Direct Action of Non-Digestible Oligosaccharides against a Leaky Gut
Abstract
1. Introduction
2. Sealing the Paracellular Route of the Intestinal Epithelial Barrier
2.1. Junctional Network of the Intestinal Epithelial Layer
2.2. Importance of TJ Network
3. Tight Junctions: Components, Regulation, and Gut Flora
3.1. Basic Components of the TJ Complex
3.1.1. Claudins
3.1.2. Occludin
3.1.3. Tricellulin
3.1.4. Zonula Occludens and Cingulin
3.1.5. JAMs
3.2. Signaling Pathways Involved in the Regulation of TJs
3.2.1. AMPK Mediated Regulation of TJs
3.2.2. Myosin Light Chain-2-Mediated Regulation of TJs
3.2.3. PKC-Mediated Regulation of TJs
3.2.4. MAPK-Mediated Regulation of TJs
3.2.5. Other Signaling Pathways Involved in the Regulation of TJs
3.3. Factors Involved in the (Patho)physiological Regulation of TJs
3.3.1. Cytokines and Growth Factor-Mediated Regulation of TJs
3.3.2. Commensal Microbiota and Their Role in TJ Regulation
The Gut Flora
Dysbiosis
Regulation of TJ via the Gut Flora
4. Non-Digestible Oligosaccharides: Their Role in TJ Modulation
4.1. Fructooligosaccharides
4.1.1. Structure and Sources
4.1.2. Microbiota-Independent Effects on TJ
FOS Directly Modulate TJs via Stimulation of Intracellular Calcium Signaling
FOS Protect TJ Integrity via TLR2/PKC/MAPK Associated Pathways
Other Direct Effects of FOS on Epithelial Barrier Integrity
4.1.3. Microbiota-Dependent Effects on TJ
| Treatment Characteristics | [FOS] | Model/Experimental Setup | Type of Study | Observed Effects on PP and/or TJs | Type of Effect | References |
|---|---|---|---|---|---|---|
| FOS (Sigma-Aldrich, St. Louis, MO, USA) | 0.1 mg/mL | T84 monolayers | In vitro | Ca2+ switch assay under normal and LPS-challenged conditions: ↑ TEER/acceleration of TJ re-assembly (better effect with basolateral application) | MID | [166] |
| Re-localization of ZO-1, occludin and claudin-1, no alterations on TJ proteins expression | ||||||
| FOS (Nutraflora®, Nutrition GTC, Golden, CO, USA ), DP 2–9 | 10% w/v | EHEC-exposed Caco-2Bbe1 monolayers | In vitro | Pre-incubation/ challenged cells: ↑ TEER, redistribution of ZO-1, ↑ mRNA ZO-1, ↑ occludin protein but not mRNA | MID | [81] |
| Unchallenged cells: no effect on TEER, ↑ mRNA & protein ZO-1, ↑ occludin protein but not mRNA, no effect on claudin-1 | ||||||
| FOS (Nutraflora®), DP 2–9 | 10% w/v | Duodenal organoids | In vitro | Pre-incubation/challenged organoids: ↑ TEER, ↓ PP of FITC-D | MID | [81] |
| Unchallenged organoids: ↑ TEER | ||||||
| FOS Frutalose (OFP; Sensus), DP ≤ 10 | 100 mg/L | PMA-exposed T84 monolayers | In vitro | Pre-incubation: ↑ TEER | MID | [179] |
| FOS Orafti® L95, 75% w/w syrup, FOS:94.8 w/w ds | 2% v/v | Caco-2 monolayers | In vitro | ↑ TEER, no effect on TJ gene or mRNA expression | MID | [171] |
| FOS (Orafti®, Beneo Orafti, Tienen, Belgium) P95, DP 7–8 | 2% | DON-exposed Caco-2 monolayers | In vitro | Apical & basolateral pre-treatment: ↑ TEER, ↓ PP of LY dose-dependently | MID | [23] |
| Total treatment: ↑ TEER during calcium-switch assay at highest C | ||||||
| FOS (Nutraflora®), DP 2–9 | 10% w/v | Caco-2Bbe1 monolayers | In vitro | Pre-incubation/challenged cells: unsuccessful attenuation of F-actin microfilaments rearrangement, no effect on TEER, PP of FITC-D and ZO-1 redistribution | MID | [180] |
| Unchallenged cells: no effect on FITC-D PP | ||||||
| FOS (34% 1-kestose, 53% nystose, 9% 1F-β-fructofuranosylnystose) | 100 mmol/L | Caco-2 monolayers | In vitro | ↓ TEER, ↑ PP of LY | MID | [175] |
| FOS (Meioligo W, Meiji Co., Tokyo, Japan) | 5% | MCD mice (NASH) | In vivo | Improvement of ZO-1 abundance | MD | [184] |
| FOS (Nutraflora®) | 6% | 5′FU-exposed mice (Mucositis) | In vivo | Pretreatment and total treatment: ↑ mRNA ZO-1 & occludin, ↓ PP of 99mTc-DTPA | MD | [185] |
| FOS (Nutraflora®) | 6% | 5′FU-exposed mice (Mucositis) | In vivo | ↓ PP of 99mTc-DTPA | MD | [186] |
| FOS (P95S, Quantum Hi-Tech Biological Co. Ltd., Guangdong, China) | 1.2% | Chronic stress exposed mice | In vivo | ↑ mRNA & proteins Claudin-1, Occludin & ZO-1 | MD | [187] |
| FOS | 4 g/kg/day | HFD-fed mice (NAFLD) | In vivo | FOS alone and as synbiotic (Lactobacillus paracasei N111): ↑ occludin-1 & claudin-1 proteins | MD | [188] |
| FOS (Solarbio Biotechnology, Beijing, China), 95.93% | 2%/day | OVA-exposed mice (Food allergy) | In vivo | Enhancement of TJ complex- electron density | MD | [189] |
| PB (Center for Anti-aging Research, Nu Skin Enterprises, Shanghai, China) composed of GOS, FOS, inulin, and anthocyanins | 1.26 mg/g/day | Trichinella spiralis-exposed mice (IBS) | In vivo | Pretreatment and total treatment: ↑ occludin | MD | [163] |
| FOS (Nutraflora®), DP 2–9 | 10% w/v | Citrobacter rodentium-exposed mice | In vivo | No effect on FITC-D PP | MD | [180] |
| FOS (Meiji Seika Kaisha, Ltd., Tokyo, Japan), 6.5% GF5, 43.4% GF4, 40.9% GF3, 7.1% 1-kestose GF2, 2.1% glucose and fructose. | 4 g/kg/day | Healthy weaned piglets | In vivo | ↑ mRNA ZO-1, occludin & claudin-1 | MD | [170] |
| Shanghai Lanpu Biotechnology Co., Ltd., Shanghai, China; FOS ≥ 20% | 2.5 mg/kg/day | ETEC-exposed weaned piglets | In vivo | ↑ mRNA ZO-1 & occludin (exceeding control) | MD | [190] |
| FOS, GOS, MFGM | 7.5 g/L/day | Weaned piglets | In vivo | ↑ mRNA ZO-1, claudin-1, occludin & E-cadherin | MD | [191] |
| FOS purity 93%g; Raftilose P95, Orafti® | 60 g/kg/day | Salmonella enterica-exposed rats | In vivo | ↑ urinary CrEDTA excretion (through TJ) | MD | [195] |
| FOS purity 93%g; Raftilose P95, Orafti® | 60 g/kg/day | Healthy rats | In vivo | ↑ urinary CrEDTA excretion (through TJ), no alterations on cadherins, ZO-1 claudin 2 & 4 genes expression | MD | [196] |
| Enzymatically synthesized FOS, DP 3.5, MW 550Da | 5 g/day | SHIME® inoculated with fecal sample from IBD patient and coupled with co-cultures of Caco-2 cells and THP1 macrophages | In vitro | ↑ TEER | MD | [192] |
| FOS Orafti® were boiled for 20min, following in vitro digestion and human fecal fermentation | 50 mg of an equivalent carbohydrate was fermented using 5% of fecal inoculum | Caco-2 cells incubated with FOS ferment supernatant | In vitro | ↑ TEER | MD | [193] |
| FOS (Sigma-Aldrich), chicory root-originated, ≥90% | 5g/L | SHIME® inoculated with fecal sample from healthy donors and coupled with co-cultures of Caco2:HT29-MTX-E12 | In vitro | ↑ TEER | MD | [194] |
4.2. Galactooligosaccharides
4.2.1. Structure and Sources
4.2.2. Microbiota-Independent Effects on TJs
GOS Reinforce the Integrity of TJ under Normal and Pathological Conditions
Postulated Underlying Mechanisms
4.2.3. Microbiota-Dependent Effects on TJ
| Treatment Characteristics | [GOS] | Model/Experimental Setup | Type of Study | Observed Effects on PP and/or TJs | Type of Effect | References |
|---|---|---|---|---|---|---|
| Vivinal® GOS syrup (FrieslandCampina Domo, The Netherlands), 45% GOS, DP 2–8 | 2% | DON-exposed Caco-2 monolayers | In vitro | ↑ TEER (Acceleration of TJ reassembly) during Ca2+ switch, Pre-incubation: ↑ TEER, ↓ PP of LY, FITC-dextran, attenuation of claudin-3 disturbed expression and localization | MID | [204] |
| Vivinal® GOS syrup, (FrieslandCampina Domo, The Netherlands), 59% GOS, DP 2–6 | 0.5, 1, 2% w/w | DON-exposed Caco-2 monolayers | In vitro | ↑ TEER during Ca2+ switch (2% time dependently), Pre-incubation: ↑ TEER, ↓ PP of LY dose dependently | MID | [23] |
| Individual DP2, DP3 from Vivinal® GOS syrup | 0.75% | ↑ TEER (both), ↓ PP of LY (only DP2), combination: ↑ TEER, ↓ PP of LY | ||||
| Purified Vivinal® GOS, (FrieslandCampina Domo, The Netherlands), 97% GOS | 2% w/v | ↑ TEER during Ca2+ switch, Pre-incubation: ↑ TEER, ↓ PP of LY | ||||
| Vivinal® GOS syrup (FrieslandCampinaDomo, The Netherlands), DP 2–8, 59% w/w GOS | 1, 2.5% | Heat stress-exposed Caco-2 monolayers | In vitro | Pre-treatment: ↑ TEER, ↓ PP of LY | MID | [205] |
| Nutrabiotic® GOS (Dairy Crest Ltd., Esher, Surrey, UK), 66.5% w/w dry solids GOS, DP 2–7 | 2% v/v = 1.4% w/v GOS | Caco-2 monolayers | In vitro | ↑ TEER after 24 h-exposure, no effect on TJ gene or mRNA expression | MID | [171] |
| Vivinal® GOS-WPC (FrieslandCampina, Amersfoort, The Netherlands), 27.5% GOS | 100 μg/mL | Caco-2 monolayers | In vitro | Non-significant increase in TJ mRNA/protein levels | MID | [206] |
| HT-29-MTX monolayers | ↑ claudin-1, occludin, ZO-1 mRNA, ↑ claudin-1, -3, occludin, ZO-1 proteins | |||||
| TNF-α-exposed Caco-2/HT-29-MTX co-culture monolayers | Pre-treatment: ↑ TEER | |||||
| Vivinal® GOS syrup (FrieslandCampina) with GOS- plant sterol enriched milk-based fruit beverages (Global Technology Center, Alcantarilla, Murcia, Spain), 1.8 g/100 mL | 1:5 v/v | Caco-2 monolayers | In vitro | ↑ TEER under oxidative stress-challenged and unchallenged conditions | MID | [207] |
| Vivinal® GOS syrup (FrieslandCampina Domo, The Netherlands), 45% GOS, DP 2–8 | 1% GOS (1 kg containing 22.22 g/kg VGOS) | DON-exposed challenged mice | In vivo | Prevention of claudin-3 mRNA overexpression and maintenance of its cellular distribution, ↓ claudin-2 mRNA without attenuation of FITC-D permeability | MD | [204] |
| Yuanye Biotechnology Co. (Shanghai, China) | 0.2 g/100 g BW | E. coli O157-exposed mice | In vivo | ↑ mRNA occludin, claudin, ZO-1 | MD | [202] |
| GOS 100%: 52.86% trisaccharide, 36.39% tetrasaccharide, 10.75% oligosaccharides with DP ≥ 5 (patent application no. 202011427659.7, China) | 0.25, 0.5g/kg BW | LPS-exposed mice | In vivo | Pre-treatment: ↑ mRNA ZO-1, occludin, claudin-1 | MD | [214] |
| Vivinal® GOS syrup (FrieslandCampin Domo, Borculo, The Netherlands), DP 2–8, 59% w/w GOS | 1, 2.5% | Heat stress-exposed broilers | In vivo | Total treatment: attenuation of ↑ mRNA expression of claudin-5 and ZO-1 dose dependently | MD | [213] |
| GOS-90S Quantum Hi-Tech Biological Co., Ltd. (China), DP 2–8, 90% (w/w) GOS | 1 g/kg BW/day | Suckling piglets | In vivo | ↑ occludin mRNA, ↑ ZO-1, occludin protein levels | MD | [203] |
| Quantum Hi-Tech Biological Co., Ltd. (China), 90% GOS | 1 g/kg BW/day | Suckling piglets | In vivo | ↑ ZO-1, claudin-1 but not occludin protein levels | MD | [200] |
| Vivinal® GOS syrup 75% DM (FrieslandCampinaDomo, Borculo, The Netherlands), DP 2–8, 59% w/w GOS | 600–1600 mL/piglet/day (0.8% FOS) | Weaned piglets | In vivo | ↑ claudin-1, ZO-2 mRNA and ZO-1, occludin mRNA and protein levels | MD | [217] |
| GOS/CGMP 2:1 (Beijing Sanyuan Foods Co., Ltd., Beijing, China), 90% GOS w/w, DM 3 | 10 g/day | Piglets fed by GOS treated sows | In vivo | ↑ mRNA claudin-1, claudin-2, occludin | MD | [215] |
| Xi’an Deshipu Bio-industry Company, China, 90% GOS | 1 g/day | Sodium taurocholate-exposed rats (SAP) | In vivo | ↑ occludin mRNA and protein (not significant but positively correlated to mRNA increase) | MD | [216] |
| Vivinal® GOS (Friesland Foods Domo, Zwolle, The Netherlands) i.e., 4.9 g/L GOS/Inulin (Beneo HP, Orafti, France) i.e., 0.70 g/L Inulin, (88/12, 5.6 g/L) | 10.9 g/L/day (V dependent of age) | Newborn rats | In vivo | No alterations in mRNA claudin-2, -3, no effect on PP of Dextran, ↓ mRNA ZO-1 | MD | [218] |
4.3. Alginate Oligosaccharides
4.3.1. Structure and Sources
4.3.2. Microbiota-Independent Effects on TJs
AOS Interact with the Mannose Receptor and Reinforce the Intestinal Epithelial Barrier
AOS Rescue the Intestinal Epithelial Integrity via Their Anti-Inflammatory and Anti-Apoptotic Properties
4.3.3. Microbiota-Dependent Effects on TJs
| Treatment Characteristics | [AOS] | Model/Experimental Setup | Type of Study | Observed Effects on PP and/or TJs | Type of Effect | References |
|---|---|---|---|---|---|---|
| AOS (Qingdao Bozhihuili Co., Ltd., Qingdao, China) | 10 μg/mL, 100 μg/mL | IPEC/J2 monolayers | In vitro | ↑ claudin protein levels | MID | [229] |
| AOS (Qingdao Bozhihuili Co., Ltd.) | 10 μg/mL, 100 μg/mL | IPEC/J2 monolayers | In vitro | ↑ TEER time dependently, ↑ claudin, occludin protein levels dose dependently | MID | [233] |
| AOS prepared by depolymerization of alginate (Qingdao Bright Moon Seaweed Group Co., Ltd., Qingdao, China) | 600 μg/mL | TNF-α-exposed IPEC/J2 monolayers | In vitro | ↑ occludin mRNA and protein levels and ↑ occludin protein levels of unchallenged cells | MID | [227] |
| AOS prepared by depolymerization of alginate (Qingdao Bright Moon Seaweed Group Co., Ltd., Qingdao, China), DP 2–8 | 600 μg/mL | LPS-exposed IPEC/J2 mono-layers | In vitro | ↑ occludin protein abundance | MID | [228] |
| AOS prepared by depolymerization of alginate (Qingdao Bright Moon Seaweed Group Co., Ltd., Qingdao, China), DP 2–8 | 10 mg/kg BW | ETEC-exposed weaned pigs | In vivo | ↑ occludin protein abundance | MID | [228] |
| ALGO (Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian, China), DP 4.4 | 100 mg/kg BW | Weaned pigs | In vivo | ↑ occludin, ZO-1 mRNA | MD | [238] |
| AOS (-) | 100 mg/kg BW | Weaned pigs | In vivo | ↑ occludin, claudin-1 mRNA, no alteration for ZO-1, 2 mRNA | MD | [225] |
| UAOS prepared according to Li et al. [242], MW 420.4 | 400 mg/kg BW/day | HFD-fed mice | In vivo | ↑ ZO-1, occludin protein abundance | MD | [240] |
| UAOS prepared according to Li et al. [242] | 200, 400 mg/kg BW/day | DSS-exposed mice (UC) | In vivo | ↑ ZO-1, occludin protein abundance dose dependently | MD | [239] |
