Resveratrol in Liquor Exacerbates Alcoholic Liver Injury with a Reduced Therapeutic Effect in Mice: An Unsupervised Herbal Wine Habit Is Risky
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. RSV Treatment in ALD Mice
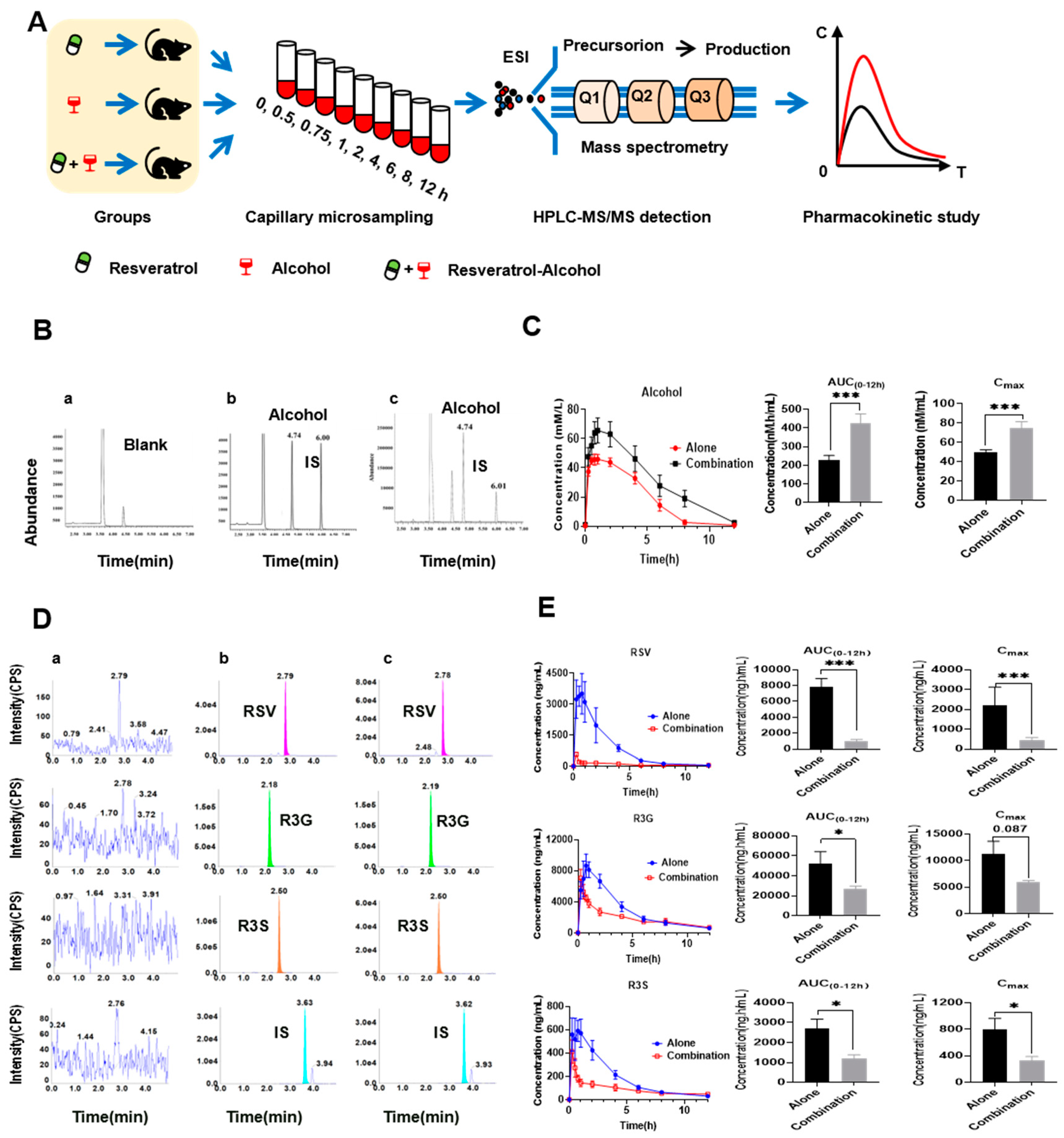
2.3. Pharmacokinetics of RSV and Alcohol in Mice
2.4. Cell Culture and Viability Assay
2.5. Liver Function Analysis
2.6. Liver Histology
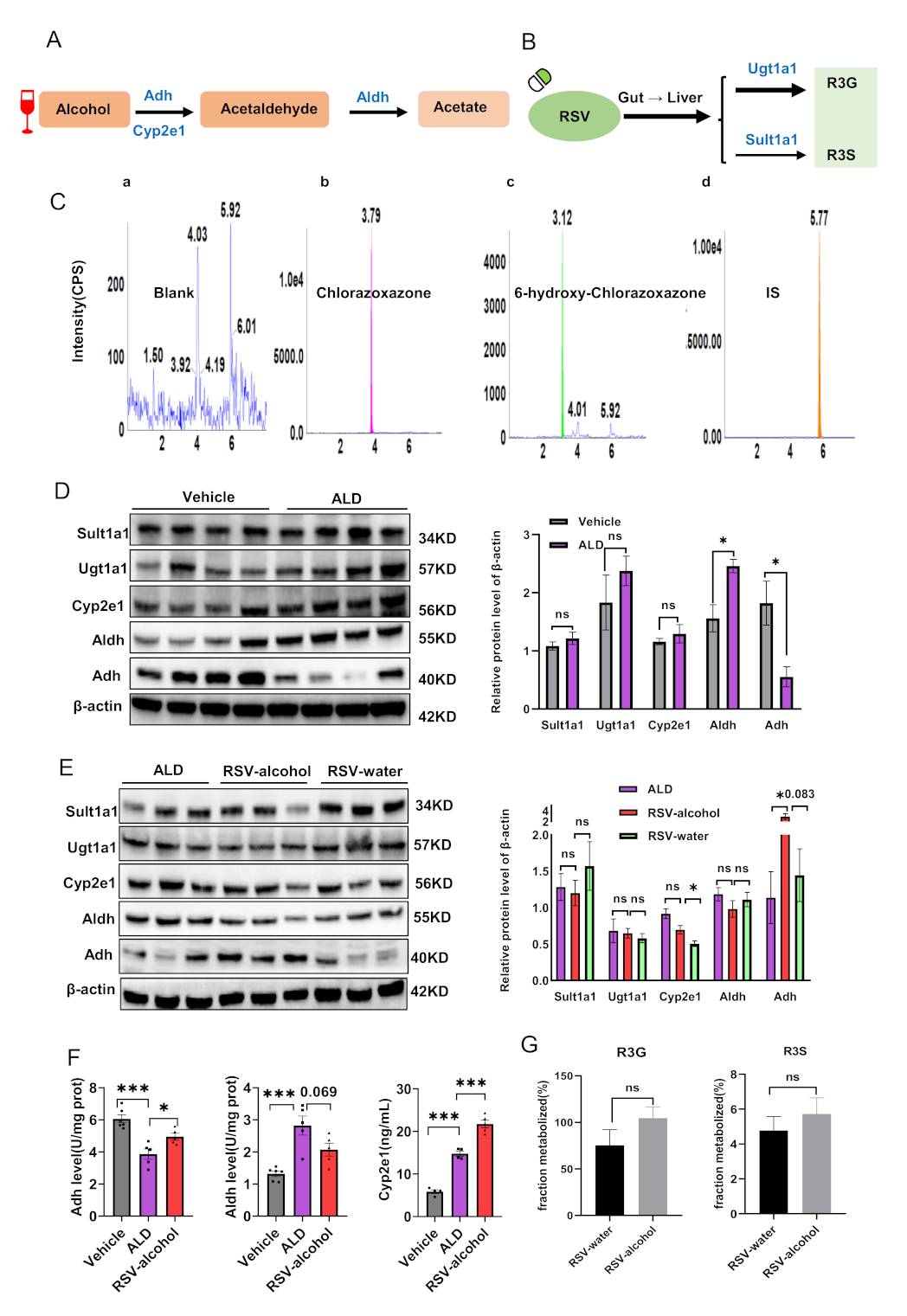
2.7. Enzymatic Activities of Adh, Aldh and Cyp2e1
2.8. Immunoblotting
2.9. Statistical Analysis
3. Results
3.1. Development of ALD in Mice
3.2. RSV-Alcohol Treatment Worsened ALD in Mice
3.3. Increased Cytotoxicity with RSV-Alcohol Combination Treatment
3.4. Pharmacokinetic Interaction between RSV and Alcohol
3.5. Activity and Expression of Alcohol- and RSV-Metabolising Enzymes in ALD Mice
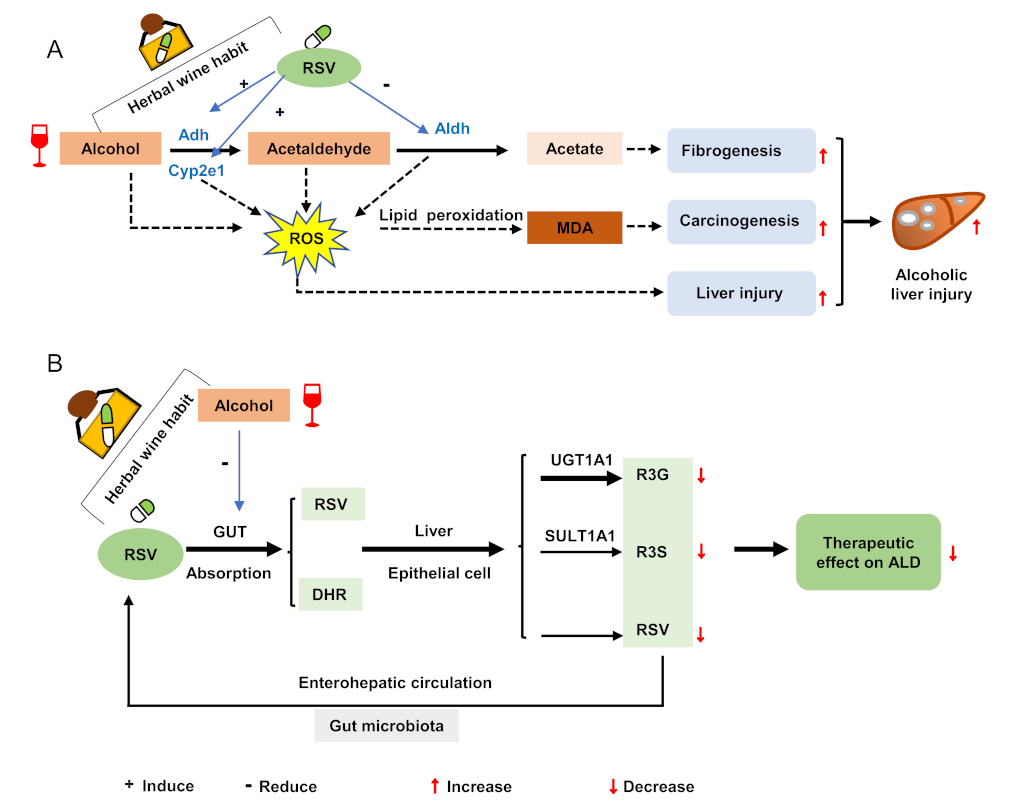
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Seitz, H.K.; Bataller, R.; Cortez-Pinto, H.; Gao, B.; Gual, A.; Lackner, C.; Mathurin, P.; Mueller, S.; Szabo, G.; Tsukamoto, H. Alcoholic liver disease. Nat. Rev. Dis. Primers. 2018, 4, 16. [Google Scholar] [CrossRef] [PubMed]
- Rehm, J.; Mathers, C.; Popova, S.; Thavorncharoensap, M.; Teerawattananon, Y.; Patra, J. Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. Lancet 2009, 373, 2223–2233. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, D.; Lin, X.X.; Zhao, Q.; Guo, J.; Chen, L.J.; Zhang, W.; Xiao, J.; Lian, G.H.; Peng, S.F.; et al. The LRP6 functional mutation rs2302685 contributes to individual susceptibility to alcoholic liver injury related to the Wnt/beta-catenin-TCF1-CYP2E1 signaling pathway. Arch. Toxicol. 2019, 93, 1679–1695. [Google Scholar] [CrossRef] [PubMed]
- Avila, M.A.; Dufour, J.F.; Gerbes, A.L.; Zoulim, F.; Bataller, R.; Burra, P.; Cortez-Pinto, H.; Gao, B.; Gilmore, I.; Mathurin, P.; et al. Recent advances in alcohol-related liver disease (ALD): Summary of a Gut round table meeting. Gut 2020, 69, 764–780. [Google Scholar] [CrossRef] [PubMed]
