Abstract
Background: Paradoxically epidemiological data illustrate a negative relationship between dietary folate intake and colorectal cancer (CRC) risk. The occurrence and progression of CRC may be influenced by variants in some key enzyme coding genes in the folate metabolic pathway. We investigated the correlation between genetic variants in methionine synthase reductase (MTRR) and methylenetetrahydrofolate reductase (MTHFR) and CRC survival. Methods: This study used data collected from the Newfoundland Familial Colorectal Cancer Study. A total of 532 patients diagnosed with CRC for the first time from 1999 to 2003 were enrolled, and their mortality were tracked until April 2010. DNA samples were genotyped by Illumina’s integrated quantum 1 million chip. Cox models were established to assess 33 tag single-nucleotide polymorphisms in MTRR and MTHFR in relation to overall survival (OS), disease-free survival (DFS) and CRC-specific survival. Results: The MTRR and MTHFR genes were associated with DFS and CRC-specific survival in CRC patients at the gene level. After multiple comparison adjustment, MTRR rs1801394 A (vs. G) allele was associated with increased DFS (p = 0.024), while MTHRT rs3737966 (G vs. A), rs4846049 (T vs. G), rs1476413 (A vs. G), rs1801131 (C vs. A), rs12121543 (A vs. C), rs1801133 (C vs. T), rs4846052 (T vs. C), rs2066471 (A vs. G) and rs7533315 (T vs. C) were related to worse CRC-specific survival. Additionally, significant interactions were seen among pre-diagnostic alcohol consumption with MTRR rs1801394, rs3776467, rs326124, rs162040, and rs3776455, with superior OS associated with those protective variant alleles limited to patients with alcohol consumption under the median. The MTHFR rs3737966 (G vs. A) allele seemed to be detrimental to CRC survival only among subjects with fruit intake below the median. Conclusions: Polymorphic variants in MTRR and MTHFR genes that code for key enzymes for folate metabolism may be associated with survival in patients with CRC. The gene-CRC outcome association seems modulated by alcohol drinking and fruit intake.
1. Introduction
Worldwide, more than 1.9 million new cases of colorectal cancer (CRC) were reported in 2020, with a 50% death rate, making CRC the third most common malignancy in adults [1]. Folate is an essential B vitamin involved in DNA methylation, synthesis, cellular growth, and repair, aberrations of which have been implicated in various neoplasms [2,3], the most frequently reported being CRC [4,5,6]. Paradoxically epidemiological evidence suggests a dual role of folate in CRC in which moderate dietary increases before the establishment of neoplastic foci suppress the development of tumors in normal tissues, whereas supraphysiologic doses of supplementation once early lesions are established enhances tumorigenesis [7].
Polymorphisms in the genes encoding folate metabolism enzymes add an additional layer to the complexity of the association between folate and CRC. Methylenetetrahydrofolate reductase (MTHFR) and methionine synthase reductase (MTRR) are key enzymes in homocysteine and folate metabolic pathways [8]. Two common studied single nucleotide polymorphisms (SNP) of MTHFR are C677T (Ala222Val, rs1801133) and A1298C (Glu429Ala, rs1801131); both mutations variably reduce MTHFR enzyme activity [9], resulting in elevated levels of 5,10-methylenetetrahydrofolate and thymidine, thereby triggering an increase in DNA synthesis and repair [10]. Most studies [11,12,13,14,15,16,17], but not all [18,19], reported protective effects of the C677T and A1298C mutations against CRC, with odds ratios (ORs) ranging from 0.54 to 0.80 [11,12,13,14] and 0.6 to 0.8 [15,16,17], respectively, whereas studies of the A66G (rs1801394) polymorphism of MTRR noted its detrimental influence, with an increase in CRC risk for GG homozygotes compared to AA homozygotes [20,21,22,23,24].
In contrast to CRC risk, the prognosis value of MTHFR and MTRR mutations has been examined in minimal research [25,26,27], one of which were conducted by members of our team [27]. The findings from these studies have generally been inconsistent. Some studies have demonstrated null association [25] or poorer survival rate related to the MTHFR A1298C CA/CC mutation than the wild AA genotype [28]. Others, including our previous analysis based on the Newfoundland Familial Colorectal Cancer Study (hazard ratio (HR) = 1.72) [27] and a recent meta-analysis (HR = 1.85) [26], have reported a shorter overall survival in patients with MTHFR A1298C CC genotype compared to those with CA/AA genotypes. No relationship between MTHFR C677T polymorphism and CRC prognosis has been found [26,27]. However, previous research on MTHFR and MTRR has focused on limited candidate SNPs for their relevance to CRC prognosis, including our prior analysis in which only two candidate SNPs in MTHFR (i.e., C677T and A1298C) were evaluated; none have used a gene-wide tag SNP panels that cover the majority of all common variations in the MTHFR and MTRR genes to detect risk alleles that are associated with CRC survival. Additionally, the interactions occurring between genetics and dietary factors are seldom assessed.
Starting from our preliminary results that evidenced an association between MTHFR A1298C and CRC prognosis, the present study as an outgrowth further examined allelic variations in MTHFR and MTRR in relation to CRC survival using a gene-wide tag SNP approach. Effect modifications by dietary intakes of B vitamins, alcohol, and fruits were further explored.
2. Materials and Methods
2.1. Study Population
The study participants were CRC patients drawn from the Newfoundland Colorectal Cancer Registry, a resource for studies on genetic and environmental risk factors of CRC that was initiated in 1999 and is described in detail elsewhere [29,30,31]. In brief, population-based case patients with a newly diagnosed CRC from 1999 to 2003 and aged 20–75 years were eligible for inclusion. From the Newfoundland Colorectal Cancer Registry, a total of 1126 eligible patients were identified; of these, 737 consenting patients completed and returned detailed epidemiological questionnaires. A germline DNA sample and disease outcome data were available on 532 confirmed CRC patients. Informed consent was obtained from all participants. Ethical approval for this study was obtained from the Research Ethics Board of the Memorial University of Newfoundland (No. 40001511).
