Genome-Wide Transcriptional Profiling Reveals PHACTR1 as a Novel Molecular Target of Resveratrol in Endothelial Homeostasis
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Total RNA Extraction, RNA-Sequencing, and Quantitative Real-Time PCR
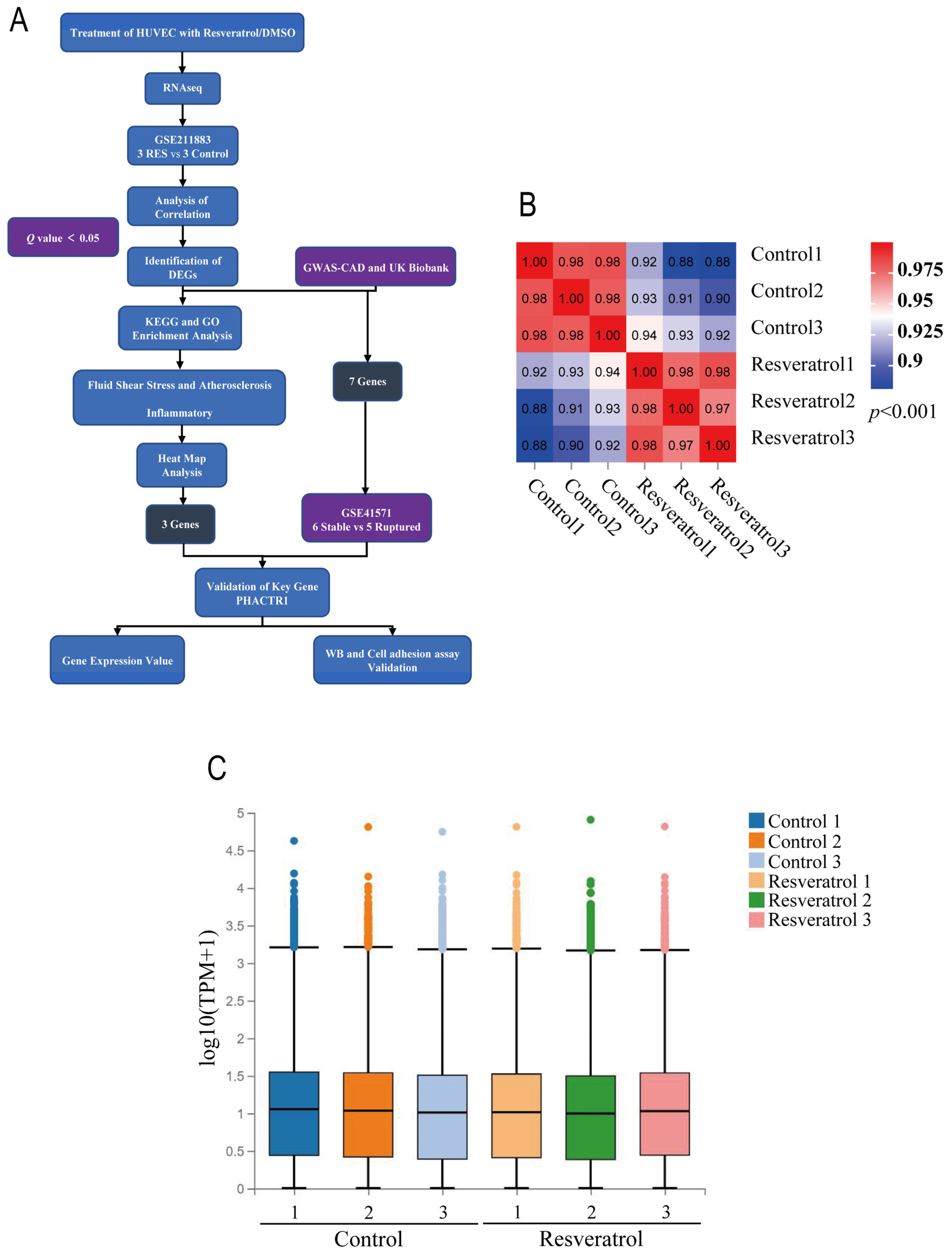
2.3. Test for Correlation and Variation of Samples
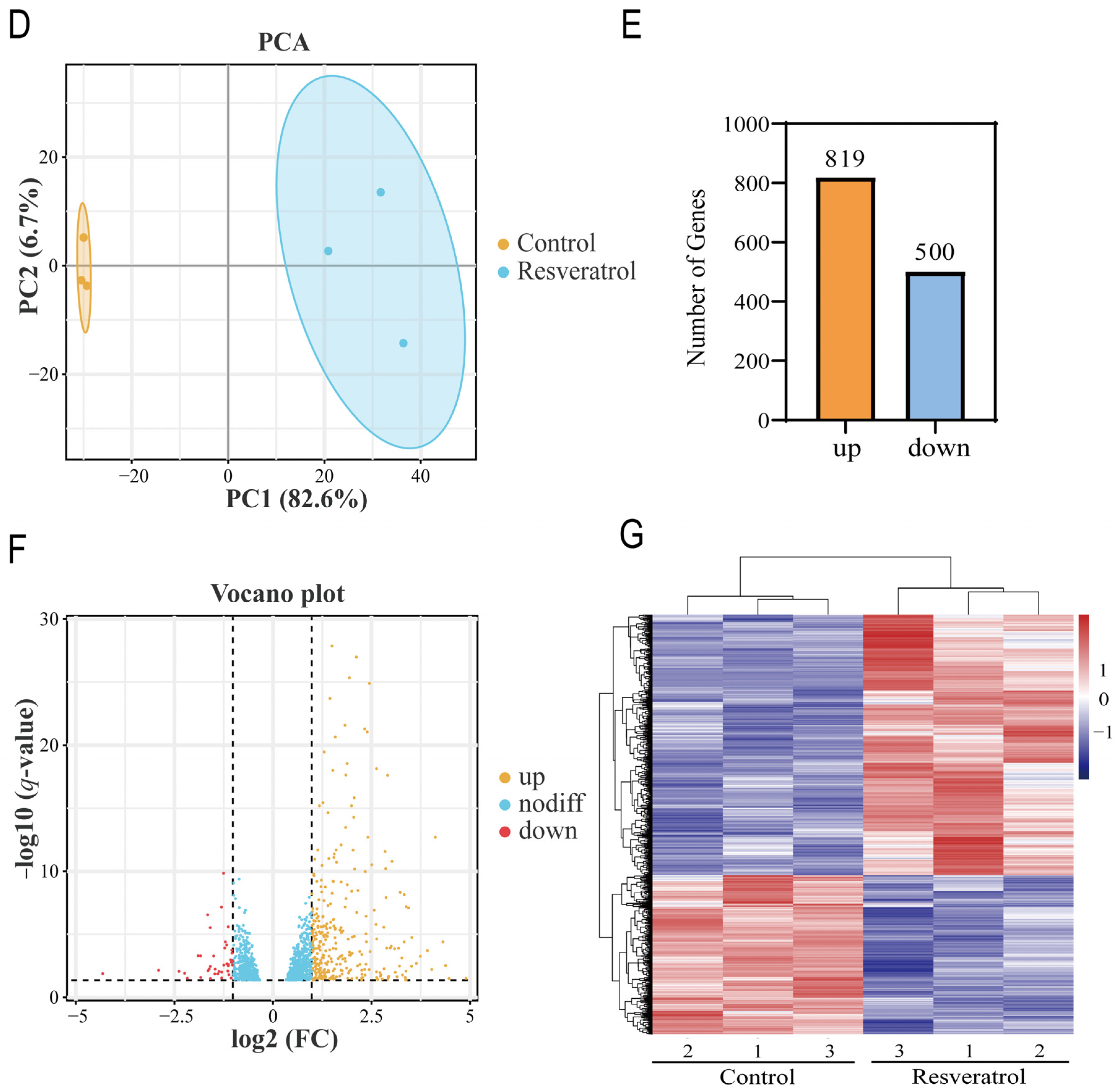
2.4. Identification of DEGs
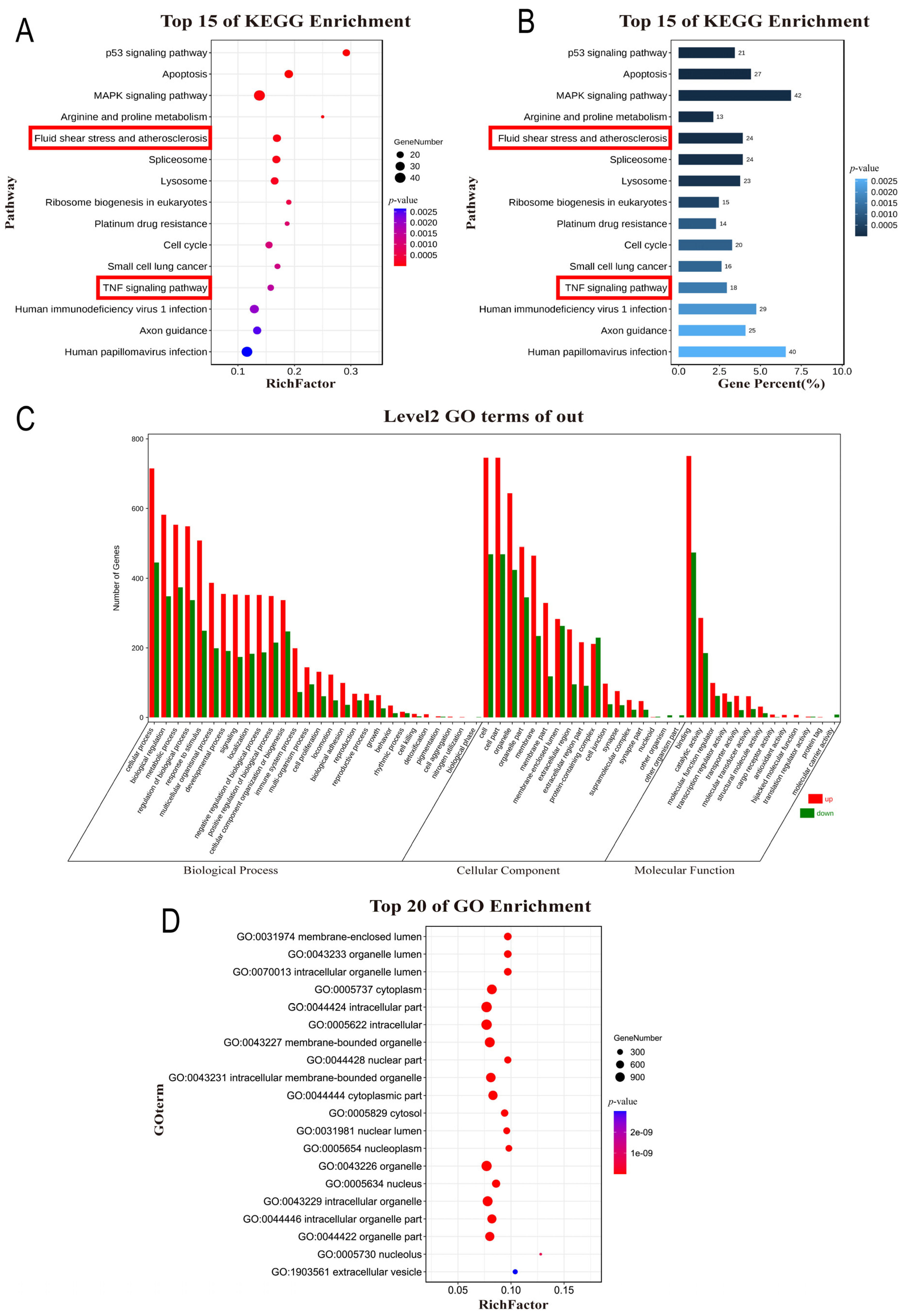
2.5. Pathway Enrichment Analysis (KEGG) and Gene Ontology (GO) Analysis
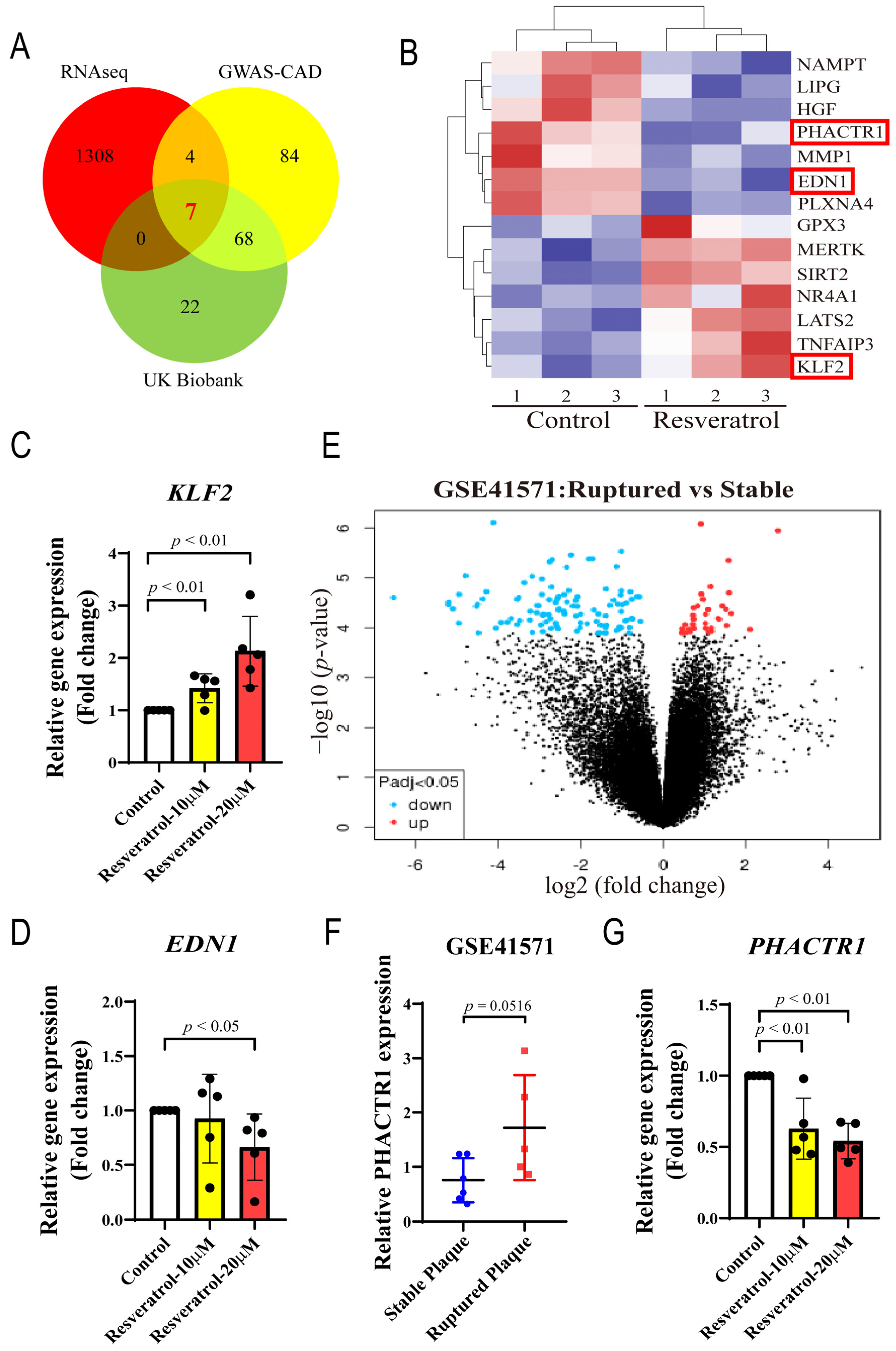
2.6. DEGs Analysis in Discovery Cohorts
2.7. Protein Extraction and Western Blotting Analysis
2.8. Statistical Analysis
3. Results
3.1. Dataset Validation and Identification of DEGs
3.2. Functional and Pathway Enrichment Analysis
3.3. Multi-Dataset Screening Showed That PHACTR1 Might Be Associated with the Vascular Protective Effects of RES
3.4. PHACTR1 Expression Was Downregulated in RES-Mediated Anti-Inflammatory Effects
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vos, T.; Lim, S.S.; Abbafati, C.; Abbas, K.M.; Abbasi, M.; Abbasifard, M.; Abbasi-Kangevari, M.; Abbastabar, H.; Abd-Allah, F.; Abdelalim, A.; et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef]
- Mensah, G.A.; Roth, G.A.; Fuster, V. The Global Burden of Cardiovascular Diseases and Risk Factors: 2020 and Beyond. J. Am. Coll. Cardiol. 2019, 74, 2529–2532. [Google Scholar] [CrossRef] [PubMed]
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update from the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef] [PubMed]
- Björkegren, J.L.M.; Lusis, A.J. Atherosclerosis: Recent developments. Cell 2022, 185, 1630–1645. [Google Scholar] [CrossRef]
- Xu, S.; Ilyas, I.; Little, P.J.; Li, H.; Kamato, D.; Zheng, X.; Luo, S.; Li, Z.; Liu, P.; Han, J.; et al. Endothelial Dysfunction in Atherosclerotic Cardiovascular Diseases and Beyond: From Mechanism to Pharmacotherapies. Pharmacol. Rev. 2021, 73, 924–967. [Google Scholar] [CrossRef] [PubMed]
- Xu, S. Therapeutic potential of blood flow mimetic compounds in preventing endothelial dysfunction and atherosclerosis. Pharmacol. Res. 2020, 155, 104737. [Google Scholar] [CrossRef]
- De la Lastra, C.A.; Villegas, I. Resveratrol as an anti-inflammatory and anti-aging agent: Mechanisms and clinical implications. Mol. Nutr. Food Res. 2005, 49, 405–430. [Google Scholar] [CrossRef]
- Öztürk, E.; Arslan, A.K.K.; Yerer, M.B.; Bishayee, A. Resveratrol and diabetes: A critical review of clinical studies. Biomed. Pharmacother. 2017, 95, 230–234. [Google Scholar] [CrossRef]
- Pan, M.-H.; Wu, J.-C.; Ho, C.-T.; Lai, C.-S. Antiobesity molecular mechanisms of action: Resveratrol and pterostilbene. Biofactors 2018, 44, 50–60. [Google Scholar] [CrossRef]
- Parsamanesh, N.; Asghari, A.; Sardari, S.; Tasbandi, A.; Jamialahmadi, T.; Xu, S.; Sahebkar, A. Resveratrol and endothelial function: A literature review. Pharmacol. Res. 2021, 170, 105725. [Google Scholar] [CrossRef]
- Su, M.; Zhao, W.; Xu, S.; Weng, J. Resveratrol in Treating Diabetes and Its Cardiovascular Complications: A Review of Its Mechanisms of Action. Antioxidants 2022, 11, 1085. [Google Scholar] [CrossRef] [PubMed]
