Supplementation of Probiotics in Pregnant Women Targeting Group B Streptococcus Colonization: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Study Selection
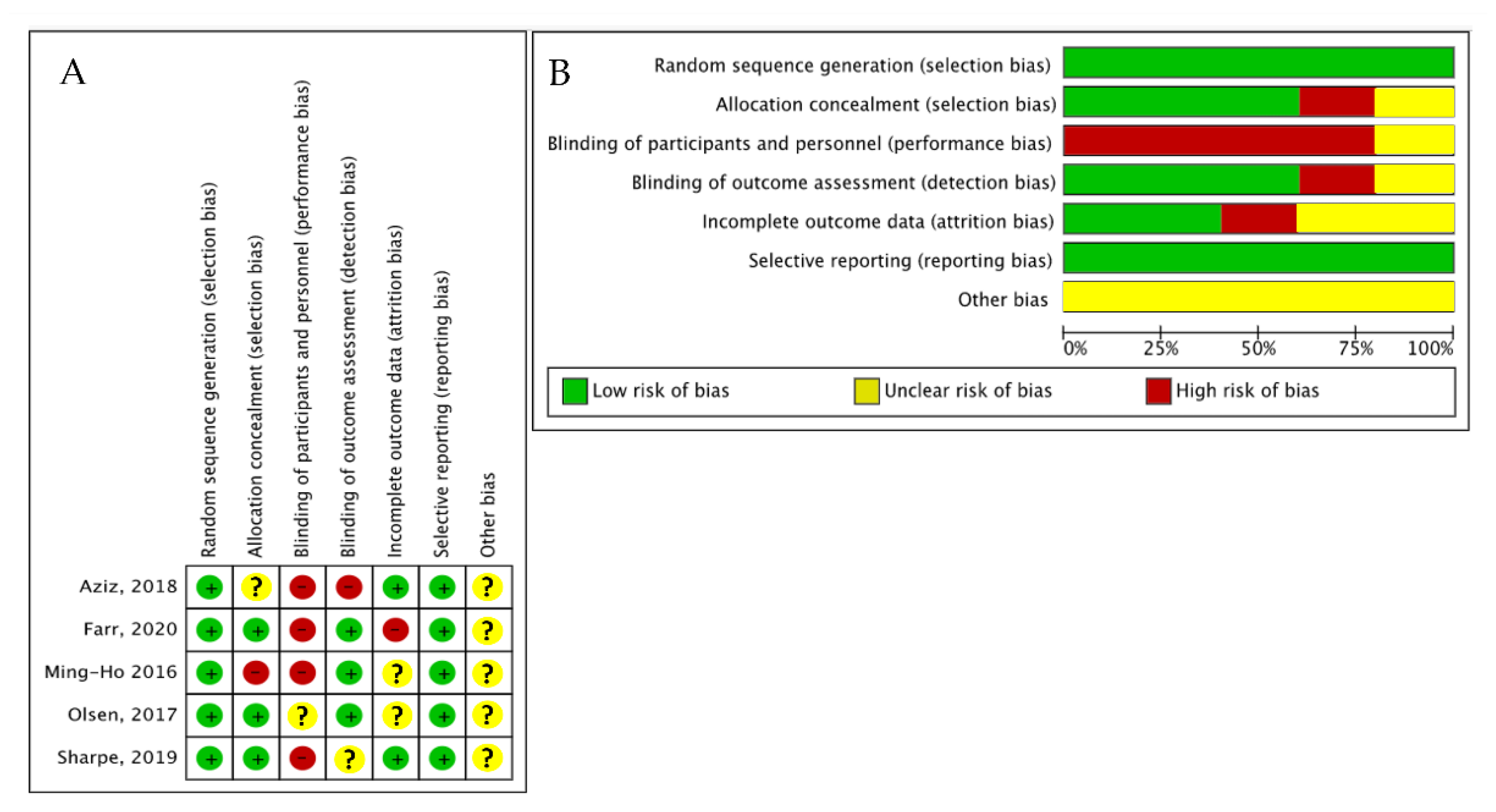
2.3. Data Extraction and Risk-of-Bias Assessment
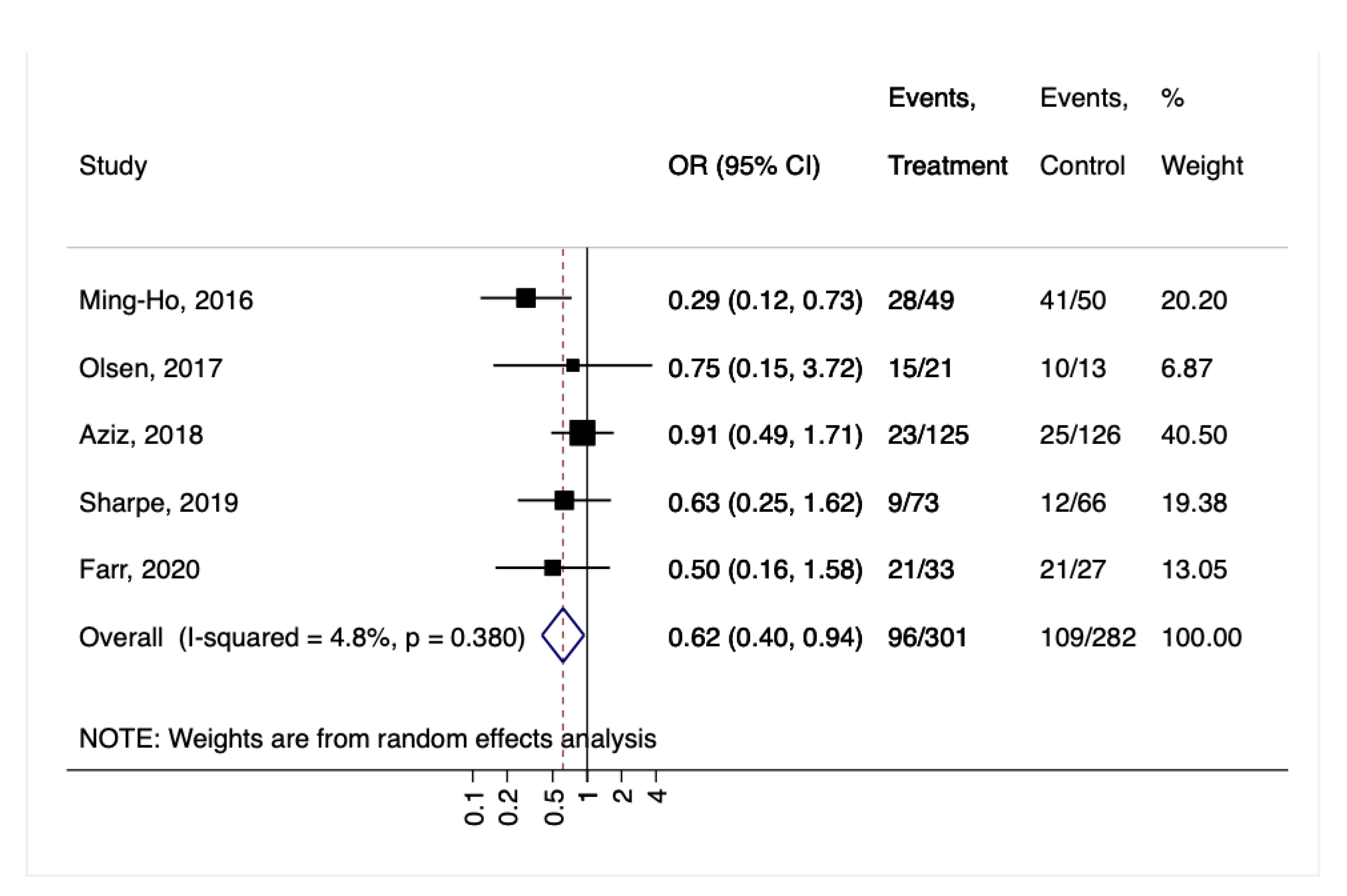
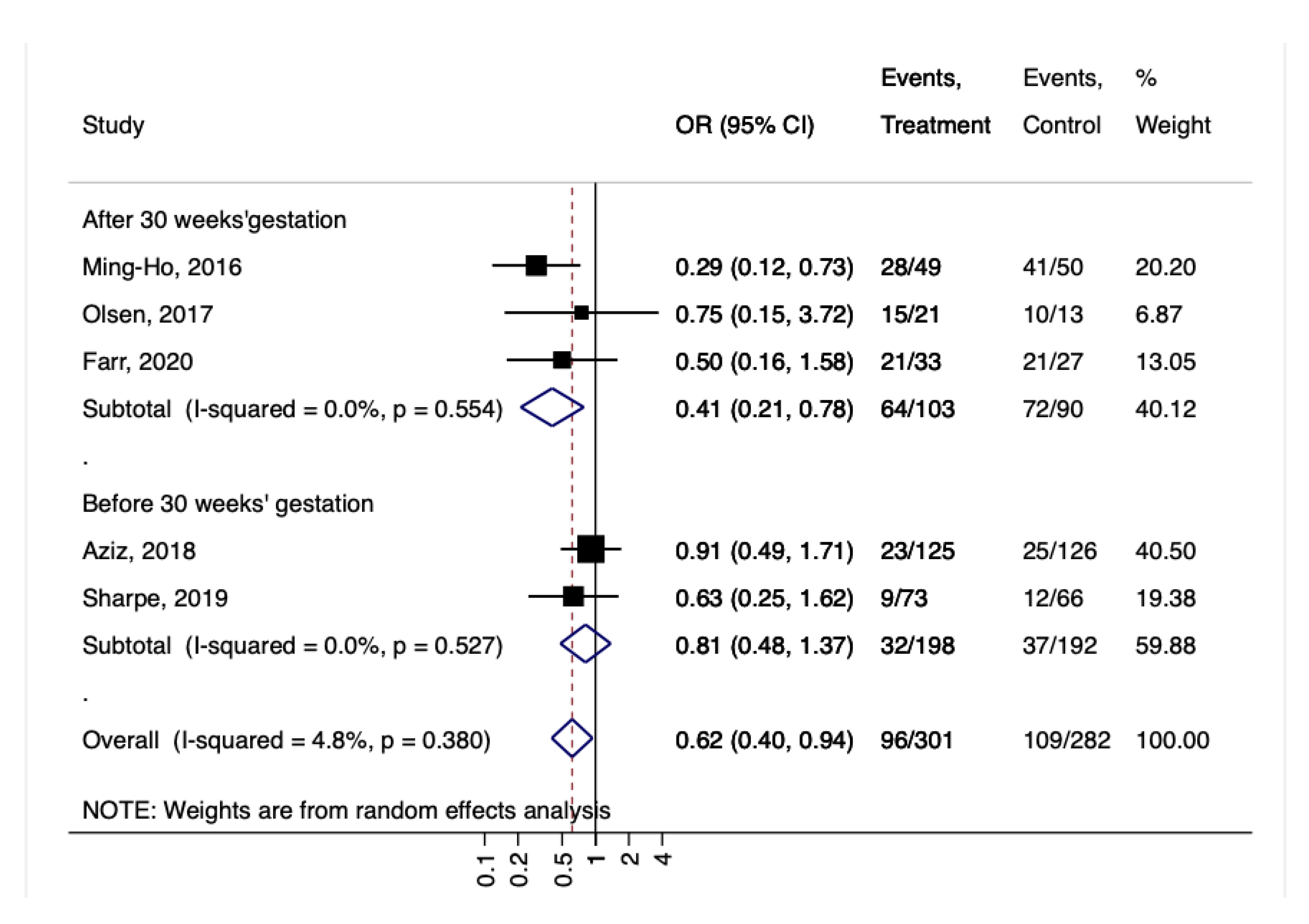
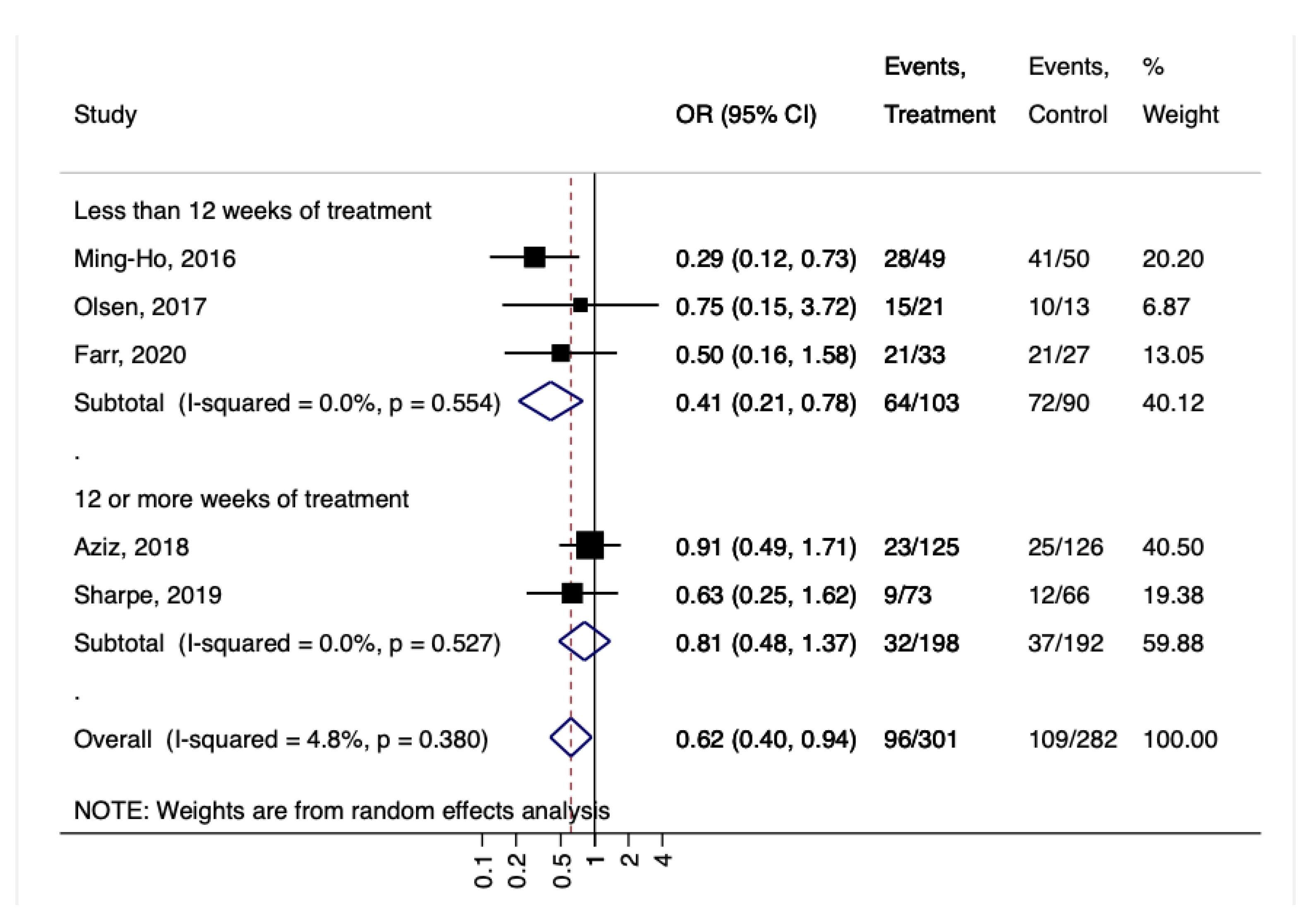
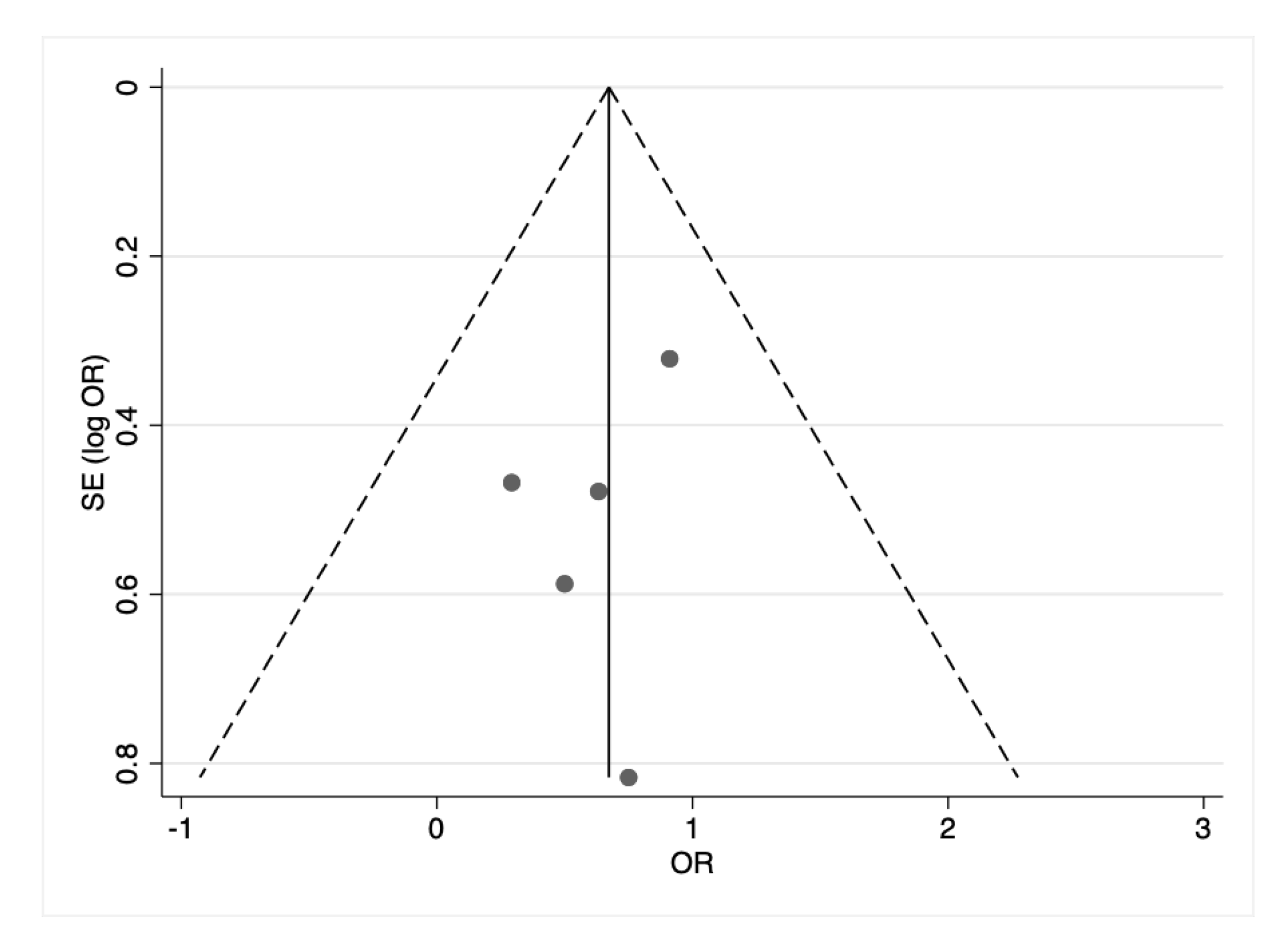
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barcaite, E.; Bartusevicius, A.; Tameliene, R.; Kliucinskas, M.; Maleckiene, L.; Nadisauskiene, R. Prevalence of maternal group B streptococcal colonisation in European countries. Acta Obs. Gynecol. Scand. 2008, 87, 260–271. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.; Hack Adams, N.; Bartlett, L.; Seale, A.; Lamagni, T.; Bianchi-Jassir, F.; Lawn, E.J.; Baker, C.J.; Cutland, C.; Heath, P.T.; et al. Maternal Disease With Group B Streptococcus and Serotype Distribution Worldwide: Systematic Review and Meta-analyses. Clin. Infect. Dis. 2017, 65, S112–S124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Kadri, H.; Bamuhair, S.; Al Johani, S.; Al-Buriki, N.; Tamim, H. Maternal and neonatal risk factors for early-onset group B streptococcal disease: A case control study. Int. J. Womens Health 2013, 5, 729–735. [Google Scholar] [CrossRef] [Green Version]
- Bianchi-Jassir, F.; Seale, A.; Kohli-Lynch, M.; Lawn, J.; Baker, C.; Bartlett, L.; Cutland, C.; Gravett, M.G.; Heath, P.T.; Ip, M.; et al. Preterm Birth Associated With Group B Streptococcus Maternal Colonization Worldwide: Systematic Review and Meta-analyses. Clin. Infect. Dis. 2017, 65, S133–S142. [Google Scholar] [CrossRef] [Green Version]
- Collin, M.; Shetty, N.; Guy, R.; Nyaga, V.; Bull, A.; Richards, M.; van der Kooi, T.; Koek, M.B.G.; De Almeida, M.; Roberts, S.A.; et al. Group B Streptococcus in surgical site and non-invasive bacterial infections worldwide: A systematic review and meta-analysis. Int. J. Infect. Dis. 2019, 83, 116–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baker, C.; Edwards, M. Group B Streptococcal Infections. In Infectious Diseases of the Fetus and Newborn Infant, 4th ed.; Remington, J., Klein, J.O., Eds.; WB Saunders: Philadelphia, PA, USA, 1995; pp. 980–1054. [Google Scholar]
