The Contribution of Diet Therapy and Probiotics in the Treatment of Sarcopenia Induced by Prolonged Immobilization Caused by the COVID-19 Pandemic
Abstract
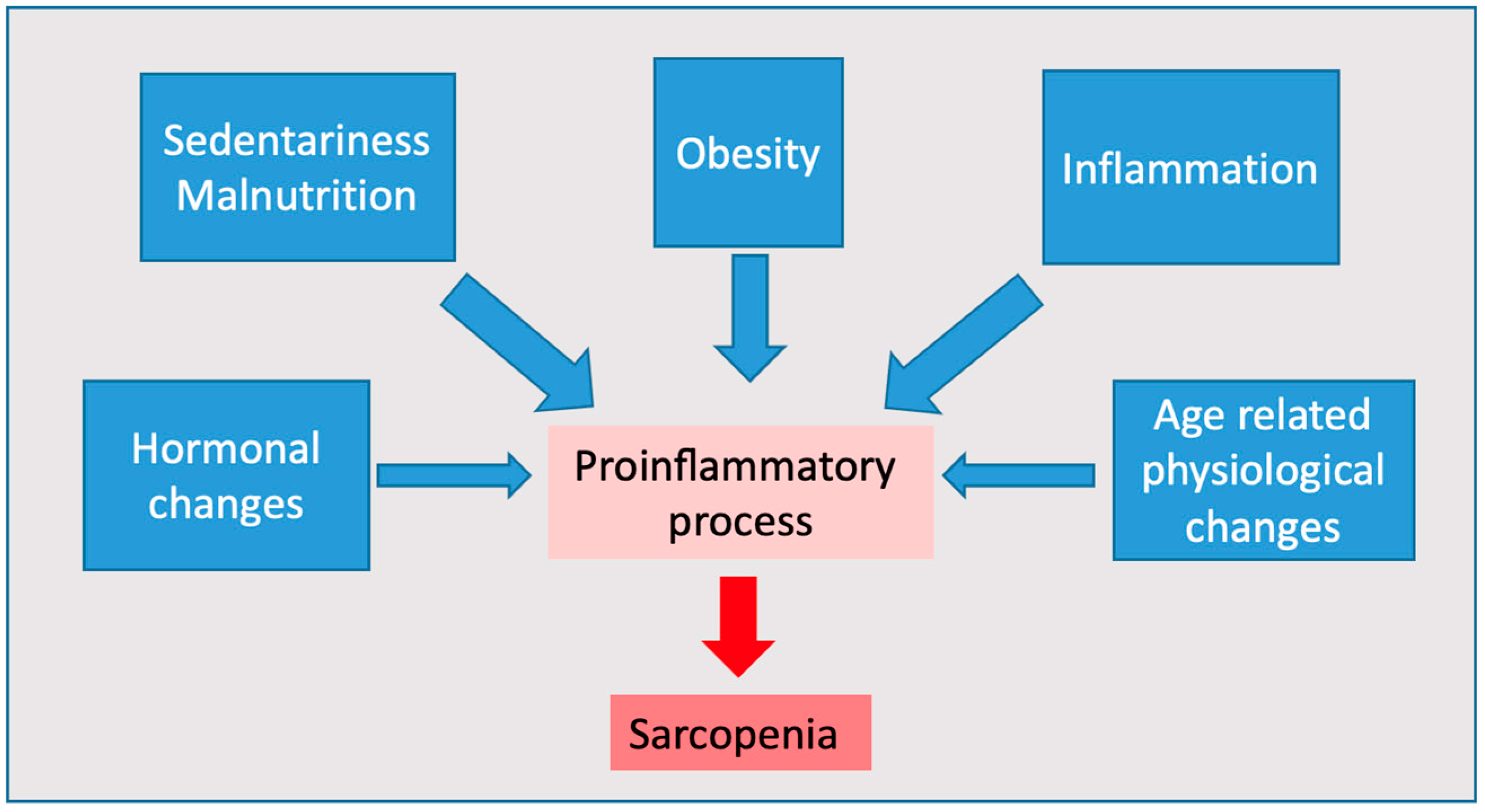
1. Introduction
2. Materials and Methods
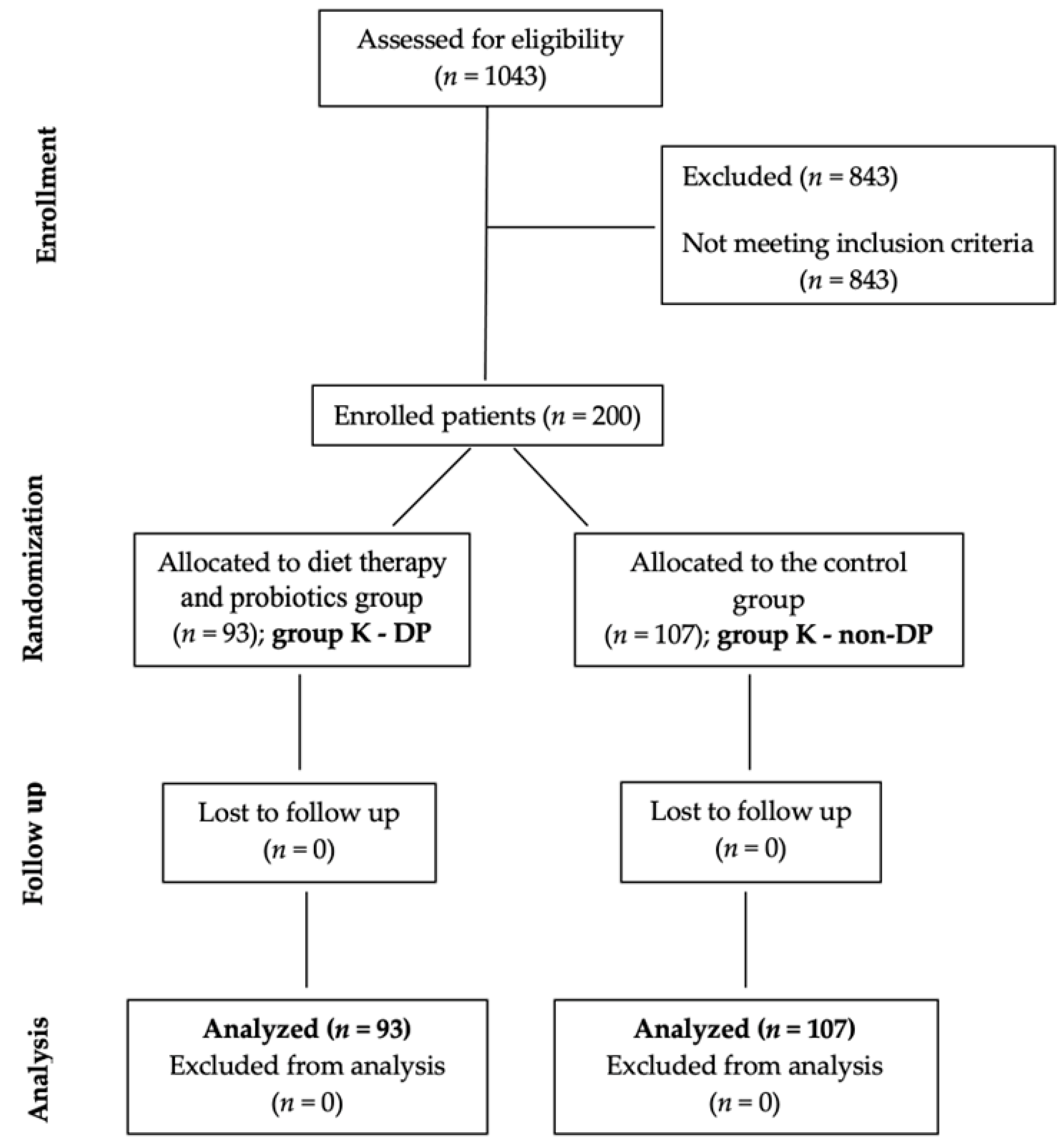
2.1. Study Design
- Group K—DP included 93 patients with dietary therapy and probiotics, and
- Group K—non-DP, 107 patients without diet therapy and probiotics.
2.2. Study Tools
2.3. Statistical Analysis
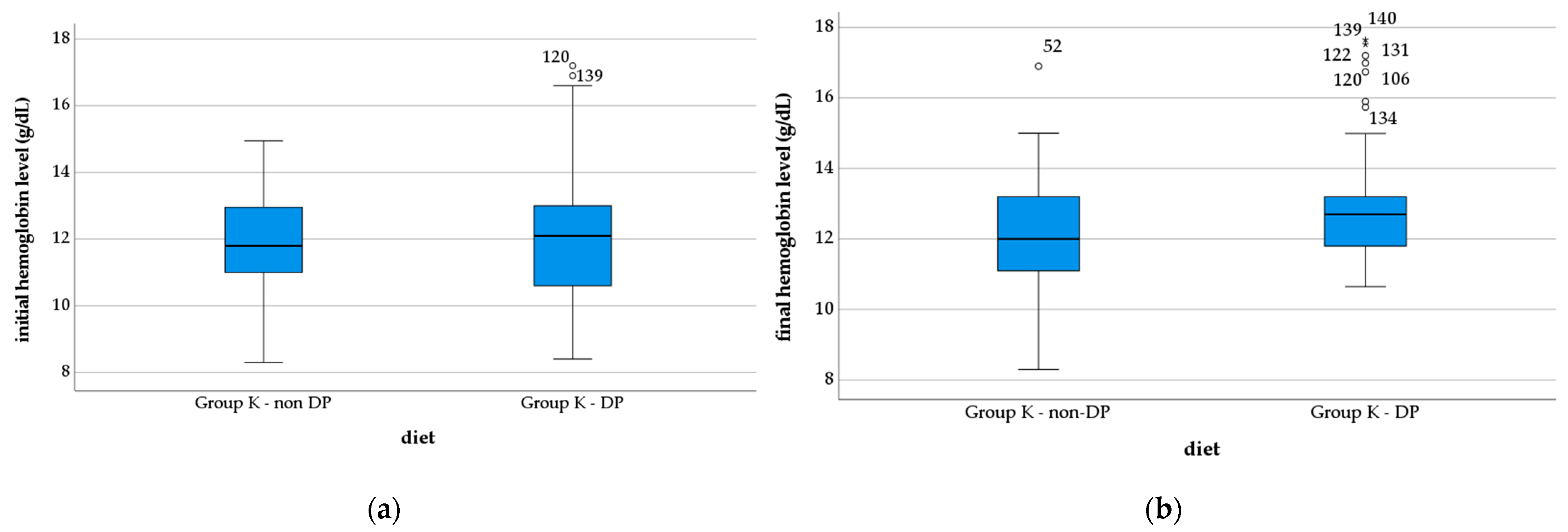
3. Results
4. Discussion
Limitations and Strengths of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Santilli, V.; Bernetti, A.; Mangone, M.; Paoloni, M. Clinical definition of sarcopenia. Clin. Cases Min. Bone Metab. 2014, 11, 177–180. [Google Scholar] [CrossRef]
- Rogeri, P.S.; Zanella, R., Jr.; Martins, G.L.; Garcia, M.D.A.; Leite, G.; Lugaresi, R.; Gasparini, S.O.; Sperandio, G.A.; Ferreira, L.H.B.; Souza-Junior, T.P.; et al. Strategies to Prevent Sarcopenia in the Aging Process: Role of Protein Intake and Exercise. Nutrients 2021, 14, 52. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.; Panton, L.B.; Dutton, G.R.; Ilich, J.Z. Relationship of Physical Performance with Body Composition and Bone Mineral Density in Individuals over 60 Years of Age: A Systematic Review. J. Aging Res. 2011, 2011, 191896. [Google Scholar] [CrossRef] [PubMed]
- Xia, Z.; Cholewa, J.; Zhao, Y.; Shang, H.-Y.; Yang, Y.-Q.; Araújo Pessôa, K.; Su, Q.-S.; Lima-Soares, F.; Zanchi, N.E. Targeting Inflammation and Downstream Protein Metabolism in Sarcopenia: A Brief Up-Dated Description of Concurrent Exercise and Leucine-Based Multimodal Intervention. Front. Physiol. 2017, 8, 434. [Google Scholar] [CrossRef] [PubMed]
- Molfino, A.; Imbimbo, G.; Rizzo, V.; Muscaritoli, M.; Alampi, D. The link between nutritional status and outcomes in COVID-19 patients in ICU: Is obesity or sarcopenia the real problem? Eur. J. Intern. Med. 2021, 91, 93–95. [Google Scholar] [CrossRef]