- Sen-Banerjee, S.; Mir, S.; Lin, Z.; Hamik, A.; Atkins, G.B.; Das, H.; Banerjee, P.; Kumar, A.; Jain, M.K. Kruppel-like factor 2 as a novel mediator of statin effects in endothelial cells. Circulation 2005, 112, 720–726. [Google Scholar] [CrossRef] [PubMed]
- Parmar, K.M.; Nambudiri, V.; Dai, G.; Larman, H.B.; Gimbrone, M.A., Jr.; García-Cardeña, G. Statins exert endothelial atheroprotective effects via the KLF2 transcription factor. J. Biol. Chem. 2005, 280, 26714–26719. [Google Scholar] [CrossRef]
- Gupta, R.M.; Hadaya, J.; Trehan, A.; Zekavat, S.M.; Roselli, C.; Klarin, D.; Emdin, C.A.; Hilvering, C.R.; Bianchi, V.; Mueller, C.; et al. A Genetic Variant Associated with Five Vascular Diseases Is a Distal Regulator of Endothelin-1 Gene Expression. Cell 2017, 170, 522–533. [Google Scholar] [CrossRef] [PubMed]
- Debette, S.; International Stroke Genetics Consortium; Kamatani, Y.; Metso, T.M.; Kloss, M.; Chauhan, G.; Engelter, S.T.; Pezzini, A.; Thijs, V.; Markus, H.S.; et al. Common variation in PHACTR1 is associated with susceptibility to cervical artery dissection. Nat. Genet. 2015, 47, 78–83. [Google Scholar] [CrossRef]
- Kikuchi, N.; Moreland, E.; Homma, H.; Semenova, E.A.; Saito, M.; Larin, A.K.; Kobatake, N.; Yusupov, R.A.; Okamoto, T.; Nakazato, K.; et al. Genes and Weightlifting Performance. Genes 2021, 13, 25. [Google Scholar] [CrossRef]
- Ito, H.; Mizuno, M.; Noguchi, K.; Morishita, R.; Iwamoto, I.; Hara, A.; Nagata, K.-I. Expression analyses of Phactr1 (phosphatase and actin regulator 1) during mouse brain development. Neurosci. Res. 2018, 128, 50–57. [Google Scholar] [CrossRef]
- Allain, B.; Jarray, R.; Borriello, L.; Leforban, B.; Dufour, S.; Liu, W.-Q.; Pamonsinlapatham, P.; Bianco, S.; Larghero, J.; Hadj-Slimane, R.; et al. Neuropilin-1 regulates a new VEGF-induced gene, Phactr-1, which controls tubulogenesis and modulates lamellipodial dynamics in human endothelial cells. Cell. Signal. 2012, 24, 214–223. [Google Scholar] [CrossRef]
- Jarray, R.; Allain, B.; Borriello, L.; Biard, D.; Loukaci, A.; Larghero, J.; Hadj-Slimane, R.; Garbay, C.; Lepelletier, Y.; Raynaud, F. Depletion of the novel protein PHACTR-1 from human endothelial cells abolishes tube formation and induces cell death receptor apoptosis. Biochimie 2011, 93, 1668–1675. [Google Scholar] [CrossRef]
- Kasikara, C.; Schilperoort, M.; Gerlach, B.; Xue, C.; Wang, X.; Zheng, Z.; Kuriakose, G.; Dorweiler, B.; Zhang, H.; Fredman, G.; et al. Deficiency of macrophage PHACTR1 impairs efferocytosis and promotes atherosclerotic plaque necrosis. J. Clin. Investig. 2021, 131, e145275. [Google Scholar] [CrossRef]
- Rubin, S.; Bougaran, P.; Martin, S.; Abelanet, A.; Delobel, V.; Pernot, M.; Jeanningros, S.; Bats, M.-L.; Combe, C.; Dufourcq, P.; et al. PHACTR-1 (Phosphatase and Actin Regulator 1) Deficiency in Either Endothelial or Smooth Muscle Cells Does Not Predispose Mice to Nonatherosclerotic Arteriopathies in 3 Transgenic Mice. Arterioscler. Thromb. Vasc. Biol. 2022, 42, 597–609. [Google Scholar] [CrossRef] [PubMed]
- Aherrahrou, R.; Aherrahrou, Z.; Schunkert, H.; Erdmann, J. Coronary artery disease associated gene Phactr1 modulates severity of vascular calcification in vitro. Biochem. Biophys. Res. Commun. 2017, 491, 396–402. [Google Scholar] [CrossRef]