- Demianczuk, N.; Halperin, S.; McMillan, D. Prevention of perinatal group B streptococcal infection: Management strategies. Can. J. Infect. Dis. 1997, 8, 68–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verani, J.; McGee, L.; Schrag, S.J. Prevention of perinatal group B streptococcal disease-revised guidelines from CDC. MMWR. Recommendations and reports: Morbidity and mortality weekly report Recommendations and reports. Centers Dis. Control Prev. 2010, 59, 1–32. [Google Scholar]
- ACOG Committee. Committee Opinion No. 797. Obstet Gynecol. 2020, 135, 978–979. [Google Scholar] [CrossRef]
- Zimmermann, P.; Curtis, N. Effect of intrapartum antibiotics on the intestinal microbiota of infants: A systematic review. Arch. Dis. Child.-Fetal Neonatal Ed. 2020, 105, 201–208. [Google Scholar] [CrossRef]
- Tapiainen, T.; Koivusaari, P.; Brinkac, L.; Lorenzi, H.; Salo, J.; Renko, M.; Pruikkonen, H.; Pokka, T.; Li, W.; Nelson, K.; et al. Impact of intrapartum and postnatal antibiotics on the gut microbiome and emergence of antimicrobial resistance in infants. Sci. Rep. 2019, 9, 10635. [Google Scholar] [CrossRef] [Green Version]
- Jašarević, E.; Howerton, C.L.; Howard, C.D.; Bale, T.L. Alterations in the Vaginal Microbiome by Maternal Stress Are Associated With Metabolic Reprogramming of the Offspring Gut and Brain. Endocrinology 2015, 156, 3265–3276. [Google Scholar] [CrossRef]
- Back, E.E.; O’Grady, E.J.; Back, J.D. High rates of perinatal group B Streptococcus clindamycin and erythromycin resistance in an Upstate New York Hospital. Antimicrob. Agents Chemother. 2012, 56, 739–742. [Google Scholar] [CrossRef] [Green Version]
- Spaetgens, R. Perinatal antibiotic usage and changes in colonization and resistance rates of group B streptococcus and other pathogens. Obstet. Gynecol. 2002, 100, 525–533. [Google Scholar]
