Multidisciplinary Prehabilitation and Postoperative Rehabilitation for Avoiding Complications in Patients Undergoing Resection of Colon Cancer: Rationale, Design, and Methodology of the ONCOFIT Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants and Selection Criteria
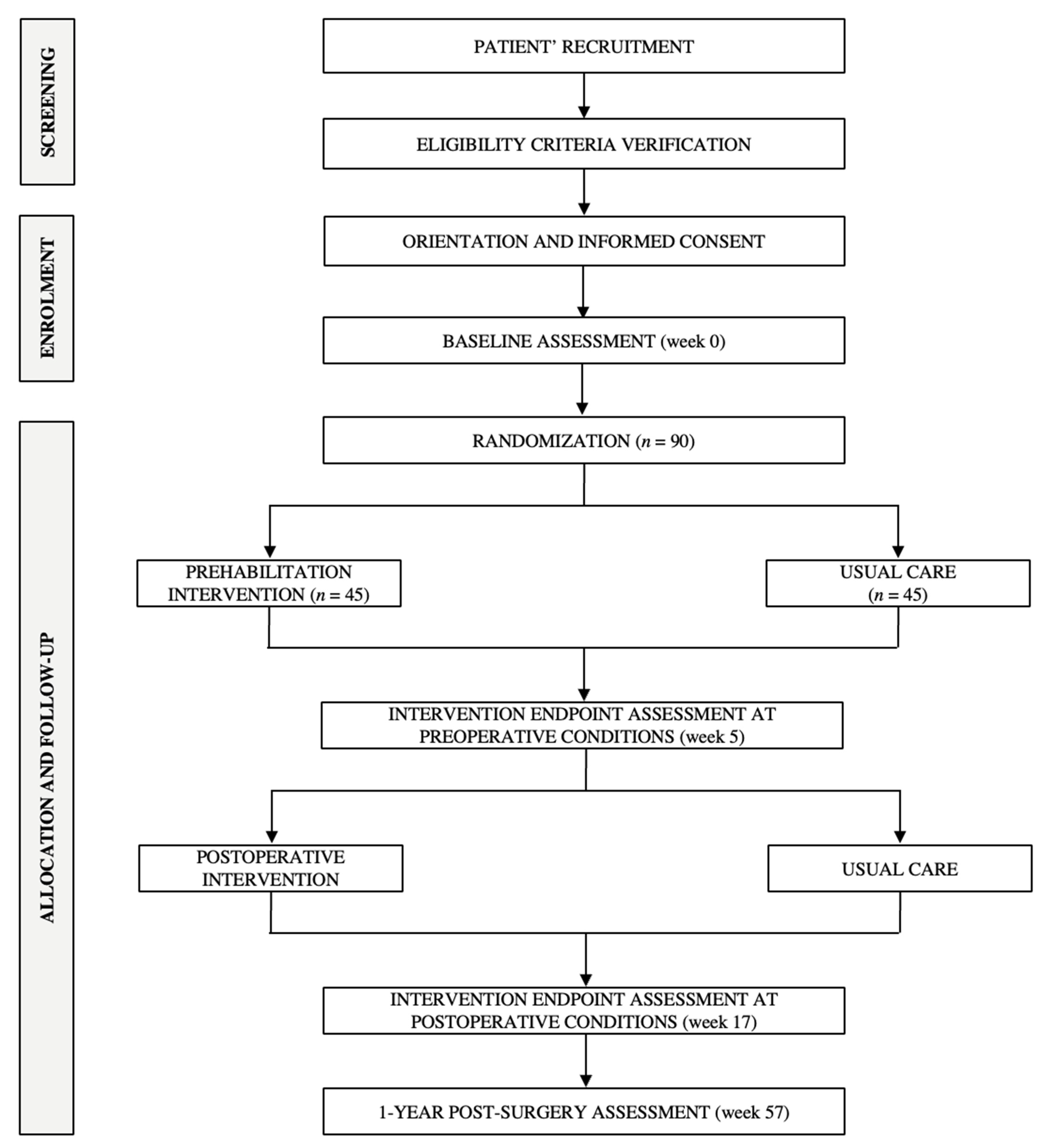
2.3. Recruitment and Randomization
Evaluation of Integrity, Compliance of the Intervention and Patients’ Retention/Adherence
2.4. Intervention Description
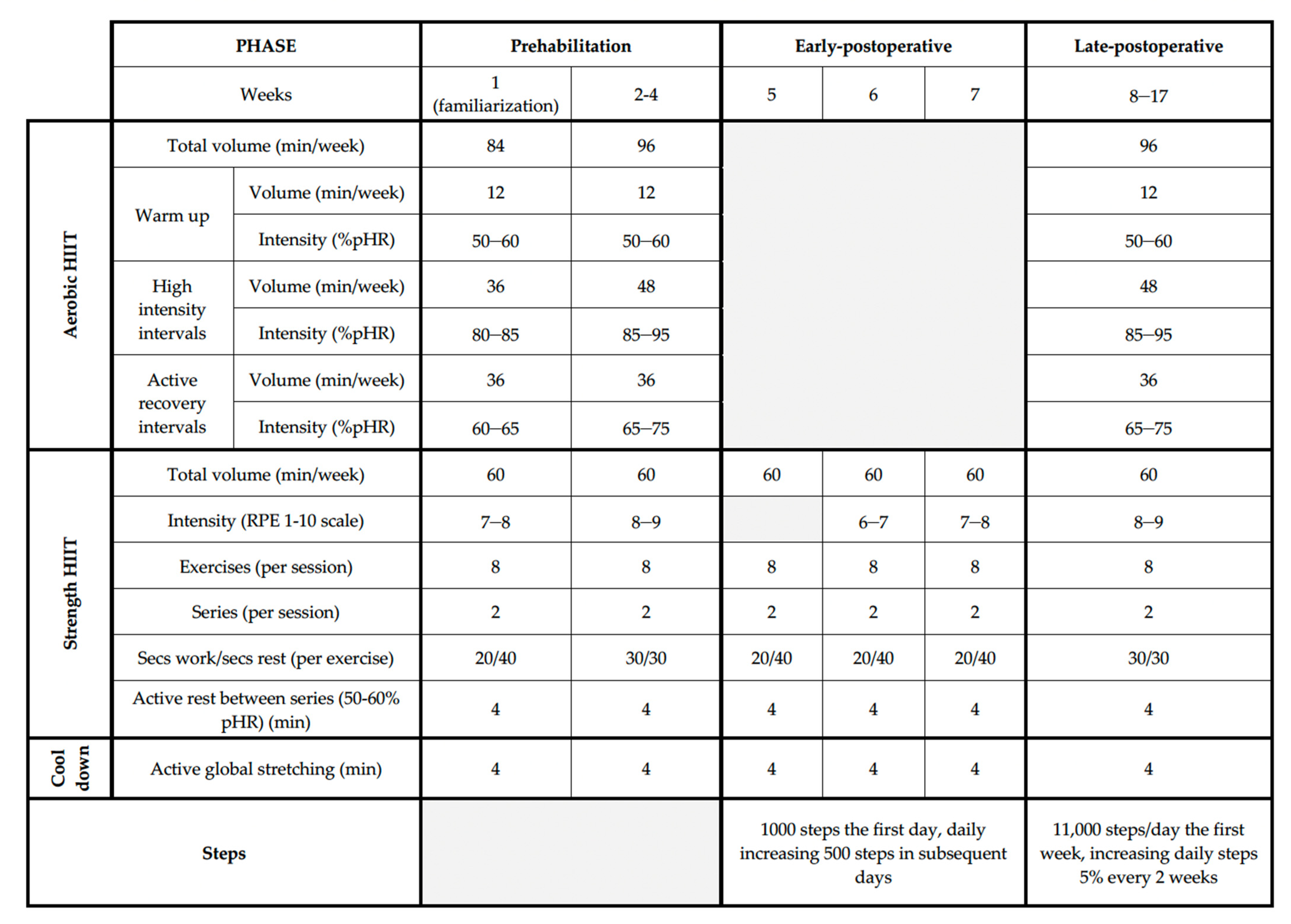
2.4.1. Physical Exercise Intervention
Volume
Intensity
Frequency
Type of Exercise
Training Load Variation
Training Periodization
Prehabilitation Phase
Early-Postoperative Phase
Late-Postoperative Phase
2.4.2. Dietary Behavior Change
Prehabilitation Phase
Postoperative Phase
2.4.3. Psychological Support
Prehabilitation Phase
Postoperative Phase
2.5. Usual Care/Control Group
2.6. Study Endpoints
2.6.1. Primary Endpoint
2.6.2. Secondary Endpoints
Additional Surgery-Derived Events
Functional Capacity
Patients-Reported Outcome Measures
Anthropometry and Body Composition
Clinical/Tumor Parameters
Physical Activity and Sedentariness
Dietary Habits
Others Unhealthy Habits
Sleep Quality
Fecal Microbiota Analysis
2.7. Cost-Effectiveness Analysis Outcome
2.8. Sample Size
2.9. Analytical Approach and Statistical Power/Data Management
3. Potential Impact of the ONCOFIT Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA. Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Boeding, J.R.E.; Ramphal, W.; Rijken, A.M.; Crolla, R.M.P.H.; Verhoef, C.; Gobardhan, P.D.; Schreinemakers, J.M.J. A Systematic Review Comparing Emergency Resection and Staged Treatment for Curable Obstructing Right-Sided Colon Cancer. Ann. Surg. Oncol. 2021, 28, 3545–3555. [Google Scholar] [CrossRef] [PubMed]
- Desborough, J.P. The stress response to trauma and surgery. Br. J. Anaesth. 2000, 85, 109–117. [Google Scholar] [CrossRef]
- Fagard, K.; Leonard, S.; Deschodt, M.; Devriendt, E.; Wolthuis, A.; Prenen, H.; Flamaing, J.; Milisen, K.; Wildiers, H.; Kenis, C. The impact of frailty on postoperative outcomes in individuals aged 65 and over undergoing elective surgery for colorectal cancer: A systematic review. J. Geriatr. Oncol. 2016, 7, 479–491. [Google Scholar] [CrossRef] [PubMed]
- McDermott, F.D.; Heeney, A.; Kelly, M.E.; Steele, R.J.; Carlson, G.L.; Winter, D.C. Systematic review of preoperative, intraoperative and postoperative risk factors for colorectal anastomotic leaks. Br. J. Surg. 2015, 102, 462–479. [Google Scholar] [CrossRef]
- Cheng, E.; Ou, F.-S.; Ma, C.; Spiegelman, D.; Zhang, S.; Zhou, X.; Bainter, T.M.; Saltz, L.B.; Niedzwiecki, D.; Mayer, R.J.; et al. Diet- and Lifestyle-Based Prediction Models to Estimate Cancer Recurrence and Death in Patients With Stage III Colon Cancer (CALGB 89803/Alliance). J. Clin. Oncol. 2022, 40, 740–751. [Google Scholar] [CrossRef]
- Gomez, D.; Jimenez-Fonseca, P.; Fernández, A.M.; Castellanos, P.C.; Arbizu, M.V.; Cabañes, R.M.; Estellés, D.L.; Ferreira, E.; del Rio, J.; García, T.G.; et al. Impact of Obesity on Quality of Life, Psychological Distress, and Coping on Patients with Colon Cancer. Oncologist 2021, 26, e874–e882. [Google Scholar] [CrossRef]
- Jeong, S.; Lee, G.; Choi, S.; Kim, K.H.; Chang, J.; Kim, S.M.; Kim, K.; Son, J.S.; Cho, Y.; Park, S.M. Estimating Risk of Cardiovascular Disease Among Long-Term Colorectal Cancer Survivors: A Nationwide Cohort Study. Front. Cardiovasc. Med. 2022, 8, 721107. [Google Scholar] [CrossRef]
- Fulop, A.; Lakatos, L.; Susztak, N.; Szijarto, A.; Banky, B. The effect of trimodal prehabilitation on the physical and psychological health of patients undergoing colorectal surgery: A randomised clinical trial. Anaesthesia 2021, 76, 82–90. [Google Scholar] [CrossRef]
- Suen, M.; Liew, A.; Turner, J.D.; Khatri, S.; Lin, Y.; Raso, K.L.; Vardy, J.L. Short-term multimodal prehabilitation improves functional capacity for colorectal cancer patients prior to surgery. Asia. Pac. J. Clin. Oncol. 2021, 18, e103–e110. [Google Scholar] [CrossRef]
- Shelton, E.; Barreto, N.B.; Bidwell, S.; Folk-Tolbert, M.; Shelton, A.; Trickey, A.W.; Kin, C.J. Engagement and Adherence with a Web-Based Prehabilitation Program for Patients Awaiting Abdominal Colorectal Surgery. J. Gastrointest. Surg. 2021, 25, 3198–3207. [Google Scholar] [CrossRef] [PubMed]
- Minnella, E.M.; Bousquet-Dion, G.; Awasthi, R.; Scheede-Bergdahl, C.; Carli, F. Multimodal prehabilitation improves functional capacity before and after colorectal surgery for cancer: A five-year research experience. Acta Oncol. 2017, 56, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Bull, F.C.; Al-Ansari, S.S.; Biddle, S.; Borodulin, K.; Buman, M.P.; Cardon, G.; Carty, C.; Chaput, J.-P.; Chastin, S.; Chou, R.; et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br. J. Sports Med. 2020, 54, 1451–1462. [Google Scholar] [CrossRef] [PubMed]