- Jarray, R.; Pavoni, S.; Borriello, L.; Allain, B.; Lopez, N.; Bianco, S.; Liu, W.-Q.; Biard, D.; Demange, L.; Hermine, O.; et al. Disruption of phactr-1 pathway triggers pro-inflammatory and pro-atherogenic factors: New insights in atherosclerosis development. Biochimie 2015, 118, 151–161. [Google Scholar] [CrossRef]
- Ma, X.; Su, M.; He, Q.; Zhang, Z.; Zhang, F.; Liu, Z.; Sun, L.; Weng, J.; Xu, S. PHACTR1, a coronary artery disease risk gene, mediates endothelial dysfunction. Front. Immunol. 2022, 13, 4716. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Xu, M.; Liu, Z.; Zhang, Z.; Liu, Y.; Luo, S.; Zheng, X.; Little, P.J.; Xu, S.; Weng, J. Pharmacological inhibition of IRAK1 and IRAK4 prevents endothelial inflammation and atherosclerosis in ApoE(-)/(-) mice. Pharmacol. Res. 2022, 175, 106043. [Google Scholar] [CrossRef]
- Onat, D.; Brillon, D.; Colombo, P.C.; Schmidt, A.M. Human Vascular Endothelial Cells: A Model System for Studying Vascular Inflammation in Diabetes and Atherosclerosis. Curr. Diabetes Rep. 2011, 11, 193–202. [Google Scholar] [CrossRef]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene Ontology: Tool for the unification of biology. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, D.; Zhang, H. Investigation of the Underlying Genes and Mechanism of Macrophage-Enriched Ruptured Atherosclerotic Plaques Using Bioinformatics Method. J. Atheroscler. Thromb. 2019, 26, 636–658. [Google Scholar] [CrossRef]
- Lee, K.; Santibanez-Koref, M.; Polvikoski, T.; Birchall, D.; Mendelow, A.D.; Keavney, B. Increased expression of fatty acid binding protein 4 and leptin in resident macrophages characterises atherosclerotic plaque rupture. Atherosclerosis 2013, 226, 74–81. [Google Scholar] [CrossRef]
- Tcheandjieu, C.; Zhu, X.; Hilliard, A.T.; Clarke, S.L.; Napolioni, V.; Ma, S.; Lee, K.M.; Fang, H.; Chen, F.; Lu, Y.; et al. Large-scale genome-wide association study of coronary artery disease in genetically diverse populations. Nat. Med. 2022, 28, 1679–1692. [Google Scholar] [CrossRef] [PubMed]
- Klarin, D.; Zhu, Q.M.; Emdin, C.A.; Chaffin, M.; Horner, S.; McMillan, B.J.; Leed, A.; Weale, M.; Spencer, C.C.A.; Aguet, F.; et al. Genetic analysis in UK Biobank links insulin resistance and transendothelial migration pathways to coronary artery disease. Nat. Genet. 2017, 49, 1392. [Google Scholar] [CrossRef] [PubMed]
- Santos, L.M.; Rodrigues, D.; Alemi, M.; Silva, S.C.; Ribeiro, C.A.; Cardoso, I. Resveratrol administration increases Transthyretin protein levels ameliorating AD features- importance of transthyretin tetrameric stability. Mol. Med. 2016, 22, 597–607. [Google Scholar] [CrossRef]
- Das, H.; Kumar, A.; Lin, Z.; Patino, W.D.; Hwang, P.M.; Feinberg, M.W.; Majumder, P.K.; Jain, M.K. Kruppel-like factor 2 (KLF2) regulates proinflammatory activation of monocytes. Proc. Natl. Acad. Sci. USA 2006, 103, 6653–6658. [Google Scholar] [CrossRef]
- Chu, H.; Li, H.; Guan, X.; Yan, H.; Zhang, X.; Cui, X.; Li, X.; Cheng, M. Resveratrol protects late endothelial progenitor cells from TNF--induced inflammatory damage by upregulating Kruppel-like factor-2. Mol. Med. Rep. 2018, 17, 5708–5715. [Google Scholar]