- Facchinetti, F.; Piccinini, F.; Mordini, B.; Volpe, A. Chlorhexidine vaginal flushings versus systemic ampicillin in the prevention of vertical transmission of neonatal group B streptococcus, at term. J. Matern. Fetal Neonatal Med. 2002, 11, 84–88. [Google Scholar] [CrossRef]
- World Health Organization. WHO Preferred Product Characteristics for Group B Streptococcus Vaccines. In Initiative for Vaccine Research (IVR) of the Department of Immunization, Vaccines and Biologicals; Vaccines and BiologicalsCH-1211; World Health Organization Department of Immunization: Geneva, Switzerland, 2014; pp. 5–16. [Google Scholar]
- Wijesundara, N.M.; Lee, S.F.; Cheng, Z.; Davidson, R.; Rupasinghe, H.P.V. Carvacrol exhibits rapid bactericidal activity against Streptococcus pyogenes through cell membrane damage. Sci. Rep. 2021, 11, 1487. [Google Scholar] [CrossRef]
- Khan, R.; Adil, M.; Danishuddin, M.; Verma, P.K.; Khan, A.U. In vitro and in vivo inhibition of Streptococcus mutans biofilm by Trachyspermum ammi seeds: An approach of alternative medicine. Phytomedicine 2012, 19, 747–755. [Google Scholar] [CrossRef]
- Adil, M.; Baig, M.H.; Rupasinghe, H.V. Impact of citral and phloretin, alone and in combination, on major virulence traits of Streptococcus pyogenes. Molecules 2019, 24, 4237. [Google Scholar] [CrossRef] [Green Version]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [Green Version]
- Chin-Lee, B.; Curry, W.J.; Fetterman, J.; Graybill, M.A.; Karpa, K. Patient experience and use of probiotics in community-based health care settings. Patient Prefer. Adherence 2014, 8, 1513–1520. [Google Scholar]
- Betz, M.; Uzueta, A.; Rasmussen, H.; Gregoire, M.; Vanderwall, C.; Witowich, G. Knowledge, use and perceptions of probiotics and prebiotics in hospitalised patients. Nutr. Diet. 2015, 72, 261–266. [Google Scholar] [CrossRef]
- Rosen, G.H.; Randis, T.M.; Desai, P.V.; Sapra, K.J.; Ma, B.; Gajer, P.; Humphrys, M.; Ravel, J.; Gelber, S.E.; Ratner, A.J. Group B Streptococcus and the Vaginal Microbiota. J. Infect. Dis. 2017, 216, 744–751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Altoparlak, U.; Kadanali, A.; Kadanali, S. Genital flora in pregnancy and its association with group B streptococcal colonization. Int. J. Gynecol. Obstet. 2004, 87, 245–246. [Google Scholar] [CrossRef] [PubMed]