| AOS (-) | 10, 100 mg/kg BW | FMT (fecal microbiota transplantation)-treated mice exposed to busulfan (mucositis) | In vivo | ↑ ZO-1, claudin protein abundance, ↑ occludin protein levels | MD | [241] |
4.4. Chitooligosaccharides
4.4.1. Structure and Sources
4.4.2. Microbiota-Independent Effects on TJs
COS Facilitate TJ Re-Assembly via AMPK Stimulation and Promote TJ Integrity
COS Alleviate Inflammation and Regulate TJ Abundance
4.4.3. Microbiota-Dependent Effects on TJs
| Treatment Characteristics | [COS] | Model/Experimental Setup | Type of Study | Observed effects on PP and/or TJs | Type of Effect | References |
|---|---|---|---|---|---|---|
| COS (Kitto Life Co., Ltd., Kyungki-do, Seoul, Korea), MW 5–10 kDa, >70% COS content, DD > 70% | 0.5-4 mg/mL | Caco-2 monolayers | In vitro | No effect on TEER nor on [14C] mannitol flux | MID | [253] |
| COS prepared by enzymatic hydrolysis of shrimp shell chitosan, MW 5000 Da, DD > 90%, | 20, 100, 500 μg/mL | LPS-exposed T84 monolayers | In vitro | ↑ TEER (best effect with 100 μg/mL) | MID | [248] |
| TNF-α-exposed T84 monolayers | ||||||
| COS prepared according to [248], MW 5000 Da, DD > 90% | 100 μg/mL | T84 monolayers | In vitro | ↑ TEER/acceleration of TJ re-assembly during Ca2+ assay | MID | [251] |
| TNF-α-exposed T84 monolayers | ↓ FITC-D flux | |||||
| COS (Beijing Zhong Tai He technology (ZTH tech, Beijing, China), MW < 1000 Da, DD > 90%, DP 2–7 | 50–100 μg/mL | TNF-γα-exposed IPEC-J2 monolayers | In vitro | Suppression of ↑ claudin-1 mRNA, tendency to ↓ ZO-1 mRNA concentration-dependently, no effect on TEER | MID | [256] |
| COS (Zhong Tai He Technology (Beijing, China), MW < 1000, DP 2–7, DD > 90% | 800 μg/mL | IPEC-J2 monolayers | In vitro | ↑ TEER concentration-dependently and ↓ FITC-D flux dose-dependently | MID | [249] |
| LPS-exposed IPEC-J2 monolayers | ||||||
| COS (GlycoBio (GlycoBio, Dalian, China), MW 363-1329 Da, DD > 95% HWCOS (Sigma (St. Louis, MO, USA), MW 4000–6000 Da, DD > 90% | 200 μg/mL | DSS-exposed Caco-2 monolayers | In vitro | ↑ occludin protein abundance and mRNA post-challenge (HWCOS less effectively) | MID | [254] |
| COS (GlycoBio (GlycoBio, Dalian, China), MW 363–1329 Da, DD > 95% | 200 mg/kg BW/day | DSS-exposed mice (UC) | In vivo | ↑ occludin protein abundance | Not determined | [259] |
| NACOS prepared as described in [270], DP 2–6, DA = 97% | 200 mg/kg BW/day (1 mg/mL NACOS) | HFD-fed mice (Metabolic syndrome) | In vivo | ↑ ZO-1, occludin mRNA | MD | [270] |
| LMW-COS enzymatically produced as described in [269], DD = 93% LMW-COS-H, MW 879.6 Da LMW-COS-W, MW 360.9 Da | 400 mg/kg BW/day | HFD-fed mice (Obesity-Metabolic syndrome) | In vivo | ↑ ZO-1, occludin mRNA and protein levels (LMW-COS-H) | MD | [269] |
| ↑ occludin mRNA and protein levels (LMW-COS-L) | ||||||
| COS prepared by enzymatic hydrolysis as described by [276], DD = 88%, DP 2–6% | 200 mg/kg BW/day | Loperamide-exposed mice | In vivo | ↑ occludin, claudin-1 mRNA, ↑ ZO-1 and claudin-1 protein levels | MD | [273] |
| COS prepared as described in [268], DD = 88%, DP 2–6 | 200 mg/kg BW/day (1 mg/mL COS) | Leprdb mutation (db/db) mice | In vivo | ↑ occludin protein levels, no effect on ZO-1 | MD | [268] |
| COS23 prepared by enzymatic degradation of COS as described in [277,278] | 4% in drinking water | HFD-fed mice (NAFLD) | In vivo | ↑ ZO-1, ZO-2 mRNA, tendency for ↑ occludin mRNA | MD | [271] |
| COS (MedChem Express, Shanghai, China), MW < 1 kDa, 91.0% COS | 200 mg/kg BW/day (1 mg/mL COS) | Carulein-exposed mice (SAP) | In vivo | ↑ occludin, claudin-1, no effect on ZO-1 abundance, ↓ FITC-Dextran flux | MD | [272] |
| COS, MW 1000–2000 Da, COS content >85% | 30 mg/kg BW/day | Healthy weaned piglets | In vivo | ↓ occludin and ZO-1 mRNA | Not determined | [275] |
| COS prepared by enzymatic hydrolysis as described in [267], DD > 95%, MW ≤ 1000 Da, DP 2–8 | 100 mg/kg BW/day | Healthy weaned piglets | In vivo | ↑ claudin-1 and occludin mRNA (jejunum only) | MD | [267] |
| COS (Zhongkerongxin Biotechnology Co., Ltd., Suzhou, China), MW 1000-2000 Da, COS > 90% | 30 mg/kg BW/day | Healthy broilers | In vivo | ↑ claudin-3 mRNA, no alteration on occludin, claudin-2 and ZO-1 mRNA | Not deter-mined | [274] |
4.5. Mannan-Oligosaccharides
4.5.1. Structure and Sources
4.5.2. Microbiota-Dependent and Independent Effects on TJs
| Treatment Characteristics | [MOS] | Model/Experimental Setup | Type of Study | Observed Effects on PP and/or TJs | Type of Effect | References |
|---|---|---|---|---|---|---|
| MOS prepared by enzymatic hydrolysis of copra milk galactomannan, DP = 5 | 10, 20 μM | T84 monolayers | In vitro | ↑ TEER | MID | [282,284] |
| 10 μM | ↑ TEER/acceleration of TJ assembly during Ca2+ assay | |||||
| KMOS/+B. subtilis | 2 g/L | LPS-exposed Caco-2 monolayers | In vitro | ↑ ZO-1, claudin-1 mRNA | MID | [283] |
| KMOS/+B. subtilis | 2 g/L | LPS-exposed mice | In vivo | ↑ claudin-1 | Not determined | [283] |
| KMOS (Xi’an Yuanseng biological technology Corporation, Xi’an, China), DP 2–6 | 400 mg/kg BW/day | DSS-exposed mice (UC) | In vivo | ↓ FITC-D flux, ↑ ZO-1, occludin | Not determined | [285] |
| MOS (Sichuan Junzheng Bio. Co., Ltd.), 98.5% MOS | 3 g/kg BW/day (0.3%) | ETEC-exposed weaned piglets | In vivo | ↑ ZO-1 expression and localization | Not determined | [287] |
| MOS (Shanghai Lanpu Bio. Co. Ltd.), MOS ≥ 20%, DP 2–9 | 0.6 g/kg BW/day | ETEC-exposed weaned piglets | In vivo | ↑ ZO-1, claudin-1 mRNA | MD | [280] |
| MOS (Safmannan, Phileo Lesaffre Animal Care, Marcq-en-Baroeul, France) | 0.5 g/kg BW/day | ETEC-exposed broilers | In vivo | ↑ occludin mRNA, no effect in ZO-1, claudin-1 mRNA | MID | [288] |
| MOS (Safmannan, Phileo Lesaffre Animal Care, Marcq-en-Baroeul, France) | 250 mg/kg BW/day | Heat stress-exposed broilers | In vivo | ↑ occludin mRNA (jejunum), ↑ occludin, ZO-1 mRNA (ileum) | Not determined | [286] |
4.6. Xylo-Oligosaccharides
4.6.1. Structure and Sources
4.6.2. Microbiota-Dependent and Independent Effects on TJs
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| [Ca2+]I | Intracellular Ca2+ |
| A. muciniphila | Akkermansia muciniphila |
| AhR | Aryl hydrocarbon receptor |
| AJs | Adherens junctions |
| AmEVs | A. muciniphila-originated EVs |
| AMP | Antimicrobial peptide |
| AMPK | Adenosine monophosphate (AMP)-activated protein kinase |
| AOS | Alginate-oligosaccharides |
| B. fragilis | Bacteroides fragilis |
| BiCM | B. infantis conditioned medium |
| CaMKKβ | Ca2+/calmodulin-dependent kinase kinase β |
| CaSR | Calcium-sensing receptor |
| CD | Crohn’s disease |
| CDX2 | Caudal type homeobox 2 |
| CLR | C-type lectin-like receptor |
| COS | Chitin/chitosan-oligosaccharides |
| CRC | Colorectal cancer |
| DA | Degree of acetylation |
| DD | Degree of deacetylation |
| DON | Mycotoxin deoxynivalenol |
| DP | Degree of polymerization |
| DSS | Dextran sulfate sodium |
| E. coli | Escherichia coli |
| EcN | Escherichia coli Nissle 1917 |
| EHEC | Enterohemorrhagic E. coli |
| ERK | Extracellular signal-regulated kinase |
| ETEC | Enteropathogenic E. coli |
| EVs | Extracellular vesicles |
| F. prausnitzii | Faecalibacterium prausnitzii |
| F-actin | Filamentous actin |
| FITC-D | Fluorescein isothiocyanate–dextran |
| FMT | Fecal microbiota transplantation |
| FOS | Fructooligosaccharides |
| GAPs | GTPase-activating proteins |
| GDIs | Guanine nucleotide dissociation inhibitors |
| GEFs | Guanine nucleotide exchange factors |
| GI | Gastrointestinal |
| GJs | Gap junctions |
| GlcNAc | N-acetyl-d-glucosamine |
| GOS | Galactooligosaccharides |
| HDAC | Histone deacetylase |
| HFD | High-fat diet |
| HMOs | Human milk oligosaccharides |
| HMW | High MW COS |
| IAPS | γ-irradiated Astragalus polysaccharide |
| IBD | Inflammatory bowel disease |
| IBS | Irritable bowel syndrome |
| ICAM-1 | Intercellular adhesion molecule-1 |
| IECs | Intestinal epithelial cells |
| IgA | Immunoglobulin A |
| IL | Interleukin |
| INF-γ | Interferon-γ |
| IP3 | D-myo-inositol 1,4,5-trisphosphate |
| JAM | Junctional adhesion molecules |
| JNK | c-Jun N-terminal kinase |
| KMOS | Konjac MOS |
| LKB1 | Liver Kinase B1 |
| LMW | Low MW |
| LPS | Lipopolysaccharide |
| LY | Lucifer yellow |
| MAMPs | Microbial-associated molecular patterns |
| MAPK | Mitogen-activated protein kinase |
| MCD | Methionine-choline-deficient |
| MD | Microbiota-dependent |
| MID | Microbiota-independent |
| MLC-2 | Myosin II regulatory light chain |
| MLCK | Myosin light chain kinase |
| MOS | Mannan-oligosaccharides |
| MR | Mannose receptor |
| mTOR | Mammalian target of rapamycin |
| MW | Molecular weight |
| NAFLD | Non-alcoholic fatty liver disease |
| NASH | Non-alcoholic steatohepatitis |
| NDOs | Non-digestible oligosaccharides |
| NM 2 | Non-muscle myosin 2 |
| NOD | Non-obese diabetic |
| Nrf2 | Nuclear factor erythroid 2-related factor 2 |
| OMVs | Outer membrane vesicles |
| PD | Polydispersity |
| PDZ | PSD95–DlgA–ZO-1 homology |
| PI3K | Phosphatidyl inositol-3 kinase/phosphoinositide 3-kinases |
| PKC | Protein kinase C |
| PLC | Phospholipase C |
| PMA | Phorbol 12-myristate 13-acetate |
| POS | Pectic-oligosaccharides |
| PP | Paracellular permeability |
| PP | Protein phosphatase |
| PRRs | Pattern recognition receptors |
| ROCK | Rho-associated coiled coil containing protein kinase |
| SAP | Severe acute pancreatitis |
| SCFAs | Short-chain fatty acids |
| SHIME | Simulator of the Human Intestinal Microbial Ecosystem |
| SIGNR1 | Specific intercellular adhesion molecule-3-grabbing nonintegrin-related 1 |
| SLPs | Surface layer proteins |
| SOS | Soybean oligosaccharides |
| TAMPS | TJ-associated MARVEL proteins |
| TEER | Transepithelial electrical resistance |
| TGF-β | Transforming growth factor-β |
| TJ | Tight junction |
| TLR | Toll-like receptor |
| UAOS | Unsaturated AOS |
| UC | Ulcerative colitis |
| UroA | Urolithin A |
| XOS | Xylooligosaccharides |
| ZO | Zonula occludens |
References
- Schoultz, I.; Keita, Å.V. The Intestinal Barrier and Current Techniques for the Assessment of Gut Permeability. Cells 2020, 9, 1909. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T. Regulation of intestinal epithelial permeability by tight junctions. Cell Mol. Life Sci. 2013, 70, 631–659. [Google Scholar] [CrossRef] [PubMed]
- Allam-Ndoul, B.; Castonguay-Paradis, S.; Veilleux, A. Gut microbiota and intestinal trans-epithelial permeability. Int. J. Mol. Sci. 2020, 21, 6402. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, M.; Madsen, K.; Spiller, R.; Van Meerveld, B.G.; Verne, G.N. Intestinal barrier function in health and gastrointestinal disease. Neurogastroenterol. Motil. 2012, 24, 503–512. [Google Scholar] [CrossRef]
- Camilleri, M. What is the leaky gut? Clinical considerations in humans. Curr. Opin. Clin. Nutr. Metab. Care 2021, 24, 473–482. [Google Scholar] [CrossRef]
- Bron, P.A.; Kleerebezem, M.; Brummer, R.-J.; Cani, P.D.; Mercenier, A.; Macdonald, T.T.; Garcia-Ródenas, C.L.; Wells, J.M. Can probiotics modulate human disease by impacting intestinal barrier function? Br. J. Nutr. 2017, 117, 93–107. [Google Scholar] [CrossRef]
- Hiippala, K.; Jouhten, H.; Ronkainen, A.; Hartikainen, A.; Kainulainen, V.; Jalanka, J.; Satokari, R. The potential of gut commensals in reinforcing intestinal barrier function and alleviating inflammation. Nutrients 2018, 10, 988. [Google Scholar] [CrossRef]
- Odenwald, M.A.; Turner, J.R. The intestinal epithelial barrier: A therapeutic target? Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 9–21. [Google Scholar] [CrossRef]
- Ulluwishewa, D.; Anderson, R.C.; McNabb, W.C.; Moughan, P.J.; Wells, J.M.; Roy, N.C. Regulation of tight junction permeability by intestinal bacteria and dietary components. J. Nutr. 2011, 141, 769–776. [Google Scholar] [CrossRef]
- McCarty, M.F.; Lerner, A. Perspective: Prospects for Nutraceutical Support of Intestinal Barrier Function. Adv. Nutr. 2021, 12, 316–324. [Google Scholar] [CrossRef]
- Hollander, D.; Kaunitz, J.D. The “Leaky Gut”: Tight Junctions but Loose Associations? Dig. Dis. Sci. 2020, 65, 1277–1287. [Google Scholar] [CrossRef] [PubMed]
- Rose, E.C.; Odle, J.; Blikslager, A.T.; Ziegler, A.L. Probiotics, prebiotics and epithelial tight junctions: A promising approach to modulate intestinal barrier function. Int. J. Mol. Sci. 2021, 22, 6729. [Google Scholar] [CrossRef] [PubMed]
- Kurilshikov, A.; Wijmenga, C.; Fu, J.; Zhernakova, A. Host Genetics and Gut Microbiome: Challenges and Perspectives. Trends Immunol. 2017, 38, 633–647. [Google Scholar] [CrossRef]
- Kuhn, K.A.; Schulz, H.M.; Regner, E.H.; Severs, E.L.; Hendrickson, J.D.; Mehta, G.; Whitney, A.; Ir, D.; Ohri, N.; Robertson, C.E.; et al. Bacteroidales recruit IL-6-producing intraepithelial lymphocytes in the colon to promote barrier integrity. Mucosal Immunol. 2018, 11, 357–368. [Google Scholar] [CrossRef] [PubMed]