- Ihling, C.; Szombathy, T.; Bohrmann, B.; Brockhaus, M.; Schaefer, H.E.; Loeffler, B.M. Coexpression of endothelin-converting enzyme-1 and endothelin-1 in different stages of human atherosclerosis. Circulation 2001, 104, 864–869. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Park, K.; Li, C.; Rask-Madsen, C.; Mima, A.; Qi, W.; Mizutani, K.; Huang, P.; King, G.L. Induction of Vascular Insulin Resistance and Endothelin-1 Expression and Acceleration of Atherosclerosis by the Overexpression of Protein Kinase C- Isoform in the Endothelium. Circ. Res. 2013, 113, 418–427. [Google Scholar] [CrossRef]
- El-Mowafy, A.M.; White, R.E. Resveratrol inhibits MAPK activity and nuclear translocation in coronary artery smooth muscle: Reversal of endothelin-1 stimulatory effects. FEBS Lett. 1999, 451, 63–67. [Google Scholar] [CrossRef]
- Liu, J.-C.; Chen, J.-J.; Chan, P.; Cheng, C.-F.; Cheng, T.-H. Inhibition of cyclic strain-induced endothelin-1 gene expression by resveratrol. Hypertension 2003, 42, 1198–1205. [Google Scholar] [CrossRef]
- Mueller, P.A.; Zhu, L.; Tavori, H.; Huynh, K.; Giunzioni, I.; Stafford, J.M.; Linton, M.F.; Fazio, S. Deletion of Macrophage Low-Density Lipoprotein Receptor-Related Protein 1 (LRP1) Accelerates Atherosclerosis Regression and Increases C-C Chemokine Receptor Type 7 (CCR7) Expression in Plaque Macrophages. Circulation 2018, 138, 1850–1863. [Google Scholar] [CrossRef]
- Yancey, P.G.; Ding, Y.; Fan, D.; Blakemore, J.L.; Zhang, Y.; Ding, L.; Zhang, J.; Linton, M.F.; Fazio, S. Low-Density Lipoprotein Receptor-Related Protein 1 Prevents Early Atherosclerosis by Limiting Lesional Apoptosis and Inflammatory Ly-6C(high) Monocytosis Evidence That the Effects Are Not Apolipoprotein E Dependent. Circulation 2011, 124, 454–464. [Google Scholar] [CrossRef] [PubMed]
- Mao, H.; Lockyer, P.; Li, L.; Ballantyne, C.M.; Patterson, C.; Xie, L.; Pi, X. Endothelial LRP1 regulates metabolic responses by acting as a co-activator of PPAR gamma. Nat. Commun. 2017, 8, 14960. [Google Scholar] [CrossRef]
- Xiao, L.; Shi, D.; Zhang, H.; Zhang, Y.; Liu, Y.; Lu, H.; Zheng, Y. Association between single nucleotide polymorphism rs11057401 of CCDC92 gene and the risk of coronary heart disease (CHD). Lipids Health Dis. 2018, 17, 28. [Google Scholar] [CrossRef] [PubMed]
- Johnston, J.M.; Angyal, A.; Bauer, R.C.; Hamby, S.; Suvarna, S.K.; Baidžajevas, K.; Hegedus, Z.; Dear, T.N.; Turner, M.; Wilson, H.L.; et al. Myeloid Tribbles 1 induces early atherosclerosis via enhanced foam cell expansion. Sci. Adv. 2019, 5, eaax9183. [Google Scholar] [CrossRef] [PubMed]
- Mallat, Z.; Gojova, A.; Marchiol-Fournigault, C.; Esposito, B.; Kamaté, C.; Merval, R.; Fradelizi, D.; Tedgui, A. Inhibition of transforming growth factor-beta signaling accelerates atherosclerosis and induces an unstable plaque phenotype in mice. Circ. Res. 2001, 89, 930–934. [Google Scholar] [CrossRef]
- Groyer, E.; Nicoletti, A.; Ait-Oufella, H.; Khallou-Laschet, J.; Varthaman, A.; Gaston, A.-T.; Thaunat, O.; Kaveri, S.V.; Blatny, R.; Stockinger, H.; et al. Atheroprotective effect of CD31 receptor globulin through enrichment of circulating regulatory T-cells. J. Am. Coll. Cardiol. 2007, 50, 344–350. [Google Scholar] [CrossRef]
- Vendrov, A.E.; Madamanchi, N.R.; Hakim, Z.S.; Rojas, M.; Runge, M.S. Thrombin and NAD(P)H oxidase-mediated regulation of CD44 and BMP4-Id pathway in VSMC, restenosis, and atherosclerosis. Circ. Res. 2006, 98, 1254–1263. [Google Scholar] [CrossRef]