- Picca, A.; Coelho-Junior, H.J.; Calvani, R.; Marzetti, E.; Vetrano, D.L. Biomarkers shared by frailty and sarcopenia in older adults: A systematic review and meta-analysis. Ageing Res. Rev. 2022, 73, 101530. [Google Scholar] [CrossRef]
- Jung, H.-W.; Park, J.H.; Kim, D.A.; Jang, I.-Y.; Park, S.J.; Lee, J.Y.; Lee, S.; Kim, J.H.; Yi, H.-S.; Lee, E.; et al. Association between serum FGF21 level and sarcopenia in older adults. Bone 2021, 145, 115877. [Google Scholar] [CrossRef]
- Evans, W.J. Skeletal muscle loss: Cachexia, sarcopenia, and inactivity. Am. J. Clin. Nutr. 2010, 91, 1123S–1127S. [Google Scholar] [CrossRef]
- Granacher, U.; Lacroix, A.; Muehlbauer, T.; Roettger, K.; Gollhofer, A. Effects of core instability strength training on trunk muscle strength, spinal mobility, dynamic balance and functional mobility in older adults. Gerontology 2013, 59, 105–113. [Google Scholar] [CrossRef]
- Kosek, D.J.; Kim, J.-S.; Petrella, J.K.; Cross, J.M.; Bamman, M.M. Efficacy of 3 days/wk resistance training on myofiber hypertrophy and myogenic mechanisms in young vs. older adults. J. Appl. Physiol. 2006, 101, 531–544. [Google Scholar] [CrossRef]
- Steffl, M.; Bohannon, R.W.; Sontakova, L.; Tufano, J.J.; Shiells, K.; Holmerova, I. Relationship between sarcopenia and physical activity in older people: A systematic review and meta-analysis. Clin. Interv. Aging 2017, 12, 835–845. [Google Scholar] [CrossRef]
- Kirwan, R.; McCullough, D.; Butler, T.; Perez de Heredia, F.; Davies, I.G.; Stewart, C. Sarcopenia during COVID-19 lockdown restrictions: Long-term health effects of short-term muscle loss. Geroscience 2020, 42, 1547–1578. [Google Scholar] [CrossRef]
- Moga, T.D.; Nistor-Cseppento, C.D.; Bungau, S.G.; Tit, D.M.; Sabau, A.M.; Behl, T.; Nechifor, A.C.; Bungau, A.F.; Negrut, N. The Effects of the ‘Catabolic Crisis’ on Patients’ Prolonged Immobility after COVID-19 Infection. Medicina 2022, 58, 828. [Google Scholar] [CrossRef]