- Waddell, A.; Vallance, J.; Moore, P.D.; Hummel, A.; Wu, D.; Shanmukhappa, S.K.; Fei, L.; Washington, M.K.; Minar, P.; Coburn, L.A.; et al. IL-33 Signaling Protects from Murine Oxazolone Colitis by Supporting Intestinal Epithelial Function. Inflamm. Bowel Dis. 2015, 21, 2737–2746. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.S.; Kao, C.Y. Current understanding of the gut microbiota shaping mechanisms. J. Biomed. Sci. 2019, 26, 59. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Yu, Z.; Tian, F.; Zhao, J.; Zhang, H.; Zhai, Q.; Chen, W. Surface components and metabolites of probiotics for regulation of intestinal epithelial barrier. Microb. Cell Fact. 2020, 19, 23. [Google Scholar] [CrossRef]
- Vidanarachchi, J.K.; Kurukulasuriya, M.S.; Samaraweera, A.M.; Silva, K.F.S.T. Applications of Marine Nutraceuticals in Dairy Products, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2012; Volume 65. [Google Scholar] [CrossRef]
- Cai, Y.; Folkerts, J.; Folkerts, G.; Maurer, M.; Braber, S. Microbiota-dependent and -independent effects of dietary fibre on human health. Br. J. Pharmacol. 2020, 177, 1363–1381. [Google Scholar] [CrossRef]
- Camilleri, M. Human Intestinal Barrier: Effects of Stressors, Diet, Prebiotics, and Probiotics. Clin. Transl. Gastroenterol. 2021, 12, e00308. [Google Scholar] [CrossRef]
- Swennen, K.; Courtin, C.M.; Delcour, J.A. Non-digestible oligosaccharides with prebiotic properties. Crit. Rev. Food Sci. Nutr. 2006, 46, 459–471. [Google Scholar] [CrossRef]
- Kong, C.; Faas, M.M.; De Vos, P.; Akkerman, R. Impact of dietary fibers in infant formulas on gut microbiota and the intestinal immune barrier. Food Funct. 2020, 11, 9445–9467. [Google Scholar] [CrossRef] [PubMed]
- Akbari, P.; Fink-Gremmels, J.; Willems, R.H.A.M.; DiFilippo, E.; Schols, H.A.; Schoterman, M.H.C.; Garssen, J.; Braber, S. Characterizing microbiota-independent effects of oligosaccharides on intestinal epithelial cells: Insight into the role of structure and size: Structure–activity relationships of non-digestible oligosaccharides. Eur. J. Nutr. 2017, 56, 1919–1930. [Google Scholar] [CrossRef] [PubMed]
- Palaniappan, A.; Antony, U.; Emmambux, M.N. Current status of xylooligosaccharides: Production, characterization, health benefits and food application. Trends Food Sci. Technol. 2021, 111, 506–519. [Google Scholar] [CrossRef]
- Zihni, C.; Mills, C.; Matter, K.; Balda, M.S. Tight junctions: From simple barriers to multifunctional molecular gates. Nat. Rev. Mol. Cell Biol. 2016, 17, 564–580. [Google Scholar] [CrossRef]
- Lee, B.; Moon, K.M.; Kim, C.Y. Tight junction in the intestinal epithelium: Its association with diseases and regulation by phytochemicals. J. Immunol. Res. 2018, 2018, 2645465. [Google Scholar] [CrossRef]
- Suzuki, T. Regulation of the intestinal barrier by nutrients: The role of tight junctions. Anim. Sci. J. 2020, 91, e13357. [Google Scholar] [CrossRef]
- Vermette, D.; Hu, P.; Canarie, M.F.; Funaro, M.; Glover, J.; Pierce, R.W. Tight junction structure, function, and assessment in the critically ill: A systematic review. Intensive Care Med. Exp. 2018, 6, 1–18. [Google Scholar] [CrossRef]
- Buckley, A.; Turner, J.R. Cell biology of tight junction barrier regulation and mucosal disease. Cold Spring Harb. Perspect. Biol. 2018, 10, a029314. [Google Scholar] [CrossRef]
- Anderson, J.M.; Van Itallie, C.M. Physiology and function of the tight junction. Cold Spring Harb. Perspect. Biol. 2009, 1, a002584. [Google Scholar] [CrossRef]
- Sugita, K.; Kabashima, K. Tight junctions in the development of asthma, chronic rhinosinusitis, atopic dermatitis, eosinophilic esophagitis, and inflammatory bowel diseases. J. Leukoc. Biol. 2020, 107, 749–762. [Google Scholar] [CrossRef]
- Chelakkot, C.; Ghim, J.; Ryu, S.H. Mechanisms regulating intestinal barrier integrity and its pathological implications. Exp. Mol. Med. 2018, 50, e450. [Google Scholar] [CrossRef] [PubMed]
- Slifer, Z.M.; Blikslager, A.T. The integral role of tight junction proteins in the repair of injured intestinal epithelium. Int. J. Mol. Sci. 2020, 21, 972. [Google Scholar] [CrossRef] [PubMed]
- Ikenouchi, J.; Furuse, M.; Furuse, K.; Sasaki, H.; Tsukita, S.; Tsukita, S. Tricellulin constitutes a novel barrier at tricellular contacts of epithelial cells. J. Cell Biol. 2005, 171, 939–945. [Google Scholar] [CrossRef] [PubMed]
- Olivier, S.; Leclerc, J.; Grenier, A.; Viollet, M.F.B.; Tamburini, J. Ampk activation promotes tight junction assembly in intestinal epithelial caco-2 cells. Int. J. Mol. Sci. 2019, 20, 5171. [Google Scholar] [CrossRef]
- Rowart, P.; Wu, J.; Caplan, M.J.; Jouret, F. Implications of AMPK in the formation of epithelial tight junctions. Int. J. Mol. Sci. 2018, 19, 2040. [Google Scholar] [CrossRef]
- Zhu, M.J.; Sun, X.; Du, M. AMPK in regulation of apical junctions and barrier function of intestinal epithelium. Tissue Barriers 2018, 6, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wang, J.; You, Q.; He, S.; Meng, Q.; Gao, J.; Wu, X.; Shen, Y.; Sun, Y.; Wu, X.; et al. Activating AMPK to restore tight junction assembly in intestinal epithelium and to attenuate experimental colitis by metformin. Front. Pharmacol. 2018, 9, 761. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Zeng, L.; Lai, X.; Li, J.; Liu, L.; Lin, Q.; Chen, Y. Metformin protects against intestinal barrier dysfunction via AMPKα1-dependent inhibition of JNK signalling activation. J. Cell Mol. Med. 2018, 22, 546–557. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Zhang, H.; Sun, X.; Zhu, M.J. Metformin improves ileal epithelial barrier function in interleukin-10 deficient mice. PLoS ONE 2016, 11, e0168670. [Google Scholar] [CrossRef]
- Sun, X.; Yang, Q.; Rogers, C.J.; Du, M.; Zhu, M.J. AMPK improves gut epithelial differentiation and barrier function via regulating Cdx2 expression. Cell Death Differ. 2017, 24, 819–831. [Google Scholar] [CrossRef]
- Zhang, L.; Li, J.; Young, L.H.; Caplan, M.J. AMP-activated protein kinase regulates the assembly of epithelial tight junctions. Proc. Natl. Acad. Sci. USA 2006, 103, 17272–17277. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; Cantley, L.C. Regulation of epithelial tight junction assembly and disassembly by AMP-activated protein kinase. Proc. Natl. Acad. Sci. USA 2007, 104, 819–822. [Google Scholar] [CrossRef] [PubMed]
- Castanares-Zapatero, D.; Bouleti, C.; Sommereyns, C.; Gerber, B.; Lecut, C.; Mathivet, T.; Horckmans, M.; Communi, D.; Foretz, M.; Vanoverschelde, J.-L.; et al. Connection between cardiac vascular permeability, myocardial edema, and inflammation during sepsis: Role of the α1amp-activated protein kinase isoform. Crit. Care Med. 2013, 41, 411–422. [Google Scholar] [CrossRef] [PubMed]
- TTakata, F.; Dohgu, S.; Matsumoto, J.; Machida, T.; Kaneshima, S.; Matsuo, M.; Sakaguchi, S.; Takeshige, Y.; Yamauchi, A.; Kataoka, Y. Metformin induces up-regulation of blood-brain barrier functions by activating AMP-activated protein kinase in rat brain microvascular endothelial cells. Biochem. Biophys. Res. Commun. 2013, 433, 586–590. [Google Scholar] [CrossRef]
- Zhang, L.; Jouret, F.; Rinehart, J.; Sfakianos, J.; Mellman, I.; Lifton, R.P.; Young, L.H.; Caplan, M.J. AMP-activated protein kinase (AMPK) activation and glycogen synthase kinase-3β(GSK-3β) inhibition induce Ca2+-independent deposition of tight junction components at the plasma membrane. J. Biol. Chem. 2011, 286, 16879–16890. [Google Scholar] [CrossRef]
- Kalsi, K.K.; Garnett, J.P.; Patkee, W.; Weekes, A.; Dockrell, M.E.; Baker, E.H.; Baines, D.L. Metformin attenuates the effect of Staphylococcus aureus on airway tight junctions by increasing PKCζ-mediated phosphorylation of occludin. J. Cell Mol. Med. 2019, 23, 317–327. [Google Scholar] [CrossRef]
- Tang, X.X.; Chen, H.; Yu, S.; Zhang, L.; Caplan, M.J.; Chan, H.C. Lymphocytes accelerate epithelial tight junction assembly: Role of AMP-activated protein kinase (AMPK). PLoS ONE 2010, 5, e12343. [Google Scholar] [CrossRef]
- Rowart, P.; Erpicum, P.; Krzesinski, J.-M.; Sebbagh, M.; Jouret, F. Mesenchymal Stromal Cells Accelerate Epithelial Tight Junction Assembly via the AMP-Activated Protein Kinase Pathway, Independently of Liver Kinase B1. Stem Cells Int. 2017, 2017, 11–16. [Google Scholar] [CrossRef]
- Mandel, L.J.; Bacallaot, R.; Zampighi, G. Uncoupling of the molecular “fence” and paracellular “gate” functions in epithelial tight junctions. Nature. 1993, 361, 552–555. [Google Scholar] [CrossRef]
- Canfield, P.E.; Geerdes, A.M.; Molitoris, B.A. Effect of reversible ATP depletion on tight-junction integrity in LLC-PK1 cells. Am. J. Physiol. Ren. Fluid Electrolyte Physiol. 1991, 261, F1038–F1045. [Google Scholar] [CrossRef]
- Xiong, Y.; Wang, C.; Shi, L.; Wang, L.; Zhou, Z.; Chen, D.; Wang, J.; Guo, H. Myosin light chain kinase: A potential target for treatment of inflammatory diseases. Front. Pharmacol. 2017, 8, 292. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Kim, J.J.; Shen, J.; Dai, N. Crosstalk between Inflammation and ROCK/MLCK Signaling Pathways in Gastrointestinal Disorders with Intestinal Hyperpermeability. Gastroenterol. Res. Pract. 2016, 2016, 7374197. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Graham, W.V.; Wang, Y.; Witkowski, E.D.; Schwarz, B.T.; Turner, J.R. Interferon-γ and tumor necrosis factor-α synergize to induce intestinal epithelial barrier dysfunction by up-regulating myosin light chain kinase expression. Am. J. Pathol. 2005, 166, 409–419. [Google Scholar] [CrossRef]
- Jin, Y.; Blikslager, A.T. The regulation of intestinal mucosal barrier by myosin light chain Kinase/Rho kinases. Int. J. Mol. Sci. 2020, 21, 3550. [Google Scholar] [CrossRef]
- Cunningham, K.E.; Turner, J.R. Myosin light chain kinase: Pulling the strings of epithelial tight junction function. Ann. N. Y. Acad. Sci. 2012, 1258, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Nighot, M.; Rawat, M.; Al-Sadi, R.; Castillo, E.F.; Nighot, P.; Ma, T.Y. Lipopolysaccharide-Induced Increase in Intestinal Permeability Is Mediated by TAK-1 Activation of IKK and MLCK/MYLK Gene. Am. J. Pathol. 2019, 189, 797–812. [Google Scholar] [CrossRef]
- Gu, L.; Li, N.; Gong, J.; Li, Q.; Zhu, W.; Li, J. Berberine ameliorates intestinal epithelial tight-junction damage and down-regulates myosin light chain kinase pathways in a mouse model of endotoxinemia. J. Infect. Dis. 2011, 203, 1602–1612. [Google Scholar] [CrossRef] [PubMed]
- Zahs, A.; Bird, M.D.; Ramirez, L.; Turner, J.R.; Choudhry, M.A.; Kovacs, E.J. Inhibition of long myosin light-chain kinase activation alleviates intestinal damage after binge ethanol exposure and burn injury. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 303, 705–712. [Google Scholar] [CrossRef]
- Nighot, M.; Al-Sadi, R.; Guo, S.; Rawat, M.; Nighot, P.; Watterson, M.D.; Ma, T.Y. Lipopolysaccharide-Induced Increase in Intestinal Epithelial Tight Permeability Is Mediated by Toll-Like Receptor 4/Myeloid Differentiation Primary Response 88 (MyD88) Activation of Myosin Light Chain Kinase Expression. Am. J. Pathol. 2017, 187, 2698–2710. [Google Scholar] [CrossRef]
- Clayburgh, D.; Barrett, T.; Tang, Y.; Meddings, J.B.; Van Eldik, L.J.; Watterson, D.; Clarke, L.L.; Mrsny, R.J.; Turner, J.R. Epithelial myosin light chain kinase-dependent barrier dysfunction mediates T cell activation-induced diarrhea in vivo. J. Clin. Investig. 2005, 115, 2702–2715. [Google Scholar] [CrossRef]
- Al-Sadi, R.; Ye, D.; Dokladny, K.; Ma, T.Y. Mechanism of IL-1β-Induced Increase in Intestinal Epithelial Tight Junction Permeability. J. Immunol. 2008, 180, 5653–5661. [Google Scholar] [CrossRef] [PubMed]
- Feighery, L.M.; Cochrane, S.W.; Quinn, T.; Baird, A.W.; O’Toole, D.; Owens, S.-E.; O’Donoghue, D.; Mrsny, R.J.; Brayden, D.J. Myosin light chain kinase inhibition: Correction of increased intestinal epithelial permeability in vitro. Pharm. Res. 2008, 25, 1377–1386. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhai, N.; Chen, Y.; Fu, C.; Huang, K. OTA induces intestinal epithelial barrier dysfunction and tight junction disruption in IPEC-J2 cells through ROS/Ca2+-mediated MLCK activation. Environ. Pollut. 2018, 242, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Miao, W.; Wu, X.; Wang, K.; Wang, W.; Wang, Y.; Li, Z.; Liu, J.; Li, L.; Peng, L. Sodium butyrate promotes reassembly of tight junctions in Caco-2 monolayers involving inhibition of MLCK/MLC2 pathway and phosphorylation of PKCβ2. Int. J. Mol. Sci. 2016, 17, 1696. [Google Scholar] [CrossRef]
- Al Nabhani, Z.; Montcuquet, N.; Roy, M.; Dussaillant, M.; Hugot, J.-P.; Barreau, F. Complementary Roles of Nod2 in Hematopoietic and Nonhematopoietic Cells in Preventing Gut Barrier Dysfunction Dependent on MLCK Activity. Inflamm. Bowel Dis. 2017, 23, 1109–1119. [Google Scholar] [CrossRef]
- Suzuki, M.; Nagaishi, T.; Yamazaki, M.; Onizawa, M.; Watabe, T.; Sakamaki, Y.; Ichinose, S.; Totsuka, M.; Oshima, S.; Okamoto, R.; et al. Myosin light chain kinase expression induced via tumor necrosis factor receptor 2 signaling in the epithelial cells regulates the development of colitis-associated carcinogenesis. PLoS ONE 2014, 9, e88369. [Google Scholar] [CrossRef]
- Clayburgh, D.R.; Rosen, S.; Witkowski, E.D.; Wang, F.; Blair, S.; Dudek, S.; Garcia, J.G.N.; Alverdy, J.C.; Turner, J.R. A differentiation-dependent splice variant of myosin light chain kinase, MLCK1, regulates epithelial tight junction permeability. J. Biol. Chem. 2004, 279, 55506–55513. [Google Scholar] [CrossRef]