- Zhou, J.; Werstuck, G.H.; Lhoták, S.; de Koning, A.B.L.; Sood, S.K.; Hossain, G.S.; Møller, J.; Ritskes-Hoitinga, M.; Falk, E.; Dayal, S.; et al. Association of multiple cellular stress pathways with accelerated atherosclerosis in hyperhomocysteinemic apolipoprotein E-deficient mice. Circulation 2004, 110, 207–213. [Google Scholar] [CrossRef]
- Rocnik, E.; Chow, L.H.; Pickering, J.G. Heat shock protein 47 is expressed in fibrous regions of human atheroma and is regulated by growth factors and oxidized low-density lipoprotein. Circulation 2000, 101, 1229–1233. [Google Scholar] [CrossRef]
- Adlam, D.; Olson, T.M.; Combaret, N.; Kovacic, J.C.; Iismaa, S.E.; Al-Hussaini, A.; O’Byrne, M.M.; Bouajila, S.; Georges, A.; Mishra, K.; et al. Association of the PHACTR1/EDN1 Genetic Locus With Spontaneous Coronary Artery Dissection. J. Am. Coll. Cardiol. 2019, 73, 58–66. [Google Scholar] [CrossRef]
- Pendurthi, U.R.; Rao, L.V.M. Resveratrol suppresses agonist-induced monocyte adhesion to cultured human endothelial cells. Thromb. Res. 2002, 106, 243–248. [Google Scholar] [CrossRef]
- Shyy, Y.J.; Wickham, L.L.; Hagan, J.P.; Hsieh, H.J.; Hu, Y.L.; Telian, S.H. Human monocyte colony-stimulating factor stimulates the gene expression of monocyte chemotactic protein-1 and increases the adhesion of monocytes to endothelial monolayers. J. Clin. Investig. 1993, 92, 1745–1751. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.-M.; Chin, Y.-T.; Ho, Y.; Chen, Y.-R.; Yang, Y.-N.; Yang, Y.-C.; Shih, Y.-J.; Lin, T.-I.; Lin, H.-Y.; Davis, P.J. Resveratrol induces sumoylated COX-2-dependent anti-proliferation in human prostate cancer LNCaP cells. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2018, 112, 67–75. [Google Scholar] [CrossRef]
- Chong, E.; Chang, S.-L.; Hsiao, Y.-W.; Singhal, R.; Liu, S.-H.; Leha, T.; Lin, W.-Y.; Hsu, C.-P.; Chen, Y.-C.; Chen, Y.-J.; et al. Resveratrol, a red wine antioxidant, reduces atrial fibrillation susceptibility in the failing heart by PI3K/AKT/eNOS signaling pathway activation. Heart Rhythm 2015, 12, 1046–1056. [Google Scholar] [CrossRef] [PubMed]
- Taubert, D.; Berkels, R. Upregulation and activation of eNOS by resveratrol. Circulation 2003, 107, e78–e79. [Google Scholar] [CrossRef]
- Gracia-Sancho, J.; Villarreal, G., Jr.; Zhang, Y.; García-Cardeña, G. Activation of SIRT1 by resveratrol induces KLF2 expression conferring an endothelial vasoprotective phenotype. Cardiovasc. Res. 2010, 85, 514–519. [Google Scholar] [CrossRef]
- Luo, J.-Y.; Cheng, C.K.; He, L.; Pu, Y.; Zhang, Y.; Lin, X.; Xu, A.; Lau, C.W.; Tian, X.Y.; Ma, R.C.W.; et al. Endothelial UCP2 Is a Mechanosensitive Suppressor of Atherosclerosis. Circ. Res. 2022, 131, 424–441. [Google Scholar] [CrossRef]
- Dembic, M.; Andersen, H.S.; Bastin, J.; Doktor, T.K.; Corydon, T.J.; Sass, J.O.; Costa, A.L.; Djouadi, F.; Andresen, B.S. Next generation sequencing of RNA reveals novel targets of resveratrol with possible implications for Canavan disease. Mol. Genet. Metab. 2019, 126, 64–76. [Google Scholar] [CrossRef]
- Wang, X.L.; Greco, M.; Sim, A.S.; Duarte, N.; Wang, J.; Wilcken, D.E. Effect of CYP1A1 MspI polymorphism on cigarette smoking related coronary artery disease and diabetes. Atherosclerosis 2002, 162, 391–397. [Google Scholar] [CrossRef]