- GBD 2016 Alcohol Collaborators. Alcohol use and burden for 195 countries and territories, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2018, 392, 1015–1035. [Google Scholar] [CrossRef]
- Rumgay, H.; Shield, K.; Charvat, H.; Ferrari, P.; Sornpaisarn, B.; Obot, I.; Islami, F.; Lemmens, V.E.P.P.; Rehm, J.; Soerjomataram, I. Global burden of cancer in 2020 attributable to alcohol consumption: A population-based study. Lancet Oncol. 2021, 22, 1071–1080. [Google Scholar] [CrossRef]
- Bao, X.Y.; Xu, B.B.; Fang, K.; Li, Y.; Hu, Y.H.; Yu, G.P. Changing trends of hospitalisation of liver cirrhosis in Beijing, China. BMJ Open Gastroenterol. 2015, 2, e000051. [Google Scholar] [CrossRef]
- Singal, A.K.; Bataller, R.; Ahn, J.; Kamath, P.S.; Shah, V.H. ACG Clinical Guideline: Alcoholic Liver Disease. Am. J. Gastroenterol. 2018, 113, 175–194. [Google Scholar] [CrossRef]
- Li, S.; Xu, Y.; Guo, W.; Chen, F.; Zhang, C.; Tan, H.Y.; Wang, N.; Feng, Y. The Impacts of Herbal Medicines and Natural Products on Regulating the Hepatic Lipid Metabolism. Front. Pharmacol. 2020, 11, 351. [Google Scholar] [CrossRef]
- Liu, Y.S.; Yuan, M.H.; Zhang, C.Y.; Liu, H.M.; Liu, J.R.; Wei, A.L.; Ye, Q.; Zeng, B.; Li, M.F.; Guo, Y.P.; et al. Puerariae Lobatae radix flavonoids and puerarin alleviate alcoholic liver injury in zebrafish by regulating alcohol and lipid metabolism. Biomed. Pharmacother. 2021, 134, 111121. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, N.; Zhao, L.; Wu, W.; Zhang, L.; Zhou, F.; Li, J. Astragalus Polysaccharides and Saponins Alleviate Liver Injury and Regulate Gut Microbiota in Alcohol Liver Disease Mice. Foods. 2021, 10, 2688. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y.; Zhu, S.; Wang, J.; Burstein, E.; Jia, D. Natural compounds in the chemoprevention of alcoholic liver disease. Phytother. Res. 2019, 33, 2192–2212. [Google Scholar] [CrossRef] [PubMed]
