Immunomodulatory Effects of Chicken Broth and Histidine Dipeptides on the Cyclophosphamide-Induced Immunosuppression Mouse Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Preparation of the CB
2.2.1. Extracting the HD from the Chicken Breast
2.2.2. Traditional CB
2.2.3. Enzymolysis of the CB
2.3. Protein and Free Amino Acid Analysis of the CB
2.4. UPLC Analysis of the HD
2.5. Animals
2.6. Experimental Procedures
2.7. Determination of the Body Weight and Immune Organ Index
2.8. Determination of Splenic Lymphocytes Proliferation
2.9. Hematological Analyses
2.10. Assay of Immunoglobulins in the Serum
2.11. Measurement of the Cytokines
2.12. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR) Analysis
2.13. Measurement of cAMP/cGMP Levels and PKA Activity
2.14. Statistical Analysis
3. Results and Discussion
3.1. Characterization of the CBH
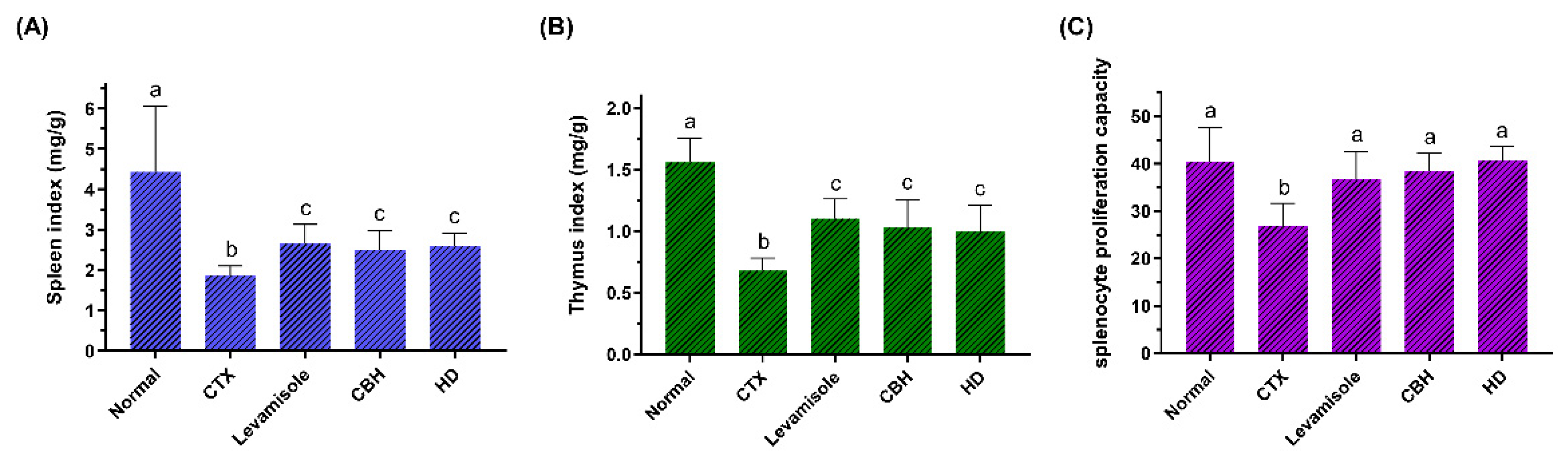
3.2. The Effect of CBH on the Body Weight and Immune Organ Index
3.3. The Effect of CBH on the Proliferation of Splenic LYM
3.4. The Effect of CBH on the Hematological Parameters
3.5. The Effect of CBH on the Immunoglobulin Levels
3.6. The Effect of CBH on the Cytokine Levels and Gene Expression
3.7. The Effect of CBH on the NO/cGMP and cAMP/PKA Signaling Pathways
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jayasena, D.D.; Jung, S.; Alahakoon, A.U.; Nam, K.C.; Lee, J.H.; Jo, C. Bioactive and Taste-related Compounds in Defatted Freeze-dried Chicken Soup Made from Two Different Chicken Breeds Obtained at Retail. J. Poultry Sci. 2015, 52, 156–165. [Google Scholar] [CrossRef]
- Yang, Y.; Feng, Y.; Li, Z.; Xie, X.; Miao, F.; Fang, G.; Tu, H.; Wen, J. Effect of sex and diet nutrition on the contents of flavor precursors in fujian hetian chicken. Acta Vet. Zootech. Sin. 2006, 37, 242–249. [Google Scholar] [CrossRef]
- Zhang, L.Z.; Rong, J.H.; Jian, H.U.; Zhao, S.M. Effect of Pre-treatment on Nutritional Characteristics of Chicken Soup. Food Sci. 2009, 30, 83–87. [Google Scholar] [CrossRef]
- Caruso, G.; Fresta, C.G.; Grasso, M.; Santangelo, R.; Lazzarino, G.; Lunte, S.M.; Caraci, F. Inflammation as the Common Biological Link Between Depression and Cardiovascular Diseases: Can Carnosine Exert a Protective Role? Curr. Med. Chem. 2020, 27, 1782–1800. [Google Scholar] [CrossRef] [PubMed]
- Gil-Agusti, M.; Esteve-Romero, J.; Carda-Broch, S. Anserine and carnosine determination in meat samples by pure micellar liquid chromatography. J. Chromatogr. A 2008, 1189, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Peiretti, P.G.; Medana, C.; Visentin, S.; Giancotti, V.; Zunino, V.; Meineri, G. Determination of carnosine, anserine, homocarnosine, pentosidine and thiobarbituric acid reactive substances contents in meat from different animal species. Food Chem. 2011, 126, 1939–1947. [Google Scholar] [CrossRef]
- Kong, Y.; Yang, X.; Ding, Q.; Zhang, Y.; Sun, B.; Chen, H.; Sun, Y. Comparison of non-volatile umami components in chicken soup and chicken enzymatic hydrolysate. Food Res. Int. 2017, 102, 559–566. [Google Scholar] [CrossRef]