- Li, Z.; Gao, M.; Yang, B.; Zhang, H.; Wang, K.; Liu, Z.; Xiao, X.; Yang, M. Naringin attenuates MLC phosphorylation and NF-κB activation to protect sepsis-induced intestinal injury via RhoA/ROCK pathway. Biomed. Pharmacother. 2018, 103, 50–58. [Google Scholar] [CrossRef]
- Zolotarevsky, Y.; Hecht, G.; Koutsouris, A.; Gonzalez, D.E.; Quan, C.; Tom, J.; Mrsny, R.J.; Turner, J.R. A membrane-permeant peptide that inhibits MLC kinase restores barrier function in in vitro models of intestinal disease. Gastroenterology 2002, 123, 163–172. [Google Scholar] [CrossRef]
- Berglund, J.J.; Riegler, M.; Zolotarevsky, Y.; Wenzl, E.; Turner, J.R. Regulation of human jejunal transmucosal resistance and MLC phosphorylation by Na+-glucose cotransport. Am. J. Physiol. Liver Physiol. 2001, 281, G1487–G1493. [Google Scholar] [CrossRef]
- Shen, L.; Black, E.D.; Witkowski, E.D.; Lencer, W.; Guerriero, V.; Schneeberger, E.E.; Turner, J.R. Myosin light chain phosphorylation regulates barrier function by remodeling tight junction structure. J. Cell Sci. 2006, 119, 2095–2106. [Google Scholar] [CrossRef] [PubMed]
- Terry, S.; Nie, M.; Matter, K.; Balda, M.S. Rho Signaling and Tight Junction Functions. Physiology 2010, 25, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Citi, S.; Guerrera, D.; Spadaro, D.; Shah, J. Epithelial junctions and Rho family GTPases: The zonular signalosome. Small GTPases 2014, 5, e972861. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, S.; Wang, J.; Shan, A.; Xu, L. Changes in intestinal barrier functions and gut microbiota in rats exposed to zearalenone. Ecotoxicol. Environ. Saf. 2020, 204, 111072. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shou, Z.; Fan, H.; Xu, M.; Chen, Q.; Tang, Q.; Liu, X.; Wu, H.; Zhang, M.; Yu, T.; et al. Protective effects of oxymatrine against DSS-induced acute intestinal inflammation in mice via blocking the RhoA/ROCK signaling pathway. Biosci. Rep. 2019, 39, BSR20182297. [Google Scholar] [CrossRef]
- Tong, J.; Wang, Y.; Chang, B.; Zhang, D.; Wang, B. Evidence for the involvement of RhoA signaling in the ethanol-induced increase in intestinal epithelial barrier permeability. Int. J. Mol. Sci. 2013, 14, 3946. [Google Scholar] [CrossRef]
- Schlegel, N.; Meir, M.; Spindler, V.; Germer, C.T.; Waschke, J. Differential role of Rho GTPases in intestinal epithelial barrier regulation in vitro. J. Cell Physiol. 2011, 226, 1196–1203. [Google Scholar] [CrossRef] [PubMed]
- Shen, Q.; Gan, H.; Yang, M.; Huang, Y.; Du, X.-G.; Chen, D.; Zhang, H. Improving RhoA-mediated intestinal epithelial permeability by continuous blood purification in patients with severe acute pancreatitis. Int. J. Artif. Organs 2013, 36, 812–820. [Google Scholar] [CrossRef] [PubMed]
- Le Dréan, G.; Haure-Mirande, V.; Ferrier, L.; Bonnet, C.; Hulin, P.; de Coppet, P.; Segain, J. Visceral adipose tissue and leptin increase colonic epithelial tight junction permeability via a RhoA-ROCK-dependent pathway. FASEB J. 2014, 28, 1059–1070. [Google Scholar] [CrossRef]
- Wu, R.Y.; Abdullah, M.; Määttänen, P.; Pilar, A.V.C.; Scruten, E.; Johnson-Henry, K.C.; Napper, S.; O’Brien, C.; Jones, N.L.; Sherman, P.M. Protein kinase Cσ signaling is required for dietary prebiotic-induced strengthening of intestinal epithelial barrier function. Sci. Rep. 2017, 7, 40820. [Google Scholar] [CrossRef]
- González-Mariscal, L.; Tapia, R.; Chamorro, D. Crosstalk of tight junction components with signaling pathways. Biochim. Biophys. Acta Biomembr. 2008, 1778, 729–756. [Google Scholar] [CrossRef] [PubMed]
- Eckert, J.J.; McCallum, A.; Mears, A.; Rumsby, M.G.; Cameron, I.T.; Fleming, T.P. Relative contribution of cell contact pattern, specific PKC isoforms and gap junctional communication in tight junction assembly in the mouse early embryo. Dev. Biol. 2005, 288, 234–247. [Google Scholar] [CrossRef] [PubMed]
- Matter, K.; Balda, M.S. Signalling to and from tight junctions. Nat. Rev. Mol. Cell Biol. 2003, 4, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Cario, E.; Gerken, G.; Podolsky, D.K. Toll-like receptor 2 enhances ZO-1-associated intestinal epithelial barrier integrity via protein kinase C. Gastroenterology 2004, 127, 224–238. [Google Scholar] [CrossRef]
- Song, J.C.; Hanson, C.M.; Tsai, V.; Farokhzad, O.C.; Lotz, M.; Matthews, J.B. Regulation of epithelial transport and barrier function by distinct protein kinase C isoforms. Am. J. Physiol. Physiol. 2001, 281, C649–C661. [Google Scholar] [CrossRef] [PubMed]
- Banan, A.; Zhang, L.J.; Shaikh, M.; Fields, J.Z.; Choudhary, S.; Forsyth, C.B.; Farhadi, A.; Keshavarzian, A. θ isoform of protein kinase C alters barrier function in intestinal epithelium through modulation of distinct claudin isotypes: A novel mechanism for regulation of permeability. J. Pharmacol. Exp. Ther. 2005, 313, 962–982. [Google Scholar] [CrossRef]
- Yoo, J.; Nichols, A.; Mammen, J.; Calvo, I.; Song, J.C.; Worrell, R.T.; Matlin, K.; Matthews, J.B. Bryostatin-1 enhances barrier function in T84 epithelia through PKC-dependent regulation of tight junction proteins. Am. J. Physiol. Cell Physiol. 2003, 285, 300–309. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Guo, Y.; Pan, W.; Liu, S.; Shen, Z.; Xu, Y.; Hu, L. ERK/MAPK signalling pathway and tumorigenesis (Review). Exp. Ther. Med. 2020, 19, 1997–2007. [Google Scholar] [CrossRef]
- Cuenda, A. Mitogen-Activated Protein Kinases (MAPK) in Cancer, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 2. [Google Scholar] [CrossRef]
- Basuroy, S.; Seth, A.; Elias, B.; Naren, A.P.; Rao, R. MAPK interacts with occludin and mediates EGF-induced prevention of tight junction disruption by hydrogen peroxide. Biochem. J. 2006, 393, 69–77. [Google Scholar] [CrossRef]
- Dai, C.; Zhao, D.H.; Jiang, M. VSL#3 probiotics regulate the intestinal epithelial barrier in vivo and in vitro via the p38 and ERK signaling pathways. Int. J. Mol. Med. 2012, 29, 202–208. [Google Scholar] [CrossRef]
- Kinugasa, T.; Sakaguchi, T.; Gu, X. Claudins Regulate the Intestinal Barrier in Response. Gastroenterology 2000, 108, 1001–1011. [Google Scholar] [CrossRef]
- Yang, R.; Harada, T.; Li, J.; Uchiyama, T.; Han, Y.; Englert, J.; Fink, M.P. Bile modulates intestinal epithelial barrier function via an extracellular signal related kinase 1/2 dependent mechanism. Intensive Care Med. 2005, 31, 709–717. [Google Scholar] [CrossRef] [PubMed]
- Howe, K.L.; Reardon, C.; Wang, A.; Nazli, A.; McKay, D.M. Transforming growth factor-β regulation of epithelial tight junction proteins enhances barrier function and blocks enterohemorrhagic Escherichia coli O157:H7-induced increased permeability. Am. J. Pathol. 2005, 167, 1587–1597. [Google Scholar] [CrossRef]
- Serreli, G.; Melis, M.P.; Zodio, S.; Naitza, M.R.; Casula, E.; Peñalver, P.; Lucas, R.; Loi, R.; Morales, J.C.; Deiana, M. Altered paracellular permeability in intestinal cell monolayer challenged with lipopolysaccharide: Modulatory effects of pterostilbene metabolites. Food Chem. Toxicol. 2020, 145, 111729. [Google Scholar] [CrossRef]
- Liu, Z.; Zou, D.; Yang, X.; Xue, X.; Zuo, L.; Zhou, Q.; Hu, R.; Wang, Y. Melatonin inhibits colon cancer RKO cell migration by downregulating Rho-associated protein kinase expression via the p38/MAPK signaling pathway. Mol. Med. Rep. 2017, 16, 9383–9392. [Google Scholar] [CrossRef]
- Al-Sadi, R.; Youssef, M.; Rawat, M.; Guo, S.; Dokladny, K.; Haque, M.; Watterson, M.D.; Ma, T.Y. MMP-9-induced increase in intestinal epithelial tight permeability is mediated by p38 kinase signaling pathway activation of MLCK gene. Am. J. Physiol Gastrointest. Liver Physiol. 2019, 316, G278–G290. [Google Scholar] [CrossRef]
- Jeong, C.H.; Seok, J.S.; Petriello, M.C.; Han, S.G. Arsenic downregulates tight junction claudin proteins through p38 and NF-κB in intestinal epithelial cell line, HT-29. Toxicology 2017, 379, 31–39. [Google Scholar] [CrossRef]
- Yin, M.; Shen, Z.; Yang, L.; Zheng, W.; Song, H. Protective effects of CXCR3/HO-1 gene-modified BMMSCs on damaged intestinal epithelial cells: Role of the p38-MAPK signaling pathway. Int. J. Mol. Med. 2019, 43, 2086–2102. [Google Scholar] [CrossRef]
- Ouyang, J.; Zhang, Z.-H.; Zhou, Y.-X.; Niu, W.-C.; Zhou, F.; Shen, C.-B.; Chen, R.-G.; Li, X. Up-regulation of Tight-Junction Proteins by p38 Mitogen-Activated Protein Kinase/p53 Inhibition Leads to a Reduction of Injury to the Intestinal Mucosal Barrier in Severe Acute Pancreatitis. Pancreas 2016, 45, 1136–1144. [Google Scholar] [CrossRef]
- Oshima, T.; Sasaki, M.; Kataoka, H.; Miwa, H.; Takeuchi, T.; Joh, T. Wip1 protects hydrogen peroxide-induced colonic epithelial barrier dysfunction. Cell Mol. Life Sci. 2007, 64, 3139–3147. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Q.; Li, F.; Xin, Y.; Duan, Z. Contributions of ho-1-dependent mapk to regulating intestinal barrier disruption. Biomol. Ther. 2021, 29, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Junyuan, Z.; Hui, X.; Chunlan, H.; Junjie, F.; Qixiang, M.; Yingying, L.; Lihong, L.; Xingpeng, W.; Yue, Z. Quercetin protects against intestinal barrier disruption and inflammation in acute necrotizing pancreatitis through TLR4/MyD88/p38 MAPK and ERS inhibition. Pancreatology 2018, 18, 742–752. [Google Scholar] [CrossRef]
- Liu, Z.; Tian, Y.; Jiang, Y.; Chen, S.; Liu, T.; Moyer, M.P.; Qin, H.; Zhou, X. Protective Effects of Let-7b on the Expression of Occludin by Targeting P38 MAPK in Preventing Intestinal Barrier Dysfunction. Cell Physiol. Biochem. 2018, 45, 343–355. [Google Scholar] [CrossRef] [PubMed]
- Reiche, J.; Huber, O. Post-translational modifications of tight junction transmembrane proteins and their direct effect on barrier function. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183330. [Google Scholar] [CrossRef] [PubMed]
- Al-Sadi, R. Mechanism of cytokine modulation of epithelial tight junction barrier. Front. Biosci. 2009, 14, 2765. [Google Scholar] [CrossRef] [PubMed]
- Andrews, C.; McLean, M.H.; Durum, S.K. Cytokine tuning of intestinal epithelial function. Front. Immunol. 2018, 9, 1270. [Google Scholar] [CrossRef]
- Wang, Y.; Mumm, J.B.; Herbst, R.; Kolbeck, R.; Wang, Y. IL-22 Increases Permeability of Intestinal Epithelial Tight Junctions by Enhancing Claudin-2 Expression. J. Immunol. 2017, 199, 3316–3325. [Google Scholar] [CrossRef]
- Rosen, M.J.; Chaturvedi, R.; Washington, M.K.; Kuhnhein, L.A.; Moore, P.D.; Coggeshall, S.S.; McDonough, E.M.; Weitkamp, J.-H.; Singh, A.B.; Coburn, L.A.; et al. STAT6 Deficiency Ameliorates Severity of Oxazolone Colitis by Decreasing Expression of Claudin-2 and Th2-Inducing Cytokines. J. Immunol. 2013, 190, 1849–1858. [Google Scholar] [CrossRef]
- Ryu, W.-I.; Lee, H.; Bae, H.C.; Jeon, J.; Ryu, H.J.; Kim, J.; Kim, J.H.; Son, J.W.; Imai, Y.; Yamanishi, K.; et al. IL-33 down-regulates CLDN1 expression through the ERK/STAT3 pathway in keratinocytes. J. Dermatol. Sci. 2018, 90, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Carding, S.; Verbeke, K.; Vipond, D.T.; Corfe, B.M.; Owen, L.J. Dysbiosis of the gut microbiota in disease. Microb. Ecol. Health Dis. 2015, 26, 26191. [Google Scholar] [CrossRef]
- Rook, G.; Bäckhed, F.; Levin, B.R.; McFall-Ngai, M.J.; McLean, A.R. Evolution, human-microbe interactions, and life history plasticity. Lancet 2017, 390, 521–530. [Google Scholar] [CrossRef]
- Gareau, M.G.; Sherman, P.M.; Walker, W.A. Probiotics and the gut microbiota in intestinal health and disease. Nat. Rev. Gastroenterol. Hepatol. 2010, 7, 503–514. [Google Scholar] [CrossRef] [PubMed]
- Michie, L.; Tucker, H.O. Influence of Commensal Microbiota in Barrier Function of Intestinal Mucosal Epithelium. Adv. Res. Endocrinol. Metab. 2019, 1, 33–36. [Google Scholar] [PubMed]
- Belizário, J.E.; Faintuch, J. Microbiome and Gut Dysbiosis. Exp. Suppl. 2018, 109, 459–476. [Google Scholar] [CrossRef]
- Ottman, N.; Reunanen, J.; Meijerink, M.; Pietilä, T.E.; Kainulainen, V.; Klievink, J.; Huuskonen, L.; Aalvink, S.; Skurnik, M.; Boeren, S.; et al. Pili-like proteins of Akkermansia muciniphila modulate host immune responses and gut barrier function. PLoS ONE 2017, 12, e0173004. [Google Scholar] [CrossRef] [PubMed]
- Venkatesh, M.; Mukherjee, S.; Wang, H.; Li, H.; Sun, K.; Benechet, A.P.; Qiu, Z.; Maher, L.; Redinbo, M.R.; Phillips, R.S.; et al. Symbiotic bacterial metabolites regulate gastrointestinal barrier function via the xenobiotic sensor PXR and toll-like receptor 4. Immunity 2014, 41, 296–310. [Google Scholar] [CrossRef]
- Bansal, T.; Alaniz, R.C.; Wood, T.K.; Jayaraman, A. The bacterial signal indole increases epithelial-cell tight-junction resistance and attenuates indicators of inflammation. Proc. Natl. Acad. Sci. USA 2010, 107, 228–233. [Google Scholar] [CrossRef]
- Rautava, S.; Walker, W.A. Commensal bacteria and epithelial cross talk in the developing intestine. Curr. Gastroenterol. Rep. 2007, 9, 385–392. [Google Scholar] [CrossRef]
- Ratajczak, W.; Rył, A.; Mizerski, A.; Walczakiewicz, K.; Sipak, O.; Laszczyńska, M. Immunomodulatory potential of gut microbiome-derived shortchain fatty acids (SCFAs). Acta Biochim. Pol. 2019, 66, 1–12. [Google Scholar] [CrossRef]
- Littman, D.R.; Pamer, E.G. Role of the commensal microbiota in normal and pathogenic host immune responses. Cell Host Microbe 2011, 10, 311–323. [Google Scholar] [CrossRef]