- Behl, T.; Kaur, I.; Bungau, S.; Kumar, A.; Uddin, M.S.; Kumar, C.; Pal, G.; Shrivastava, K.; Zengin, G.; Arora, S. The dual impact of ACE2 in COVID-19 and ironical actions in geriatrics and pediatrics with possible therapeutic solutions. Life Sci. 2020, 257, 118075. [Google Scholar] [CrossRef]
- Kemmler, W.; Kohl, M.; Fröhlich, M.; Jakob, F.; Engelke, K.; von Stengel, S.; Schoene, D. Effects of high-intensity resistance training on osteopenia and sarcopenia parameters in older men with osteosarcopenia—One-year results of the randomized controlled Franconian Osteopenia and Sarcopenia Trial (FrOST). J. Bone Miner. Res. 2020, 35, 1634–1644. [Google Scholar] [CrossRef]
- Piotrowicz, K.; Gąsowski, J.; Michel, J.-P.; Veronese, N. Post-COVID-19 acute sarcopenia: Physiopathology and management. Aging Clin. Exp. Res. 2021, 33, 2887–2898. [Google Scholar] [CrossRef]
- Maynard, C.; Weinkove, D. The gut microbiota and ageing. Biochem. Cell Biol. Ageing Part I Biomed. Sci. 2018, 90, 351–371. [Google Scholar]
- Vaira, L.A.; Salzano, G.; Fois, A.G.; Piombino, P.; De Riu, G. Potential pathogenesis of ageusia and anosmia in COVID-19 patients. Int. Forum Allergy Rhinol. 2020, 10, 1103–1104. [Google Scholar] [CrossRef]
- Sandler, C.X.; Wyller, V.B.; Moss-Morris, R.; Buchwald, D.; Crawley, E.; Hautvast, J.; Katz, B.Z.; Knoop, H.; Little, P.; Taylor, R. Long COVID and post-infective fatigue syndrome: A review. Open Forum Infect. Dis. 2021, 8, ofab440. [Google Scholar] [CrossRef]
- Moga, T.D.; Ioana, M.; Sabau, M.; Nistor-Cseppento, C.D.; Iovanovici, D.C.; Cavalu, S.; Dogaru, B.G. Sarcopenia, a major clinical problem in old age, potential cau-ses, clinical consequences and therapeutic possibilities. Balneo PRM Res. J. 2022, 13, 492. [Google Scholar] [CrossRef]