- Rock, C.L.; Doyle, C.; Demark-Wahnefried, W.; Meyerhardt, J.; Courneya, K.S.; Schwartz, A.L.; Bandera, E.V.; Hamilton, K.K.; Grant, B.; McCullough, M.; et al. Nutrition and physical activity guidelines for cancer survivors. CA. Cancer J. Clin. 2012, 62, 242–274. [Google Scholar] [CrossRef]
- Mentella, M.C.; Scaldaferri, F.; Ricci, C.; Gasbarrini, A.; Miggiano, G.A.D. Cancer and Mediterranean Diet: A Review. Nutrients 2019, 11, 2059. [Google Scholar] [CrossRef]
- Cheville, A.L.; Mustian, K.; Winters-Stone, K.; Zucker, D.S.; Gamble, G.L.; Alfano, C.M. Cancer Rehabilitation. Phys. Med. Rehabil. Clin. N. Am. 2017, 28, 1–17. [Google Scholar] [CrossRef]
- Frawley, H.C.; Lin, K.-Y.; Granger, C.L.; Higgins, R.; Butler, M.; Denehy, L. An allied health rehabilitation program for patients following surgery for abdomino-pelvic cancer: A feasibility and pilot clinical study. Support. Care Cancer 2020, 28, 1335–1350. [Google Scholar] [CrossRef]
- Mohammed, T.; Parekh, T.; Desai, A. Cardiovascular risk management in cancer survivors: Are we doing it right? World J. Clin. Oncol. 2021, 12, 144–149. [Google Scholar] [CrossRef]
- Carli, F.; Bousquet-Dion, G.; Awasthi, R.; Elsherbini, N.; Liberman, S.; Boutros, M.; Stein, B.; Charlebois, P.; Ghitulescu, G.; Morin, N.; et al. Effect of Multimodal Prehabilitation vs Postoperative Rehabilitation on 30-Day Postoperative Complications for Frail Patients Undergoing Resection of Colorectal Cancer. JAMA Surg. 2020, 155, 233. [Google Scholar] [CrossRef] [PubMed]
- Schulz, K.F.; Grimes, D.; Schulz, K.F.; Grimes, D.A. Generation of allocation sequences in randomised trials: Chance, not choice. Lancet 2002, 359, 515–519. [Google Scholar] [CrossRef]
- Friedberg, J.P.; Lipsitz, S.R.; Natarajan, S. Challenges and recommendations for blinding in behavioral interventions illustrated using a case study of a behavioral intervention to lower blood pressure. Patient Educ. Couns. 2010, 78, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Borrelli, B. The assessment, monitoring, and enhancement of treatment fidelity in public health clinical trials. J. Public Health Dent. 2011, 71, S52–S63. [Google Scholar] [CrossRef] [PubMed]
- Carneiro-Barrera, A.; Amaro-Gahete, F.J.; Díaz-Román, A.; Guillén-Riquelme, A.; Jurado-Fasoli, L.; Sáez-Roca, G.; Martín-Carrasco, C.; Ruiz, J.R.; Buela-Casal, G. Interdisciplinary weight loss and lifestyle intervention for obstructive sleep apnoea in adults: Rationale, design and methodology of the INTERAPNEA study. Nutrients 2019, 11, 2227. [Google Scholar] [CrossRef] [PubMed]
- Prochaska, J.O.; Velicer, W.F. The Transtheoretical Model of Health Behavior Change. Am. J. Health Promot. 1997, 12, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Buchheit, M.; Laursen, P.B. High-intensity interval training, solutions to the programming puzzle. Part II: Anaerobic energy, neuromuscular load and practical applications. Sports Med. 2013, 43, 927–954. [Google Scholar] [CrossRef]
- Buchheit, M.; Laursen, P.B. High-intensity interval training, solutions to the programming puzzle: Part I: Cardiopulmonary emphasis. Sport. Med. 2013, 43, 313–338. [Google Scholar] [CrossRef]
- Gibala, M.J.; Little, J.P.; MacDonald, M.J.; Hawley, J. Physiological adaptations to low-volume, high-intensity interval training in health and disease. J. Physiol. 2012, 590, 1077–1084. [Google Scholar] [CrossRef]
- Hwang, C.L.; Yoo, J.K.; Kim, H.K.; Hwang, M.H.; Handberg, E.M.; Petersen, J.W.; Christou, D. Novel all-extremity high-intensity interval training improves aerobic fitness, cardiac function and insulin resistance in healthy older adults. Exp. Gerontol. 2016, 82, 112–119. [Google Scholar] [CrossRef]
- Amaro-Gahete, F.J.; De-la-O, A.; Jurado-Fasoli, L.; Espuch-Oliver, A.; Robles-Gonzalez, L.; Navarro-Lomas, G.; de Haro, T.; Femia, P.; Castillo, M.J.; Gutierrez, A. Exercise training as S-Klotho protein stimulator in sedentary healthy adults: Rationale, design, and methodology. Contemp. Clin. Trials Commun. 2018, 11, 10–19. [Google Scholar] [CrossRef]
- Haidari, F.; Abiri, B.; Iravani, M.; Razavi, S.-M.; Sarbakhsh, P.; Ahmadi-Angali, K.; Vafa, M. Effects of vitamin D and omega-3 fatty acids co-supplementation on inflammatory biomarkers, tumor marker CEA, and nutritional status in patients with colorectal cancer: A study protocol for a double blind randomized controlled trial. Trials 2019, 20, 682. [Google Scholar] [CrossRef]