- Whitney, C.; Daly, S.; Limpongsanurak, S.; Festin, M.; Thinn, K.; Chipato, T.; Lumbiganon, P.; Sauvarin, J.; Andrews, W.; Tolosa, J.E.; et al. The International Infections in Pregnancy Study: Group B streptococcal colonization in pregnant women. J. Matern. Neonatal Med. 2004, 15, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Tachedjian, G.; Aldunate, M.; Bradshaw, C.S.; Cone, R.A. The role of lactic acid production by probiotic Lactobacillus species in vaginal health. Res. Microbiol. 2017, 168, 782–792. [Google Scholar] [CrossRef]
- Petrova, M.I.; Imholz, N.C.E.; Verhoeven, T.L.A.; Balzarini, J.; Van Damme, E.J.M.; Schols, D.; Vanderleyden, J.; Lebeer, S. Lectin-Like Molecules of Lactobacillus rhamnosus GG Inhibit Pathogenic Escherichia coli and Salmonella Biofilm Formation. PLoS ONE 2016, 11, e0161337. [Google Scholar] [CrossRef] [Green Version]
- Allonsius, C.N.; Vandenheuvel, D.; Oerlemans, E.F.M.; Petrova, M.I.; Donders, G.G.G.; Cos, P.; Delputte, P.; Lebeer, S. Inhibition of Candida albicans morphogenesis by chitinase from Lactobacillus rhamnosus GG. Sci. Rep. 2019, 2900, 1–12. [Google Scholar] [CrossRef]
- Wijgert, J.; Verwijs, M. Lactobacilli-containing vaginal probiotics to cure or prevent bacterial or fungal vaginal dysbiosis: A systematic review and recommendations for future trial designs. BJOG An Int. J. Obstet. Gynaecol. 2020, 127, 287–299. [Google Scholar] [CrossRef]
- Sheyholislami, H.; Connor, K.L. Are Probiotics and Prebiotics Safe for Use during Pregnancy and Lactation? A Systematic Review and Meta-Analysis. Nutrients 2021, 13, 2382. [Google Scholar] [CrossRef]
- Higgins, J.P.; Green, S. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0; The Cochrane Collaboration: London, UK; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2011. [Google Scholar]