- Wakabayashi, H. Rehabilitation nutrition in general and family medicine. J. Gen. Fam. Med. 2017, 18, 153–154. [Google Scholar] [CrossRef] [PubMed]
- Frățilă, O.; Mihele, A.I.; Hodisan-Pap, E.; Hocopan, S.C.; Brata, R.; Iliaș, T. Comparative hepatoprotective efficacy of silymarin-phyllanthus-choline combination versus silymarin alone in liver diseases with different destruction and inflammation stages. Farmacia 2020, 68, 299–306. [Google Scholar] [CrossRef]
- Corb Aron, R.A.; Abid, A.; Vesa, C.M.; Nechifor, A.C.; Behl, T.; Ghitea, T.C.; Munteanu, M.A.; Fratila, O.; Andronie-Cioara, F.L.; Toma, M.M.; et al. Recognizing the Benefits of Pre-/Probiotics in Metabolic Syndrome and Type 2 Diabetes Mellitus Considering the Influence of Akkermansia muciniphila as a Key Gut Bacterium. Microorganisms 2021, 9, 618. [Google Scholar] [CrossRef] [PubMed]
- Jäger, R.; Zaragoza, J.; Purpura, M.; Iametti, S.; Marengo, M.; Tinsley, G.M.; Anzalone, A.J.; Oliver, J.M.; Fiore, W.; Biffi, A. Probiotic administration increases amino acid absorption from plant protein: A placebo-controlled, randomized, double-blind, multicenter, crossover study. Probiotics Antimicrob. Proteins 2020, 12, 1330–1339. [Google Scholar] [CrossRef]
- Uchitomi, R.; Oyabu, M.; Kamei, Y. Vitamin D and sarcopenia: Potential of vitamin D supplementation in sarcopenia prevention and treatment. Nutrients 2020, 12, 3189. [Google Scholar] [CrossRef]
- Global Database on Body Mass Index, World Health Organization. Available online: http://www.assessmentpsychology.com/icbmi.htm (accessed on 10 October 2022).
- Beaudart, C.; McCloskey, E.; Bruyère, O.; Cesari, M.; Rolland, Y.; Rizzoli, R.; Araujo de Carvalho, I.; Amuthavalli Thiyagarajan, J.; Bautmans, I.; Bertière, M.-C. Sarcopenia in daily practice: Assessment and management. BMC Geriatr. 2016, 16, 170. [Google Scholar] [CrossRef]
- Tanita.eu. Available online: https://www.tanita.com/data/Manuals/MC-780manual_R0.pdf?rev=C87A (accessed on 22 August 2022).