- Morishima, T.; Sato, A.; Nakata, K.; Miyashiro, I. Geriatric assessment domains to predict overall survival in older cancer patients: An analysis of functional status, comorbidities, and nutritional status as prognostic factors. Cancer Med. 2020, 9, 5839–5850. [Google Scholar] [CrossRef] [PubMed]
- Tabung, F.K.; Liu, L.; Wang, W.; Fung, T.T.; Wu, K.; Smith-Warner, S.A.; Cao, Y.; Hu, F.B.; Ogino, S.; Fuchs, C.S.; et al. Association of Dietary Inflammatory Potential With Colorectal Cancer Risk in Men and Women. JAMA Oncol. 2018, 4, 366. [Google Scholar] [CrossRef] [PubMed]
- Short, V.; Atkinson, C.; Ness, A.R.; Thomas, S.; Burden, S.; Sutton, E. Patient experiences of perioperative nutrition within an Enhanced Recovery After Surgery programme for colorectal surgery: A qualitative study. Color. Dis. 2016, 18, O74–O80. [Google Scholar] [CrossRef] [PubMed]
- Martínez-González, M.A.; Salas-Salvadó, J.; Estruch, R.; Corella, D.; Fitó, M.; Ros, E. Benefits of the Mediterranean Diet: Insights From the PREDIMED Study. Prog. Cardiovasc. Dis. 2015, 58, 50–60. [Google Scholar] [CrossRef]
- Fuchs, M.A.; Sato, K.; Niedzwiecki, D.; Ye, X.; Saltz, L.B.; Mayer, R.J.; Mowat, R.B.; Whittom, R.; Hantel, A.; Benson, A.; et al. Sugar-Sweetened Beverage Intake and Cancer Recurrence and Survival in CALGB 89803 (Alliance). PLoS ONE 2014, 9, e99816. [Google Scholar] [CrossRef]
- Winkels, R.M.; Heine-Bröring, R.C.; van Zutphen, M.; van Harten-Gerritsen, S.; Kok, D.E.; van Duijnhoven, F.J.; Kampman, E. The COLON study: Colorectal cancer: Longitudinal, Observational study on Nutritional and lifestyle factors that may influence colorectal tumour recurrence, survival and quality of life. BMC Cancer 2014, 14, 374. [Google Scholar] [CrossRef]
- Harvard, T.-H. Healthy Eating Plate; University of Hardvard: Cambridge, MA, USA, 2011. [Google Scholar]
- Carbajal, A. Manual de nutrición y dietética: Dieta en españa. In Consumo de Alimentos; Universidad Complutense de Madrid: Madrid, Spain, 2019. [Google Scholar]
- Metcalfe, J.J.; Leonard, D. The relationship between culinary skills and eating behaviors: Challenges and opportunities for parents and families. Physiol. Behav. 2018, 191, 95–99. [Google Scholar] [CrossRef]
- Gonzalez-Ayora, S.; Pastor, C.; Guadalajara, H.; Ramirez, J.M.; Royo, P.; Redondo, E.; Arroyo, A.; Moya, P.; Garcia-Olmo, D. Enhanced recovery care after colorectal surgery in elderly patients. Compliance and outcomes of a multicenter study from the Spanish working group on ERAS. Int. J. Colorectal Dis. 2016, 31, 1625–1631. [Google Scholar] [CrossRef]
- Miller, T.E.; Roche, A.M.; Mythen, M. Fluid management and goal-directed therapy as an adjunct to Enhanced Recovery After Surgery (ERAS). Can. J. Anesth. Can. D’anesthésie 2015, 62, 158–168. [Google Scholar] [CrossRef]
- Moya, P.; Soriano-Irigaray, L.; Ramirez, J.M.; Garcea, A.; Blasco, O.; Blanco, F.J.; Brugiotti, C.; Miranda, E.; Arroyo, A. Perioperative Standard Oral Nutrition Supplements Versus Immunonutrition in Patients Undergoing Colorectal Resection in an Enhanced Recovery (ERAS) Protocol. Medicine (Baltimore) 2016, 95, e3704. [Google Scholar] [CrossRef]
- Van Rooijen, S.; Carli, F.; Dalton, S.; Thomas, G.; Bojesen, R.; Le Guen, M.; Barizien, N.; Awasthi, R.; Minnella, E.; Beijer, S.; et al. Multimodal prehabilitation in colorectal cancer patients to improve functional capacity and reduce postoperative complications: The first international randomized controlled trial for multimodal prehabilitation. BMC Cancer 2019, 19, 98. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.F.; Ho, J.W.C.; Fong, D.Y.T.; Macfarlane, D.J.; Cerin, E.; Lee, A.M.; Leung, S.; Chan, W.Y.Y.; Leung, I.P.F.; Lam, S.H.S.; et al. Dietary and Physical Activity Interventions for Colorectal Cancer Survivors: A Randomized Controlled Trial. Sci. Rep. 2018, 8, 5731. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.G.A.; Molinger, J.; Wischmeyer, P.E. The malnourished surgery patient. Curr. Opin. Anaesthesiol. 2019, 32, 405–411. [Google Scholar] [CrossRef]
- Gómez Sánchez, M.B.; García-Talavera Espín, N.V.; Sánchez Álvarez, C.; Zomeño Ros, A.I.; Hernández, M.N.; Gómez Ramos, M.J.; Parra Baños, P.; González Valverde, F.M. Perioperative nutritional support in patients with colorectal neoplasms. Nutr. Hosp. 2010, 25, 797–805. [Google Scholar] [PubMed]
- Garth, A.K.; Newsome, C.M.; Simmance, N.; Crowe, T.C. Nutritional status, nutrition practices and post—operative complications in patients with gastrointestinal cancer. J. Hum. Nutr. Diet. 2010, 23, 393–401. [Google Scholar] [CrossRef]
- Uçar, A.; Yilmaz, M.V.; Çakiroglu, F.P. Food Safety—Problems and Solutions. In Significance, Prevention and Control of Food Related Diseases; InTech: Istanbul, Turkey, 2016. [Google Scholar]
- Garrett, W.S. The gut microbiota and colon cancer. Science 2019, 364, 1133–1135. [Google Scholar] [CrossRef]