- Hsiao, E.Y.; McBride, S.W.; Hsien, S.; Sharon, G.; Hyde, E.R.; McCue, T.; Codelli, J.A.; Chow, J.; Reisman, S.E.; Petrosino, J.F.; et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell 2013, 155, 1451–1463. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, N.; Emoto, T.; Yamashita, T.; Watanabe, H.; Hayashi, T.; Tabata, T.; Hoshi, N.; Hatano, N.; Ozawa, G.; Sasaki, N.; et al. Bacteroides vulgatus and Bacteroides dorei Reduce Gut Microbial Lipopolysaccharide Production and Inhibit Atherosclerosis. Circulation 2018, 138, 2486–2498. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhao, X.; Zhu, Y.; Ma, J.; Ma, H.; Zhang, H. Probiotic mixture protects dextran sulfate sodium-induced colitis by altering tight junction protein expressions and increasing tregs. Mediat. Inflamm. 2018, 2018, 9416391. [Google Scholar] [CrossRef]
- Srutkova, D.; Schwarzer, M.; Hudcovic, T.; Zakostelska, Z.; Drab, V.; Spanova, A.; Rittich, B.; Kozakova, H.; Schabussova, I. Bifidobacterium longum CCM 7952 promotes epithelial barrier function and prevents acute dss-induced colitis in strictly strain-Specific manner. PLoS ONE 2015, 10, e0134050. [Google Scholar] [CrossRef] [PubMed]
- Zyrek, A.A.; Cichon, C.; Helms, S.; Enders, C.; Sonnenborn, U.; Schmidt, M.A. Molecular mechanisms underlying the probiotic effects of Escherichia coli Nissle 1917 involve ZO-2 and PKCζ redistribution resulting in tight junction and epithelial barrier repair. Cell Microbiol. 2007, 9, 804–816. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Chen, S.; Ma, J.; Ma, Y.; Zhu, J.; Ma, Y.; Liu, Y.; Wang, P.; Pan, Y. Escherichia coli nissle 1917 protects intestinal barrier function by inhibiting NF-κB-mediated activation of the MLCK-P-MLC signaling pathway. Mediat. Inflamm. 2019, 2019, 5796491. [Google Scholar] [CrossRef]
- Martín, R.; Miquel, S.; Chain, F.; Natividad, J.M.; Jury, J.; Lu, J.; Sokol, H.; Theodorou, V.; Bercik, P.; Verdu, E.F.; et al. Faecalibacterium prausnitzii prevents physiological damages in a chronic low-grade inflammation murine model. BMC Microbiol. 2015, 15, 67. [Google Scholar] [CrossRef]
- Yi, H.; Wang, L.; Xiong, Y.; Wang, Z.; Qiu, Y.; Wen, X.; Jiang, Z.; Yang, X.; Ma, X. Lactobacillus reuteri LR1 Improved Expression of Genes of Tight Junction Proteins via the MLCK Pathway in IPEC-1 Cells during Infection with Enterotoxigenic Escherichia coli K88. Mediat. Inflamm. 2018, 2018, 6434910. [Google Scholar] [CrossRef]
- Kinashi, Y.; Hase, K. Partners in Leaky Gut Syndrome: Intestinal Dysbiosis and Autoimmunity. Front. Immunol. 2021, 12, 673708. [Google Scholar] [CrossRef]
- Plovier, H.; Everard, A.; Druart, C.; Depommier, C.; Van Hul, M.; Geurts, L.; Chilloux, J.; Ottman, N.; Duparc, T.; Lichtenstein, L.; et al. A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat. Med. 2017, 23, 107–113. [Google Scholar] [CrossRef]
- Ewaschuk, J.B.; Diaz, H.; Meddings, L.; Diederichs, B.; Dmytrash, A.; Backer, J.; Langen, M.L.-V.; Madsen, K.L. Secreted bioactive factors from Bifidobacterium infantis enhance epithelial cell barrier function. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 295, 1025–1034. [Google Scholar] [CrossRef] [PubMed]
- Shimada, Y.; Kinoshita, M.; Harada, K.; Mizutani, M.; Masahata, K.; Kayama, H.; Takeda, K. Commensal bacteria-dependent indole production enhances epithelial barrier function in the colon. PLoS ONE 2013, 8, e80604. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.-S.; Ban, M.; Choi, E.-J.; Moon, H.-G.; Jeon, J.-S.; Kim, D.-K.; Park, S.-K.; Jeon, S.G.; Roh, T.-Y.; Myung, S.-J.; et al. Extracellular Vesicles Derived from Gut Microbiota, Especially Akkermansia muciniphila, Protect the Progression of Dextran Sulfate Sodium-Induced Colitis. PLoS ONE 2013, 8, e76520. [Google Scholar] [CrossRef] [PubMed]
- Schwechheimer, C.; Kuehn, M.J. Outer-membrane vesicles from Gram-negative bacteria: Biogenesis and functions. Nat. Rev. Microbiol. 2015, 13, 605–619. [Google Scholar] [CrossRef]
- Kim, M.; Lee, S.-W.; Kim, J.; Shin, Y.; Chang, F.; Kim, J.M.; Cong, X.; Yu, G.-Y.; Park, K. LPS-induced epithelial barrier disruption via hyperactivation of CACC and ENaC. Am. J. Physiol. Cell Physiol. 2021, 320, C448–C461. [Google Scholar] [CrossRef]
- Chelakkot, C.; Choi, Y.; Kim, D.-K.; Park, H.T.; Ghim, J.; Kwon, Y.; Jeon, J.; Kim, M.-S.; Jee, Y.-K.; Gho, Y.S.; et al. Akkermansia muciniphila-derived extracellular vesicles influence gut permeability through the regulation of tight junctions. Exp. Mol. Med. 2018, 50, e450. [Google Scholar] [CrossRef]
- Alvarez, C.S.; Badia, J.; Bosch, M.; Giménez, R.; Baldomà, L. Outer membrane vesicles and soluble factors released by probiotic escherichia coli nissle 1917 and commensal ECOR63 enhance barrier function by regulating expression of tight junction proteins in intestinal epithelial cells. Front. Microbiol. 2016, 7, 1981. [Google Scholar] [CrossRef]
- Hering, N.A.; Richter, J.F.; Fromm, A.; Wieser, A.; Hartmann, S.; Günzel, D.; Bücker, R.; Fromm, M.; Schulzke, J.D.; Troeger, H. TcpC protein from E. coli Nissle improves epithelial barrier function involving PKCζ and ERK1/2 signaling in HT-29/B6 cells. Mucosal Immunol. 2014, 7, 369–378. [Google Scholar] [CrossRef]
- Carlsson, A.H.; Yakymenko, O.; Olivier, I.; Håkansson, F.; Postma, E.; Keita, V.; Söderholm, J.D. Faecalibacterium prausnitzii supernatant improves intestinal barrier function in mice DSS colitis. Scand. J. Gastroenterol. 2013, 48, 1136–1144. [Google Scholar] [CrossRef]
- Han, X.; Lee, A.; Huang, S.; Gao, J.; Spence, J.R.; Owyang, C. Lactobacillus rhamnosus GG prevents epithelial barrier dysfunction induced by interferon-gamma and fecal supernatants from irritable bowel syndrome patients in human intestinal enteroids and colonoids. Gut Microbes 2019, 10, 59–76. [Google Scholar] [CrossRef]
- Liu, H.; Wang, J.; He, T.; Becker, S.; Zhang, G.; Li, D.; Ma, X. Butyrate: A double-edged sword for health? Adv. Nutr. 2018, 9, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Bordin, M.; D’Atri, F.; Guillemot, L.; Citi, S. Histone deacetylase inhibitors up-regulate the expression of tight junction proteins. Mol. Cancer Res. 2004, 2, 692–701. [Google Scholar] [CrossRef] [PubMed]
- Ohata, A.; Usami, M.; Miyoshi, M. Short-chain fatty acids alter tight junction permeability in intestinal monolayer cells via lipoxygenase activation. Nutrition 2005, 21, 838–847. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; He, Z.; Chen, W.; Holzman, I.R.; Lin, J. Effects of butyrate on intestinal barrier function in a caco-2 cell monolayer model of intestinal barrier. Pediatr. Res. 2007, 61, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Li, Z.R.; Green, R.S.; Holzman, I.R.; Lin, J. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. J. Nutr. 2009, 139, 1619–1625. [Google Scholar] [CrossRef]
- Wang, H.B.; Wang, P.Y.; Wang, X.; Wan, Y.L.; Liu, Y.C. Butyrate enhances intestinal epithelial barrier function via up-regulation of tight junction protein claudin-1 transcription. Dig. Dis. Sci. 2012, 57, 3126–3135. [Google Scholar] [CrossRef]
- Zheng, L.; Kelly, C.J.; Battista, K.D.; Schaefer, R.; Lanis, J.M.; Alexeev, E.E.; Wang, R.X.; Onyiah, J.C.; Kominsky, D.J.; Colgan, S.P. Microbial-Derived Butyrate Promotes Epithelial Barrier Function through IL-10 Receptor–Dependent Repression of Claudin-2. J. Immunol. 2017, 199, 2976–2984. [Google Scholar] [CrossRef]
- Pham, V.T.; Seifert, N.; Richard, N.; Raederstorff, D.; Steinert, R.; Prudence, K.; Mohajeri, M.H. The effects of fermentation products of prebiotic fibres on gut barrier and immune functions in vitro. PeerJ 2018, 2018, e5288. [Google Scholar] [CrossRef]
- Ma, X.; Fan, P.X.; Li, L.S.; Qiao, S.Y.; Zhang, G.L.; Li, D.F. Butyrate promotes the recovering of intestinal wound healing through its positive effect on the tight junctions. J. Anim. Sci. 2012, 90, 266–268. [Google Scholar] [CrossRef]
- Suzuki, T.; Yoshida, S.; Hara, H. Physiological concentrations of short-chain fatty acids immediately suppress colonic epithelial permeability. Br. J. Nutr. 2008, 100, 297–305. [Google Scholar] [CrossRef]
- Grilli, E.; Tugnoli, B.; Foerster, C.J.; Piva, A. Butyrate modulates inflammatory cytokines and tight junctions components along the gut of weaned pigs. J. Anim. Sci. 2016, 94, 433–436. [Google Scholar] [CrossRef]
- Feng, W.; Wu, Y.; Chen, G.; Fu, S.; Li, B.; Huang, B.; Wang, D.; Wang, W.; Liu, J. Sodium Butyrate Attenuates Diarrhea in Weaned Piglets and Promotes Tight Junction Protein Expression in Colon in a GPR109A-Dependent Manner. Cell Physiol. Biochem. 2018, 47, 1617–1629. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.-H.; Gao, C.-L.; Guo, H.-L.; Zhang, W.-Q.; Huang, W.; Tang, S.-S.; Gan, W.-J.; Xu, Y.; Zhou, H.; Zhu, Q. Sodium butyrate supplementation ameliorates diabetic inflammation in db/db mice. J. Endocrinol. 2018, 238, 231–244. [Google Scholar] [CrossRef]
- Elamin, E.E.; Masclee, A.A.; Dekker, J.; Pieters, H.J.; Jonkers, D.M. Short-chain fatty acids activate AMP-activated protein kinase and ameliorate ethanol-induced intestinal barrier dysfunction in Caco-2 cell monolayers. J. Nutr. 2013, 143, 1872–1881. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Ajuwon, K.M. Butyrate modifies intestinal barrier function in IPEC-J2 cells through a selective upregulation of tight junction proteins and activation of the Akt signaling pathway. PLoS ONE 2017, 12, e0179586. [Google Scholar] [CrossRef]
- Tong, L.-C.; Wang, Y.; Wang, Z.-B.; Liu, W.-Y.; Sun, S.; Li, L.; Su, D.-F.; Zhang, L.-C. Propionate ameliorates dextran sodium sulfate-induced colitis by improving intestinal barrier function and reducing inflammation and oxidative stress. Front. Pharmacol. 2016, 7, 253. [Google Scholar] [CrossRef]
- Singh, R.; Chandrashekharappa, S.; Bodduluri, H.; Baby, B.V.; Hegde, B.; Kotla, N.G.; Hiwale, A.A.; Saiyed, T.; Patel, P.; Vijay-Kumar, M.; et al. Enhancement of the gut barrier integrity by a microbial metabolite through the Nrf2 pathway. Nat. Commun. 2019, 10, 89. [Google Scholar] [CrossRef]
- Guarino, M.P.L.; Altomare, A.; Emerenziani, S.; Di Rosa, C.; Ribolsi, M.; Balestrieri, P.; Iovino, P.; Rocchi, G.; Cicala, M. Mechanisms of action of prebiotics and their effects on gastro-intestinal disorders in adults. Nutrients 2020, 12, 1037. [Google Scholar] [CrossRef]
- Asadpoor, M.; Peeters, C.; Henricks, P.A.J.; Varasteh, S.; Pieters, R.J.; Folkerts, G.; Braber, S. Anti-Pathogenic Functions of Non-Digestible Oligosaccharides In Vitro. Nutrients 2020, 12, 1789. [Google Scholar] [CrossRef]
- Singh, S.P.; Jadaun, J.S.; Narnoliya, L.K.; Pandey, A. Prebiotic Oligosaccharides: Special Focus on Fructooligosaccharides, Its Biosynthesis and Bioactivity. Appl. Biochem. Biotechnol. 2017, 183, 613–635. [Google Scholar] [CrossRef]
- Chen, Q.; Ren, Y.; Lu, J.; Bartlett, M.; Chen, L.; Zhang, Y.; Guo, X.; Liu, C. A novel prebiotic blend product prevents irritable bowel syndrome in mice by improving gut microbiota and modulating immune response. Nutrients 2017, 9, 1341. [Google Scholar] [CrossRef] [PubMed]
- Davani-Davari, D.; Negahdaripour, M.; Karimzadeh, I.; Seifan, M.; Mohkam, M.; Masoumi, S.; Berenjian, A.; Ghasemi, Y. Prebiotics: Definition, types, sources, mechanisms, and clinical applications. Foods 2019, 8, 92. [Google Scholar] [CrossRef] [PubMed]
- Asadpoor, M.; Ithakisiou, G.N.; Henricks, P.A.; Pieters, R.; Folkerts, G.; Braber, S. Non-digestible oligosaccharides and short chain fatty acids and their toxins. Toxins 2021, 13, 175. [Google Scholar] [CrossRef] [PubMed]
- Wongkrasant, P.; Pongkorpsakol, P.; Ariyadamrongkwan, J.; Meesomboon, R.; Satitsri, S.; Pichyangkura, R.; Barrett, K.E.; Muanprasat, C. A prebiotic fructo-oligosaccharide promotes tight junction assembly in intestinal epithelial cells via an AMPK-dependent pathway. Biomed. Pharmacother. 2020, 129, 110415. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Chen, D.; Yu, B.; Huang, Z.; Mao, X.; Zheng, P.; Luo, Y.; Yu, J.; Luo, J.; Yan, H.; et al. Effects of dietary inulin supplementation on growth performance, intestinal barrier integrity and microbial populations in weaned pigs. Br. J. Nutr. 2020, 124, 296–305. [Google Scholar] [CrossRef]
- Sivieri, K.; Morales, M.L.V.; Saad, S.M.I.; Adorno, M.A.T.; Sakamoto, I.K.; Rossi, E.A. Prebiotic effect of fructooligosaccharide in the simulator of the human intestinal microbial ecosystem (SHIME® model). J. Med. Food. 2014, 17, 894–901. [Google Scholar] [CrossRef]
- Liu, T.-W.; Cephas, K.D.; Holscher, H.D.; Kerr, K.R.; Mangian, H.F.; A Tappenden, K.; Swanson, K.S. Nondigestible fructans alter gastrointestinal barrier function, gene expression, histomorphology, and themicrobiota profiles of diet-induced obese C57BL/6J Mice. J. Nutr. 2016, 146, 949–956. [Google Scholar] [CrossRef]
- Zhao, W.; Yuan, M.; Li, P.; Yan, H.; Zhang, H.; Liu, J. Short-chain fructo-oligosaccharides enhances intestinal barrier function by attenuating mucosa inflammation and altering colonic microbiota composition of weaning piglets. Ital. J. Anim. Sci. 2019, 18, 976–986. [Google Scholar] [CrossRef]
- Lafontaine, G.M.F.; Fish, N.M.; Connerton, I.F. In vitro evaluation of the effects of commercial prebiotic GOS and FOS products on human colonic caco–2 cells. Nutrients 2020, 12, 1281. [Google Scholar] [CrossRef]