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Lee, J.K.; Park, Y.S.; Kim, K.; Oh, T.J.; Chang, W. Comparison of Bioelectrical Impedance Analysis and Computed Tomography on Body Composition changes including Visceral Fat after Bariatric Surgery in Asian Patients with Obesity. Obes. Surg. 2021, 31, 4243–4250. [Google Scholar] [CrossRef]
- van den Helder, J.; Verreijen, A.M.; van Dronkelaar, C.; Memelink, R.G.; Engberink, M.F.; Engelbert, R.H.H.; Weijs, P.J.M.; Tieland, M. Bio-Electrical Impedance Analysis: A Valid Assessment Tool for Diagnosis of Low Appendicular Lean Mass in Older Adults? Front. Nutr. 2022, 9, 874980. [Google Scholar] [CrossRef]
- Chen, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Chou, M.Y.; Iijima, K.; Jang, H.C.; Kang, L.; Kim, M.; Kim, S.; et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J. Am. Med. Dir. Assoc. 2020, 21, 300–307.e2. [Google Scholar] [CrossRef]
- Sbrignadello, S.; Göbl, C.; Tura, A. Bioelectrical Impedance Analysis for the Assessment of Body Composition in Sarcopenia and Type 2 Diabetes. Nutrients 2022, 14, 1864. [Google Scholar] [CrossRef]
- Khanal, P.; He, L.; Stebbings, G.; Onambele-Pearson, G.L.; Degens, H.; Williams, A.; Thomis, M.; Morse, C.I. Prevalence and association of single nucleotide polymorphisms with sarcopenia in older women depends on definition. Sci. Rep. 2020, 10, 2913. [Google Scholar] [CrossRef]
- Khanal, P.; Williams, A.G.; He, L.; Stebbings, G.K.; Onambele-Pearson, G.L.; Thomis, M.; Degens, H.; Morse, C.I. Sarcopenia, obesity, and sarcopenic obesity: Relationship with skeletal muscle phenotypes and single nucleotide polymorphisms. J. Clin. Med. 2021, 10, 4933. [Google Scholar] [CrossRef]
- Shafiee, G.; Keshtkar, A.; Soltani, A.; Ahadi, Z.; Larijani, B.; Heshmat, R. Prevalence of sarcopenia in the world: A systematic review and meta-analysis of general population studies. J. Diabetes Metab. Disord. 2017, 16, 21. [Google Scholar] [CrossRef]
- Presbyphagia, W.H. Sarcopenic dysphagia: Association between aging, sarcopenia, and deglutition disorders. J. Frailty Aging 2014, 3, 97–103. [Google Scholar]
- Tit, D.M.; Bungau, S.; Iovan, C.; Nistor Cseppento, D.C.; Endres, L.; Sava, C.; Sabau, A.M.; Furau, G.; Furau, C. Effects of the hormone replacement therapy and of soy isoflavones on bone resorption in postmenopause. J. Clin. Med. 2018, 7, 297. [Google Scholar] [CrossRef]
- Guralnik, J.; Ferrucci, L.; Simonsick, E.; Salive, M.; Wallace, R. Short Physical Performance Battery Protocol and Score Sheet. 1994. Available online: https://www.mcroberts.nl/wp-content/uploads/2016/11/SPPB_form.pdf (accessed on 21 September 2022).
- Pallag, A.; Roşca, E.; Ţiţ, D.M.; MuŢiu, G.; Bungău, S.G.; Pop, O.L. Monitoring the effects of treatment in colon cancer cells using immunohistochemical and histoenzymatic techniques. Rom. J. Morphol. Embryol. 2015, 56, 1103–1109. [Google Scholar]
- Ghitea, T.C.; Vlad, S.; Birle, D.; Tit, D.M.; Lazar, L.; Nistor-Cseppento, C.; Behl, T.; Bungau, S. The influence of diet therapeutic intervention on the sarcopenic index of patients with metabolic syndrome. Acta Endocrinol. 2020, 16, 470–478. [Google Scholar] [CrossRef]
- Kumar, S.; Behl, T.; Sachdeva, M.; Sehgal, A.; Kumari, S.; Kumar, A.; Kaur, G.; Yadav, H.N.; Bungau, S. Implicating the effect of ketogenic diet as a preventive measure to obesity and diabetes mellitus. Life Sci. 2021, 264, 118661. [Google Scholar] [CrossRef]