- Helmink, B.A.; Khan, M.A.W.; Hermann, A.; Gopalakrishnan, V.; Wargo, J.A. The microbiome, cancer, and cancer therapy. Nat. Med. 2019, 25, 377–388. [Google Scholar] [CrossRef]
- Veintimilla, D.R.; Frías Toral, E. Microbiota intestinal y cáncer. Rev. Nutr. Clínica y Metab. 2021, 4, 94–102. [Google Scholar] [CrossRef]
- Ames, B.N.; Wakimoto, P. Are vitamin and mineral deficiencies a major cancer risk? Nat. Rev. Cancer 2002, 2, 694–704. [Google Scholar] [CrossRef]
- Nissensohn, M.; Sánchez-Villegas, A.; Ortega, R.; Aranceta-Bartrina, J.; Gil, Á.; González-Gross, M.; Varela-Moreiras, G.; Serra-Majem, L. Beverage Consumption Habits and Association with Total Water and Energy Intakes in the Spanish Population: Findings of the ANIBES Study. Nutrients 2016, 8, 232. [Google Scholar] [CrossRef]
- Shaukat, A.; Dostal, A.; Menk, J.; Church, T.R. BMI Is a Risk Factor for Colorectal Cancer Mortality. Dig. Dis. Sci. 2017, 62, 2511–2517. [Google Scholar] [CrossRef] [PubMed]
- Nestares, T.; Martín-Masot, R.; Flor-Alemany, M.; Bonavita, A.; Maldonado, J.; Aparicio, V.A. Influence of Ultra-Processed Foods Consumption on Redox Status and Inflammatory Signaling in Young Celiac Patients. Nutrients 2021, 13, 156. [Google Scholar] [CrossRef] [PubMed]
- Fiolet, T.; Srour, B.; Sellem, L.; Kesse-Guyot, E.; Allès, B.; Méjean, C.; Deschasaux, M.; Fassier, P.; Latino-Martel, P.; Beslay, M.; et al. Consumption of ultra-processed foods and cancer risk: Results from NutriNet-Santé prospective cohort. BMJ 2018, 360, k322. [Google Scholar] [CrossRef] [PubMed]
- Burgess, C.; Cornelius, V.; Love, S.; Graham, J.; Richards, M.; Ramirez, A. Depression and anxiety in women with early breast cancer: Five year observational cohort study. BMJ 2005, 330, 702. [Google Scholar] [CrossRef]
- Sheard, T.; Maguire, P. The effect of psychological interventions on anxiety and depression in cancer patients: Results of two meta-analyses. Br. J. Cancer 1999, 80, 1770–1780. [Google Scholar] [CrossRef]
- Dunn, J.; Ng, S.K.; Holland, J.; Aitken, J.; Youl, P.; Baade, P.D.; Chambers, S.K. Trajectories of psychological distress after colorectal cancer. Psychooncology 2013, 22, 1759–1765. [Google Scholar] [CrossRef]
- Wells, K.B.; Stewart, A.; Hays, R.D.; Burnam, M.A.; Rogers, W.; Daniels, M.; Berry, S.; Greenfield, S.; Ware, J. The functioning and well-being of depressed patients. Results from the Medical Outcomes Study. JAMA 1989, 262, 914–919. [Google Scholar] [CrossRef]
- Lynch, B.M.; Steginga, S.K.; Hawkes, A.L.; Pakenham, K.I.; Dunn, J. Describing and predicting psychological distress after colorectal cancer. Cancer 2008, 112, 1363–1370. [Google Scholar] [CrossRef]
- Faller, H.; Schuler, M.; Richard, M.; Heckl, U.; Weis, J.; Küffner, R. Effects of Psycho-Oncologic Interventions on Emotional Distress and Quality of Life in Adult Patients With Cancer: Systematic Review and Meta-Analysis. J. Clin. Oncol. 2013, 31, 782–793. [Google Scholar] [CrossRef]
- Mosher, C.E.; Winger, J.G.; Given, B.A.; Shahda, S.; Helft, P.R. A systematic review of psychosocial interventions for colorectal cancer patients. Support. Care Cancer 2017, 25, 2349–2362. [Google Scholar] [CrossRef]
- Pugliese, P.; Perrone, M.; Nisi, E.; Garufi, C.; Giannarelli, D.; Bottomley, A.; Terzoli, E. An integrated psychological strategy for advanced colorectal cancer patients. Health Qual. Life Outcomes 2006, 4, 9. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, U.O.; Scott, M.J.; Hubner, M.; Nygren, J.; Demartines, N.; Francis, N.; Rockall, T.A.; Young-Fadok, T.M.; Hill, A.G.; Soop, M.; et al. Guidelines for Perioperative Care in Elective Colorectal Surgery: Enhanced Recovery After Surgery (ERAS®) Society Recommendations: 2018. World J. Surg. 2019, 43, 659–695. [Google Scholar] [CrossRef] [PubMed]
- Weimann, A.; Braga, M.; Carli, F.; Higashiguchi, T.; Hübner, M.; Klek, S.; Laviano, A.; Ljungqvist, O.; Lobo, D.N.; Martindale, R.; et al. ESPEN guideline: Clinical nutrition in surgery. Clin. Nutr. 2017, 36, 623–650. [Google Scholar] [CrossRef] [PubMed]
- Slankamenac, K.; Nederlof, N.; Pessaux, P.; de Jonge, J.; Wijnhoven, B.P.L.; Breitenstein, S.; Oberkofler, C.E.; Graf, R.; Puhan, M.A.; Clavien, P.-A. The Comprehensive Complication Index. Ann. Surg. 2014, 260, 757–763. [Google Scholar] [CrossRef]
- Dindo, D.; Demartines, N.; Clavien, P.-A. Classification of Surgical Complications. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef]
- Amaro-Gahete, F.J.; De-la-O, A.; Jurado-Fasoli, L.; Martinez-Tellez, B.; Ruiz, J.R.; Castillo, M.J. Exercise Training as a Treatment for Cardiometabolic Risk in Sedentary Adults: Are Physical Activity Guidelines the Best Way to Improve Cardiometabolic Health? The FIT-AGEING Randomized Controlled Trial. J. Clin. Med. 2019, 8, 2097. [Google Scholar] [CrossRef]