- Mirhadi, E.; Roufogalis, B.D.; Banach, M.; Barati, M.; Sahebkar, A. Resveratrol: Mechanistic and therapeutic perspectives in pulmonary arterial hypertension. Pharmacol. Res. 2021, 163, 105287. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.D.; Luo, M.; Huang, S.Y.; Saimaiti, A.; Shang, A.; Gan, R.Y.; Li, H.B. Effects and Mechanisms of Resveratrol on Aging and Age-Related Diseases. Oxid. Med. Cell. Longev. 2021, 2021, 9932218. [Google Scholar] [CrossRef] [PubMed]
- Izzo, C.; Annunziata, M.; Melara, G.; Sciorio, R.; Dallio, M.; Masarone, M.; Federico, A.; Persico, M. The Role of Resveratrol in Liver Disease: A Comprehensive Review from In Vitro to Clinical Trials. Nutrients 2021, 13, 933. [Google Scholar] [CrossRef] [PubMed]
- Chambers, K.F.; Day, P.E.; Aboufarrag, H.T.; Kroon, P.A. Polyphenol Effects on Cholesterol Metabolism via Bile Acid Biosynthesis, CYP7A1: A Review. Nutrients 2019, 11, 2588. [Google Scholar] [CrossRef]
- Yu, B.; Qin, S.Y.; Hu, B.L.; Qin, Q.Y.; Jiang, H.X.; Luo, W. Resveratrol improves CCL4-induced liver fibrosis in mouse by upregulating endogenous IL-10 to reprogramme macrophages phenotype from M(LPS) to M(IL-4). Biomed. Pharmacother. 2019, 117, 109110. [Google Scholar] [CrossRef]
- Trepiana, J.; Krisa, S.; Renouf, E.; Portillo, M.P. Resveratrol Metabolites Are Able to Reduce Steatosis in Cultured Hepatocytes. Pharmaceuticals 2020, 13, 285. [Google Scholar] [CrossRef]
- Wan, J.; Benkdane, M.; Teixeira-Clerc, F.; Bonnafous, S.; Louvet, A.; Lafdil, F.; Pecker, F.; Tran, A.; Gual, P.; Mallat, A.; et al. M2 Kupffer cells promote M1 Kupffer cell apoptosis: A protective mechanism against alcoholic and nonalcoholic fatty liver disease. Hepatology 2014, 59, 130–142. [Google Scholar] [CrossRef]