2.2. Baseline Information
Data on demographics (e.g., age, sex and race), living habits (e.g., smoke, drink status and exercise), medical history, and personal and familial history of cancer were collected from each subject through a self-administered family history questionnaires and a personal history questionnaire (the median time from date of diagnosis to study enrollment was 1.8 years). Dietary intake from one year prior to diagnosis was collected via a 169-item food frequency questionnaire, which had previously been validated in the Newfoundland population [32]. The average nutrient intakes were calculated by multiplying the frequencies of consumption of each item by the nutrient content per unit.
2.3. Study Outcomes
Participants in this study were followed from cancer diagnosis until April 2010. The main outcomes were overall survival (OS), defined as the time from CRC diagnosis until all-cause death; disease-free survival (DFS), defined as time from CRC diagnosis until death from any causes, CRC recurrence or metastasis, whichever came first; and CRC-specific survival, measured from the date of cancer diagnosis to death from CRC. Patients alive and free of all these events at the end of study were censored at the date of last contact.
2.4. SNP Genotyping and Selection
Genotyping was performed on DNA from peripheral blood via the Illumina Human Omni-Quad Bead chip that contains about 1.1 million SNPs at Centrillion Biosciences (Palo Alto, CA, USA). For quality control purpose, 200 duplicates were genotyped using the Affymetrix Axiom my Design GW Array Plate (Thermo Fisher Scientific, Waltham, MA, USA), which contains 1.3 million probes. SNPs with genotyping concordance smaller than 97% were removed from the analysis.
We used a SNP tagging approach to minimize the number of SNPs to be examined. A subset of SNPs (tag SNPs) capturing most of the common variation in the MTRR and MTHFR genes were selected using Plink v1.07 (http://www.cog-genomics.org/plink/1.9/ accessed 20 March 2022) according to following criteria: the minor allele frequency of SNP ≥ 5%; pairwise r2 > 0.9; and distance from any adjacent SNPs greater than 50 base pairs [33]. We selected 17 SNPs in the MTRR gene and 16 SNPs in the MTHFR gene. Several hotspot SNPs reported in previous research were additionally included (i.e., MTRR rs1801394, MTHFR rs1801131 and MTHFR rs1801133. Genotype distributions of all SNP were in line with the expected Hardy–Weinberg equilibrium.
2.5. Statistical Analysis
Comparisons of baseline variables between groups were performed with Log-Rank test. Further analysis was stratified by anatomical site of cancer.
We used a principal component analysis accounting for linkage disequilibrium (LD) among SNPs to examine overall association of a gene with CRC survival [34]. Uncorrelated linear combinations of original SNPs that explain the greatest amount of variance in the entire gene were calculated. The number of principal components retained in the Cox model was determined by 80% explained-variance threshold. A global p-value for an overall association between gene and CRC survival was computed via a likelihood ratio test comparing models with and without principal components with degrees of freedom equal to the total number of principal components.
Cox proportional hazard models estimated the HRs and 95% confidence intervals (CIs) for the association between individual SNPs and disease outcomes. Covariates were selected using the stepwise approach based on a p value less than 0.05. The final models adjusted for sex, race, age at diagnosis, disease stage at diagnosis, marital status, microsatellite instability (MSI) status, alcohol drinking and folate intake. p values for all SNPs were adjusted for multiple comparisons accounting for correlated SNPs with a modified test of Conneely and Boehnke [35].
For haplotype analysis, haplotype blocks and LD plots were generated by Haploview v4.2 (https://www.broadinstitute.org/haploview/downloads accessed 10 April 2022). Haplotypes for individual study subjects were assembled a “best” reconstruction from the genotyped data by PHASE v2.1 program [36]. The relationship between individual haplotypes and CRC survival was assessed by modeling all haplotypes simultaneously using the most frequent haplotype as the reference. The global p-value was computed for each haplotype block with a likelihood ratio test. Haplotypes with a frequency less than 0.01 and their subjects were removed from the analysis. Bonferroni correction for multiple comparisons was performed in haplotype analysis where individual tests each used a p value threshold of 0.05/adjusted p value = 0.0016.
We estimated stratum-specific HRs to evaluate potential effect modification of genetic variants by dietary factors in CRC survival. Heterogeneity of the HRs by dietary intake was evaluated by way of a two-sided Wald test for regression models that included interaction terms between SNPs and dietary factors. All analyses were performed using SAS v9.4 (SAS Institute, Cary, NC, USA) and RStudio (R Foundation for Statistical Computing, Vienna, Austria), and figures were produced with Graphpad Prism 9.0 (GraphPad Software, San Diego, CA, USA).
3. Results
3.1. Patient Baseline Characteristics
Of the 532 CRC patients enrolled in the current study, 330 (62.03%) were men, and 440 (96.92%) were from white ethnic groups (Table 1). The mean age at study enrollment among participants was 60.06 (±9.23) years. Three hundred and three (56.96%) CRC patients were diagnosed at an early stage (stage I/II), and the majority (65.83%) had cancers at colon subsite. Of the 504 patients with MSI information, 446 (88.49%) were classified as microsatellite stable or microsatellite instability-low. During follow-up (median follow-up time, 6.4 years), 183 patients died from all causes; 213 patients died from any cause or experienced a cancer recurrence or metastasis; and 94 patients died from CRC. Log-rank test indicated that sex, race, stage at diagnosis, MSI status and red meat intakes were significantly associated with overall survival.

Table 1.
Baseline characteristics of patients in the Newfoundland Familial Colorectal Cancer Study (NFCCS).
3.2. Gene-Level Association with CRC Survival
Principal component analysis was applied to assess the overall gene-level association of MTRR and MTHFR with survival among CRC patients (Table 2). Although MTRR was not related to survival of CRC patients as a whole, significant site-specific associations were observed for the MTRR gene with DFS (Global p = 0.015) and CRC-specific survival (Global p = 0.025) among colon cancer patients. With respect to MTHFR, gene-level association was seen for CRC-specific survival in total CRC patients (Global p = 0.0005) and colonic cancer patients (Global p = 0.0004).

Table 2.
Associations between MTRR and MTHFR genes and colorectal cancer overall, disease-free and CRC-specific survival.