- Ten Bruggencate, S.J.M.; Bovee-Oudenhoven, I.M.J.; Lettink-Wissink, M.L.G.; Katan, M.B.; Van Der Meer, R. Dietary fructooligosaccharides affect intestinal barrier function in healthy men. J. Nutr. 2006, 136, 70–74. [Google Scholar] [CrossRef]
- Krupa-Kozak, U.; Świątecka, D.; Bączek, N.; Brzóska, M.M. Inulin and fructooligosaccharide affect: In vitro calcium uptake and absorption from calcium-enriched gluten-free bread. Food Funct. 2016, 7, 1950–1958. [Google Scholar] [CrossRef]
- Gama, L.; Baxendale-Cox, L.M.; Breitwieser, G.E. Ca2+-sensing receptors in intestinal epithelium. Am. J. Physiol. 1997, 273, C1168–C1175. [Google Scholar] [CrossRef]
- Suzuki, T.; Hara, H. Various nondigestible saccharides open a paracellular calcium transport pathway with the induction of intracellular calcium signaling in human intestinal Caco-2 cells. J. Nutr. 2004, 134, 1935–1941. [Google Scholar] [CrossRef]
- Vogt, L.; Ramasamy, U.; Meyer, D.; Pullens, G.; Venema, K.; Faas, M.M.; Schols, H.A.; De Vos, P. Immune Modulation by Different Types of β2→1-Fructans Is Toll-Like Receptor Dependent. PLoS ONE 2013, 8, e68367. [Google Scholar] [CrossRef] [PubMed]
- Cario, E.; Gerken, G.; Podolsky, D. Toll-Like Receptor 2 Controls Mucosal Inflammation by Regulating Epithelial Barrier Function. Gastroenterology 2007, 132, 1359–1374. [Google Scholar] [CrossRef] [PubMed]
- Cario, E. Barrier-protective function of intestinal epithelial toll-like receptor 2. Mucosal Immunol. 2008, 1, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Vogt, L.M.; Meyer, D.; Pullens, G.; Faas, M.M.; Venema, K.; Ramasamy, U.; Schols, H.A.; de Vos, P. Toll-like receptor 2 activation by β2→1-fructans protects barrier function of t84 human intestinal epithelial cells in a chain length-dependent manner. J. Nutr. 2014, 144, 1002–1008. [Google Scholar] [CrossRef]
- Johnson-Henry, K.; Pinnell, L.J.; Waskow, A.M.; Irrazabal, T.; Martin, A.; Hausner, M.; Sherman, P.M. Short-chain fructo-oligosaccharide and inulin modulate inflammatory responses and microbial communities in Caco2-bbe cells and in a mouse model of intestinal injury. J. Nutr. 2014, 144, 1725–1733. [Google Scholar] [CrossRef]
- Wu, R.Y.; Määttänen, P.; Napper, S.; Scruten, E.; Li, B.; Koike, Y.; Johnson-Henry, K.C.; Pierro, A.; Rossi, L.; Botts, S.R.; et al. Non-digestible oligosaccharides directly regulate host kinome to modulate host inflammatory responses without alterations in the gut microbiota. Microbiome 2017, 5, 135. [Google Scholar] [CrossRef]
- Zenhom, M.; Hyder, A.; De Vrese, M.; Heller, K.J.; Roeder, T.; Schrezenmeir, J. Prebiotic oligosaccharides reduce proinflammatory cytokines in intestinal Caco-2 cells via activation of PPARγ and peptidoglycan recognition protein 3. J. Nutr. 2011, 141, 971–977. [Google Scholar] [CrossRef]
- Jiang, C.; Ting, A.T.; Seed, B. PPAR-γ agonists inhibit production of monocyte inflammatory cytokines. Nature 1998, 391, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Ichimura, M.; Tsuneyama, K.; Moritoki, Y.; Tsunashima, H.; Omagari, K.; Hara, M.; Yasuda, I.; Miyakawa, H.; Kikuchi, K. Fructo-oligosaccharides and intestinal barrier function in a methionine–choline-deficient mouse model of nonalcoholic steatohepatitis. PLoS ONE 2017, 12, e0175406. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, P.L.A.; Andrade, M.E.R.; Trindade, L.M.; Leocádio, P.C.L.; Alvarez-Leite, J.I.; dos Reis, D.C.; Cassali, G.D.; Melo, L.D.S.S.E.; Martins, F.D.S.; Fernandes, S.O.A.; et al. Prophylactic and therapeutic supplementation using fructo-oligosaccharide improves the intestinal homeostasis after mucositis induced by 5- fluorouracil. Biomed. Pharmacother. 2021, 133, 111012. [Google Scholar] [CrossRef] [PubMed]
- Galdino, F.M.P.; Andrade, M.E.R.; de Barros, P.A.V.; Generoso, S.D.V.; Alvarez-Leite, J.I.; de Almeida-Leite, C.M.; Peluzio, M.D.C.G.; Fernandes, S.O.A.; Cardoso, V.N. Pretreatment and treatment with fructo-oligosaccharides attenuate intestinal mucositis induced by 5-FU in mice. J. Funct. Foods 2018, 49, 485–492. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, P.; Xia, C.; Wu, Z.; Zhong, Z.; Xu, Y.; Zeng, Y.; Liu, H.; Liu, R.; Liao, M. Fructooligosaccharides supplementation mitigated chronic stress-induced intestinal barrier impairment and neuroinflammation in mice. J. Funct. Foods 2020, 72, 104060. [Google Scholar] [CrossRef]
- Yao, F.; Jia, R.; Huang, H.; Yu, Y.; Mei, L.; Bai, L.; Ding, Y.; Zheng, P. Effect of Lactobacillus paracasei N1115 and fructooligosaccharides in nonalcoholic fatty liver disease. Arch. Med. Sci. 2019, 15, 1336–1344. [Google Scholar] [CrossRef]
- Yan, X.; Yan, J.; Xiang, Q.; Wang, F.; Dai, H.; Huang, K.; Fang, L.; Yao, H.; Wang, L.; Zhang, W. Fructooligosaccharides protect against OVA-induced food allergy in mice by regulating the Th17/Treg cell balance using tryptophan metabolites. Food Funct. 2021, 12, 3191–3205. [Google Scholar] [CrossRef]
- Liu, L.; Chen, D.; Yu, B.; Yin, H.; Huang, Z.; Luo, Y.; Zheng, P.; Mao, X.; Yu, J.; Luo, J.; et al. Fructooligosaccharides improve growth performance and intestinal epithelium function in weaned pigs exposed to enterotoxigenic: Escherichia coli. Food Funct. 2020, 11, 9599–9612. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhang, X.; Han, D.; Ye, H.; Tao, S.; Pi, Y.; Zhao, J.; Chen, L.; Wang, J. Short administration of combined prebiotics improved microbial colonization, gut barrier, and growth performance of neonatal piglets. ACS Omega 2020, 5, 20506–20516. [Google Scholar] [CrossRef]
- Daguet, D.; Pinheiro, I.; Verhelst, A.; Possemiers, S.; Marzorati, M. Arabinogalactan and fructooligosaccharides improve the gut barrier function in distinct areas of the colon in the Simulator of the Human Intestinal Microbial Ecosystem. J. Funct. Foods 2016, 20, 369–379. [Google Scholar] [CrossRef]
- Chen, T.; Kim, C.Y.; Kaur, A.; Lamothe, L.; Shaikh, M.; Keshavarzian, A.; Hamaker, B.R. Dietary fibre-based SCFA mixtures promote both protection and repair of intestinal epithelial barrier function in a Caco-2 cell model. Food Funct. 2017, 8, 1166–1173. [Google Scholar] [CrossRef] [PubMed]
- Pham, V.; Calatayud, M.; Rotsaert, C.; Seifert, N.; Richard, N.; Abbeele, P.V.D.; Marzorati, M.; Steinert, R. Antioxidant Vitamins and Prebiotic FOS and XOS Differentially Shift Microbiota Composition and Function and Improve Intestinal Epithelial Barrier In Vitro. Nutrients 2021, 13, 1125. [Google Scholar] [CrossRef]
- Bruggencate, S.J.M.T.; Bovee-Oudenhoven, I.M.J.; Lettink-Wissink, M.L.G.; Van Der Meer, R. Dietary fructooligosaccharides increase intestinal permeability in rats. J. Nutr. 2005, 135, 837–842. [Google Scholar] [CrossRef] [PubMed]
- Rodenburg, W.; Keijer, J.; Kramer, E.; Vink, C.; van der Meer, R.; Bovee-Oudenhoven, I.M. Impaired barrier function by dietary fructo-oligosaccharides (FOS) in rats is accompanied by increased colonic mitochondrial gene expression. BMC Genom. 2008, 9, 144. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.M.; Fahey, G.C.; Wolf, B.W. Selected indigestible oligosaccharides affect large bowel mass, cecal and fecal short-chain fatty acids, pH and microflora in rats. J. Nutr. 1997, 127, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Bovee-Oudenhoven, I.M.J.; Ten Bruggencate, S.J.M.; Lettink-Wissink, M.L.G.; Van Der Meer, R. Dietary fructo-oligosaccharides dose-dependently increase translocation of salmonella in rats. Nutr. Immunol. 2003, 52, 1572–1578. [Google Scholar] [CrossRef]
- Fehlbaum, S.; Prudence, K.; Kieboom, J.; Heerikhuisen, M.; Broek, T.V.D.; Schuren, F.H.J.; Steinert, R.E.; Raederstorff, D. In vitro fermentation of selected prebiotics and their effects on the composition and activity of the adult gut microbiota. Int. J. Mol. Sci. 2018, 19, 3097. [Google Scholar] [CrossRef]
- Weng, J.; Tian, S.; Yu, H.; Wang, J.; Zhu, W. Response of Colonic Mucosa-Associated Microbiota Composition, Mucosal Immune Homeostasis, and Barrier Function to Early Life Galactooligosaccharides Intervention in Suckling Piglets. J. Agric. Food Chem. 2019, 67, 578–588. [Google Scholar] [CrossRef]
- Belorkar, S.A.; Gupta, A.K. Oligosaccharides: A boon from nature’s desk. AMB Express 2016, 6, 1–11. [Google Scholar] [CrossRef]
- Zou, Y.; Wang, J.; Wang, Y.; Peng, B.; Liu, J.; Zhang, B.; Wang, S. Protection of Galacto-Oligosaccharide against E. coli O157 Colonization through Enhancing Gut Barrier Function and Modulating Gut Microbiota. Foods 2020, 9, 1710. [Google Scholar] [CrossRef]
- Tian, S.; Wang, J.; Yu, H.; Wang, J.; Zhu, W. Effects of galacto-oligosaccharides on growth and gut function of newborn suckling piglets. J. Anim. Sci. Biotechnol. 2018, 9, 75. [Google Scholar] [CrossRef] [PubMed]
- Akbari, P.; Braber, S.; Alizadeh, A.; Verheijden, K.A.; Schoterman, M.H.; Kraneveld, A.D.; Garssen, J.; Fink-Gremmels, J. Galacto-oligosaccharides protect the intestinal barrier by maintaining the tight junction network and modulating the inflammatory responses after a challenge with the mycotoxin deoxynivalenol in human Caco-2 cell monolayers and B6C3F1 mice. J. Nutr. 2015, 145, 1604–1613. [Google Scholar] [CrossRef] [PubMed]
- Varasteh, S.; Braber, S.; Garssen, J.; Fink-Gremmels, J. Galacto-oligosaccharides exert a protective effect against heat stress in a Caco-2 cell model. J. Funct. Foods 2015, 16, 265–277. [Google Scholar] [CrossRef]
- Arbizu, S.; Chew, B.; Mertens-Talcott, S.U.; Noratto, G. Commercial whey products promote intestinal barrier function with glycomacropeptide enhanced activity in downregulating bacterial endotoxin lipopolysaccharides (LPS)-induced inflammation: In vitro. Food Funct. 2020, 11, 5842–5852. [Google Scholar] [CrossRef]
- López-García, G.; Cilla, A.; Barbera, R.; Alegria, A. Anti-Inflammatory and Cytoprotective Effect of Plant Sterol and Galactooligosaccharides-Enriched Beverages in Caco-2 Cells. J. Agric. Food Chem. 2020, 68, 1862–1870. [Google Scholar] [CrossRef]
- Ortega, M.D.S.; Ocón, B.; Calvo, I.R.; Anzola, A.; Guadix, E.M.; Zarzuelo, A.; Suárez, M.D.; de Medina, F.S.; Martínez-Augustin, O. Nondigestible oligosaccharides exert nonprebiotic effects on intestinal epithelial cells enhancing the immune response via activation of TLR4-NFκB. Mol. Nutr. Food Res. 2014, 58, 384–393. [Google Scholar] [CrossRef]
- Bermudez-Brito, M.; Sahasrabudhe, N.M.; Rösch, C.; Schols, H.A.; Faas, M.M.; de Vos, P. The impact of dietary fibers on dendritic cell responses in vitro is dependent on the differential effects of the fibers on intestinal epithelial cells. Mol. Nutr. Food Res. 2015, 59, 698–710. [Google Scholar] [CrossRef]
- Newburg, D.S.; Ko, J.S.; Leone, S.; Nanthakumar, N.N. Human milk oligosaccharides and synthetic galactosyloligosaccharides contain 3’-, 4-, and 6’-galactosyllactose and attenuate inflammation in human T84, NCM-460, and H4 cells and intestinal tissue ex vivo1,2. J. Nutr. 2016, 146, 358–367. [Google Scholar] [CrossRef]
- Sun, J.; Liang, W.; Yang, X.; Li, Q.; Zhang, G. Cytoprotective effects of galacto-oligosaccharides on colon epithelial cells via up-regulating miR-19b. Life Sci. 2019, 231, 116589. [Google Scholar] [CrossRef]
- Perdijk, O.; van Baarlen, P.; Fernandez-Gutierrez, M.M.; Brink, E.V.D.; Schuren, F.H.J.; Brugman, S.; Savelkoul, H.F.J.; Kleerebezem, M.; van Neerven, R.J.J. Sialyllactose and galactooligosaccharides promote epithelial barrier functioning and distinctly modulate microbiota composition and short chain fatty acid production in vitro. Front. Immunol. 2019, 10, 94. [Google Scholar] [CrossRef]
- Varasteh, S.; Braber, S.; Akbari, P.; Garssen, J.; Fink-Gremmels, J. Differences in susceptibility to heat stress along the chicken intestine and the protective effects of galacto- oligosaccharides. PLoS ONE 2015, 10, e0138975. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Sun, W.; Pei, X.; Jin, Y.; Wang, H.; Tao, W.; Xiao, Z.; Liu, L.; Wang, M. Galactooligosaccharide pretreatment alleviates damage of the intestinal barrier and inflammatory responses in LPS-challenged mice. Food Funct. 2021, 12, 1569–1579. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, X.; Tao, S.; Pi, Y.; Han, D.; Ye, H.; Feng, C.; Zhao, J.; Chen, L.; Wang, J. Maternal supplementation with combined galactooligosaccharides and casein glycomacropeptides modulated microbial colonization and intestinal development of neonatal piglets. J. Funct. Foods 2020, 74, 104170. [Google Scholar] [CrossRef]
- Zhong, Y.; Cai, D.; Cai, W.; Geng, S.; Chen, L.; Han, T. Protective effect of galactooligosaccharide-supplemented enteral nutrition on intestinal barrier function in rats with severe acute pancreatitis. Clin. Nutr. 2009, 28, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, A.; Akbari, P.; Difilippo, E.; Schols, H.A.; Ulfman, L.H.; Schoterman, M.H.C.; Garssen, J.; Fink-Gremmels, J.; Braber, S. The piglet as a model for studying dietary components in infant diets: Effects of galacto-oligosaccharides on intestinal functions. Br. J. Nutr. 2016, 115, 605–618. [Google Scholar] [CrossRef] [PubMed]