- Ho, M.; Chang, Y.Y.; Chang, W.C.; Lin, H.C.; Wang, M.H.; Lin, W.C.; Wang, M.H.; Lin, W.C.; Chiu, T.H. Oral Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14 to reduce Group B Streptococcus colonization in pregnant women: A randomized controlled trial. Taiwan J. Obstet. Gynecol. 2016, 55, 515–518. [Google Scholar] [CrossRef] [Green Version]
- Olsen, P.; Williamson, M.; Traynor, T.; Georgiou, C. The impact of oral probiotics on vaginal Group B Streptococcal colonisation rates in pregnant women: A pilot randomised control study. Women Birth 2017, 31, 31–37. [Google Scholar] [CrossRef] [Green Version]
- Aziz, N.; Spiegel, A.; Bentley, J.; Yoffe, P.; Klikoff, A.; Ehrlich, K.; El-Sayed, Y.; Norton, M.; Taslimi, M. Evaluation of Probiotic Oral Supplementation Effects on Group B Streptococcus Rectovaginal Colonization in Pregnant Women: A Randomized Double-Blind Placebo-Controlled Trial. Am. J. Obstet. Gynecol. 2018, 218, S509–S510. [Google Scholar] [CrossRef] [Green Version]
- Sharpe, M.; Shah, V.; Freire-Lizama, T.; Cates, E.; McGrath, K.; David, I.; Cowan, S.; Letkeman, J.; Steward-Wilson, E. Effectiveness of oral intake of Lactobacillus rhamnosusGR-1 and Lactobacillus reuteri RC-14 on Group B Streptococcus colonization during pregnancy: A midwifery-led double-blind randomized controlled pilot trial. J. Matern. Neonatal Med. 2019, 34, 1814–1821. [Google Scholar] [CrossRef] [PubMed]
- Farr, A.; Sustr, V.; Kiss, H.; Rosicky, I.; Graf, A.; Makristathis, A.; Makristathis, A.; Petricevic, L. Oral probiotics to reduce vaginal group B streptococcal colonization in late pregnancy. Sci. Rep. 2020, 10, 19745. [Google Scholar] [CrossRef] [PubMed]
- Marziali, G.; Foschi, C.; Parolin, C.; Vitali, B.; Marangoni, A. In-vitro effect of vaginal lactobacilli against group B Streptococcus. Microb. Pathog. 2019, 136, 103692. [Google Scholar] [CrossRef]
- De Gregorio, P.R.; Tomás, M.S.J.; Terraf, M.C.L.; Nader-Macías, M.E.F. In vitro and in vivo effects of beneficial vaginal lactobacilli on pathogens responsible for urogenital tract infections. J. Med. Microbiol. 2014, 63, 685–696. [Google Scholar] [CrossRef]