- Kervio, G.; Carre, F.; Ville, N.S. Reliability and Intensity of the Six-Minute Walk Test in Healthy Elderly Subjects. Med. Sci. Sport. Exerc. 2003, 35, 169–174. [Google Scholar] [CrossRef]
- ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS Statement. Am. J. Respir. Crit. Care Med. 2002, 166, 111–117. [Google Scholar] [CrossRef]
- Pecorelli, N.; Fiore, J.F.; Gillis, C.; Awasthi, R.; Mappin-Kasirer, B.; Niculiseanu, P.; Fried, G.M.; Carli, F.; Feldman, L.S. The six-minute walk test as a measure of postoperative recovery after colorectal resection: Further examination of its measurement properties. Surg. Endosc. 2016, 30, 2199–2206. [Google Scholar] [CrossRef]
- Antonescu, I.; Scott, S.; Tran, T.T.; Mayo, N.E.; Feldman, L.S. Measuring postoperative recovery: What are clinically meaningful differences? Surgery 2014, 156, 319–327. [Google Scholar] [CrossRef]
- Studenski, S.; Perera, S.; Patel, K.; Rosano, C.; Faulkner, K.; Inzitari, M.; Brach, J.; Chandler, J.; Cawthon, P.; Connor, E.B.; et al. Gait speed and survival in older adults. JAMA 2011, 305, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Guralnik, J.M.; Ferrucci, L.; Pieper, C.F.; Leveille, S.G.; Markides, K.S.; Ostir, G.V.; Studenski, S.; Berkman, L.F.; Wallace, R.B. Lower extremity function and subsequent disability: Consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J. Gerontol. A. Biol. Sci. Med. Sci. 2000, 55, M221–M231. [Google Scholar] [CrossRef] [PubMed]
- Bruyère, O.; Beaudart, C.; Reginster, J.Y.; Buckinx, F.; Schoene, D.; Hirani, V.; Cooper, C.; Kanis, J.A.; Rizzoli, R.; McCloskey, E.; et al. Assessment of muscle mass, muscle strength and physical performance in clinical practice: An international survey. Eur. Geriatr. Med. 2016, 7, 243–246. [Google Scholar] [CrossRef]
- Cesari, M.; Kritchevsky, S.B.; Newman, A.B.; Simonsick, E.M.; Harris, T.B.; Penninx, B.W.; Brach, J.S.; Tylavsky, F.A.; Satterfield, S.; Bauer, D.C.; et al. Added Value of Physical Performance Measures in Predicting Adverse Health-Related Events: Results from the Health, Aging and Body Composition Study. J. Am. Geriatr. Soc. 2009, 57, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Ruiz-Ruiz, J.; Mesa, J.L.M.; Gutiérrez, A.; Castillo, M.J. Hand size influences optimal grip span in women but not in men. J. Hand Surg. Am. 2002, 27, 897–901. [Google Scholar] [CrossRef]
- Beaudart, C.; McCloskey, E.; Bruyère, O.; Cesari, M.; Rolland, Y.; Rizzoli, R.; Araujo de Carvalho, I.; Amuthavalli Thiyagarajan, J.; Bautmans, I.; Bertière, M.-C.; et al. Sarcopenia in daily practice: Assessment and management. BMC Geriatr. 2016, 16, 170. [Google Scholar] [CrossRef]
- Alcazar, J.; Kamper, R.S.; Aagaard, P.; Haddock, B.; Prescott, E.; Ara, I.; Suetta, C. Relation between leg extension power and 30-s sit-to-stand muscle power in older adults: Validation and translation to functional performance. Sci. Rep. 2020, 10, 16337. [Google Scholar] [CrossRef]
- Arraras, J.I.; Suárez, J.; Arias de la Vega, F.; Vera, R.; Asín, G.; Arrazubi, V.; Rico, M.; Teijeira, L.; Azparren, J. The EORTC quality of life questionnaire for patients with colorectal cancer: EORTC QLQ-CR29 validation study for Spanish patients. Clin. Transl. Oncol. 2011, 13, 50–56. [Google Scholar] [CrossRef]
- Wang, Y.; Gorenstein, C. Assessment of depression in medical patients: A systematic review of the utility of the Beck Depression Inventory-II. Clinics 2013, 68, 1274–1287. [Google Scholar] [CrossRef]
- Buela-Casal, G.; Guillén-Riquelme, A.; Seisdedos Cubero, N. Cuestionario de Ansiedad Estado-Rasgo: Adaptación Española; TEA Edicio: Madrid, Spain, 2011. [Google Scholar]
- López-Roig, S.; Terol, M.C.; Pastor, M.A.; Neipp, M.C.; Massutí, B. Ansiedad y depresión. Validación de la escala HAD en pacientes oncológicos. Rev. Psicol. Salud 2000, 12, 127–155. [Google Scholar] [CrossRef]
- Berlanga, J.F.; Aliaga, M.T.; Martín, M.P.B. Evaluación cognitiva y afrontamiento como predictores del bienestar futuro de las pacientes con cáncer de mama. Rev. Latinoam. Psicol. 1995, 27, 87–102. [Google Scholar]
- Marfell-Jones, M.J.; Stewart, A.D.; de Ridder, J.H. International Standards for Anthropometric Assessment; International Society for the Advancement of Kinanthropometry: Wellington, New Zealand, 2012. [Google Scholar]
- Whelton, P.K.; Williams, B. The 2018 European Society of Cardiology/European Society of Hypertension and 2017 American College of Cardiology/American Heart Association Blood Pressure Guidelines. JAMA 2018, 320, 1749. [Google Scholar] [CrossRef] [PubMed]