3.3. Single SNPs and CRC Survival
The associations between individual SNPs within each gene for any, colon, and rectal cancers were examined using additive models for each SNP. Of the 17 SNPs in the MTRR gene, the rs1801394 homozygous mutant GG genotype was associated with decreased DFS in colon cancer patients, even after the adjustment for multiple comparisons (A vs. G allele, HR = 0.60, 95%CI: 0.44, 0.81) (Supplementary Table S1 and Figure 1C). Of the 16 SNPs in the MTHFR gene, 8 SNPs specifically, rs3737966 (G vs. A allele), rs4846049 (T vs. G allele), rs1476413 (A vs. G allele), rs1801131 (C vs. A allele), rs12121543 (A vs. C allele), rs4846052 (T vs. C allele), rs2066471 (A vs. G allele), and rs7533315 (T vs. C allele), were correlated with decreased CRC-specific survival in all cases (Supplementary Table S2 and Figure 1A). Further, 7 SNPs, including the hotspot SNP in prior research, rs1801133 (Figure 1B), demonstrated some suggestion of association with colon cancer-specific mortality.
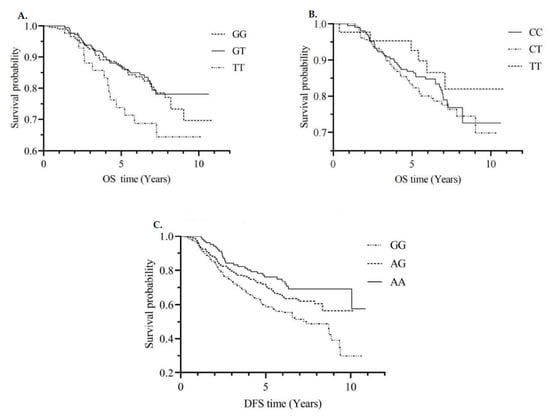
Figure 1.
CRC-specific survival curves by MTHFR rs4846049 genotype (A); CRC-specific survival curves by MTHFR rs1801133 genotype (B); DFS curves by MTRR rs1801394 genotype (C). Abbreviations: CRC = colorectal cancer; MTHFR = methylenetetrahydrofolate reductase; OS = overall survival; DFS = disease-free survival. GG, GT and TT; CC, CT and TT; GG, AG and AA are genotypes of SNPs.
3.4. Associations between Haplotypes and CRC Survival
To detect possible epistatic effects, we constructed haplotype blocks formed by the markers that are in disequilibrium. A total of three major haplotype blocks on MTRR and four major blocks on MTHFR were identified (Figure 2, Supplementary Materials Figure S1). In the MTRR gene, a G-C haplotype in LD block 1 (rs1801394, rs13181011) was associated with a poorer OS (HR = 1.63, 95%CI: 1.08–2.48), while the haplotypes designated G-T and G-C (rs1801394, rs13181011) were associated with a reduced DFS (HR = 1.44, 95%CI: 1.04–2.01, HR = 1.68, 95%CI: 1.14–2.46, Global p = 0.038) for colon cancer patients. For the MTHFR gene, a G-A haplotype (rs4846048, rs2184226) led to worse OS in colon cancer (HR = 1.45, 95%CI: 1.01–2.06). Patients with the haplotype T-G (rs4846049 and rs1476413) were more likely to experience a reduction in OS for colon cancer patients (HR = 1.71, 95%CI: 1.00–2.91) and CRC-specific survival (HR = 2.73, 95%CI: 1.30–5.74). A C-A haplotype in block 3 (rs1801131 and rs12121543) of MTHFR was related to an increased odds of all-cause death of colon cancer (HR = 1.50, 95%CI: 1.05–2.13) and CRC-specific death (HR = 1.54, 95%CI: 1.01–2.35), while a C-C haplotype (rs1801131 and rs12121543) was associated with a higher risk of CRC-specific mortality (HR = 2.18, 95%CI: 1.06–4.51). However, none of the above results were significant after adjusting for multiple comparisons.
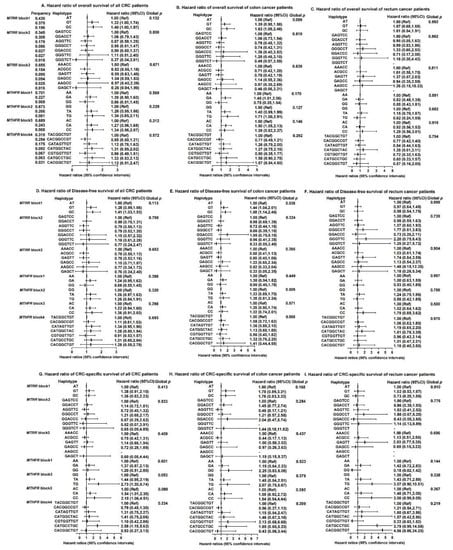
Figure 2.
Haplotypes on MTRR and MTHFR genes and associations with overall survival, disease-free survival and CRC-specific survival among colorectal cancer patients (A–I). Abbreviations: CRC = colorectal cancer; MTRR = methionine synthase reductase; MTHFR = methylenetetrahydrofolate reductase; Ref = Reference; CI = confidence interval. MTRR, block1 includes rs1801394 and rs13181011. MTRR, block2 includes rs326124, rs1532268, rs7703033, rs6555501, rs162031 and rs162033. MTRR, block3 includes rs161871, rs162040, rs3776455, rs10380 and rs9332. MTHFR, block1 includes rs4846048 and rs2184226. MTHFR, block2 includes rs4846049 and rs1476413. MTHFR, block3 includes rs1801131 and rs12121543. MTHFR, block4 includes rs1801133, rs1572151, rs4846052, rs2066471, rs13306567, rs7533315, rs9651118, rs7553194 and rs13306561. Haplotype frequency is calculated based on the n = 532 sample. Cox proportional hazard models were adjusted for age at diagnosis, sex, stage at diagnosis, race, drink status, body mass index (BMI), screen status, BRAF2, microsatellite instability (MSI) status and marital status, where appropriate.