- Sawada, S.; Ozaki, H.; Natsume, T.; Nakano, D.; Deng, P.; Yoshihara, T.; Osawa, T.; Kobayashi, H.; Machida, S.; Naito, H. Serum albumin levels as a predictive biomarker for low-load resistance training programs’ effects on muscle thickness in the community-dwelling elderly Japanese population: Interventional study result. BMC Geriatr. 2021, 21, 464. [Google Scholar] [CrossRef]
- Liu, C.; Cheung, W.H.; Li, J.; Chow, S.K.H.; Yu, J.; Wong, S.H.; Ip, M.; Sung, J.J.Y.; Wong, R.M.Y. Understanding the gut microbiota and sarcopenia: A systematic review. J. Cachexia Sarcopenia Muscle 2021, 12, 1393–1407. [Google Scholar] [CrossRef] [PubMed]
- Daily, J.W.; Park, S. Sarcopenia Is a Cause and Consequence of Metabolic Dysregulation in Aging Humans: Effects of Gut Dysbiosis, Glucose Dysregulation, Diet and Lifestyle. Cells 2022, 11, 338. [Google Scholar] [CrossRef] [PubMed]
- Sousa-Santos, A.R.; Barros, D.; Montanha, T.L.; Carvalho, J.; Amaral, T.F. Which is the best alternative to estimate muscle mass for sarcopenia diagnosis when DXA is unavailable? Arch. Gerontol. Geriatr. 2021, 97, 104517. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.M.; Jebb, S.A.; Oke, J.; Piernas, C. Reference values for skeletal muscle mass and fat mass measured by bioelectrical impedance in 390 565 UK adults. J. Cachexia Sarcopenia Muscle 2020, 11, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Holdoway, A. Nutritional management of patients during and after COVID-19 illness. Br. J. Community Nurs. 2020, 25, S6–S10. [Google Scholar] [CrossRef]
- Cawood, A.L.; Walters, E.R.; Smith, T.R.; Sipaul, R.H.; Stratton, R.J. A Review of Nutrition Support Guidelines for Individuals with or Recovering from COVID-19 in the Community. Nutrients 2020, 12, 3230. [Google Scholar] [CrossRef]
- Negrut, N.; Codrean, A.; Hodisan, I.; Bungau, S.; Tit, D.M.; Marin, R.; Behl, T.; Banica, F.; Diaconu, C.C.; Nistor-Cseppento, D.C. Efficiency of antiviral treatment in COVID-19. Exp. Ther. Med. 2021, 21, 648. [Google Scholar] [CrossRef]
- Barazzoni, R.; Bischoff, S.C.; Breda, J.; Wickramasinghe, K.; Krznaric, Z.; Nitzan, D.; Pirlich, M.; Singer, P. ESPEN expert statements and practical guidance for nutritional management of individuals with SARS-CoV-2 infection. Clin. Nutr. 2020, 39, 1631–1638. [Google Scholar] [CrossRef]
- Martínez-Arnau, F.M.; Fonfría-Vivas, R.; Cauli, O. Beneficial Effects of Leucine Supplementation on Criteria for Sarcopenia: A Systematic Review. Nutrients 2019, 11, 2504. [Google Scholar] [CrossRef]
- Jayanama, K.; Theou, O. Effects of probiotics and prebiotics on frailty and ageing: A narrative review. Curr. Clin. Pharmacol. 2020, 15, 183–192. [Google Scholar]
- Varian, B.J.; Goureshetti, S.; Poutahidis, T.; Lakritz, J.R.; Levkovich, T.; Kwok, C.; Teliousis, K.; Ibrahim, Y.M.; Mirabal, S.; Erdman, S.E. Beneficial bacteria inhibit cachexia. Oncotarget 2016, 7, 11803–11816. [Google Scholar] [CrossRef]
- Tanaka, M.; Ikeji, T.; Nakanishi, R.; Hirabayashi, T.; Ono, K.; Hirayama, Y.; Tategaki, A.; Kondo, H.; Ishihara, A.; Fujino, H. Protective effects of Enterococcus faecium strain R30 supplementation on decreased muscle endurance under disuse in rats. Exp. Physiol. 2021, 106, 1961–1970. [Google Scholar] [CrossRef]