- Huang, K.; Zhang, P.; Zhang, Z.; Youn, J.Y.; Wang, C.; Zhang, H.; Cai, H. Traditional Chinese Medicine (TCM) in the treatment of COVID-19 and other viral infections: Efficacies and mechanisms. Pharmacol. Ther. 2021, 225, 107843. [Google Scholar] [CrossRef]
- Wang, W.Y.; Zhou, H.; Wang, Y.F.; Sang, B.S.; Liu, L. Current Policies and Measures on the Development of Traditional Chinese Medicine in China. Pharmacol. Res. 2021, 163, 105187. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.L.; Verpoorte, R.; Yen, H.R.; Peng, W.H.; Cheng, Y.C.; Chao, J.; Pao, L.H. Effects of processing adjuvants on traditional Chinese herbs. J. Food Drug Anal. 2018, 26, S96–S114. [Google Scholar] [CrossRef] [PubMed]
- Li, R.L.; Zhang, Q.; Liu, J.; He, L.Y.; Huang, Q.W.; Peng, W.; Wu, C.J. Processing methods and mechanisms for alkaloid-rich Chinese herbal medicines: A review. J. Integr. Med. 2021, 19, 89–103. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Shan, B.; Zeng, S.; Zhang, J.; Jin, C.; Liao, Z.; Wang, T.; Zeng, Q.; He, H.; Wei, F.; et al. Raw and wine processed Schisandra chinensis attenuate anxiety like behavior via modulating gut microbiota and lipid metabolism pathway. J. Ethnopharmacol. 2021, 266, 113426. [Google Scholar] [CrossRef] [PubMed]
- Ya, Y.; Zhixiang, Z.; Chao, L.; Wei, Z.; Zhiyong, W.; Huafeng, C.; Shaohua, Z.; Hongfei, X. Reflections on the aconitine poisoning. J. Forensic Sci. 2021, 66, 2035–2040. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, S.X.; Guo, J.; Chen, L.J.; Liou, Y.L.; Rao, T.; Peng, J.B.; Guo, Y.; Huang, W.H.; Tan, Z.R.; et al. A Joint Technology Combining the Advantages of Capillary Microsampling with Mass Spectrometry Applied to the Trans-Resveratrol Pharmacokinetic Study in Mice. J. Anal. Methods Chem. 2022, 2022, 5952436. [Google Scholar] [CrossRef]