- Yeum, K.-J.; Orioli, M.; Regazzoni, L.; Carini, M.; Rasmussen, H.; Russell, R.M.; Aldini, G. Profiling histidine dipeptides in plasma and urine after ingesting beef, chicken or chicken broth in humans. Amino Acids 2010, 38, 847–858. [Google Scholar] [CrossRef]
- Min, J.; Senut, M.-C.; Rajanikant, K.; Greenberg, E.; Bandagi, R.; Zemke, D.; Mousa, A.; Kassab, M.; Farooq, M.U.; Gupta, R.; et al. Differential neuroprotective effects of carnosine, anserine, and N-acetyl carnosine against permanent focal ischemia. J. Neurosci. Res. 2008, 86, 2984–2991. [Google Scholar] [CrossRef]
- Binguel, I.; Yilmaz, Z.; Aydin, A.F.; Coban, J.; Dogru-Abbasoglu, S.; Uysal, M. Antiglycation and anti-oxidant efficiency of carnosine in the plasma and liver of aged rats. Geriatr. Gerontol. Int. 2017, 17, 2610–2614. [Google Scholar] [CrossRef]
- Antonini, F.M.; Petruzzi, E.; Pinzani, P.; Orlando, C.; Poggesi, M.; Serio, M.; Pazzagli, M.; Masotti, G. The meat in the diet of aged subjects and the antioxidant effects of carnosine. Arch. Gerontol. Geriatr. 2002, 35, 7–14. [Google Scholar] [CrossRef]
- Hipkiss, A.R.; Baye, E.; de Courten, B. Carnosine and the processes of ageing. Maturitas 2016, 93, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kim, E.; Kim, Y. L-histidine and L-carnosine accelerate wound healing via regulation of corticosterone and PI3K/Akt phosphorylation in D-galactose-induced aging models in vitro and in vivo. J. Funct. Foods 2019, 58, 227–237. [Google Scholar] [CrossRef]
- Caruso, G.; Caraci, F.; Jolivet, R.B. Pivotal role of carnosine in the modulation of brain cells activity: Multimodal mechanism of action and therapeutic potential in neurodegenerative disorders. Prog. Neurobiol. 2019, 175, 35–53. [Google Scholar] [CrossRef] [PubMed]
- Naghshvar, F.; Abianeh, S.M.; Ahmadashrafi, S.; Hosseinimehr, S.J. Chemoprotective effects of carnosine against genotoxicity induced by cyclophosphamide in mice bone marrow cells. Cell Biochem. Funct. 2012, 30, 569–573. [Google Scholar] [CrossRef]
- Caruso, G.; Fresta, C.G.; Fidilio, A.; O’Donnell, F.; Musso, N.; Lazzarino, G.; Grasso, M.; Amorini, A.M.; Tascedda, F.; Bucolo, C.; et al. Carnosine Decreases PMA-Induced Oxidative Stress and Inflammation in Murine Macrophages. Antioxidants 2019, 8, 281. [Google Scholar] [CrossRef]
- Fresta, C.G.; Fidilio, A.; Lazzarino, G.; Musso, N.; Grasso, M.; Merlo, S.; Amorini, A.M.; Bucolo, C.; Tavazzi, B.; Lazzarino, G.; et al. Modulation of Pro-Oxidant and Pro-Inflammatory Activities of M1 Macrophages by the Natural Dipeptide Carnosine. Int. J. Mol. Sci. 2020, 21, 776. [Google Scholar] [CrossRef]
- Li, X.; Yang, K.; Gao, S.; Zhao, J.; Liu, G.; Chen, Y.; Lin, H.; Zhao, W.; Hu, Z.; Xu, N. Carnosine Stimulates Macrophage-Mediated Clearance of Senescent Skin Cells Through Activation of the AKT2 Signaling Pathway by CD36 and RAGE. Front. Pharmacol. 2020, 11, 593832. [Google Scholar] [CrossRef]
- Guiotto, A.; Calderan, A.; Ruzza, P.; Borin, G. Carnosine and carnosine-related antioxidants: A review. Curr. Med. Chem. 2005, 12, 2293–2315. [Google Scholar] [CrossRef]