- Huang, W.-C.; Lee, M.-C.; Lee, C.-C.; Ng, K.-S.; Hsu, Y.-J.; Tsai, T.-Y.; Young, S.-L.; Lin, J.-S.; Huang, C.-C. Effect of Lactobacillus plantarum TWK10 on exercise physiological adaptation, performance, and body composition in healthy humans. Nutrients 2019, 11, 2836. [Google Scholar] [CrossRef]
- Buigues, C.; Fernández-Garrido, J.; Pruimboom, L.; Hoogland, A.J.; Navarro-Martínez, R.; Martínez-Martínez, M.; Verdejo, Y.; Mascarós, M.C.; Peris, C.; Cauli, O. Effect of a prebiotic formulation on frailty syndrome: A randomized, double-blind clinical trial. Int. J. Mol. Sci. 2016, 17, 932. [Google Scholar] [CrossRef]
- Valentini Neto, J.; De Melo, C.M.; Lima Ribeiro, S.M. Effects of three-month intake of synbiotic on inflammation and body composition in the elderly: A pilot study. Nutrients 2013, 5, 1276–1286. [Google Scholar] [CrossRef]
- Vikberg, S.; Sörlén, N.; Brandén, L.; Johansson, J.; Nordström, A.; Hult, A.; Nordström, P. Effects of Resistance Training on Functional Strength and Muscle Mass in 70-Year-Old Individuals With Pre-sarcopenia: A Randomized Controlled Trial. J. Am. Med. Dir. Assoc. 2019, 20, 28–34. [Google Scholar] [CrossRef]
- Papadopoulou, S.K. Rehabilitation Nutrition for Injury Recovery of Athletes: The Role of Macronutrient Intake. Nutrients 2020, 12, 2449. [Google Scholar] [CrossRef]
- Verlaan, S.; Aspray, T.J.; Bauer, J.M.; Cederholm, T.; Hemsworth, J.; Hill, T.R.; McPhee, J.S.; Piasecki, M.; Seal, C.; Sieber, C.C. Nutritional status, body composition, and quality of life in community-dwelling sarcopenic and non-sarcopenic older adults: A case-control study. Clin. Nutr. 2017, 36, 267–274. [Google Scholar] [CrossRef]
- Liberman, K.; Njemini, R.; Luiking, Y.; Forti, L.N.; Verlaan, S.; Bauer, J.M.; Memelink, R.; Brandt, K.; Donini, L.M.; Maggio, M.; et al. Thirteen weeks of supplementation of vitamin D and leucine-enriched whey protein nutritional supplement attenuates chronic low-grade inflammation in sarcopenic older adults: The PROVIDE study. Aging Clin. Exp. Res. 2019, 31, 845–854. [Google Scholar] [CrossRef]
- Ottestad, I.; Ulven, S.M.; Øyri, L.K.L.; Sandvei, K.S.; Gjevestad, G.O.; Bye, A.; Sheikh, N.A.; Biong, A.S.; Andersen, L.F.; Holven, K.B. Reduced plasma concentration of branched-chain amino acids in sarcopenic older subjects: A cross-sectional study. Br. J. Nutr. 2018, 120, 445–453. [Google Scholar] [CrossRef]
- Tseng, S.-H.; Lee, W.-J.; Peng, L.-N.; Lin, M.-H.; Chen, L.-K. Associations between hemoglobin levels and sarcopenia and its components: Results from the I-Lan longitudinal study. Exp. Gerontol. 2021, 150, 111379. [Google Scholar] [CrossRef] [PubMed]
- Alkahtani, S.A. A cross-sectional study on sarcopenia using different methods: Reference values for healthy Saudi young men. BMC Musculoskelet Disord. 2017, 18, 119. [Google Scholar] [CrossRef] [PubMed]
- Aleixo, G.F.; Shachar, S.S.; Nyrop, K.A.; Muss, H.B.; Battaglini, C.L.; Williams, G.R. Bioelectrical impedance analysis for the assessment of sarcopenia in patients with cancer: A systematic review. Oncology 2020, 25, 170–182. [Google Scholar] [CrossRef]
- Walowski, C.O.; Braun, W.; Maisch, M.J.; Jensen, B.; Peine, S.; Norman, K.; Müller, M.J.; Bosy-Westphal, A. Reference Values for Skeletal Muscle Mass—Current Concepts and Methodological Considerations. Nutrients 2020, 12, 755. [Google Scholar] [CrossRef] [PubMed]