- Guo, J.; Xu, Y.; Chen, L.J.; Zhang, S.X.; Liou, Y.L.; Chen, X.P.; Tan, Z.R.; Zhou, H.H.; Zhang, W.; Chen, Y. Gut microbiota and host Cyp450s co-contribute to pharmacokinetic variability in mice with non-alcoholic steatohepatitis: Effects vary from drug to drug. J. Adv. Res. 2022, 39, 319–332. [Google Scholar] [CrossRef]
- Chen, L.J.; Lin, X.X.; Guo, J.; Xu, Y.; Zhang, S.X.; Chen, D.; Zhao, Q.; Xiao, J.; Lian, G.H.; Peng, S.F.; et al. Lrp6 Genotype affects Individual Susceptibility to Nonalcoholic Fatty Liver Disease and Silibinin Therapeutic Response via Wnt/beta-catenin-Cyp2e1 Signaling. Int. J. Biol. Sci. 2021, 17, 3936–3953. [Google Scholar] [CrossRef]
- Luo, G.; Huang, B.; Qiu, X.; Xiao, L.; Wang, N.; Gao, Q.; Yang, W.; Hao, L. Resveratrol attenuates excessive ethanol exposure induced insulin resistance in rats via improving NAD (+)/NADH ratio. Mol. Nutr. Food Res. 2017, 61, 1700087. [Google Scholar] [CrossRef]
- Bujanda, L.; García-Barcina, M.; Gutiérrez de Juan, V.; Bidaurrazaga, J.; de Luco, M.F.; Gutiérrez-Stampa, M.; Larzabal, M.; Hijona, E.; Sarasqueta, C.; Echenique-Elizondo, M.; et al. Effect of resveratrol on alcohol-induced mortality and liver lesions in mice. BMC Gastroenterol. 2006, 6, 35. [Google Scholar] [CrossRef]
- Ma, Z.; Zhang, Y.; Li, Q.; Xu, M.; Bai, J.; Wu, S. Resveratrol improves alcoholic fatty liver disease by downregulating HIF-1α expression and mitochondrial ROS production. PLoS ONE 2017, 12, e0183426. [Google Scholar] [CrossRef] [PubMed]
- Ajmo, J.M.; Liang, X.; Rogers, C.Q.; Pennock, B.; You, M. Resveratrol alleviates alcoholic fatty liver in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 295, G833–G842. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.M.; Shaw, L.H.; Chang, P.J.; Tung, S.Y.; Chang, T.S.; Shen, C.H.; Hsieh, Y.Y.; Wei, K.L. Hepatoprotective effect of resveratrol against ethanol-induced oxidative stress through induction of superoxide dismutase in vivo and in vitro. Exp. Ther. Med. 2016, 11, 1231–1238. [Google Scholar] [CrossRef] [PubMed]