3.5. Gene-Diet Interactions
To evaluate the possibility of gene-diet interactions, participants were grouped as high versus low intakes of vitamin B, fruits and alcohol based on the respective median value (Figure 3 and Figure 4). We observed significant interactions among pre-diagnostic alcohol consumption with several SNPs in MTRR (i.e., rs1801394, rs3776467, rs326124, rs162040, and rs3776455), with increased OS time associated with those protective variant alleles limited to patients consuming alcohol below the median. In addition, the MTHFR rs3737966 (G vs. A) allele seemed to be detrimental to CRC survival only among subjects with fruit intake below the median (p = 0.041). No significant interactions were detected between dietary vitamin B and any of these SNPs examined in this analysis.
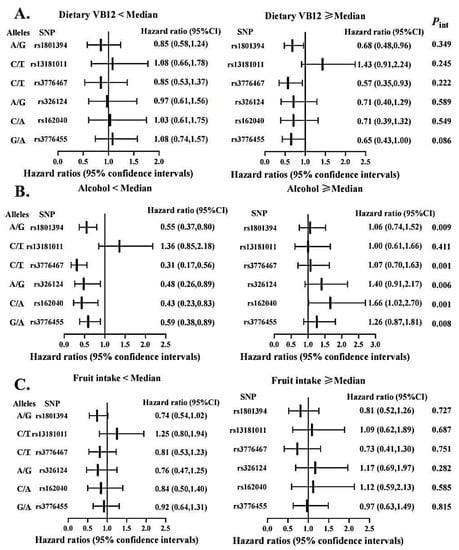
Figure 3.
Association between selected genetic variations in MTRR and CRC overall survival stratified by dietary vitamin B12 (A), alcohol intake (B), and fruit consumption (C). Abbreviations: SNP = single nucleotide polymorphisms; VB12 = vitamin B12. Two variants at the locus presented as: Minor/Major allele. Hazard ratios were calculated in reference to the allele underlined. Cox proportional hazard models were age at diagnosis, sex, stage at diagnosis, race, BMI, marital status and MSI status, where appropriate. The medians of dietary vitamin B12, alcohol drinking, and fruit intake were 7.30 µg/day, 2.17 g/day, and 7 pieces/week, respectively.
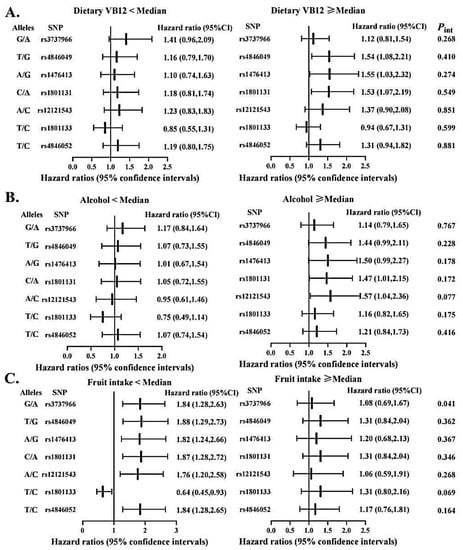
Figure 4.
Association between selected genetic variations in MTHFR and CRC overall survival stratified by dietary vitamin B12 (A), alcohol intake (B), and fruit consumption (C). Abbreviations: SNP = single nucleotide polymorphisms. Two variants at the locus presented as: Minor/Major allele. Hazard ratios were calculated in reference to the allele underlined. Cox proportional hazard models were adjusted for age at diagnosis, sex, stage at diagnosis, race, BMI, BRAF2, smoke status, marital status and MSI status, where appropriate. The medians of dietary vitamin B12, alcohol drinking, and fruit intake were 7.30 µg/day, 2.17 g/day, and 7 pieces/week, respectively.
4. Discussion
In the current work, MTRR and MTHFR genes were related to DFS and CRC-specific survival at the gene level. The MTRR rs1801394 (A66G) was associated with reduced DFS, while in the MTHRT gene, rs3737966 (G vs. A), rs4846049 (T vs. G), rs1476413 (A vs. G), rs1801131 (C vs. A), rs12121543 (A vs. C), rs1801133 (C vs. T), rs4846052 (T vs. C), rs2066471 (A vs. G) and rs7533315 (T vs. C) were related to worse CRC-specific survival. Potential effect modifications were observed between MTRR and alcohol drinking, and between MTHFR and fruit intake.
The direction of our estimate that MTRR rs1801394 (A66G) conferred detrimental effect on DFS is concordant with the findings of many observational studies [21,24] and two meta-analyses [37,38] employing CRC incidence as an outcome. The MTRR A66G variant, located in 5p15.31, harbors a missense mutation arising from a 66 A-to-G substitution that changes isoleucine to methionine at MTRR position 22. The derived variant was predicted to influence splicing and transcriptional regulation, and thus, increase homocysteine concentrations [39,40]. Elevated homocysteine levels are thought to induce a higher risk for colorectal polyps [41]. This might help explain the allelic association between rs1801394 and DFS observed in this study. It should be noted that results from a meta-analysis by ethnicity demonstrated no significant relationship between the rs1801394 and CRC susceptibility among Asian, Caucasians, Japanese, and mixed populations [42]. However, three out of five meta-analysis [38,43,44,45,46] reported an increased risk of CRC development for MTRR G allele and/or GG genotype in Caucasians [38,44] and in Asians [46], suggesting a race-specific effect of MTRR rs1801394 on CRC. Nevertheless, the majority of participants in this study were white (96.92%), and therefore we were unable to assess the possibility of different patterns of effects across race. Further research is required to decipher the race-specific effect of MTRR rs1801394 on CRC prognosis. With respect to the MTHFR gene, two non-synonymous MTHFR variants, rs1801133 (C677T) and rs1801131 (A1298C) are among the most studied genetic markers for folate metabolism-related health outcomes. The C677T, resulting in a substitution of the amino acid alanine by valine, and the A1298C, resulting in a substitution of glutamate by alanine [44], were in high LD and were related to a reduced enzyme activity, an elevated level of homocysteine and a lower level of plasma folate [20,47]. Our previous analysis based on the same population using OS and DFS as outcomes [27] has reported a poorer OS in patients with MTHFR A1298C CC genotype compared to those with CA/AA genotypes (HR = 1.72), whereas no relationship of MTHFR C677T with either DFS or OS has been found. As an extension, this study additionally assessed these variants in relation to CRC-specific survival and, intriguingly, MTHFR 677T polymorphism was related to prolonged colon cancer-specific survival. These results are consistent with most previous reports [21,22,48] indicating a protective role of C677T against CRC.