- Fresta, C.G.; Chakraborty, A.; Wijesinghe, M.B.; Amorini, A.M.; Lazzarino, G.; Lazzarino, G.; Tavazzi, B.; Lunte, S.M.; Caraci, F.; Dhar, P.; et al. Non-toxic engineered carbon nanodiamond concentrations induce oxidative/nitrosative stress, imbalance of energy metabolism, and mitochondrial dysfunction in microglial and alveolar basal epithelial cells. Cell Death Dis. 2018, 9, 245. [Google Scholar] [CrossRef]
- Caruso, G.; Fresta, C.G.; Martinez-Becerra, F.; Antonio, L.; Johnson, R.T.; de Campos, R.P.S.; Siegel, J.M.; Wijesinghe, M.B.; Lazzarino, G.; Lunte, S.M. Carnosine modulates nitric oxide in stimulated murine RAW 264.7 macrophages. Mol. Cell. Biochem. 2017, 431, 197–210. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; He, R.; Tsoi, B.; Li, X.; Li, W.; Abe, K.; Kurihara, H. Anti-Stress Effects of Carnosine on Restraint-Evoked Immunocompromise in Mice through Spleen Lymphocyte Number Maintenance. PLoS ONE 2012, 7, e33190. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Zhong, Y.; Wu, Y.; Luo, Z.; Sun, Y.; Wang, G.; Kurihara, H.; Li, Y.; He, R. Carnosine attenuates cyclophosphamide-induced bone marrow suppression by reducing oxidative DNA damage. Redox Biol. 2018, 14, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Yang, F.; Zhao, Q.; Tian, D.; Tang, Y. Protective effects of pentadecapeptide derived from Cyclaina sinensis against cyclophosphamide-induced hepatotoxicity. Biochem. Biophys. Res. Commun. 2019, 520, 392–398. [Google Scholar] [CrossRef] [PubMed]
- Huyan, X.; Lin, Y.; Gao, T.; Chen, R.; Fan, Y. Immunosuppressive effect of cyclophosphamide on white blood cells and lymphocyte subpopulations from peripheral blood of Balb/c mice. Int. Immunopharmacol. 2011, 11, 1293–1297. [Google Scholar] [CrossRef]
- Gao, S.; Hong, H.; Zhang, C.; Wang, K.; Zhang, B.; Han, Q.; Liu, H.; Luo, Y. Immunomodulatory effects of collagen hydrolysates from yak (Bos grunniens) bone on cyclophosphamide-induced immunosuppression in BALB/c mice. J. Funct. Foods 2019, 60, 103420. [Google Scholar] [CrossRef]
- Stevenson, H.C.; Green, I.; Hamilton, J.M.; Calabro, B.A.; Parkinson, D.R. Levamisole-known effects on the immune-system, clinical-results, and future applications to the treatment of cancer. J. Clin. Oncol. 1991, 9, 2052–2066. [Google Scholar] [CrossRef]
- Sajid, M.S.; Iqbal, Z.; Muhammad, G.; Iqbal, M.U. Immunomodulatory effect of various anti-parasitics: A review. Parasitology 2006, 132, 301–313. [Google Scholar] [CrossRef]
- Yu, F.; He, K.; Dong, X.; Zhang, Z.; Wang, F.; Tang, Y.; Chen, Y.; Ding, G. Immunomodulatory activity of low molecular-weight peptides from Nibea japonica skin in cyclophosphamide-induced immunosuppressed mice. J. Funct. Foods 2020, 68, 103888. [Google Scholar] [CrossRef]
- Banerjee, S.; Sinha, K.; Chowdhury, S.; Sil, P.C. Unfolding the mechanism of cisplatin induced pathophysiology in spleen and its amelioration by carnosine. Chem. Biol. Interact. 2018, 279, 159–170. [Google Scholar] [CrossRef]
- Liu, Q.; Ge, M.; Xu, X.; Cheng, L.; Su, C.; Yu, S.; Huang, Z. Immunifaction Accommodation of Phenylalanine Dipeptide Compound Y101 in Normal Mice. Pharm. J. Chin. People’s Lib. Army 2011, 27, 215–217. [Google Scholar] [CrossRef]
