F.; Liu, A.Y.; Austen, K.F.; Gurish, M.F. Maturation of mast cell progenitors to mucosal mast cells during allergic pulmonary inflammation in mice. Mucosal Immunol. 2015, 8, 596–606. [Google Scholar] [CrossRef]
- Hirata, S.I.; Kunisawa, J. Gut microbiome, metabolome, and allergic diseases. Allergol. Int. Off. J. Jpn. Soc. Allergol. 2017, 66, 523–528. [Google Scholar] [CrossRef]


| Pk # | RT | Name | Height | Area | ESTD Conc/nmol | Units |
|---|---|---|---|---|---|---|
| 1 | 4.7 | Asp | 135,861 | 2,135,172 | 0.155 | mol/kg |
| 2 | 5.34 | Thr | 90,990 | 1,474,391 | 0.099 | mol/kg |
| 3 | 5.927 | Ser | 124,707 | 2,056,568 | 0.135 | mol/kg |
| 4 | 6.66 | Glu | 618,119 | 11,656,396 | 0.809 | mol/kg |
| 6 | 9.44 | Gly | 258,741 | 4,983,018 | 0.348 | mol/kg |
| 7 | 10.26 | Ala | 125,102 | 3,034,497 | 0.216 | mol/kg |
| 8 | 11.54 | Cys | 54,848 | 775,330 | 0.05 | mol/kg |
| 9 | 12.193 | Val | 133,756 | 2,104,260 | 0.154 | mol/kg |
| 11 | 13.467 | Met | 51,020 | 1,200,018 | 0.071 | mol/kg |
| 13 | 15.747 | Ile | 55,246 | 1,503,985 | 0.111 | mol/kg |
| 14 | 16.947 | Leu | 97,766 | 2,778,678 | 0.226 | mol/kg |
| 15 | 17.767 | Tyr | 31,653 | 613,296 | 0.05 | mol/kg |
| 16 | 18.680 | Phe | 71,998 | 1,519,116 | 0.114 | mol/kg |
| 19 | 20.860 | Lys | 40,882 | 626,199 | 0.041 | mol/kg |
| 20 | 22.260 | NH3 | 362,631 | 8,340,763 | 1.051 | mol/kg |
| 21 | 23.180 | His | 55,188 | 1,004,429 | 0.07 | mol/kg |
| 23 | 27.207 | Arg | 237,198 | 6,324,650 | 0.466 | mol/kg |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Liu, X.; Wang, Z.; Zhang, H.; Gao, J.; Wu, Y.; Meng, X.; Zhong, Y.; Chen, H. Potential Allergenicity Response to Moringa oleifera Leaf Proteins in BALB/c Mice. Nutrients 2022, 14, 4700. https://doi.org/10.3390/nu14214700
Zhang J, Liu X, Wang Z, Zhang H, Gao J, Wu Y, Meng X, Zhong Y, Chen H. Potential Allergenicity Response to Moringa oleifera Leaf Proteins in BALB/c Mice. Nutrients. 2022; 14(21):4700. https://doi.org/10.3390/nu14214700
Chicago/Turabian StyleZhang, Jie, Xuan Liu, Zhongliang Wang, Hua Zhang, Jinyan Gao, Yong Wu, Xuanyi Meng, Youbao Zhong, and Hongbing Chen. 2022. "Potential Allergenicity Response to Moringa oleifera Leaf Proteins in BALB/c Mice" Nutrients 14, no. 21: 4700. https://doi.org/10.3390/nu14214700
APA StyleZhang, J., Liu, X., Wang, Z., Zhang, H., Gao, J., Wu, Y., Meng, X., Zhong, Y., & Chen, H. (2022). Potential Allergenicity Response to Moringa oleifera Leaf Proteins in BALB/c Mice. Nutrients, 14(21), 4700. https://doi.org/10.3390/nu14214700