In addition to the two commonly analyzed SNPs, our study identified novel SNPs that might influence survival after a diagnosis of CRC, including MTHFR rs4846049, rs3737966, rs1476413, rs12121543, rs4846052, rs2066471, rs7533315 and rs7553194. The rs4846049 and rs3737966 polymorphisms exist on the 3′UTR of the MTHFR gene while the others are within MTHFR introns. PCR analysis revealed that subjects carrying a GG genotype of rs4846049 exhibited reduced MTHFR gene expression [49]. Current research on MTHFR rs4846049 polymorphism mainly focused on diseases such as acute lymphoblastic leukemia and preeclampsia [49,50]. This is the first study linking MTHFR rs4846049 G > T to poorer survival among CRC patients; the results are in agreement with the only few studies published on CRC risk so far, in which MTHFR rs4846049 CA + AA vs. CC was associated with a modest, significantly increased risk for this cancer [51]. Similarly, MTHFR rs3737966 has not yet been linked to CRC prognosis, but this variant exists in the miRNA binding site of MTHFR and might interfere with the binding of miRNA to target mRNA [52]. The mechanisms underlying the associations between some intronic variants and CRC survival remain undetermined, but intronic variants have been suggested to affect alternative splicing by interfering with splice site recognition [53].
For the MTRR gene, the relationship between haplotype G-C/G-T, composed of rs1801394 (exon 2) and rs13181011 (intron 3), and the worse survival of colon cancer patients provides good evidence for a harmful effect of the rs1801394 G allele on survival. For the MTHFR gene, haplotypes T-G, consisting of rs4846049 and rs1476413, C-A and C-C, consisting of rs1801131 (exon 8) and rs12121543 (intron 7), were associated with a significant reduction in CRC-specific survival. This outcome appears logical and consistent with the results of the genotype analysis for individual SNPs in the two genes. We speculate that these SNPs or those located in close proximity in the genome may harbor causal variants that in conjunction with each other affect health outcome; although not all functional, these SNPs can be considered as candidate markers for future association studies to detect health-related genetic variants.
One of the novel findings of our study is the gene-diet interactions between gene polymorphisms and CRC survival. MTRR gene polymorphisms conferred a protective effect on survival in the low alcohol drinking group, suggesting that alcohol consumption may be a modulator for the gene-survival relationship. High alcohol intake can be regarded a low-methyl diet [54] while MTRR may affect the methylation process by leading to the decreased activity of methionine synthase [46], which may jointly give rise to the risk of CRC. Nevertheless, no interaction was found between the MTRR gene and alcohol consumption in relation to the risk of colorectal adenoma in a Japanese study [55], possibly due to differences in ethnicity and gender of the study populations (e.g., only Asian men were investigated in that Japanese study). The MTHFR rs3737966 G allele was related to decreased survival only among the group with low fruit intake. If present results are validated in further research, with extended sample size, then CRC patients, especially those with unfavorable genotypes, may receive survival benefit through increasing fruit consumption.
The strengths of this study include the relatively large sample size, the long follow-up period of up to 10 years, and comprehensive gene coverage (i.e., a gene-wide tag SNP panels that cover the majority of common variations in the MTHFR and MTRR genes were studied). There are some limitations. First, some rare haplotypes may result in lower power for part of the analyses. Second, exposure to environmental factors in the cohort participants were assessed at the time of cancer diagnosis, and thus, we were unable to examine how health behavior changes following cancer diagnosis could modulate the way genes work. This provides the impetus for future replication studies using post-diagnosis exposures.
5. Conclusions
Our data demonstrated that genetic variation in MTRR and MTHFR, as measured by a tag SNP approach, seemed to play an independent role in CRC survival. The gene-CRC outcome association was modulated by alcohol drinking. Our analysis highlights the concrete value, as prognostic value, of the MTRR and MTHFR gene variants in CRC patients and sheds additional light on potentially modifiable factors that could be targeted to improve prognosis in CRC survivors.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu14214594/s1, Table S1: Association Between MTRR SNPs and Colorectal Cancer Overall, Disease-Free and CRC Specific Survival Assuming an Additive Mode of Inheritance [35], Table S2: Association Between MTHFR SNPs and Colorectal Cancer Overall, Disease-Free and CRC-Specific Survival Assuming an Additive Mode of Inheritance [35], Figure S1: The linkage disequilibrium (LD) plot of (A) MTRR and (B) MTHFR genes. LD strength between the SNPs was indicated by the standard Haploview color scheme based on both D’ and LOD values (D’< 1 and LOD < 2 in white; D’ = 1 and LOD < 2 in blue; D’ < 1 and LOD ≥ 2 in shades of pink/red; D’ = 1 and LOD ≥ 2 in bright red). Numbers in squares are D’ (×100), but those with D’ = 1 are not shown. The black triangle marks the single haplotype block within each gene.
Author Contributions
Methodology, Y.Z.; resources, P.P.W.; data curation, P.P.W.; writing—original draft preparation, Y.W.; writing—review and editing, M.D., J.V., M.S., P.S.P., J.R.M. and Y.Z.; visualization, Y.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Canadian Institutes of Health Research Team Grant (CIHR-CPT79845), Canadian Institutes of Health Research Team in Interdisciplinary Research on Colorectal Cancer Studentship (205835), the National Cancer Institutes of Health under RFA#CA-08-502, and by cooperative agreements with members of the Colon Cancer Family Registry and principal investigators: Ontario Registry for Studies of Familial Colorectal Cancer (U01 CA074783). Yun Zhu received grants from the National Natural Science Foundation of China (No. 82003533) and the CNS-ZD Tizhi and Health Fund (No. CNS-ZD2020-82).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Human Investigation Committee of Memorial University of Newfoundland (No. 40001511).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data are available upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interests.