- Almazroo, O.A.; Miah, M.K.; Venkataramanan, R. Drug Metabolism in the Liver. Clin. Liver Dis. 2017, 21, 1–20. [Google Scholar] [CrossRef]
- Gao, B.; Bataller, R. Alcoholic liver disease: Pathogenesis and new therapeutic targets. Gastroenterology 2011, 141, 1572–1585. [Google Scholar] [CrossRef]
- Ganne-Carrie, N.; Nahon, P. Hepatocellular carcinoma in the setting of alcohol-related liver disease. J. Hepatol. 2019, 70, 284–293. [Google Scholar] [CrossRef]
- Harjumaki, R.; Pridgeon, C.S.; Ingelman-Sundberg, M. CYP2E1 in Alcoholic and Non-Alcoholic Liver Injury. Roles of ROS, Reactive Intermediates and Lipid Overload. Int. J. Mol. Sci. 2021, 22, 8221. [Google Scholar] [CrossRef]
- Bedada, S.K.; Neerati, P. Resveratrol Pretreatment Affects CYP2E1 Activity of Chlorzoxazone in Healthy Human Volunteers. Phytother. Res. 2016, 30, 463–468. [Google Scholar] [CrossRef]
- Yip, L.; Bixler, D.; Brooks, D.E.; Clarke, K.R.; Datta, D.; Lind, J.N.; Melgar, M.; Schmit, C.M.; Seifert, S.; Chang, A.; et al. Serious Adverse Health Events, Including Death, Associated with Ingesting Alcohol-Based Hand Sanitizers Containing Methanol—Arizona and New Mexico, May–June 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 1070–1073. [Google Scholar] [CrossRef]
- Schicchi, A.; Besson, H.; Rasamison, R.; Berleur, M.P.; Mégarbane, B. Fomepizole to treat disulfiram-ethanol reaction: A case series. Clin. Toxicol. 2020, 58, 922–925. [Google Scholar] [CrossRef]
- Rasamison, R.; Besson, H.; Berleur, M.P.; Schicchi, A.; Mégarbane, B. Analysis of fomepizole safety based on a 16-year post-marketing experience in France. Clin. Toxicol. 2020, 58, 742–747. [Google Scholar] [CrossRef] [PubMed]
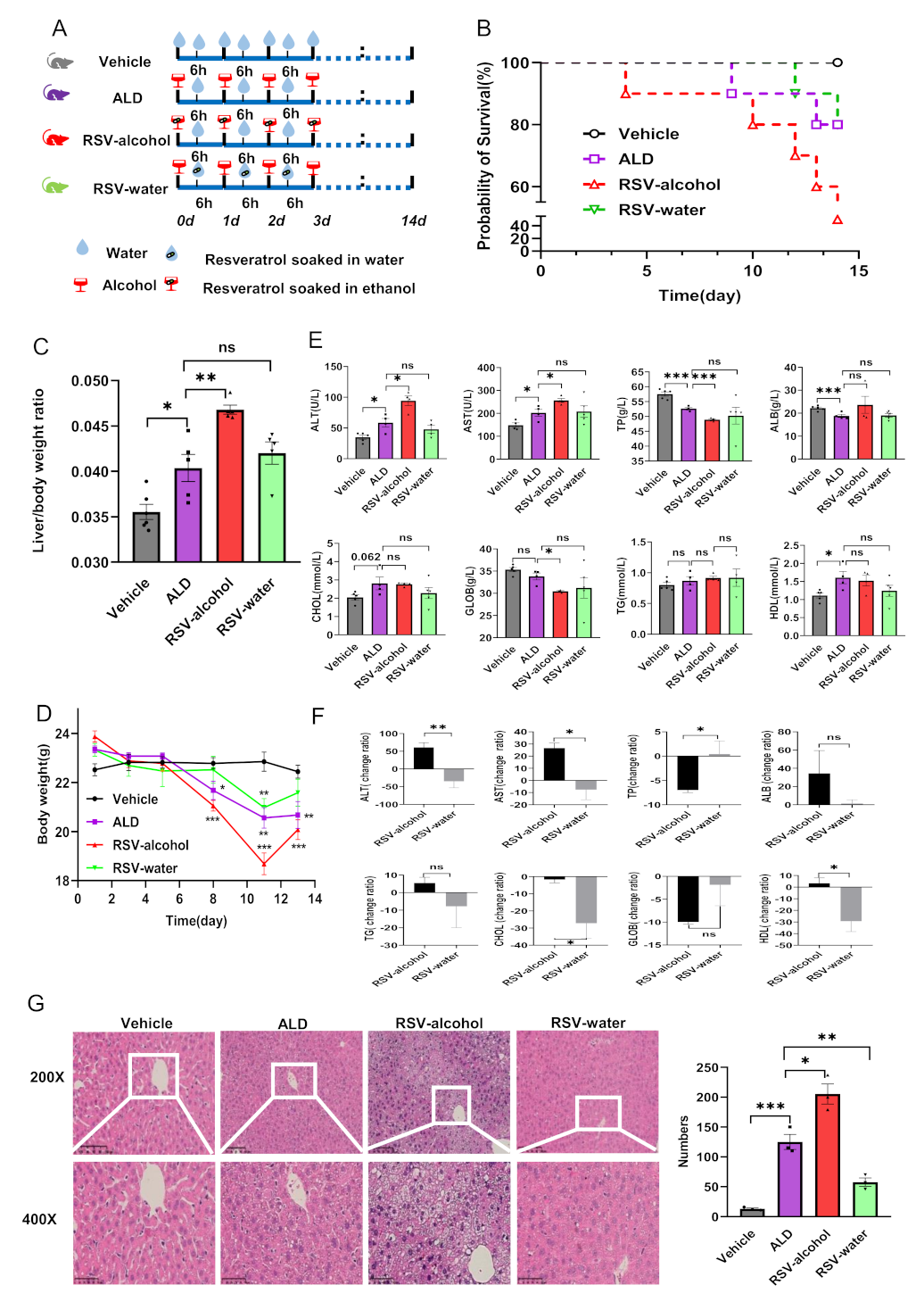

| Parameters (Unit) | Groups | |||
|---|---|---|---|---|
| Vehicle Control | ALD Model | RSV-Alcohol | RSV-Water | |