- Clerbaux, L.-A.; Fillipovska, J.; Muñoz, A.; Petrillo, M.; Coecke, S.; Amorim, M.-J.; Grenga, L. Mechanisms Leading to Gut Dysbiosis in COVID-19: Current Evidence and Uncertainties Based on Adverse Outcome Pathways. J. Clin. Med. 2022, 11, 5400. [Google Scholar] [CrossRef]
- Otrisal, P.; Bungau, C.; Obsel, V.; Melicharik, Z.; Tont, G. Selected Respiratory Protective Devices: Respirators and Significance of Some Markings. Sustainability 2021, 13, 4988. [Google Scholar] [CrossRef]



| Parameter | Group K—DP | Group K—Non-DP | p |
|---|---|---|---|
| N | 93 | 107 | 0.322 ** |
| Age, M, SD | 68.03 ± 8.09 | 66.91 ± 7.81 | 0.503 * |
| Female, N (%) | 66 (33.0) | 80 (40.0) | 0.233 ** |
| Rural area, N (%) | 47 (23.5) | 49 (24.5) | 0.430 ** |
| BMI, M, SD | 28.72 ± 4.97 | 28.19 ± 5.93 | 0.850 * |
| Smoker, N (%) | 19 (9.5) | 25 (12.5) | 0.319 ** |
| Alcohol user, N (%) | 8 (4) | 12 (6) | 0.220 ** |
| Coffee user, N (%) | 25 (12.5) | 27 (13.5) | 0.599 ** |
| Kyphosis, N (%) | 90 (45) | 102 (51) | 0.299 ** |
| Scoliosis, N (%) | 64 (32) | 68 (34) | 0.118 ** |
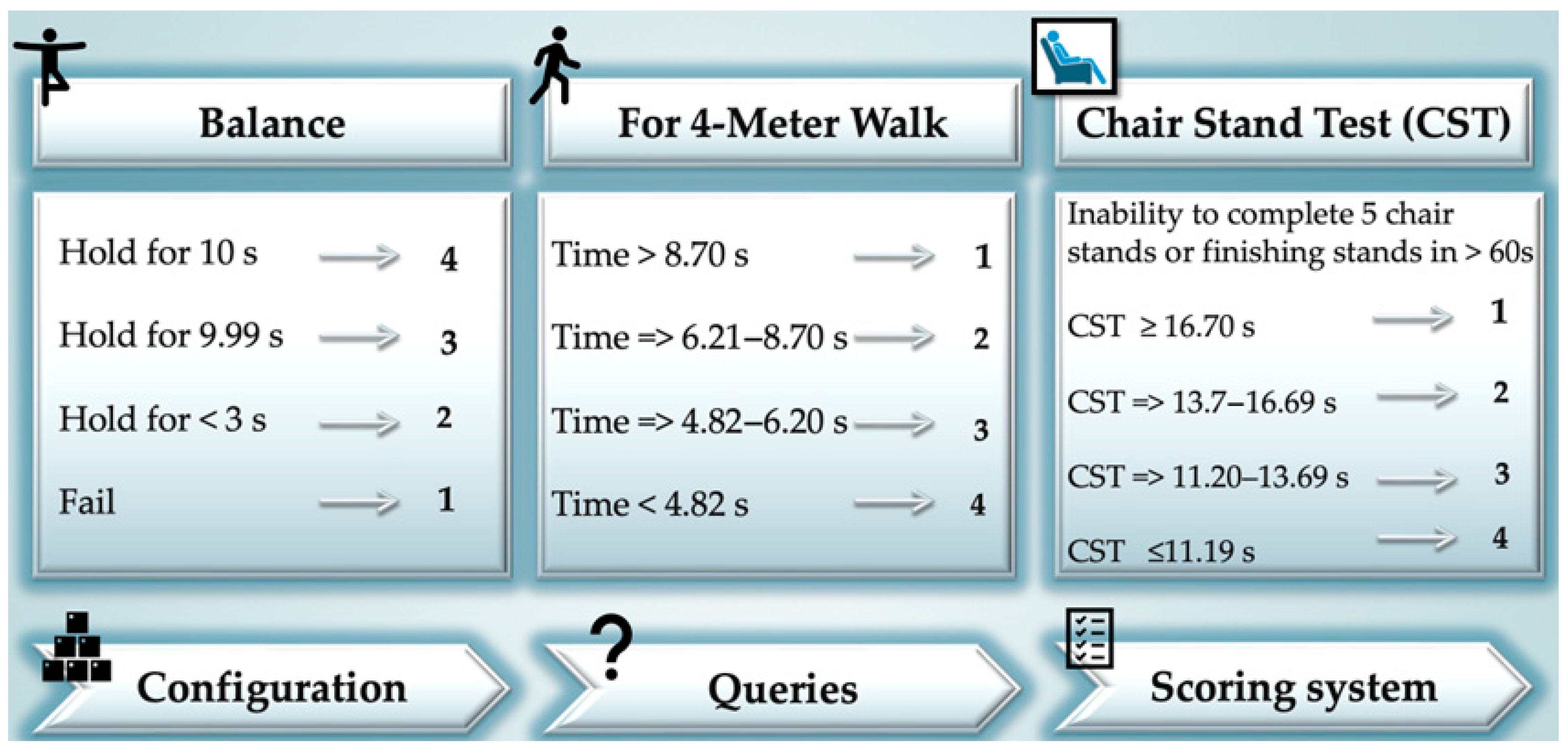
| Parameter | Scoring Points | N (%) |
|---|---|---|
| Balance | 0 | 8 (4) |
| 1 | 40 (20) | |
| 2 | 152 (76) | |
| For 4-m walk | 0 | 4 (2) |
| 1 | 60 (30) | |
| 2 | 84 (42) | |
| 3 | 40 (20) | |
| 4 | 12 (6) | |
| Chair standing Test | 0 | 24 (12) |
| 1 | 48 (24) | |
| 2 | 32 (16) | |
| 3 | 24 (12) | |
| 4 | 72 (36) | |
| Score value, M, SD | 4.86 ± 2.57 |
| Patients | N (%) | p | ||
|---|---|---|---|---|
| Initial | Final | |||
| Group K—non-DP | sarcopenia | 70 (75.26) | 56 (52.33) | 0.068 |
| normal value | 23 (24.73) | 37 (47.66) | 0.070 | |
| Group K—DP | sarcopenia | 75 (80.64) | 37 (34.57) | <0.001 |
| normal value | 32 (19.35) | 70 (65.42) | <0.001 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nistor-Cseppento, C.D.; Moga, T.D.; Bungau, A.F.; Tit, D.M.; Negrut, N.; Pasca, B.; Bochis, C.F.; Ghitea, T.C.; Jurcau, A.; Purza, A.L.; et al. The Contribution of Diet Therapy and Probiotics in the Treatment of Sarcopenia Induced by Prolonged Immobilization Caused by the COVID-19 Pandemic. Nutrients 2022, 14, 4701. https://doi.org/10.3390/nu14214701
Nistor-Cseppento CD, Moga TD, Bungau AF, Tit DM, Negrut N, Pasca B, Bochis CF, Ghitea TC, Jurcau A, Purza AL, et al. The Contribution of Diet Therapy and Probiotics in the Treatment of Sarcopenia Induced by Prolonged Immobilization Caused by the COVID-19 Pandemic. Nutrients. 2022; 14(21):4701. https://doi.org/10.3390/nu14214701
Chicago/Turabian StyleNistor-Cseppento, Carmen Delia, Titus David Moga, Alexa Florina Bungau, Delia Mirela Tit, Nicoleta Negrut, Bianca Pasca, Calin Florin Bochis, Timea Claudia Ghitea, Anamaria Jurcau, Anamaria Lavinia Purza, and et al. 2022. "The Contribution of Diet Therapy and Probiotics in the Treatment of Sarcopenia Induced by Prolonged Immobilization Caused by the COVID-19 Pandemic" Nutrients 14, no. 21: 4701. https://doi.org/10.3390/nu14214701
APA StyleNistor-Cseppento, C. D., Moga, T. D., Bungau, A. F., Tit, D. M., Negrut, N., Pasca, B., Bochis, C. F., Ghitea, T. C., Jurcau, A., Purza, A. L., & Uivarosan, D. (2022). The Contribution of Diet Therapy and Probiotics in the Treatment of Sarcopenia Induced by Prolonged Immobilization Caused by the COVID-19 Pandemic. Nutrients, 14(21), 4701. https://doi.org/10.3390/nu14214701