- Ciolino, H.P.; Daschner, P.J.; Yeh, G.C. Resveratrol inhibits transcription of CYP1A1 in vitro by preventing activation of the aryl hydrocarbon receptor. Cancer Res. 1998, 58, 5707–5712. [Google Scholar]
- Hsieh, T.C.; Juan, G.; Darzynkiewicz, Z.; Wu, J.M. Resveratrol increases nitric oxide synthase, induces accumulation of p53 and p21(WAF1/CIP1), and suppresses cultured bovine pulmonary artery endothelial cell proliferation by perturbing progression through S and G2. Cancer Res. 1999, 59, 2596–2601. [Google Scholar] [PubMed]
- She, Q.B.; Bode, A.M.; Ma, W.Y.; Chen, N.Y.; Dong, Z. Resveratrol-induced activation of p53 and apoptosis is mediated by extracellular-signal-regulated protein kinases and p38 kinase. Cancer Res. 2001, 61, 1604–1610. [Google Scholar] [PubMed]
- Zhang, Y.; Guo, L.; Law, B.Y.; Liang, X.; Ma, N.; Xu, G.; Wang, X.; Yuan, X.; Tang, H.; Chen, Q.; et al. Resveratrol decreases cell apoptosis through inhibiting DNA damage in bronchial epithelial cells. Int. J. Mol. Med. 2020, 45, 1673–1684. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Xue, Y.; Chen, H.; Meng, L.; Chen, B.; Gong, H.; Zhao, Y.; Qi, R. Resveratrol Inhibits MMP3 and MMP9 Expression and Secretion by Suppressing TLR4/NF-B/STAT3 Activation in Ox-LDL-Treated HUVECs. Oxidative Med. Cell. Longev. 2019, 2019, 9013169. [Google Scholar] [CrossRef] [PubMed]
- Mattison, J.A.; Wang, M.; Bernier, M.; Zhang, J.; Park, S.-S.; Maudsley, S.; An, S.S.; Santhanam, L.; Martin, B.; Faulkner, S.; et al. Resveratrol prevents high fat/sucrose diet-induced central arterial wall inflammation and stiffening in nonhuman primates. Cell Metab. 2014, 20, 183–190. [Google Scholar] [CrossRef]
- Kaneko, H.; Anzai, T.; Morisawa, M.; Kohno, T.; Nagai, T.; Anzai, A.; Takahashi, T.; Shimoda, M.; Sasaki, A.; Maekawa, Y.; et al. Resveratrol prevents the development of abdominal aortic aneurysm through attenuation of inflammation, oxidative stress, and neovascularization. Atherosclerosis 2011, 217, 350–357. [Google Scholar] [CrossRef]
- Deng, Y.H.; Alex, D.; Huang, H.Q.; Wang, N.; Yu, N.; Wang, Y.T.; Leung, G.P.H.; Lee, S.M.Y. Inhibition of TNF-α-mediated endothelial cell-monocyte cell adhesion and adhesion molecules expression by the resveratrol derivative, trans-3,5,4’-trimethoxystilbene. Phytother. Res. PTR 2011, 25, 451–457. [Google Scholar] [CrossRef]
- Zhang, Z.; Jiang, F.; Zeng, L.; Wang, X.; Tu, S. PHACTR1 regulates oxidative stress and inflammation to coronary artery endothelial cells via interaction with NF-κB/p65. Atherosclerosis 2018, 278, 180–189. [Google Scholar] [CrossRef]
- Gresele, P.; Pignatelli, P.; Guglielmini, G.; Carnevale, R.; Mezzasoma, A.M.; Ghiselli, A.; Momi, S.; Violi, F. Resveratrol, at concentrations attainable with moderate wine consumption, stimulates human platelet nitric oxide production. J. Nutr. 2008, 138, 1602–1608. [Google Scholar] [CrossRef]
- Marques, B.; Trindade, M.; Aquino, J.C.F.; Cunha, A.R.; Gismondi, R.O.; Neves, M.F.; Oigman, W. Beneficial effects of acute trans-resveratrol supplementation in treated hypertensive patients with endothelial dysfunction. Clin. Exp. Hypertens. 2018, 40, 218–223. [Google Scholar] [CrossRef]