References
- Xi, Y.; Xu, P. Global colorectal cancer burden in 2020 and projections to 2040. Transl. Oncol. 2021, 14, 101174. [Google Scholar] [CrossRef] [PubMed]
- Renee, P.; Stephanie, P.; Sharon, D.; Carolyn, L. Folate and Its Impact on Cancer Risk. Curr. Nutr. Rep. 2018, 7, 70–84. [Google Scholar]
- Williams, E.A. Folate, colorectal cancer and the involvement of DNA methylation. Proc. Nutr. Soc. 2012, 71, 592–597. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, D.H.; Lee, B.H.; Kang, S.H.; Lee, H.J.; Lim, S.Y.; Suh, Y.K.; Ahn, Y.O. Folate intake and the risk of colorectal cancer in a Korean population. Eur. J. Clin. Nutr. 2009, 63, 1057–1064. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, C.M. Nutrigenetics in cancer research—Folate metabolism and colorectal cancer. J. Nutr. 2005, 135, 2698–2702. [Google Scholar] [CrossRef]
- Eussen, S.J.; Vollset, S.E.; Igland, J.; Meyer, K.; Fredriksen, Å.; Ueland, P.M.; Jenab, M.; Slimani, N.; Boffetta, P.; Overvad, K.; et al. Plasma folate, related genetic variants, and colorectal cancer risk in EPIC. Cancer Epidemiol. Biomark. Prev. 2010, 19, 1328–1340. [Google Scholar] [CrossRef]
- Hubner, R.A.; Houlston, R.S. Folate and colorectal cancer prevention. Br. J. Cancer 2009, 100, 233–239. [Google Scholar] [CrossRef]
- Li, W.X.; Cheng, F.; Zhang, A.J.; Dai, S.X.; Li, G.H.; Lv, W.W.; Zhou, T.; Zhang, Q.; Zhang, H.; Zhang, T.; et al. Folate Deficiency and Gene Polymorphisms of MTHFR, MTR and MTRR Elevate the Hyperhomocysteinemia Risk. Clin. Lab. 2017, 63, 523–533. [Google Scholar] [CrossRef]
- Huang, Y.; Han, S.Z.; Li, Y.; Mao, Y.M.; Xie, Y. Different roles of MTHFR C677T and A1298C polymorphisms in colorectal adenoma and colorectal cancer: A meta-analysis. J. Hum. Genet. 2007, 52, 73–85. [Google Scholar] [CrossRef][Green Version]
- Lin, W.; Yuhong, L.; Zhengrong, Z.; Zuoli, S.; Yi, H.; Rena, L. Methylenetetrahydrofolate reductase and psychiatric diseases. Transl. Psychiatry 2018, 8, 242. [Google Scholar] [CrossRef]
- Ma, J.; Stampfer, M.J.; Giovannucci, E.; Artigas, C.; Hunter, D.J.; Fuchs, C.; Willett, W.C.; Selhub, J.; Hennekens, C.H.; Rozen, R. Methylenetetrahydrofolate reductase polymorphism, dietary interactions, and risk of colorectal cancer. Cancer Res. 1997, 57, 1098–1102. [Google Scholar] [PubMed]
- Park, K.S.; Mok, J.W.; Kim, J.C. The 677C>T mutation in 5,10-methylenetetrahydrofolate reductase and colorectal cancer risk. Genet. Test. 1999, 3, 233–236. [Google Scholar] [CrossRef] [PubMed]
- Slattery, M.L.; Potter, J.D.; Samowitz, W.; Schaffer, D.; Leppert, M. Methylenetetrahydrofolate reductase, diet, and risk of colon cancer. Cancer Epidemiol. Biomark. Prev. 1999, 8, 513–518. [Google Scholar]
- Ulrich, C.M.; Kampman, E.; Bigler, J.; Schwartz, S.M.; Chen, C.; Bostick, R.; Fosdick, L.; Beresford, S.A.; Yasui, Y.; Potter, J.D. Colorectal adenomas and the C677T MTHFR polymorphism: Evidence for gene-environment interaction? Cancer Epidemiol. Biomark. Prev. 1999, 8, 659–668. [Google Scholar]
- Le Marchand, L.; Donlon, T.; Hankin, J.H.; Kolonel, L.N.; Wilkens, L.R.; Seifried, A. B-vitamin intake, metabolic genes, and colorectal cancer risk (United States). Cancer Causes Control 2002, 13, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Ma, J.; Stampfer, M.J.; Palomeque, C.; Selhub, J.; Hunter, D.J. Linkage disequilibrium between the 677C>T and 1298A>C polymorphisms in human methylenetetrahydrofolate reductase gene and their contributions to risk of colorectal cancer. Pharmacogenetics 2002, 12, 339–342. [Google Scholar] [CrossRef]
- Keku, T.; Millikan, R.; Worley, K.; Winkel, S.; Eaton, A.; Biscocho, L.; Martin, C.; Sandler, R. 5,10-Methylenetetrahydrofolate reductase codon 677 and 1298 polymorphisms and colon cancer in African Americans and whites. Cancer Epidemiol. Biomark. Prev. 2002, 11, 1611–1621. [Google Scholar]
- Mahla, G.; Marjan, A.; Reza, K.; Javad, B.; Amin, K.M. Association of MTHFR C677T variant genotype with serum folate and Vit B12 in Iranian patients with colorectal cancer or adenomatous polyps. BMC Med. Genom. 2021, 14, 246. [Google Scholar] [CrossRef]
- Levine, A.J.; Siegmund, K.D.; Ervin, C.M.; Diep, A.; Lee, E.R.; Frankl, H.D.; Haile, R.W. The methylenetetrahydrofolate reductase 677C-->T polymorphism and distal colorectal adenoma risk. Cancer Epidemiol. Biomark. Prev. 2000, 9, 657–663. [Google Scholar]
- Jokic, M.; Brcic-Kostic, K.; Stefulj, J.; Ivkovic, T.C.; Bozo, L.; Gamulin, M.; Kapitanovic, S. Association of MTHFR, MTR, MTRR, RFC1, and DHFR Gene Polymorphisms with Susceptibility to Sporadic Colon Cancer. DNA Cell Biol. 2011, 30, 771–776. [Google Scholar] [CrossRef]