- Schootman, M.; Jeffe, D.B.; Ratnapradipa, K.L.; Eberth, J.M.; Davidson, N.O. Increased 30-Day Mortality Risk in Patients With Diabetes Mellitus After Colon Cancer Surgery: A Mediation Analysis. Dis. Colon Rectum 2020, 63, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.; Pereira, S.S.; Monteiro, M.P.; Araújo, A.; Faria, G. Effect of Metabolic Syndrome and Individual Components on Colon Cancer Characteristics and Prognosis. Front. Oncol. 2021, 11, 631257. [Google Scholar] [CrossRef]
- Chen, W.; Wang, M.; Jing, X.; Wu, C.; Zeng, Y.; Peng, J.; Cai, X. High risk of colorectal polyps in men with non-alcoholic fatty liver disease: A systematic review and meta-analysis. J. Gastroenterol. Hepatol. 2020, 35, 2051–2065. [Google Scholar] [CrossRef]
- Wang, S.C.; Schulman-Marcus, J.; Fantauzzi, J.; Bevington, T.; Sayegh, A.; Lee, E.; Ata, A.; Kambam, M.; Sidhu, M.; Lyubarova, R. Colon cancer laterality is associated with atherosclerosis and coronary artery disease. J. Gastrointest. Oncol. 2018, 10, 30–36. [Google Scholar] [CrossRef]
- Ascaso, J.F.; Romero, P.; Real, J.T.; Priego, A.; Valdecabres, C.; Carmena, R. Insulin resistance quantification by fasting insulin plasma values and HOMA index in a non-diabetic population. Med. Clin. 2001, 117, 530–533. [Google Scholar] [CrossRef]
- Bedogni, G.; Bellentani, S.; Miglioli, L.; Masutti, F.; Passalacqua, M.; Castiglione, A.; Tiribelli, C. The Fatty Liver Index: A simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006, 6, 33. [Google Scholar] [CrossRef]
- del Río-Moreno, M.; Luque, R.M.; Rangel-Zúñiga, O.A.; Alors-Pérez, E.; Alcalá-Diaz, J.F.; Roncero-Ramos, I.; Camargo, A.; Gahete, M.D.; López-Miranda, J.; Castaño, J.P. Dietary Intervention Modulates the Expression of Splicing Machinery in Cardiovascular Patients at High Risk of Type 2 Diabetes Development: From the CORDIOPREV Study. Nutrients 2020, 12, 3528. [Google Scholar] [CrossRef]
- Gahete, M.D.; del Rio-Moreno, M.; Camargo, A.; Alcala-Diaz, J.F.; Alors-Perez, E.; Delgado-Lista, J.; Reyes, O.; Ventura, S.; Perez-Martínez, P.; Castaño, J.P.; et al. Changes in Splicing Machinery Components Influence, Precede, and Early Predict the Development of Type 2 Diabetes: From the CORDIOPREV Study. EBioMedicine 2018, 37, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Gahete, M.D.; Luque, R.M.; Yubero-Serrano, E.M.; Cruz-Teno, C.; Ibañez-Costa, A.; Delgado-Lista, J.; Gracia-Navarro, F.; Perez-Jimenez, F.; Castaño, J.P.; Lopez-Miranda, J. Dietary fat alters the expression of cortistatin and ghrelin systems in the PBMCs of elderly subjects: Putative implications in the postprandial inflammatory response. Mol. Nutr. Food Res. 2014, 58, 1897–1906. [Google Scholar] [CrossRef] [PubMed]
- López-Cánovas, J.L.; del Rio-Moreno, M.; García-Fernandez, H.; Jiménez-Vacas, J.M.; Moreno-Montilla, M.T.; Sánchez-Frias, M.E.; Amado, V.; López, L.F.; Fondevila, M.F.; Ciria, R.; et al. Splicing factor SF3B1 is overexpressed and implicated in the aggressiveness and survival of hepatocellular carcinoma. Cancer Lett. 2021, 496, 72–83. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Vacas, J.M.; Herrero-Aguayo, V.; Montero-Hidalgo, A.J.; Gómez-Gómez, E.; Fuentes-Fayos, A.C.; León-González, A.J.; Sáez-Martínez, P.; Alors-Pérez, E.; Pedraza-Arévalo, S.; González-Serrano, T.; et al. Dysregulation of the splicing machinery is directly associated to aggressiveness of prostate cancer. EBioMedicine 2020, 51, 102547. [Google Scholar] [CrossRef]
- Fuentes-Fayos, A.C.; Vázquez-Borrego, M.C.; Jiménez-Vacas, J.M.; Bejarano, L.; Pedraza-Arévalo, S.; López, L.F.; Blanco-Acevedo, C.; Sánchez-Sánchez, R.; Reyes, O.; Ventura, S.; et al. Splicing machinery dysregulation drives glioblastoma development/aggressiveness: Oncogenic role of SRSF3. Brain 2020, 143, 3273–3293. [Google Scholar] [CrossRef]
- del Río-Moreno, M.; Alors-Pérez, E.; González-Rubio, S.; Ferrín, G.; Reyes, O.; Rodríguez-Perálvarez, M.; Sánchez-Frías, M.E.; Sánchez-Sánchez, R.; Ventura, S.; López-Miranda, J.; et al. Dysregulation of the Splicing Machinery Is Associated to the Development of Nonalcoholic Fatty Liver Disease. J. Clin. Endocrinol. Metab. 2019, 104, 3389–3402. [Google Scholar] [CrossRef] [PubMed]
- Roman-Viñas, B.; Serra-Majem, L.; Hagströmer, M.; Ribas-Barba, L.; Sjöström, M.; Segura-Cardona, R. International Physical Activity Questionnaire: Reliability and validity in a Spanish population. Eur. J. Sport Sci. 2010, 10, 297–304. [Google Scholar] [CrossRef]
- Kim, Y.; Park, I.; Kang, M. Convergent validity of the International Physical Activity Questionnaire (IPAQ): Meta-analysis. Public Health Nutr. 2013, 16, 440–452. [Google Scholar] [CrossRef]