| ALT (U/L) | 34.8 ± 37.0 | 58.6 ± 6.9 *↑ | 94.3 ± 7.8 #↑ | 47.8 ± 6.5 |
| AST (U/L) | 148.5 ± 10.1 | 202.5 ± 15.3 *↑ | 233.8 ± 24.8 #↑ | 208.2 ± 24.7 |
| TP (g/L) | 57.5 ± 0.7 | 52.6 ± 0.4 ***↓ | 48.9 ± 0.3 ###↓ | 50.2 ± 2.8 |
| ALB (g/L) | 22.2 ± 0.5 | 18.8 ± 0.7 ***↓ | 23.7 ± 3.7 | 19.0 ± 0.8 |
| TG (mmol/L) | 0.8 ± 0.0 | 0.9 ± 0.1 | 0.9 ± 0.0 | 1.0 ± 0.1 |
| CHOL (mmol/L) | 2.1 ± 0.1 | 2.8 ± 0.4 | 2.8 ± 0.1 | 2.3 ± 0.3 |
| HDL (mmol/L) | 1.1 ± 0.1 | 1.6 ± 0.2 *↑ | 1.5 ± 0.2 | 1.2 ± 0.2 |
| GLOB (g/L) | 35.3 ± 0.5 | 33.8 ± 0.8 | 30.4 ± 0.2 #↓ | 31.2 ± 2.3 |
| Parameters (Unit) | Groups | |
|---|---|---|
| RSV-Alcohol (%) | RSV-Water (%) | |
| ALT (U/L) | 61.0 ± 13.3 | −34.8 ± 18.4 ** |
| AST (U/L) | 26.7 ± 4.2 | −7.5 ± 8.3 * |
| TP (g/L) | −7.0 ± 1.0 | 0.4 ± 2.7 * |
| ALB (g/L) | 34.4 ± 25.1 | 1.3 ± 4.2 |
| CHOL (mmol/L) | −2.0 ± 0.5 | −27.1 ± 8.7 * |
| TG (mmol/L) | 5.5 ± 3.3 | 6.1 ± 16.3 |
| HDL (mmol/L) | 3.3 ± 4.7 | −29.2 ± 9.0 * |
| Parameters (Unit) | Groups | |
|---|---|---|
| Alone | Combination | |
| AUC(0–12h) (nmol.h/mL) | 228.3 ± 24.6 | 427.9 ± 47.2 **↑ |
| AUC(0–∞) (nmol.h/mL) | 228.6 ± 24.6 | 516.7 ± 81.0 **↑ |
| Cmax (nmol/mL) | 49.3 ± 3.1 | 74.9 ± 6.4 **↑ |
| Tmax (h) | 1.0 ± 0.2 | 1.5 ± 0.3 |
| t1/2 (h) | 0.8 ± 0.2 | 2.9 ± 1.0 |
| Parameters (Unit) | Resveratrol | R3G | R3S | |||
|---|---|---|---|---|---|---|
| Alone | Combination | Alone | Combination | Alone | Combination | |
| AUC(0–12h) (nmol.h/mL) | 7779.5 ± 1103.0 | 1036.0 ± 181.4 ***↓ | 52,550.5 ± 11,692.2 | 26,734.7 ± 3102.9 *↓ | 2716.0 ± 455.0 | 1195.0 ± 193.7 *↓ |
| AUC(0-∞) (nmol.h/mL) | 10,302.1 ± 2484.6 | 2215.5 ± 908.7 *↓ | 56,446.3 ± 11,852.6 | 31,948.1 ± 1964.0 | 2358.4 ± 390.4 | 1764.8 ± 363.9 |
| Cmax (nmol/mL) | 4986.0 ± 865.0 | 453.6 ± 134.5 ***↓ | 11,211.7 ± 2441.7 | 6012.0 ± 259.0 | 801.8 ± 160.6 | 333.8 ± 57.2 *↓ |
| Tmax (h) | 0.7 ±0.1 | 0.3 ± 0.1 ***↓ | 0.8 ± 0.1 | 0.3± 0.0 ***↓ | 0.7 ± 0.1 | 0.3 ± 0.0 *↓ |