- Pardini, B.; Kumar, R.; Naccarati, A.; Prasad, R.B.; Forsti, A.; Polakova, V.; Vodickova, L.; Novotny, J.; Hemminki, K.; Vodicka, P. MTHFR and MTRR genotype and haplotype analysis and colorectal cancer susceptibility in a case-control study from the Czech Republic. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2011, 721, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Arve, U.; Emil, V.S.; Svein, H.; Randi, G.; Egil, J.; Magne, U.P. Colorectal cancer and the methylenetetrahydrofolate reductase 677C -> T and methionine synthase 2756A -> G polymorphisms: A study of 2168 case-control pairs from the JANUS cohort. J. Cancer Epidemiol. Biomark. Prev. 2004, 13, 2175–2180. [Google Scholar]
- Zhong, S.; Yang, J.H.; Liu, K.; Jiao, B.H.; Chang, Z.J. Quantitative assessment of the association between MTHFR C677T polymorphism and colorectal cancer risk in East Asians. Tumor Biol. 2012, 33, 2041–2051. [Google Scholar] [CrossRef]
- Keitaro, M.; Nobuyuki, H.; Takashi, H.; Tomoyuki, K.; Manami, I.; Toshiro, T.; Kazuo, T. Methionine Synthase Reductase Gene A66G Polymorphism is Associated with Risk of Colorectal Cancer. Asian Pac. J. Cancer Prev. APJCP 2002, 3, 353–359. [Google Scholar]
- Zhu, L.; Wang, F.; Hu, F.L.; Wang, Y.B.N.; Li, D.D.; Dong, X.S.; Cui, B.B.; Zhao, Y.S. Association between MTHFR polymorphisms and overall survival of colorectal cancer patients in Northeast China. Med. Oncol. 2013, 30, 6. [Google Scholar] [CrossRef] [PubMed]
- Xin-Lin, C.; Yu-Mei, W.; Fei, Z.; Zheng, C.; Xiaofei, Y.; Cong, S.; Yunpeng, G.; Tian-Ge, Y.; Guo, T.; Yi-Ming, C.; et al. Methylenetetrahydrofolate reductase polymorphisms and colorectal cancer prognosis: A meta-analysis. J. Gene Med. 2019, 21, e3114. [Google Scholar] [CrossRef]
- Negandhi, A.A.; Hyde, A.; Dicks, E.; Pollett, W.; Younghusband, B.H.; Parfrey, P.; Green, R.C.; Savas, S. MTHFR Glu429Ala and ERCC5 His46His Polymorphisms Are Associated with Prognosis in Colorectal Cancer Patients: Analysis of Two Independent Cohorts from Newfoundland. PLoS ONE 2013, 8, 14. [Google Scholar] [CrossRef]
- Osian, G.; Procopciuc, L.; Vlad, L.; Iancu, C.; Mocan, T.; Mocan, L. C677T and A1298C mutations in the MTHFR gene and survival in colorectal cancer. J. Gastrointest. Liver Dis. JGLD 2009, 18, 455–460. [Google Scholar]
- Green, R.C.; Green, J.S.; Buehler, S.K.; Robb, J.D.; Daftary, D.; Gallinger, S.; McLaughlin, J.R.; Parfrey, P.S.; Younghusband, H.B. Very high incidence of familial colorectal cancer in Newfoundland: A comparison with Ontario and 13 other population-based studies. Fam. Cancer 2007, 6, 53–62. [Google Scholar] [CrossRef]
- Zhu, Y.; Yang, S.R.; Wang, P.P.; Savas, S.; Wish, T.; Zhao, J.; Green, R.; Woods, M.; Sun, Z.; Roebothan, B.; et al. Influence of pre-diagnostic cigarette smoking on colorectal cancer survival: Overall and by tumour molecular phenotype. Br. J. Cancer 2014, 110, 1359–1366. [Google Scholar] [CrossRef][Green Version]
- Woods, M.O.; Younghusband, H.B.; Parfrey, P.S.; Gallinger, S.; McLaughlin, J.; Dicks, E.; Stuckless, S.; Pollett, A.; Bapat, B.; Mrkonjic, M.; et al. The genetic basis of colorectal cancer in a population-based incident cohort with a high rate of familial disease. Gut 2010, 59, 1369–1377. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wang, P.P.; Roebothan, B.; Ryan, A.; Tucker, C.S.; Colbourne, J.; Baker, N.; Cotterchio, M.; Yi, Y.Q.; Sun, G. Assessing the validity of a self-administered food-frequency questionnaire (FFQ) in the adult population of Newfoundland and Labrador, Canada. Nutr. J. 2013, 12, 9. [Google Scholar] [CrossRef] [PubMed]
- De Bakker, P.I.; Yelensky, R.; Pe’er, I.; Gabriel, S.B.; Daly, M.J.; Altshuler, D. Efficiency and power in genetic association studies. Nat. Genet. 2005, 37, 1217–1223. [Google Scholar] [CrossRef] [PubMed]
- Gauderman, W.J.; Murcray, C.; Gilliland, F.; Conti, D.V. Testing association between disease and multiple SNPs in a candidate gene. Genet. Epidemiol. 2007, 31, 383–395. [Google Scholar] [CrossRef] [PubMed]
- Conneely, K.N.; Boehnke, M. So Many Correlated Tests, So Little Time! Rapid Adjustment of P Values for Multiple Correlated Tests. Am. J. Hum. Genet. 2007, 81, 1158–1168. [Google Scholar] [CrossRef]
- Stephens, M.; Donnelly, P. A comparison of bayesian methods for haplotype reconstruction from population genotype data. Am. J. Hum. Genet. 2003, 73, 1162–1169. [Google Scholar] [CrossRef]
- Wang, P.; Li, S.Q.; Wang, M.L.; He, J.; Xi, S.M. Association of MTRR A66G polymorphism with cancer susceptibility: Evidence from 85 studies. J. Cancer 2017, 8, 266–277. [Google Scholar] [CrossRef]