- Mataix, J.L.; Martinez de Victoria, E.; Montellano, M.; Lopez, M.; Aranda, P.L. Valoración del estado nutricional de la comunidad autónoma de Andalucía. Cons Salud. 2000. Available online: https://www.repositoriosalud.es/bitstream/10668/1215/5/ValoracionNutricional_2000.pdf (accessed on 2 October 2022).
- López, M.D.R.; Martín-Lagos, R.A.; Martin-Lagos, R.A. Guía Para Estudios Dietéticos: Álbum Fotográfico de Alimentos; Editorial Universidad de Granada: Granada, Spain, 2010; ISBN 8433851675. [Google Scholar]
- Zaragoza-Martí, A.; Cabañero-Martínez, M.; Hurtado-Sánchez, J.; Laguna-Pérez, A.; Ferrer-Cascales, R. Evaluation of Mediterranean diet adherence scores: A systematic review. BMJ Open 2018, 8, e019033. [Google Scholar] [CrossRef]
- Becoña, E.; Alvarez-Soto, E.; Gómez-Durán, B.; García, M.P. Scores of Spanish Smokers on Fagerström’s Tolerance Questionnaire. Psychol. Rep. 1992, 71, 1227–1233. [Google Scholar] [CrossRef]
- Buysse, D.J.; Reynolds, C.F.; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Ternes, D.; Karta, J.; Tsenkova, M.; Wilmes, P.; Haan, S.; Letellier, E. Microbiome in Colorectal Cancer: How to Get from Meta-omics to Mechanism? Trends Microbiol. 2020, 28, 401–423. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.H.; Yu, J. Gut microbiota in colorectal cancer: Mechanisms of action and clinical applications. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 690–704. [Google Scholar] [CrossRef]
- Sánchez-Alcoholado, L.; Ramos-Molina, B.; Otero, A.; Laborda-Illanes, A.; Ordóñez, R.; Medina, J.A.; Gómez-Millán, J.; Queipo-Ortuño, M.I. The Role of the Gut Microbiome in Colorectal Cancer Development and Therapy Response. Cancers 2020, 12, 1406. [Google Scholar] [CrossRef] [PubMed]
- Herlemann, D.; Labrenz, M.; Jü Rgens, K.; Bertilsson, S.; Waniek, J.J.; Andersson, A.F. Transitions in bacterial communities along the 2000 km salinity gradient of the Baltic Sea. ISME J. 2011, 5, 1571–1579. [Google Scholar] [CrossRef]
- Lozupone, C.A.; Knight, R. Species divergence and the measurement of microbial diversity. FEMS Microbiol. Rev. 2008, 32, 557–578. [Google Scholar] [CrossRef]
- Kim, B.-R.; Shin, J.; Guevarra, R.B.; Lee, J.H.; Kim, D.W.; Seol, K.-H.; Lee, J.-H.; Kim, H.B.; Isaacson, R.E. Deciphering Diversity Indices for a Better Understanding of Microbial Communities. J. Microbiol. Biotechnol 2017, 27, 2089–2093. [Google Scholar] [CrossRef]
- Simpson, E.H. Measurement of diversity. Nature 1949, 163, 688. [Google Scholar] [CrossRef]
- Aßhauer, K.P.; Wemheuer, B.; Daniel, R.; Meinicke, P. Tax4Fun: Predicting functional profiles from metagenomic 16S rRNA data. Bioinformatics 2015, 31, 2882–2884. [Google Scholar] [CrossRef]
- Hlatky, M.A.; Owens, D.K.; Sanders, G.D. Cost-effectiveness as an outcome in randomized clinical trials. Clin. Trials 2006, 3, 543–551. [Google Scholar] [CrossRef]
- Ramos-Goñi, J.M.; Craig, B.M.; Oppe, M.; Ramallo-Fariña, Y.; Pinto-Prades, J.L.; Luo, N.; Rivero-Arias, O. Handling Data Quality Issues to Estimate the Spanish EQ-5D-5L Value Set Using a Hybrid Interval Regression Approach. Value Health 2018, 21, 596–604. [Google Scholar] [CrossRef] [PubMed]
- Herdman, M.; Gudex, C.; Lloyd, A.; Janssen, M.; Kind, P.; Parkin, D.; Bonsel, G.; Badia, X. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual. Life Res. 2011, 20, 1727–1736. [Google Scholar] [CrossRef] [PubMed]
- Edejer, T.T.T.; Baltussen, R.; Adam, T.; Hutubessy, R.; Acharya, A.; Evans, D.B.; Murray, C.J.L. Making Choices in Health: WHO Guide to Cost-Effectiveness Analysis; World Health Organization: Geneva, Switzerland, 2003. [Google Scholar]
- Onerup, A.; Angenete, E.; Bonfre, P.; Börjesson, M.; Haglind, E.; Wessman, C.; Nilsson, H. Self-assessed preoperative level of habitual physical activity predicted postoperative complications after colorectal cancer surgery: A prospective observational cohort study. Eur. J. Surg. Oncol. 2019, 45, 2045–2051. [Google Scholar] [CrossRef] [PubMed]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 406–5823. [Google Scholar] [CrossRef]
- Orangio, G.R. The Economics of Colon Cancer. Surg. Oncol. Clin. N. Am. 2018, 27, 327–347. [Google Scholar] [CrossRef]
- Zadlo, J. Cost-effectiveness of new and emerging treatment options for the treatment of metastatic colorectal cancer. Am. J. Manag. Care 2018, 24, S118–S124. [Google Scholar]
- Rezende, L.F.M.; Ferrari, G.; Bahia, L.R.; Rosa, R.D.S.; da Rosa, M.Q.M.; de Souza, R.C.; Lee, D.H.; Giovannucci, E.; Eluf-Neto, J. Economic burden of colorectal and breast cancers attributable to lack of physical activity in Brazil. BMC Public Health 2021, 21, 1190. [Google Scholar] [CrossRef]

| Inclusion Criteria | Exclusion Criteria |
|---|---|
|
|
|
|
|
|
|
|
|
|
|
|
| Outcome | Measurement | Assessment |
|---|---|---|
| Sociodemographic data and medical history | ||