| t1/2 (h) | 1.7 ± 0.2 | 4.2 ± 0.8 *↑ | 2.1 ± 0.5 | 5.2 ± 1.1 *↑ | 2.4 ± 0.3 | 6.5 ± 1.4 ***↑ |
| Parameters (Unit) | Metabolic Compounds | Enzyme Activities | ||
|---|---|---|---|---|
| Vehicle | ALD Model | RSV-Alcohol | ||
| Adh (U/mg prot) | alcohol | 6.1 ± 0.3 | 3.9 ± 0.3 ***↓ | 4.8 ± 0.3 #↑ |
| Aldh (U/mg prot) | alcohol | 1.3 ± 0.1 | 2.8 ± 0.3 ***↑ | 2.1 ± 0.2 |
| Cyp2e1 (ng/mL) | alcohol | 5.9 ± 0.4 | 14.8 ± 0.6 ***↑ | 21.7 ± 0.9 ###↑ |
| Ugt1a1 (%) | RSV | 75.4 ± 16.8 | 89.0 ± 10.3 | / |
| Sult1a1 (%) | RSV | 4.8 ± 0.8 | 4.9 ± 0.8 | / |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Xu, Y.; Ye, M.; Ye, W.; Xiao, J.; Zhou, H.; Zhang, W.; Shu, Y.; Huang, Y.; Chen, Y. Resveratrol in Liquor Exacerbates Alcoholic Liver Injury with a Reduced Therapeutic Effect in Mice: An Unsupervised Herbal Wine Habit Is Risky. Nutrients 2022, 14, 4752. https://doi.org/10.3390/nu14224752
Zhang S, Xu Y, Ye M, Ye W, Xiao J, Zhou H, Zhang W, Shu Y, Huang Y, Chen Y. Resveratrol in Liquor Exacerbates Alcoholic Liver Injury with a Reduced Therapeutic Effect in Mice: An Unsupervised Herbal Wine Habit Is Risky. Nutrients. 2022; 14(22):4752. https://doi.org/10.3390/nu14224752
Chicago/Turabian StyleZhang, Songxia, Ying Xu, Mengling Ye, Wenli Ye, Jian Xiao, Honghao Zhou, Wei Zhang, Yan Shu, Yun Huang, and Yao Chen. 2022. "Resveratrol in Liquor Exacerbates Alcoholic Liver Injury with a Reduced Therapeutic Effect in Mice: An Unsupervised Herbal Wine Habit Is Risky" Nutrients 14, no. 22: 4752. https://doi.org/10.3390/nu14224752
APA StyleZhang, S., Xu, Y., Ye, M., Ye, W., Xiao, J., Zhou, H., Zhang, W., Shu, Y., Huang, Y., & Chen, Y. (2022). Resveratrol in Liquor Exacerbates Alcoholic Liver Injury with a Reduced Therapeutic Effect in Mice: An Unsupervised Herbal Wine Habit Is Risky. Nutrients, 14(22), 4752. https://doi.org/10.3390/nu14224752