- do Carmo, M.S.; Noronha, F.M.F.; Arruda, M.O.; da Silva Costa, Ê.P.; Bomfim, M.R.Q.; Monteiro, A.S.; Ferro, T.A.F.; Fernandes, E.S.; Giron, J.A.; Monteneiro-Neto, V. Lactobacillus fermentum ATCC 23271 displays in vitro inhibitory activities against Candida spp. Front. Microbiol. 2016, 7, 1722. [Google Scholar] [CrossRef] [Green Version]
- Foschi, C.; Salvo, M.; Cevenini, R.; Parolin, C.; Vitali, B.; Marangoni, A. Vaginal Lactobacilli Reduce Neisseria gonorrhoeae Viability through Multiple Strategies: An in Vitro Study. Front. Cell Infect. Microbiol. 2017, 7, 502. [Google Scholar] [CrossRef] [Green Version]
- Elias, J.; Bozzo, P.; Einarson, A. Are probiotics safe for use during pregnancy and lactation? Can. Fam. Physician 2011, 57, 299–301. [Google Scholar]
- Chen, X.; Jiang, X.; Huang, X.; He, H.; Zheng, J. Association between Probiotic Yogurt Intake and Gestational Diabetes Mellitus: A Case-Control Study. Iran. J. Public Health 2019, 48, 1248–1256. [Google Scholar] [CrossRef]
- Fernández, L.; Cárdenas, N.; Arroyo, R.; Manzano, S.; Jiménez, E.; Martín, V.; Rodríguez, J.M. Prevention of Infectious Mastitis by Oral Administration of Lactobacillus salivarius PS2 During Late Pregnancy. Clin. Infect. Dis. 2016, 62, 568–573. [Google Scholar] [CrossRef] [Green Version]
- Kriss, J.L.; Ramakrishnan, U.; Beauregard, J.L.; Phadke, V.K.; Stein, A.D.; Rivera, J.A.; Omer, S.B. Yogurt consumption during pregnancy and preterm delivery in Mexican women: A prospective analysis of interaction with maternal overweight status. Matern. Child. Nutr. 2018, 14, e12522. [Google Scholar] [CrossRef] [PubMed]
- Celik, V.; Beken, B.; Yazicioglu, M.; Ozdemir, P.G.; Sut, N. Do traditional fermented foods protect against infantile atopic dermatitis. Pediatr. Allergy Immunol. 2019, 30, 540–546. [Google Scholar] [CrossRef] [PubMed]
- VandeVusse, L.; Hanson, L.; Safdar, N. Perinatal Outcomes of Prenatal Probiotic and Prebiotic Administration. J. Perinat. Neonatal Nurs. 2013, 27, 288–301. [Google Scholar] [CrossRef]