- Tomé-Carneiro, J.; Larrosa, M.; Yáñez-Gascón, M.J.; Dávalos, A.; Gil-Zamorano, J.; Gonzálvez, M.; García-Almagro, F.J.; Ruiz Ros, J.A.; Tomás-Barberána, F.A.; Espin, J.C.; et al. One-year supplementation with a grape extract containing resveratrol modulates inflammatory-related microRNAs and cytokines expression in peripheral blood mononuclear cells of type 2 diabetes and hypertensive patients with coronary artery disease. Pharmacol. Res. 2013, 72, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Samsami-Kor, M.; Daryani, N.E.; Asl, P.R.; Hekmatdoost, A. Anti-Inflammatory Effects of Resveratrol in Patients with Ulcerative Colitis: A Randomized, Double-Blind, Placebo-controlled Pilot Study. Arch. Med. Res. 2015, 46, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Movahed, A.; Raj, P.; Nabipour, I.; Mahmoodi, M.; Ostovar, A.; Kalantarhormozi, M.; Netticadan, T. Efficacy and Safety of Resveratrol in Type 1 Diabetes Patients: A Two-Month Preliminary Exploratory Trial. Nutrients 2020, 12, 161. [Google Scholar] [CrossRef] [PubMed]
- Imamura, H.; Yamaguchi, T.; Nagayama, D.; Saiki, A.; Shirai, K.; Tatsuno, I. Resveratrol Ameliorates Arterial Stiffness Assessed by Cardio-Ankle Vascular Index in Patients With Type 2 Diabetes Mellitus. Int. Heart J. 2017, 58, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Ford, T.J.; Corcoran, D.; Padmanabhan, S.; Aman, A.; Rocchiccioli, P.; Good, R. Genetic dysregulation of endothelin-1 is implicated in coronary microvascular dysfunction. Eur. Heart J. 2020, 41, 3239–3252. [Google Scholar] [CrossRef]

| Gene Name | Gene Symbol | q-Value | Log2 (FC) |
|---|---|---|---|
| DEGs (upregulated) | |||
| Solute Carrier Family 52 Member 1 | SLC52A1 | 9.02 × 10−9 | 8.310 |
| Epsin 3 | EPN3 | 7.20 × 10−7 | 7.721 |
| Grainyhead-like Transcription Factor 3 | GRHL3 | 2.85 × 10−6 | 7.538 |
| Nectin Cell Adhesion Molecule 4 | NECTIN4 | 3.10 × 10−5 | 7.022 |
| Ras Related Glycolysis Inhibitor and Calcium Channel Regulator | RRAD | 1.66 × 10−27 | 6.789 |
| Triggering Receptor Expressed on Myeloid Cell-like 1 | TREML1 | 5.30 × 10−4 | 6.495 |
| Transmembrane Protein 40 | TMEM40 | 1.45 × 10−3 | 6.382 |
| Lipocalin 15 | LCN15 | 3.04 × 10−3 | 6.157 |
| Dorsal Inhibitory Axon Guidance Protein | DRAXIN | 2.17 × 10−49 | 6.147 |
| Glutaminase 2 | GLS2 | 2.23 × 10−3 | 5.824 |
| DEGs (downregulated) | |||
| Cytochrome P450 Family 1 Subfamily A Member 1 | CYP1A1 | 1.46 × 10−25 | −6.700 |
| Hepatocyte Growth Factor | HGF | 1.47 × 10−2 | −4.303 |
| Gap Junction Protein Alpha 5 | GJA5 | 8.07 × 10−3 | −2.885 |
| Shisa Family Member 2 | SHISA2 | 9.72 × 10−3 | −2.368 |
| 5-Hydroxytryptamine Receptor 2B | HTR2B | 1.40 × 10−2 | −2.225 |
| Dynein Axonemal Heavy Chain 8 | DNAH8 | 3.45 × 10−2 | −2.155 |
| Vasoactive Intestinal Peptide Receptor 1 | VIPR1 | 5.58 × 10−4 | −1.879 |
| Tolloid-like 1 | TLL1 | 5.68 × 10−4 | −1.819 |
| Matrix Metallopeptidase 1 | MMP1 | 3.04 × 10−2 | −1.814 |
| 4-Hydroxyphenylpyruvate Dioxygenase-like | HPDL | 3.17 × 10−7 | −1.640 |
| Term | Count | Gene | q-Value |
|---|---|---|---|
| p53 signaling pathway | 21 | PMAIP1, FAS, THBS1, BCL2L1, SESN2, SESN1, TP53I3, CCNB1, GADD45A, CD82, PPM1D, CDKN1A, DDB2, BBC3, BAX, AIFM2, TP53, CCND3, CDK2, PIDD1, RRM2 | 7.65 × 10−6 |
| Apoptosis | 27 | CSF2RB, PMAIP1, FOS, FAS, ITPR3, BCL2L1, CTSZ, BIRC3, GADD45A, TRAF1, ITPR2, BBC3, BAX, CTSV, NFKBIA, LMNB2, RIPK1, TP53, CTSC, CTSD, PARP3, DFFB, DDIT3, PIDD1, JUN, AKT3, NRAS | 5.82 × 10−4 |