- Barrat, E.; Michel, C.; Poupeau, G.; David-Sochard, A.; Rival, M.; Pagniez, A.; Champ, M.; Darmaun, D. Supplementation with galactooligosaccharides and inulin increases bacterial translocation in artificially reared newborn rats. Pediatr. Res. 2008, 64, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Thongaram, T.; Hoeflinger, J.L.; Chow, J.; Miller, M.J. Prebiotic Galactooligosaccharide Metabolism by Probiotic Lactobacilli and Bifidobacteria. J. Agric. Food Chem. 2017, 65, 4184–4192. [Google Scholar] [CrossRef]
- Yang, F.; Wei, J.-D.; Lu, Y.-F.; Sun, Y.-L.; Wang, Q.; Zhang, R.-L. Galacto-oligosaccharides modulate gut microbiota dysbiosis and intestinal permeability in rats with alcohol withdrawal syndrome. J. Funct. Foods 2019, 60, 103423. [Google Scholar] [CrossRef]
- Arnold, J.W.; Roach, J.; Fabela, S.; Moorfield, E.; Ding, S.; Blue, E.; Dagher, S.; Magness, S.; Tamayo, R.; Bruno-Barcena, J.M.; et al. The pleiotropic effects of prebiotic galacto-oligosaccharides on the aging gut. Microbiome 2021, 9, 31. [Google Scholar] [CrossRef]
- Vasudevan, U.M.; Lee, O.K.; Lee, E.Y. Alginate derived functional oligosaccharides: Recent developments, barriers, and future outlooks. Carbohydr. Polym. 2021, 267, 118158. [Google Scholar] [CrossRef]
- Liu, J.; Yang, S.; Li, X.; Yan, Q.; Reaney, M.J.T.; Jiang, Z. Alginate Oligosaccharides: Production, Biological Activities, and Potential Applications. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1859–1881. [Google Scholar] [CrossRef] [PubMed]
- Xing, M.; Cao, Q.; Wang, Y.; Xiao, H.; Zhao, J.; Zhang, Q.; Ji, A.; Song, S. Advances in research on the bioactivity of alginate oligosaccharides. Mar. Drugs 2020, 18, 144. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Zhang, J.; Chen, D.; Yu, B.; Huang, Z.; Mao, X.; Zheng, P.; Yu, J.; He, J. Alterations in intestinal microbiota by alginate oligosaccharide improve intestinal barrier integrity in weaned pigs. J. Funct. Foods 2020, 71, 104040. [Google Scholar] [CrossRef]
- Wan, J.; Zhang, J.; Chen, D.; Yu, B.; Mao, X.; Zheng, P.; Yu, J.; Huang, Z.; Luo, J.; Luo, Y.; et al. Alginate oligosaccharide alleviates enterotoxigenic: Escherichia coli-induced intestinal mucosal disruption in weaned pigs. Food Funct. 2018, 9, 6401–6413. [Google Scholar] [CrossRef]
- Wan, J.; Zhang, J.; Yin, H.; Chen, D.; Yu, B.; He, J. Ameliorative effects of alginate oligosaccharide on tumour necrosis factor-α-induced intestinal epithelial cell injury. Int. Immunopharmacol. 2020, 89, 107084. [Google Scholar] [CrossRef]
- Wan, J.; Zhang, J.; Xu, Q.; Yin, H.; Chen, D.; Yu, B.; He, J. Alginate oligosaccharide protects against enterotoxigenic Escherichia coli-induced porcine intestinal barrier injury. Carbohydr. Polym. 2021, 270, 118316. [Google Scholar] [CrossRef]
- Zhao, Y.; Feng, Y.; Liu, M.; Chen, L.; Meng, Q.; Tang, X.; Wang, S.; Liu, L.; Li, L.; Shen, W.; et al. Single-cell RNA sequencing analysis reveals alginate oligosaccharides preventing chemotherapy-induced mucositis. Mucosal Immunol. 2020, 13, 437–448. [Google Scholar] [CrossRef]
- East, L.; Isacke, C.M. The mannose receptor family. Biochim. Biophys. Acta Gen. Subj. 2002, 1572, 364–386. [Google Scholar] [CrossRef]
- Gazi, U.; Martinez-Pomares, L. Influence of the mannose receptor in host immune responses. Immunobiology 2009, 214, 554–561. [Google Scholar] [CrossRef]
- Martinez-Pomares, L. The mannose receptor. J. Leukoc. Biol. 2012, 92, 1177–1186. [Google Scholar] [CrossRef]
- Xiong, B.; Liu, M.; Zhang, C.; Hao, Y.; Zhang, P.; Chen, L.; Tang, X.; Zhang, H.; Zhao, Y. Alginate oligosaccharides enhance small intestine cell integrity and migration ability. Life Sci. 2020, 258, 118085. [Google Scholar] [CrossRef] [PubMed]
- Asadpoor, M.; Varasteh, S.; Pieters, R.J.; Folkerts, G.; Braber, S. Differential effects of oligosaccharides on the effectiveness of ampicillin against Escherichia coli in vitro. PharmaNutrition 2021, 16, 100264. [Google Scholar] [CrossRef]
- Zhang, P.; Feng, Y.; Li, L.; Ge, W.; Yu, S.; Hao, Y.; Shen, W.; Han, X.; Ma, D.; Yin, S.; et al. Improvement in sperm quality and spermatogenesis following faecal microbiota transplantation from alginate oligosaccharide dosed mice. Gut 2021, 70, 222–225. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, L.; Ye, C.; Yuan, J.; Qin, S. Alginate oligosaccharide improves lipid metabolism and inflammation by modulating gut microbiota in high-fat diet fed mice. Appl. Microbiol. Biotechnol. 2020, 104, 3541–3554. [Google Scholar] [CrossRef]
- Yan, G.L.; Guo, Y.M.; Yuan, J.M.; Liu, D.; Zhang, B.K. Sodium alginate oligosaccharides from brown algae inhibit Salmonella Enteritidis colonization in broiler chickens. Poult. Sci. 2011, 90, 1441–1448. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Jiang, F.; Xu, Q.; Chen, D.; He, J. Alginic acid oligosaccharide accelerates weaned pig growth through regulating antioxidant capacity, immunity and intestinal development. RSC Adv. 2016, 6, 87026–87035. [Google Scholar] [CrossRef]
- He, N.; Yang, Y.; Wang, H.; Liu, N.; Yang, Z.; Li, S. Unsaturated alginate oligosaccharides (UAOS) protects against dextran sulfate sodium-induced colitis associated with regulation of gut microbiota. J. Funct. Foods 2021, 83, 104536. [Google Scholar] [CrossRef]
- Li, S.; Wang, L.; Liu, B.; He, N. Unsaturated alginate oligosaccharides attenuated obesity-related metabolic abnormalities by modulating gut microbiota in high-fat-diet mice. Food Funct. 2020, 11, 4773–4784. [Google Scholar] [CrossRef]
- Zhang, P.; Liu, J.; Xiong, B.; Zhang, C.; Kang, B.; Gao, Y.; Li, Z.; Ge, W.; Cheng, S.; Hao, Y.; et al. Microbiota from alginate oligosaccharide-dosed mice successfully mitigated small intestinal mucositis. Microbiome 2020, 8, 112. [Google Scholar] [CrossRef]
- Li, S.-Y.; Wang, Z.-P.; Wang, L.-N.; Peng, J.-X.; Wang, Y.-N.; Han, Y.-T.; Zhao, S.-F. Combined enzymatic hydrolysis and selective fermentation for green production of alginate oligosaccharides from Laminaria japonica. Bioresour. Technol. 2019, 281, 84–89. [Google Scholar] [CrossRef]
- Kumar, A.; Kumaar, A. The virtuous potential of chitosan oligosaccharide for promising biomedical applications. J. Mater. Res. 2020, 35, 1123–1134. [Google Scholar] [CrossRef]
- Guan, G.; Azad, M.A.K.; Lin, Y.; Kim, S.W.; Tian, Y.; Liu, G.; Wang, H. Biological effects and applications of chitosan and chito-oligosaccharides. Front. Physiol. 2019, 10, 516. [Google Scholar] [CrossRef] [PubMed]
- Muanprasat, C.; Chatsudthipong, V. Chitosan oligosaccharide: Biological activities and potential therapeutic applications. Pharmacol. Ther. 2017, 170, 80–97. [Google Scholar] [CrossRef] [PubMed]
- Naveed, M.; Phil, L.; Sohail, M.; Hasnat, M.; Baig, M.M.F.A.; Ihsan, A.U.; Shumzaid, M.; Kakar, M.U.; Khan, T.M.; Akabar, M.D.; et al. Chitosan oligosaccharide (COS): An overview. Int. J. Biol. Macromol. 2019, 129, 827–843. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Santamarina, A.; Mondragon, A.D.C.; Lamas, A.; Miranda, J.M.; Franco, C.M.; Cepeda, A. Animal-origin prebiotics based on chitin: An alternative for the future? a critical review. Foods 2020, 9, 782. [Google Scholar] [CrossRef] [PubMed]
- Yousef, M.; Pichyangkura, R.; Soodvilai, S.; Chatsudthipong, V.; Muanprasat, C. Chitosan oligosaccharide as potential therapy of inflammatory bowel disease: Therapeutic efficacy and possible mechanisms of action. Pharmacol. Res. 2012, 66, 66–79. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Fang, B.; Yong, Y.; Li, X.; Gong, D.; Li, J.; Yu, T.; Gooneratne, R.; Gao, Z.; Li, S.; et al. Chitosan oligosaccharide-mediated attenuation of LPS-induced inflammation in IPEC-J2 cells is related to the TLR4/NF-κB signaling pathway. Carbohydr. Polym. 2019, 219, 269–279. [Google Scholar] [CrossRef]
- Yuan, W.-P.; Liu, B.; Liu, C.-H.; Wang, X.-J.; Zhang, M.-S.; Meng, X.-M.; Xia, X.-K. Antioxidant activity of chito-oligosaccharides on pancreatic islet cells in streptozotocin-induced diabetes in rats. World J. Gastroenterol. 2009, 15, 1339–1345. [Google Scholar] [CrossRef] [PubMed]
- Muanprasat, C.; Wongkrasant, P.; Satitsri, S.; Moonwiriyakit, A.; Pongkorpsakol, P.; Mattaveewong, T.; Pichyangkura, R.; Chatsudthipong, V. Activation of AMPK by chitosan oligosaccharide in intestinal epithelial cells: Mechanism of action and potential applications in intestinal disorders. Biochem. Pharmacol. 2015, 96, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-H.; Senevirathne, M.; Ahn, C.-B.; Kim, S.-K.; Je, J.-Y. Factors affecting anti-inflammatory effect of chitooligosaccharides in lipopolysaccharides-induced RAW264.7 macrophage cells. Bioorganic Med. Chem. Lett. 2009, 19, 6655–6658. [Google Scholar] [CrossRef]
- Bahar, B.; O’Doherty, J.V.; Maher, S.; McMorrow, J.; Sweeney, T. Chitooligosaccharide elicits acute inflammatory cytokine response through AP-1 pathway in human intestinal epithelial-like (Caco-2) cells. Mol. Immunol. 2012, 51, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wen, R.; Liu, D.; Zhang, C.; Wang, Z.; Du, Y. Exploring effects of chitosan oligosaccharides on the dss-induced intestinal barrier impairment in vitro and in vivo. Molecules 2021, 26, 2199. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Xiao, D.; Tan, B.; Xiao, H.; Wang, J.; Yin, J.; Duan, J.; Huang, R.; Yang, C.; Yin, Y. Chitosan Oligosaccharide Reduces Intestinal Inflammation That Involves Calcium-Sensing Receptor (CaSR) Activation in Lipopolysaccharide (LPS)-Challenged Piglets. J. Agric. Food. Chem. 2016, 64, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.W.; Tian, G.; Chen, D.W.; Yao, Y.; He, J.; Zheng, P.; Mao, X.B.; Yu, J.; Huang, Z.Q.; Yu, B. Involvement of PKA signalling in anti-inflammatory effects of chitosan oligosaccharides in IPEC-J2 porcine epithelial cells. J. Anim. Physiol. Anim. Nutr. 2018, 102, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Piao, X.S.; Kim, S.W.; Wang, L.; Shen, Y.B.; Lee, H.S.; Li, S.Y. Effects of chito-oligosaccharide supplementation on the growth performance, nutrient digestibility, intestinal morphology, and fecal shedding of Escherichia coli and Lactobacillus in weaning pigs. J. Anim. Sci. 2008, 86, 2609–2618. [Google Scholar] [CrossRef]
- Yang, C.M.; Ferket, P.; Hong, Q.H.; Zhou, J.; Cao, G.T.; Zhou, L.; Chen, A.G. Effect of chito-oligosaccharide on growth performance, intestinal barrier function, intestinal morphology and cecal microflora in weaned pigs. J. Anim. Sci. 2012, 90, 2671–2676. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Wang, Y.; Chen, S.; Hu, M.; Wang, Z.; Wu, G.; Ma, X.; Chen, Z.; Zheng, C. Low-molecular-weight chitosan supplementation increases the population of prevotella in the cecal contents of weanling pigs. Front. Microbiol. 2017, 8, 2182. [Google Scholar] [CrossRef]
- Kong, X.; Zhou, X.; Lian, G.; Blachier, F.; Liu, G.; Tan, B.; Nyachoti, C.; Yin, Y. Dietary supplementation with chitooligosaccharides alters gut microbiota and modifies intestinal luminal metabolites in weaned Huanjiang mini-piglets. Livest. Sci. 2014, 160, 97–101. [Google Scholar] [CrossRef]
- Li, X.J.; Piao, X.S.; Kim, S.W.; Liu, P.; Wang, L.; Shen, Y.B.; Jung, S.C.; Lee, H.S. Effects of chito-oligosaccharide supplementation on performance, nutrient digestibility, and serum composition in broiler chickens. Poult. Sci. 2007, 86, 1107–1114. [Google Scholar] [CrossRef]
- Zhang, C.; Jiao, S.; Wang, Z.A.; Du, Y. Exploring effects of chitosan oligosaccharides on mice gut microbiota in in vitro fermentation and animal model. Front. Microbiol. 2018, 9, 2388. [Google Scholar] [CrossRef]
- Koppová, I.; Bureš, M.; Šimůnek, J. Intestinal bacterial population of healthy rats during the administration of chitosan and chitooligosaccharides. Folia Microbiol. 2012, 57, 295–299. [Google Scholar] [CrossRef] [PubMed]
- Shang, W.; Si, X.; Zhou, Z.; Li, Y.; Strappe, P.; Blanchard, C. Characterization of fecal fat composition and gut derived fecal microbiota in high-fat diet fed rats following intervention with chito-oligosaccharide and resistant starch complexes. Food Funct. 2017, 8, 4374–4383. [Google Scholar] [CrossRef] [PubMed]
- Selenius, O.V.O.; Korpela, J.; Salminen, S.; Gallego, C.G. Effect of chitin and chitooligosaccharide on in vitro growth of Lactobacillus rhamnosus GG and Escherichia coli TG. Appl. Food Biotechnol. 2018, 5, 163–172. [Google Scholar] [CrossRef]
- Mateos-Aparicio, I.; Mengíbar, M.; Heras, A. Effect of chito-oligosaccharides over human faecal microbiota during fermentation in batch cultures. Carbohydr. Polym. 2016, 137, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Jiang, F.; Xu, Q.; Chen, D.; Yu, B.; Huang, Z.; Mao, X.; Yu, J.; He, J. New insights into the role of chitosan oligosaccharide in enhancing growth performance, antioxidant capacity, immunity and intestinal development of weaned pigs. RSC Adv. 2017, 7, 9669–9679. [Google Scholar] [CrossRef]
- Zheng, J.; Yuan, X.; Cheng, G.; Jiao, S.; Feng, C.; Zhao, X.; Yin, H.; Du, Y.; Liu, H. Chitosan oligosaccharides improve the disturbance in glucose metabolism and reverse the dysbiosis of gut microbiota in diabetic mice. Carbohydr. Polym. 2018, 190, 77–86. [Google Scholar] [CrossRef]
- He, N.; Wang, S.; Lv, Z.; Zhao, W.; Li, S. Low molecular weight chitosan oligosaccharides (LMW-COSs) prevent obesity-related metabolic abnormalities in association with the modification of gut microbiota in high-fat diet (HFD)-fed mice. Food Funct. 2020, 11, 9947–9959. [Google Scholar] [CrossRef]