| Anamnesis | Week 0 | |
| Physical exploration | Week 0 | |
| Sociodemographic interview | Week 0 | |
| Surgery-derived events | ||
| Post-surgery complications | Week 17 and 57 | |
| Hospital length of stay | Week 17 and 57 | |
| Readmissions | Week 17 and 57 | |
| Emergency department appointments | Week 17 and 57 | |
| Functional capacity | ||
| Cardiorespiratory fitness | 6 min walking test | Week 0, 5, 17 and 57 |
| Gait speed | 4 min usual walking speed test | Week 0, 5, 17 and 57 |
| Muscular strength | Handgrip strength | Week 0, 5, 17 and 57 |
| 5-times sit-to-stand test | Week 0, 5, 17 and 57 | |
| 30 s sit-to-stand muscle power | Week 0, 5, 17 and 57 | |
| Subjective physical fitness | International fitness scale | Week 0, 5, 17 and 57 |
| Patients-reported outcome measures | ||
| Health-related quality of life | EORTC QLQ-C30 | Week 0, 5, 17 and 57 |
| Depression symptoms | Beck Depression Inventory-II | Week 0, 5, 17 and 57 |
| Anxiety symptoms | State-Trait Anxiety Inventory | Week 0, 5, 17 and 57 |
| Hospital Anxiety and Depression Scale | Week 0, 5, 17 and 57 | |
| Mental adjustment to cancer | Mini--Mental Adjustment to Cancer | Week 0, 5, 17 and 57 |
| Anthropometry and body composition | ||
| Anthropometry | Weight and height measurement, and neck, waist, and hip circumferences | Week 0, 5, 17 and 57 |
| Body composition | Dual Energy X-ray Absorptiometry | Week 0, 5, 17 and 57 |
| Clinical/tumor parameters | ||
| Blood parameters | Glycemic profile, lipid profile, hepatic transaminases, blood cell profile, and renal function profile | Week 0, 5, 17 and 57 |
| Clinical characterization | Blood pressure, homeostatic model assessment of insulin resistance index (HOMA), fatty liver index (FLI) and the cardiometabolic risk score | Week 0, 5, 17 and 57 |
| Tumor biomarkers | Genetic and molecular biomarkers | Week 0, 5, 17 and 57 |
| Circulatory biomarkers | Inflammatory factors, immunological blood profiles, and hormones | Week 0, 5, 17 and 57 |
| Physical activity and sedentariness | ||
| Physical activity habits | International Physical Activity Questionnaire | Week 0, 5, 17 and 57 |
| Dietary habits | ||
| Food frequency questionnaire | Week 0, 5, 17 and 57 | |
| Mediterranean Diet Adherence | Mediterranean Diet Score | Week 0, 5, 17 and 57 |
| Others unhealthy habits | ||
| Tobacco dependence | The Fagerstrom Test for Nicotine Dependence | Week 0, 5, 17 and 57 |
| Tobacco consumption | Self-reported tobacco consumption logs | Week 0, 5, 17 and 57 |
| Alcohol consumption | Self-reported alcohol consumption logs | Week 0, 5, 17 and 57 |
| Sleep quality | ||
| Pittsburgh Sleep Quality Index | Week 0, 5, 17 and 57 | |
| Fecal microbiota | Week 0, 5, 17 and 57 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amaro-Gahete, F.J.; Jurado, J.; Cisneros, A.; Corres, P.; Marmol-Perez, A.; Osuna-Prieto, F.J.; Fernández-Escabias, M.; Salcedo, E.; Hermán-Sánchez, N.; Gahete, M.D.; et al. Multidisciplinary Prehabilitation and Postoperative Rehabilitation for Avoiding Complications in Patients Undergoing Resection of Colon Cancer: Rationale, Design, and Methodology of the ONCOFIT Study. Nutrients 2022, 14, 4647. https://doi.org/10.3390/nu14214647
Amaro-Gahete FJ, Jurado J, Cisneros A, Corres P, Marmol-Perez A, Osuna-Prieto FJ, Fernández-Escabias M, Salcedo E, Hermán-Sánchez N, Gahete MD, et al. Multidisciplinary Prehabilitation and Postoperative Rehabilitation for Avoiding Complications in Patients Undergoing Resection of Colon Cancer: Rationale, Design, and Methodology of the ONCOFIT Study. Nutrients. 2022; 14(21):4647. https://doi.org/10.3390/nu14214647
Chicago/Turabian StyleAmaro-Gahete, Francisco J., Javier Jurado, Andrea Cisneros, Pablo Corres, Andres Marmol-Perez, Francisco J. Osuna-Prieto, Manuel Fernández-Escabias, Estela Salcedo, Natalia Hermán-Sánchez, Manuel D. Gahete, and et al. 2022. "Multidisciplinary Prehabilitation and Postoperative Rehabilitation for Avoiding Complications in Patients Undergoing Resection of Colon Cancer: Rationale, Design, and Methodology of the ONCOFIT Study" Nutrients 14, no. 21: 4647. https://doi.org/10.3390/nu14214647
APA StyleAmaro-Gahete, F. J., Jurado, J., Cisneros, A., Corres, P., Marmol-Perez, A., Osuna-Prieto, F. J., Fernández-Escabias, M., Salcedo, E., Hermán-Sánchez, N., Gahete, M. D., Aparicio, V. A., González-Callejas, C., Mirón Pozo, B., R. Ruiz, J., Nestares, T., & Carneiro-Barrera, A. (2022). Multidisciplinary Prehabilitation and Postoperative Rehabilitation for Avoiding Complications in Patients Undergoing Resection of Colon Cancer: Rationale, Design, and Methodology of the ONCOFIT Study. Nutrients, 14(21), 4647. https://doi.org/10.3390/nu14214647