- Wu, P.P.; Tang, R.N.; An, L. A meta-analysis of MTRR A66G polymorphism and colorectal cancer susceptibility. J. Buon 2015, 20, 918–922. [Google Scholar]
- Sharp, L.; Little, J. Polymorphisms in genes involved in folate metabolism and colorectal neoplasia: A HuGE review. Am. J. Epidemiol. 2004, 159, 423–443. [Google Scholar] [CrossRef]
- Mahasweta, C.; Tanusree, S.; Subhamita, M.; Swagata, S.; Kanchan, M. Folate System Gene Variant rs1801394 66AG may have a Causal Role in Down Syndrome in the Eastern Indian Population. Int. J. Mol. Cell. Med. 2020, 9, 215–224. [Google Scholar] [CrossRef]
- Manchun, S.; Manyi, S.; Li, Z.; Songli, S. Colorectal polyp risk is linked to an elevated level of homocysteine. Biosci. Rep. 2018, 38, BSR20171699. [Google Scholar] [CrossRef]
- Haerian, M.S.; Haerian, B.S.; Molanaei, S.; Kosari, F.; Sabeti, S.; Bidari-Zerehpoosh, F.; Abdolali, E. MTRR rs1801394 and its interaction with MTHFR rs1801133 in colorectal cancer: A case-control study and meta-analysis. Pharmacogenomics 2017, 18, 1075–1084. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Shen, C.; Meng, X.; Bai, J.; Chen, F.; Yu, Y.; Jin, Y.; Fu, S. Methionine synthase reductase A66G polymorphism contributes to tumor susceptibility: Evidence from 35 case-control studies. Mol. Biol. Rep. 2012, 39, 805–816. [Google Scholar] [CrossRef]
- Zhou, D.; Mei, Q.; Luo, H.; Tang, B.; Yu, P. The polymorphisms in methylenetetrahydrofolate reductase, methionine synthase, methionine synthase reductase, and the risk of colorectal cancer. Int. J. Biol. Sci. 2012, 8, 819–830. [Google Scholar] [CrossRef]
- Figueiredo, J.C.; Levine, A.J.; Crott, J.W.; Baurley, J.; Haile, R.W. Folate-genetics and colorectal neoplasia: What we know and need to know next. Mol. Nutr. Food Res. 2013, 57, 607–627. [Google Scholar] [CrossRef] [PubMed]
- Pabalan, N.; Singian, E.; Tabangay, L.; Jarjanazi, H.; Singh, N. Associations of the A66G Methionine Synthase Reductase Polymorphism in Colorectal Cancer: A Systematic Review and Meta-Analysis. Biomark. Cancer 2015, 7, 21–28. [Google Scholar] [CrossRef]
- Marugame, T.; Tsuji, E.; Inoue, H.; Shinomiya, S.; Kiyohara, C.; Onuma, K.; Hamada, H.; Koga, H.; Handa, K.; Hayabuchi, H.; et al. Methylenetetrahydrofolate reductase polymorphism and risk of colorectal adenomas. Cancer Lett. 2000, 151, 181–186. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, X.F.; Liu, H.X.; Hao, Y.S.; Zhao, C.L. MTHFR C677T Polymorphism and Colorectal Cancer Risk in Asians, a Meta- analysis of 21 Studies. Asian Pac. J. Cancer Prev. 2012, 13, 1203–1208. [Google Scholar] [CrossRef]
- Xiaolei, L.; Shunguo, Z.; Feng, Y. Rs4846049 Polymorphism at the 3’-UTR of MTHFR Gene: Association with Susceptibility to Childhood Acute Lymphoblastic Leukemia. BioMed Res. Int. 2019, 2019, 4631091. [Google Scholar] [CrossRef]
- Abbas, M.-G.; Batool, T.; Mehrnaz, N.-N.; Mehrnaz, M.; Ramin, S.; Saeedeh, S. The association of the placental MTHFR 3’-UTR polymorphisms, promoter methylation, and MTHFR expression with preeclampsia. J. Cell. Biochem. 2018, 119, 1346–1354. [Google Scholar]
- Jeon, Y.J.; Kim, J.W.; Park, H.M.; Kim, J.O.; Jang, H.G.; Oh, J.; Hwang, S.G.; Kwon, S.W.; Oh, D.; Kim, N.K. Genetic variants in 3’-UTRs of methylenetetrahydrofolate reductase (MTHFR) predict colorectal cancer susceptibility in Koreans. Sci. Rep. 2015, 5, 12. [Google Scholar] [CrossRef] [PubMed]
- Shu-Mei, W.; Wei-Xin, Z.; Wan-Shui, W.; Lu-Lu, S.; Dan, Y. Association between MTHFR microRNA binding site polymorphisms and methotrexate concentrations in Chinese pediatric patients with acute lymphoblastic leukemia. J. Gene Med. 2017, 19, 353–359. [Google Scholar]
- Lin, H.; Hargreaves, K.A.; Li, R.; Reiter, J.L.; Wang, Y.; Mort, M.; Cooper, D.N.; Zhou, Y.; Zhang, C.; Eadon, M.T.; et al. RegSNPs-intron: A computational framework for predicting pathogenic impact of intronic single nucleotide variants. Genome Biol. 2019, 20, 254. [Google Scholar] [CrossRef] [PubMed]
- Giovannucci, E.; Rimm, E.B.; Ascherio, A.; Stampfer, M.J.; Colditz, G.A.; Willett, W.C. Alcohol, low-methionine--low-folate diets, and risk of colon cancer in men. J. Natl. Cancer Inst. 1995, 87, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Yoshimitsu, S.; Morita, M.; Hamachi, T.; Tabata, S.; Abe, H.; Tajima, O.; Uezono, K.; Ohnaka, K.; Kono, S. Methionine synthase and thymidylate synthase gene polymorphisms and colorectal adenoma risk: The self defense forces study. Mol. Carcinog. 2012, 51, E151–E157. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).