- Zheng, J.; Cheng, G.; Li, Q.; Jiao, S.; Feng, C.; Zhao, X.; Yin, H.; Du, Y.; Liu, H. Chitin oligosaccharide modulates gut microbiota and attenuates high-fat-diet-induced metabolic syndrome in mice. Mar. Drugs 2018, 16, 66. [Google Scholar] [CrossRef]
- Qian, M.; Lyu, Q.; Liu, Y.; Hu, H.; Wang, S.; Pan, C.; Duan, X.; Gao, Y.; Qi, L.-W.; Liu, W.; et al. Chitosan Oligosaccharide Ameliorates Nonalcoholic Fatty Liver Disease (NAFLD) in Diet-Induced Obese Mice. Mar. Drugs 2019, 17, 391. [Google Scholar] [CrossRef]
- Mei, Q.-X.; Hu, J.-H.; Huang, Z.-H.; Fan, J.-J.; Huang, C.-L.; Lu, Y.-Y.; Wang, X.-P.; Zeng, Y. Pretreatment with chitosan oligosaccharides attenuate experimental severe acute pancreatitis via inhibiting oxidative stress and modulating intestinal homeostasis. Acta Pharmacol. Sin. 2021, 42, 942–953. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, H.; Zheng, J.; Jiang, N.; Sun, G.; Bao, X.; Lin, A.; Liu, H. Chitosan oligosaccharides attenuate loperamide-induced constipation through regulation of gut microbiota in mice. Carbohydr. Polym. 2021, 253, 117218. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Cheng, Y.; Chen, Y.; Qu, H.; Zhao, Y.; Wen, C.; Zhou, Y. Dietary chitooligosaccharide inclusion as an alternative to antibiotics improves intestinal morphology, barrier function, antioxidant capacity, and immunity of broilers at early age. Animals 2019, 9, 493. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.; Yang, H.S.; Wang, X.C.; Hu, Q.; Liu, C.X.; Wu, X.; Deng, D.; Hou, Y.Q.; Nyachoti, C.M.; Xiao, D.; et al. Effect of low dosage of chito-oligosaccharide supplementation on intestinal morphology, immune response, antioxidant capacity, and barrier function in weaned piglets. J. Anim. Sci. 2015, 93, 1089–1097. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Du, Y.; Yu, X.; Mitsutomi, M.; Aiba, S.-I. Preparation of chitooligosaccharides from chitosan by a complex enzyme. Carbohydr. Res. 1999, 320, 257–260. [Google Scholar] [CrossRef]
- Lyu, Q.; Shi, Y.; Wang, S.; Yang, Y.; Han, B.; Liu, W.; Jones, D.N.; Liu, W. Structural and biochemical insights into the degradation mechanism of chitosan by chitosanase OU01. Biochim. Biophys. Acta Gen. Subj. 2015, 1850, 1953–1961. [Google Scholar] [CrossRef]
- Lyu, Q.; Wang, S.; Xu, W.; Han, B.; Liu, W.; Jones, D.N.M.; Liu, W. Structural insights into the substrate-binding mechanism for a novel chitosanase. Biochem. J. 2014, 461, 335–345. [Google Scholar] [CrossRef]
- Brufau, M.T.; Campo-Sabariz, J.; Carné, S.; Ferrer, R.; Martín-Venegas, R. Salmosan, a β-galactomannan-rich product, in combination with Lactobacillus plantarum contributes to restore intestinal epithelial barrier function by modulation of cytokine production. J. Nutr. Biochem. 2017, 41, 20–24. [Google Scholar] [CrossRef]
- Yu, E.; Chen, D.; Yu, B.; Huang, Z.; Mao, X.; Zheng, P.; Luo, Y.; Yin, H.; Yu, J.; Luo, J.; et al. Manno-oligosaccharide attenuates inflammation and intestinal epithelium injury in weaned pigs upon enterotoxigenic Escherichia coli K88 challenge. Br. J. Nutr. 2021, 126, 993–1002. [Google Scholar] [CrossRef]
- Yan, S.; Shi, R.; Li, L.; Ma, S.; Zhang, H.; Ye, J.; Wang, J.; Pan, J.; Wang, Q.; Jin, X.; et al. Mannan Oligosaccharide Suppresses Lipid Accumulation and Appetite in Western-Diet-Induced Obese Mice Via Reshaping Gut Microbiome and Enhancing Short-Chain Fatty Acids Production. Mol. Nutr. Food Res. 2019, 63, 1900521. [Google Scholar] [CrossRef]
- Nopvichai, C.; Charoenwongpaiboon, T.; Luengluepunya, N.; Ito, K.; Muanprasat, C.; Pichyangkura, R. Production and purification of mannan oligosaccharide with epithelial tight junction enhancing activity. PeerJ 2019, 2019, e7206. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, S.; Wu, S.; Ren, Z.; Liu, G.; Wu, J. Synergistic Protective Effect of Konjac Mannan Oligosaccharides and Bacillus subtilis on Intestinal Epithelial Barrier Dysfunction in Caco-2 Cell Model and Mice Model of Lipopolysaccharide Stimulation. Front. Immunol. 2021, 12, 696148. [Google Scholar] [CrossRef]
- Nopvichai, C.; Pongkorpsakol, P.; Wongkrasant, P.; Wangpaiboon, K.; Charoenwongpaiboon, T.; Ito, K.; Muanprasat, C.; Pichyangkura, R. Galactomannan pentasaccharide produced from copra meal enhances tight junction integration of epithelial tissue through activation of AMPK. Biomedicines 2019, 7, 81. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Liu, J.; Yan, Q.; Gu, Z.; August, A.; Huang, W.; Jiang, Z. Konjac Glucomannan Oligosaccharides Prevent Intestinal Inflammation Through SIGNR1-Mediated Regulation of Alternatively Activated Macrophages. Mol. Nutr. Food Res. 2021, 65, 2001010. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Chen, Y.; Chen, R.; Su, Y.; Zhang, R.; He, Q.; Wang, K.; Wen, C.; Zhou, Y. Dietary mannan oligosaccharide ameliorates cyclic heat stress-induced damages on intestinal oxidative status and barrier integrity of broilers. Poult. Sci. 2019, 98, 4767–4776. [Google Scholar] [CrossRef] [PubMed]
- Yu, E.; Chen, D.; Yu, B. Amelioration of Enterotoxigenic Escherichia coli- induced disruption of intestinal epithelium by Manno- oligosaccharide in weaned pigs. J. Funct. Foods. 2021, 82, 104492. [Google Scholar] [CrossRef]
- Wang, W.; Li, Z.; Han, Q.; Guo, Y.; Zhang, B.; D’Inca, R. Dietary live yeast and mannan-oligosaccharide supplementation attenuate intestinal inflammation and barrier dysfunction induced by Escherichia coli in broilers. Br. J. Nutr. 2016, 116, 1878–1888. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.D.; Banerjee, J.; Arora, A. Prebiotic potential of oligosaccharides: A focus on xylan derived oligosaccharides. Bioact. Carbohydr. Diet. Fibre 2015, 5, 19–30. [Google Scholar] [CrossRef]
- Su, J.; Zhang, W.; Ma, C.; Xie, P.; Blachier, F.; Kong, X. Dietary Supplementation with Xylo-oligosaccharides Modifies the Intestinal Epithelial Morphology, Barrier Function and the Fecal Microbiota Composition and Activity in Weaned Piglets. Front. Veter. Sci. 2021, 8, 680208. [Google Scholar] [CrossRef]
- Christensen, E.G.; Licht, T.R.; Leser, T.D.; Bahl, M.I. Dietary Xylo-oligosaccharide stimulates intestinal bifidobacteria and lactobacilli but has limited effect on intestinal integrity in rats. BMC Res. Notes 2014, 7, 660. [Google Scholar] [CrossRef]
- Chen, Y.; Xie, Y.; Ajuwon, K.M.; Zhong, R.; Li, T.; Chen, L.; Zhang, H.; Beckers, Y.; Everaert, N. Xylo-Oligosaccharides, Preparation and Application to Human and Animal Health: A Review. Front. Nutr. 2021, 8, 10430. [Google Scholar] [CrossRef]
- Sheng, K.; He, S.; Sun, M.; Zhang, G.; Kong, X.; Wang, J.; Wang, Y. Synbiotic supplementation containing: Bifidobacterium infantis and xylooligosaccharides alleviates dextran sulfate sodium-induced ulcerative colitis. Food Funct. 2020, 11, 3964–3974. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Li, F.; Kong, X.; Wen, C.; Guo, Q.; Zhang, L.; Wang, W.; Duan, Y.; Li, T.; Tan, Z.; et al. Dietary xylo-oligosaccharide improves intestinal functions in weaned piglets. Food Funct. 2019, 10, 2701–2709. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xiao, K.; Yu, C.; Wang, L.; Liang, T.; Zhu, H.; Xu, X.; Liu, Y. Xylooligosaccharide attenuates lipopolysaccharide-induced intestinal injury in piglets via suppressing inflammation and modulating cecal microbial communities. Anim. Nutr. 2021, 7, 609–620. [Google Scholar] [CrossRef] [PubMed]
- Fei, Y.; Wang, Y.; Pang, Y.; Wang, W.; Zhu, D.; Xie, M.; Lan, S.; Wang, Z. Xylooligosaccharide Modulates Gut Microbiota and Alleviates Colonic Inflammation Caused by High Fat Diet Induced Obesity. Front. Physiol. 2020, 10, 1601. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, X.; Xing, T.; Li, J.; Zhu, X.; Zhang, L.; Gao, F. The combined impact of xylo-oligosaccharides and gamma-irradiated Astragalus polysaccharides on growth performance and intestinal mucosal barrier function of broilers. Poult. Sci. 2021, 100, 1–11. [Google Scholar] [CrossRef]
- Ravn, J.L.; Thøgersen, J.C.; Eklöf, J.; Pettersson, D.; Ducatelle, R.; van Immerseel, F.; Pedersen, N.R. GH11 xylanase increases prebiotic oligosaccharides from wheat bran favouring butyrate-producing bacteria in vitro. Anim. Feed Sci. Technol. 2017, 226, 113–123. [Google Scholar] [CrossRef]
- Van den Abbeele, P.; Taminiau, B.; Pinheiro, I.; Duysburgh, C.; Jacobs, H.; Pijls, L.; Marzorati, M. Arabinoxylo-Oligosaccharides and Inulin Impact Inter-Individual Variation on Microbial Metabolism and Composition, Which Immunomodulates Human Cells. J. Agric. Food Chem. 2018, 66, 1121–1130. [Google Scholar] [CrossRef]
- Hansen, C.H.F.; Larsen, C.S.; Petersson, H.O.; Zachariassen, L.F.; Vegge, A.; Lauridsen, C.; Kot, W.; Krych, Ł.; Nielsen, D.S.; Hansen, A.K. Targeting gut microbiota and barrier function with prebiotics to alleviate autoimmune manifestations in NOD mice. Diabetologia 2019, 62, 1689–1700. [Google Scholar] [CrossRef]
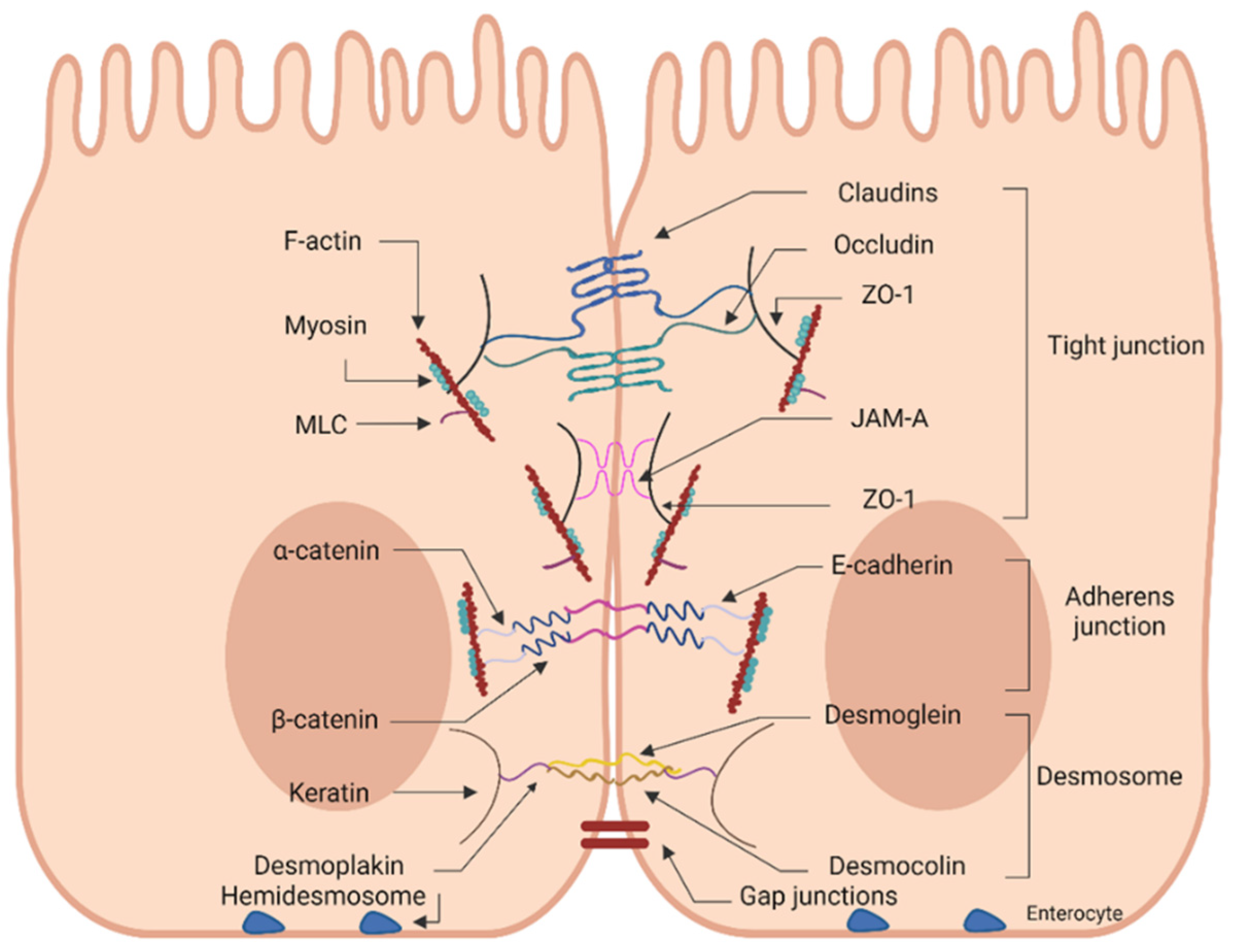
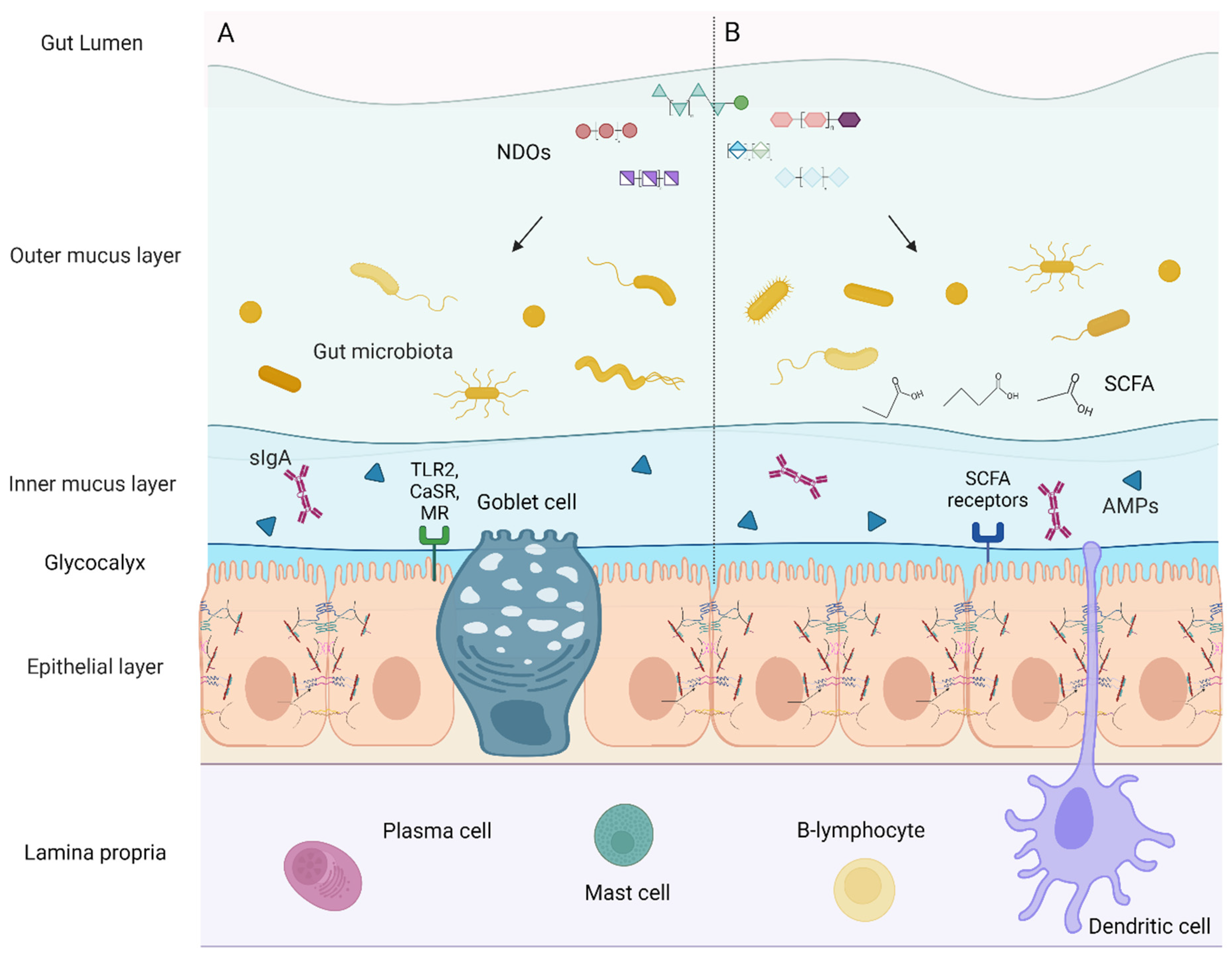
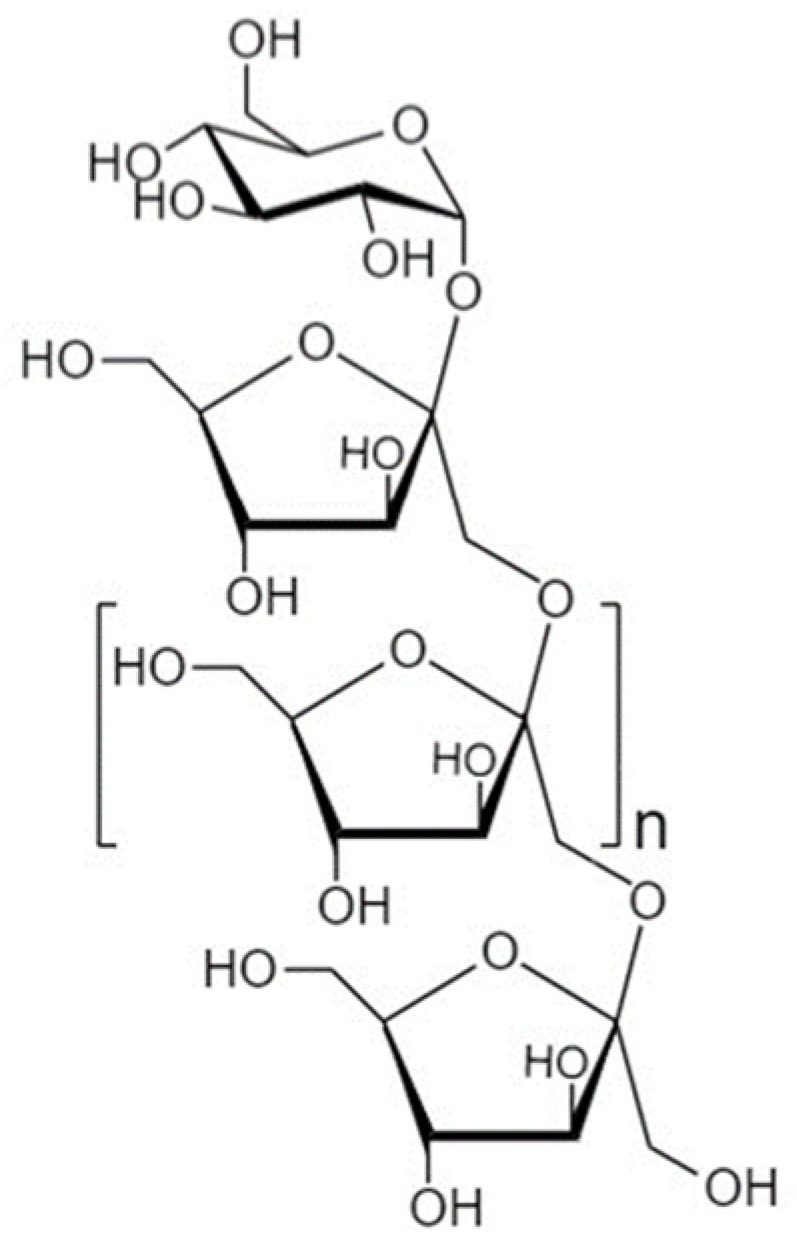


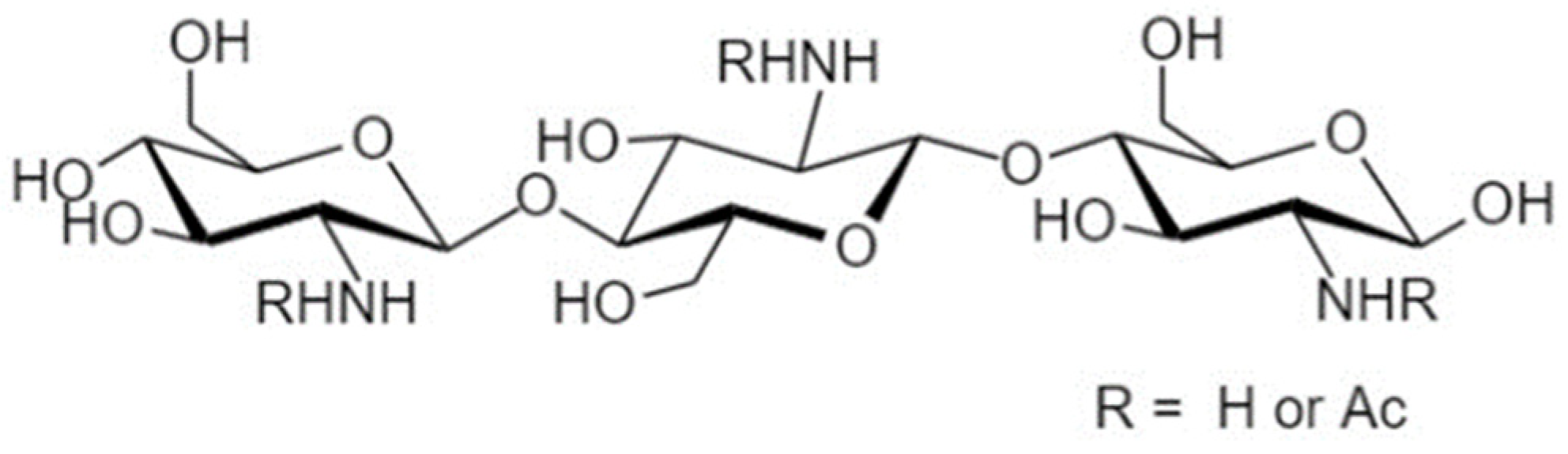
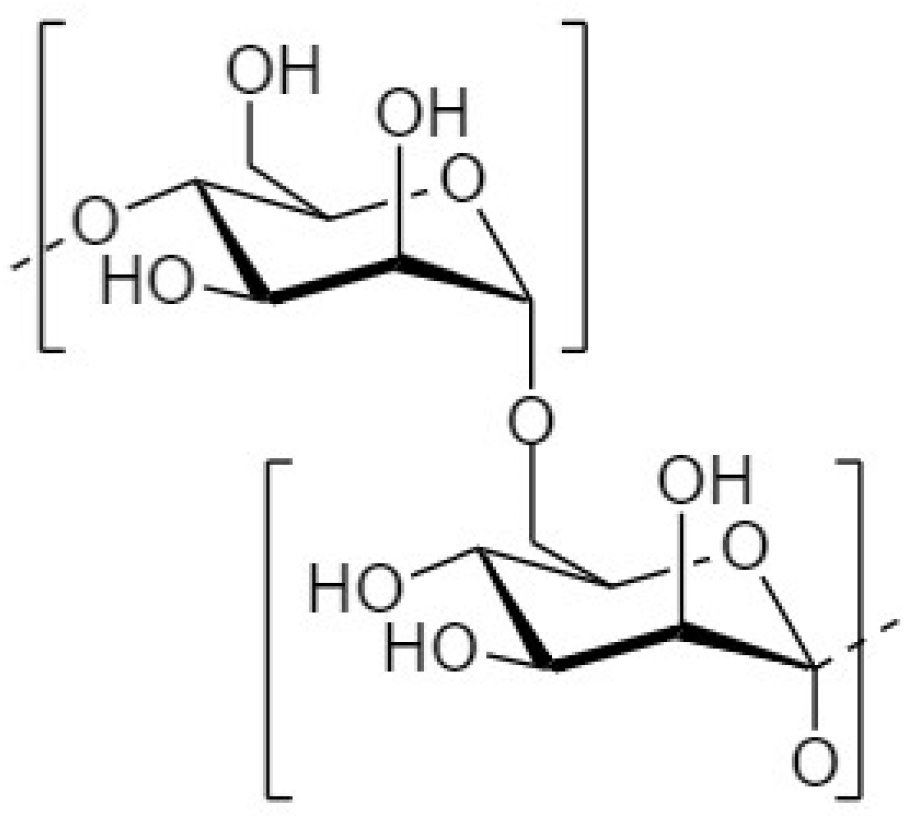
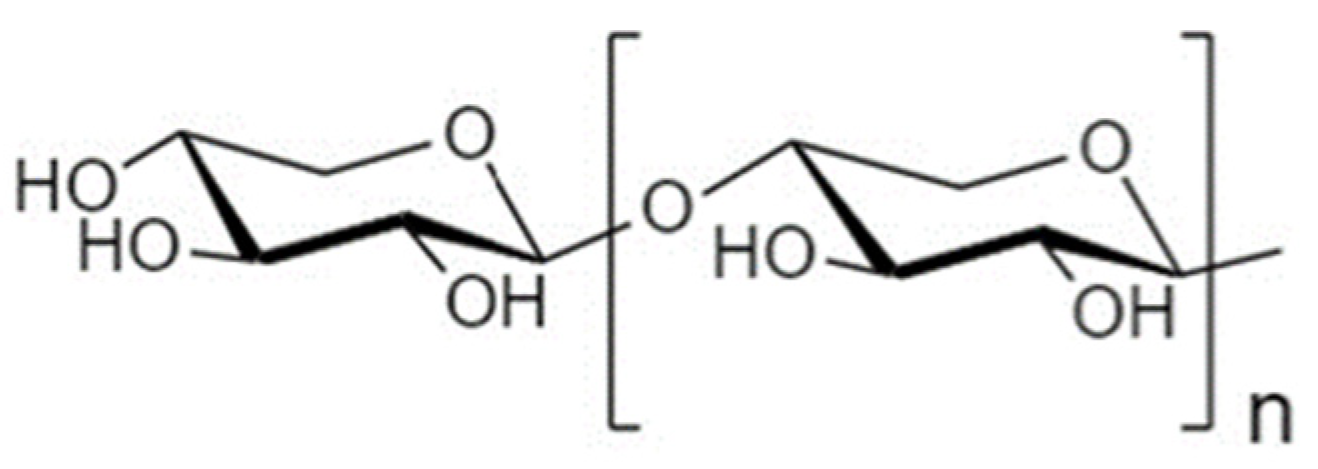
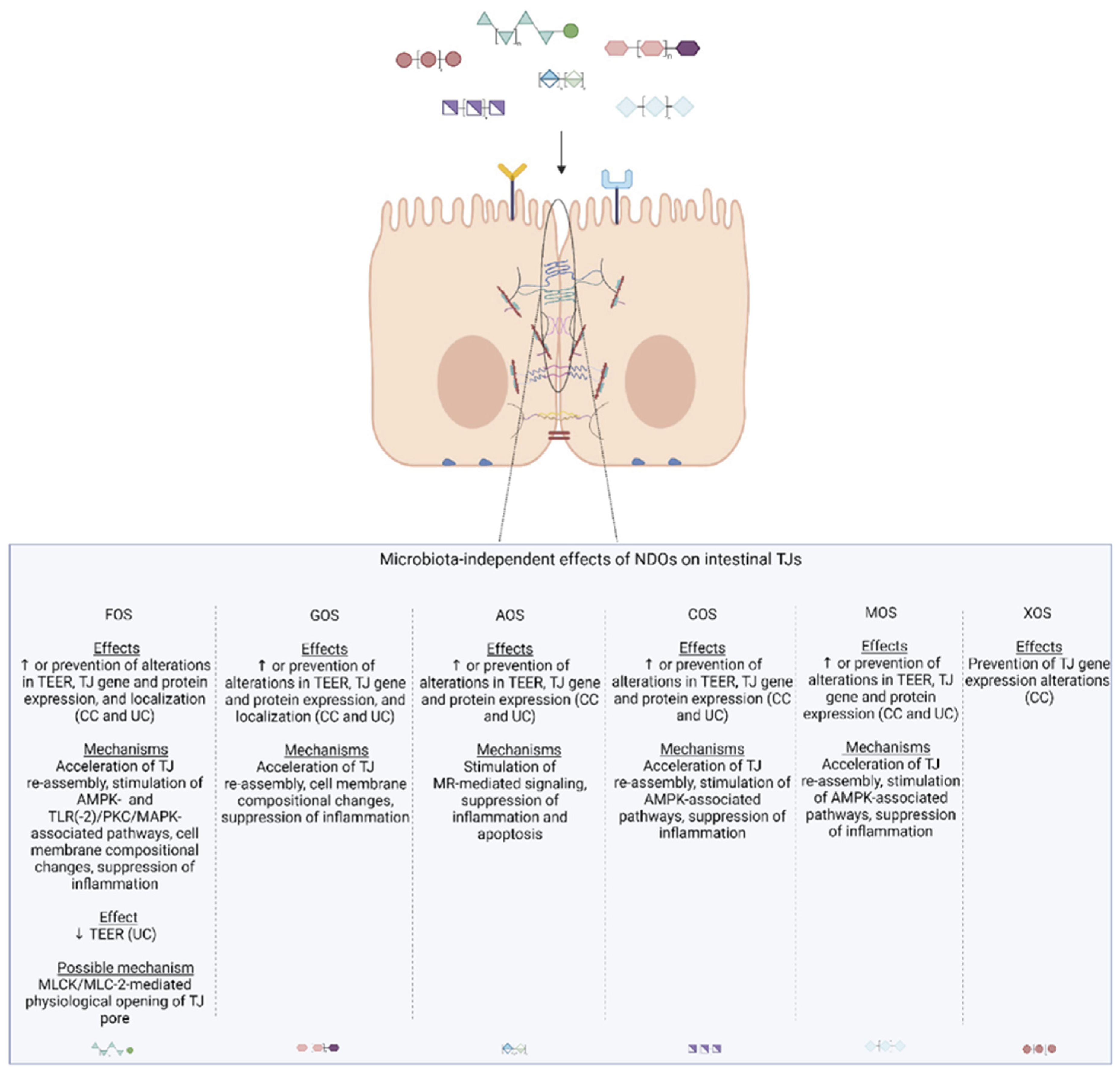
| Treatment Characteristics | [(A)XOS] | Model/Experimental Setup | Type of Study | Observed Effects on PP and/or TJs | Type of Effect | References |
|---|---|---|---|---|---|---|
| AXOS (produced upon addition of xylanase to wheat bran) | 10, 50 mg EP/kg (xylanase) | LPS-exposed Caco-2 monolayers incubated with AXOS ferment supernatant (using cecal content of broilers) | In vitro | ↑ TEER | MD | [298] |
| AXOS-rich extract (Cosucra Groupe Warcoing S.A., Pecq, Belgium), 88% dietary fiber of which 66% AXOS, DS 0.38, DP 6 | Fermentation of 5g/L AXOS | Caco2:THP-1 co-cultures incubated with AXOS ferment supernatant (using fecal batches of donors) | In vitro | ↑ TEER | MD | [299] |
| XOS derived from corn, (AIDP, Inc., City of Industry, CA, USA), 70% XOS | 0.5 g hydrated in 40 mL sterile trypticase peptone fermentation medium | Caco2:HT29-MTX co-cultures incubated with XOS ferment supernatant | In vitro | ↑ TEER after 24 h on healthy gut model, no effect on TEER after 24 h on leaky gut model but ↓ LY flux | MD | [150] |
| XOS derived from sugarcane (Prenexus Health, Gilbert, AZ, USA), XOS 84% | 3 g/day | SHIME® inoculated with fecal sample from healthy donors and coupled with co-cultures of Caco2:HT29-MTX-E12 | In vitro | ↑ TEER | MD | [194] |
| XOS (Shandong Longlive, Qingdao, China) | ad libitum | NOD/MrkTac mice | In vivo | ↓ FITC-Dextran flux | MD | [300] |
| ↑ ZO-1, occludin mRNA (only in the large intestine) | MID | |||||
| XOS alone or +B. infantis | 0.23 g/kg BW/day | DSS-exposed mice (UC) | In vivo | ↑ ZO-1, occludin, claudin-1 mRNA (better effect as synbiotic) | Not determined | [293] |
| XOS (Shandong Longlive Biotechnology Co., Ltd., Dezhou, China) | 0.02% XOS containing 35% XOS with 65% maltodextrin as carrier | LPS-exposed weaned piglets | In vivo | ↑ claudin-1 in both unchallenged and challenged animals | MD | [295] |
| XOS (Jiangsu Kangwei Biologic Co., Ltd., Dongtai, China) | 0.01% XOS | Weaned piglets | In vivo | ↑ ZO-1, tendency to ↑ occludin, no effect on claudin-1 abundance | MD | [294] |
| XOS (Shandong Longlive Bio-technology Co. Ltd., Shandong, China), containing xylobiose, xylotriose, and xylotetraose at ≥35% | 100 g/t | Weaned piglets | In vivo | ↑ claudin-2 mRNA (compared to positive control group | MD | [290] |
| 250 g/t | ↑ ZO-1 mRNA, no effect on occludin, claudin 2,3 (compared to basal diet-fed animals | |||||
| XOS (Jiangsu Kangwei Biological Co., Ltd., Nanjing, China), 35% XOS + IAPS | 100 mg/kg | Broilers | In vivo | ↑ ZO-1, occludin, claudin-1, -3 mRNA (better effect with combined treatments) | Not determined | [297] |
| 100 mg/kg + 600 mg/kg IAPS | ||||||
| XOS (Shandong Longlive Bio-Technology Co., Ltd., China), 95% pure powder extracted from corncob | 500 mg/mL (2 mL every 2nd day) | Rats | In vivo | No effect on FITC-D permeability on plasma, nor on TEER (Caco-2 cells treated with cecal water), ↑ occludin mRNA | MD | [291] |
| XOS (Shandong Longli Biotechnology Co., Shandong, China), 95% XOS | 1 g/kg BW/day | HFD-fed rats | In vivo | ↑ occludin mRNA | MD | [296] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mavrogeni, M.E.; Asadpoor, M.; Henricks, P.A.J.; Keshavarzian, A.; Folkerts, G.; Braber, S. Direct Action of Non-Digestible Oligosaccharides against a Leaky Gut. Nutrients 2022, 14, 4699. https://doi.org/10.3390/nu14214699
Mavrogeni ME, Asadpoor M, Henricks PAJ, Keshavarzian A, Folkerts G, Braber S. Direct Action of Non-Digestible Oligosaccharides against a Leaky Gut. Nutrients. 2022; 14(21):4699. https://doi.org/10.3390/nu14214699
Chicago/Turabian StyleMavrogeni, Maria Eleni, Mostafa Asadpoor, Paul A. J. Henricks, Ali Keshavarzian, Gert Folkerts, and Saskia Braber. 2022. "Direct Action of Non-Digestible Oligosaccharides against a Leaky Gut" Nutrients 14, no. 21: 4699. https://doi.org/10.3390/nu14214699
APA StyleMavrogeni, M. E., Asadpoor, M., Henricks, P. A. J., Keshavarzian, A., Folkerts, G., & Braber, S. (2022). Direct Action of Non-Digestible Oligosaccharides against a Leaky Gut. Nutrients, 14(21), 4699. https://doi.org/10.3390/nu14214699