- McDonald, B.; McCoy, K.D. Maternal microbiota in pregnancy and early life. Science 2019, 365, 984–985. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, D.A.; Peña Vélez, R.; Toro Monjaraz, E.M.; Ramirez Mayans, J.; MacDaragh Ryan, P. The Gut Microbiota: A Clinically Impactful Factor in Patient Health and Disease. SN Compr. Clin. Med. 2019, 1, 188–199. [Google Scholar] [CrossRef] [Green Version]
- Hanson, L.; VandeVusse, L.; Malloy, E.; Garnier-Villarreal, M.; Watson, L.; Fial, A.; Forgie, M.; Nerdini, K.; Safdar, N. Probiotic interventions to reduce antepartum Group B streptococcus colonization: A systematic review and meta-analysis. Midwifery 2022, 105, 103208. [Google Scholar] [CrossRef]
- Gray, K.D.; Messina, J.A.; Cortina, C.; Owens, T.; Fowler, M.; Foster, M.; Gbadegesin, S.; Clark, R.H.; Benjamin, D.K., Jr.; Zimmerman, K.O.; et al. Probiotic Use and Safety in the Neonatal Intensive Care Unit: A Matched Cohort Study. J. Pediatr. 2020, 222, 59–64.e1. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; The PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]


| Study | Country | Ethnicity | Probiotic/ Placebo | Intervention | Doses | Time |
|---|---|---|---|---|---|---|
| Ming-Ho, 2016 [32] | China | Asian | 49/50 | L. rhamnosus GR-1 and L. reuteri RC-14 | 2 × 109 CFU/day from 35–37 weeks until delivery | 2 weeks |
| Olsen, 2017 [33] | Australia | Caucasian | 21/13 | L. rhamnosus GR-1 and L. reuteri RC-14 | 1 × 108 CFU (108 viable strains) for three weeks/until delivery | 3 weeks |
| Aziz, 2018 [34] | USA | Caucasian Hispanic Other | 125/126 | L. rhamnosus GR-1 and L. reuteri RC-14 | 5.4 × 109 CFU daily in capsule started at 28 weeks | 12 weeks |
| Sharpe, 2019 [35] | Canada | Caucasian | 73/66 | L. rhamnosus GR-1 and L. reuteri RC-14 | 5 × 109 daily started at 23–25th week | 12 weeks |
| Farr, 2020 [36] | Austria | Caucasian | 33/27 | L. jensenii Lbv116; L. crispatus Lbv88; L. rhamnosus Lbv96; L. gasseri Lbv150 | 4 × 109 CFU daily oral intake started between 32–36 weeks | 2 weeks |
| Study | N | Maternal | Labor and Delivery | Intervention |