| MAPK signaling pathway | 42 | PDGFA, RAC2, FOS, FAS, NF1, RPS6KA1, HGF, EPHA2, ARRB1, PTPRR, CACNA2D1, PGF, NFKB2, HSPA8, PLA2G4C, GADD45A, KITLG, MAP3K4, RELB, DUSP1, DUSP5, RASA1, MAPK8IP1, JUND, CSF1, RRAS, FGFR3, IL1A, FLNC, TP53, TGFB1, PLA2G4A, ANGPT2, NR4A1, PRKACB, ARRB2, TAOK1, DDIT3, JUN, AKT3, NRAS, PTPN7 | 4.86 × 10−3 |
| Arginine and proline metabolism | 13 | SMOX, ALDH4A1, CKB, SMS, ALDH7A1, SAT1, AMD1, GAMT, ALDH18A1, CARNS1, P4HA2, PYCR1, SRM | 5.54 × 10−3 |
| Fluid shear stress and atherosclerosis | 24 | PDGFA, RAC2, EDN1, FOS, SDC4, GSTT2B, HSP90AB1, CYBA, GSTM2, VCAM1, PTK2, DUSP1, KLF2, SQSTM1, ICAM1, TRPV4, GPC1, IL1A, TP53, PECAM1, RHOC, SDC1, JUN, AKT3 | 5.54 × 10−3 |
| Spliceosome | 24 | GGT1, TRA2A, PPIL1, SNRPB, DHX15, PRPF19, SRSF10, SRSF3, HSPA8, HNRNPK, SRSF7, TRA2B, HNRNPC, DDX39B, HNRNPM, SNRPA1, SRSF2, SRSF1, HNRNPA1, HNRNPU, TCERG1, DDX5, PRPF40A, HNRNPA3 | 5.54 × 10−3 |
| Lysosome | 23 | NPC1, HYAL1, GUSB, NAGA, LAPTM5, LGMN, CTSZ, NPC2, ARSA, ABCA2, LAMP3, CTSV, PSAP, NEU1, AP3B1, CD68, MAN2B1, CTSC, CTSD, ABCB9, GBA, TPP1, FUCA1 | 8.19 × 10−3 |
| Ribosome biogenesis in eukaryotes | 15 | XRN2, NOP56, BMS1, NAT10, GNL3, DKC1, IMP4, RAN, WDR3, NHP2, LSG1, WDR43, GAR1, HEATR1, XPO1 | 2.13 × 10−2 |
| Platinum drug resistance | 14 | PMAIP1, FAS, BCL2L1, GSTT2B, BIRC3, BRCA1, GSTM2, CDKN1A, BBC3, BAX, POLH, TP53, TOP2B, AKT3 | 3.43 × 10−2 |
| Cell cycle | 20 | ORC6, CDC6, PCNA, CDC27, PKMYT1, CCNB1, STAG1, GADD45A, CDKN1A, MCM6, PLK1, E2F4, TP53, TGFB1, DBF4, CUL1, CCND3, CDK2, YWHAE, CDC20 | 3.47 × 10−2 |
| Gene Symbol | Log2 (FC) | q-Value | Function | Refs. |
|---|---|---|---|---|
| LRP1 | 1.757 | 8.32 × 10−6 | (+/−) Atherosclerosis (−) CCR7 (+) PPARγ | [40,41,42] |
| CCDC92 | 0.813 | 8.32 × 10−6 | (+) insulin resistance | [32,43] |
| TRIB1 | 0.679 | 4.36 × 10−2 | (+) OLR1 (+) oxLDL uptake (+) the formation of lipid-laden foam cells | [44] |
| TGFB1 | 0.556 | 2.08 × 10−2 | (+) Atherosclerosis | [45] |
| PECAM1 | 0.403 | 4.88 × 10−2 | (−) blood T-cell activation | [46] |
| SERPINH1 | −0.387 | 2.90 × 10−2 | relate to Atherosclerosis | [47,48,49] |
| PHACTR1 | −0.950 | 2.01 × 10−2 | (+) EDN1 | [14,50] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, M.; Zhao, W.; Li, Y.; Li, H.; Xu, S.; Weng, J. Genome-Wide Transcriptional Profiling Reveals PHACTR1 as a Novel Molecular Target of Resveratrol in Endothelial Homeostasis. Nutrients 2022, 14, 4518. https://doi.org/10.3390/nu14214518
Su M, Zhao W, Li Y, Li H, Xu S, Weng J. Genome-Wide Transcriptional Profiling Reveals PHACTR1 as a Novel Molecular Target of Resveratrol in Endothelial Homeostasis. Nutrients. 2022; 14(21):4518. https://doi.org/10.3390/nu14214518
Chicago/Turabian StyleSu, Meiming, Wenqi Zhao, Yujie Li, Hong Li, Suowen Xu, and Jianping Weng. 2022. "Genome-Wide Transcriptional Profiling Reveals PHACTR1 as a Novel Molecular Target of Resveratrol in Endothelial Homeostasis" Nutrients 14, no. 21: 4518. https://doi.org/10.3390/nu14214518
APA StyleSu, M., Zhao, W., Li, Y., Li, H., Xu, S., & Weng, J. (2022). Genome-Wide Transcriptional Profiling Reveals PHACTR1 as a Novel Molecular Target of Resveratrol in Endothelial Homeostasis. Nutrients, 14(21), 4518. https://doi.org/10.3390/nu14214518