|---|---|---|---|---|
| Ming-Ho, 2016 [32] | 99 | Intrapartum fever: Placebo: 0/50 Probiotic: 1/49 (2.0%) | N/A | NICU admission Placebo: 0/50 Probiotic: 1/49 (2.0%) |
| Olsen, 2017 [33] | 34 | PTB Control: 0/13 Probiotic: 0/21 | Emergency CS Control: 5/13 (38.5%) Probiotic: 0/21 * | Neonatal allergies ª Control:0/13 Probiotic: 0/21 |
| Aziz, 2018 [34] | 251 | PTB Placebo: 3/121 (2.5%) Probiotic: 4/116 (3.5%) | Chorioamnionitis Placebo: 4/116 (3.5%) Probiotic: 5/113 (4.4%) | Neonatal infections Placebo: 2/121 (1.7%) Probiotic: 4/115 (3.5%) |
| Sharpe, 2019 [35] | 139 | N/A | Intrapartum infections Placebo: 3/56 (5.3%) Probiotic: 4/57 (7.0%) | NICU admission Placebo: 3/56 (5.3%) Probiotic: 0/57 |
| Farr, 2020 [36] | 60 | PTB Placebo: 1/41 (2.4%) Probiotic: 4/41 (9.8%) | Cesarean section Placebo: 22/41 (53.7%) Probiotic: 22/41 (53.7%) | Neonatal sepsis Placebo: 0/41 Probiotic: 0/41 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Menichini, D.; Chiossi, G.; Monari, F.; De Seta, F.; Facchinetti, F. Supplementation of Probiotics in Pregnant Women Targeting Group B Streptococcus Colonization: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 4520. https://doi.org/10.3390/nu14214520
Menichini D, Chiossi G, Monari F, De Seta F, Facchinetti F. Supplementation of Probiotics in Pregnant Women Targeting Group B Streptococcus Colonization: A Systematic Review and Meta-Analysis. Nutrients. 2022; 14(21):4520. https://doi.org/10.3390/nu14214520
Chicago/Turabian StyleMenichini, Daniela, Giuseppe Chiossi, Francesca Monari, Francesco De Seta, and Fabio Facchinetti. 2022. "Supplementation of Probiotics in Pregnant Women Targeting Group B Streptococcus Colonization: A Systematic Review and Meta-Analysis" Nutrients 14, no. 21: 4520. https://doi.org/10.3390/nu14214520
APA StyleMenichini, D., Chiossi, G., Monari, F., De Seta, F., & Facchinetti, F. (2022). Supplementation of Probiotics in Pregnant Women Targeting Group B Streptococcus Colonization: A Systematic Review and Meta-Analysis. Nutrients, 14(21), 4520. https://doi.org/10.3390/nu14214520