- Zhao, L.; Liu, L.; Guo, B.; Zhu, B. Regulation of adaptive immune responses by guiding cell movements in the spleen. Front. Microbiol. 2015, 6, 645. [Google Scholar] [CrossRef] [PubMed]
- Bajenoff, M.; Glaichenhaus, N.; Germain, R.N. Fibroblastic reticular cells guide T lymphocyte entry into and migration within the splenic T cell zone. J. Immunol. 2008, 181, 3947–3954. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Ojalvo, D.; Molina, E.; Lopez-Fandino, R. Hydrolysates of egg white proteins modulate T- and B-cell responses in mitogen-stimulated murine cells. Food Funct. 2016, 7, 1048–1056. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Huang, M.; Cai, X.; Jia, L.; Wang, S. Investigation on activation in RAW264.7 macrophage cells and protection in cyclophosphamide-treated mice of Pseudostellaria heterophylla protein hydrolysate. Food Chem. Toxicol. 2019, 134, 110816. [Google Scholar] [CrossRef] [PubMed]
- Hou, H.; Fan, Y.; Li, B.; Xue, C.; Yu, G.; Zhang, Z.; Zhao, X. Purification and identification of immunomodulating peptides from enzymatic hydrolysates of Alaska pollock frame. Food Chem. 2012, 134, 821–828. [Google Scholar] [CrossRef]
- Pantoja, P.; Perez-Guzman, E.X.; Rodriguez, I.V.; White, L.J.; Gonzalez, O.; Serrano, C.; Giavedoni, L.; Hodara, V.; Cruz, L.; Arana, T.; et al. Zika virus pathogenesis in rhesus macaques is unaffected by pre-existing immunity to dengue virus. Nat. Commun. 2017, 8, 15674. [Google Scholar] [CrossRef]
- Zhou, H.; Sun, F.; Li, H.; Zhang, S.; Liu, Z.; Pei, J.; Liang, C. Effect of recombinant Ganoderma lucidum immunoregulatory protein on cyclophosphamide-induced leukopenia in mice. Immunopharmacol. Immunotoxicol. 2013, 35, 426–433. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, S.; Ying, H.; Dai, X.; Li, X.; Yu, C.; Ye, H. Chemical characterization and immunostimulatory effects of a polysaccharide from Polygoni Multiflori Radix Praeparata in cyclophosphamide-induced anemic mice. Carbohydr. Polym. 2012, 88, 1476–1482. [Google Scholar] [CrossRef]
- Arslan, S.; Ozyurek, E.; Gunduz-Demir, C. A color and shape based algorithm for segmentation of white blood cells in peripheral blood and bone marrow images. Cytom. Part A 2014, 85A, 480–490. [Google Scholar] [CrossRef]
- Juaristi, J.A.; Aguirre, M.V.; Todaro, J.S.; Alvarez, M.A.; Brandan, N.C. EPO receptor, Bax and Bcl-x(L) expressions in murine erythropoiesis after cyclophosphamide treatment. Toxicology 2007, 231, 188–199. [Google Scholar] [CrossRef] [PubMed]
- Chu, Q.; Zhang, Y.; Chen, W.; Jia, R.; Yu, X.; Wang, Y.; Li, Y.; Liu, Y.; Ye, X.; Yu, L.; et al. Apios americana Medik flowers polysaccharide (AFP) alleviate Cyclophosphamide-induced immunosuppression in ICR mice. Int. J. Biol. Macromol. 2020, 144, 829–836. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Gao, S.; Li, H.; Cao, M.; Li, W.; Liu, X. Immunomodulatory effects of selenium-enriched peptides from soybean in cyclophosphamide-induced immunosuppressed mice. Food Sci. Nutr. 2021, 9, 6322–6334. [Google Scholar] [CrossRef] [PubMed]
- Lis, M.; Obminska-Mrukowicz, B. Modulatory effects of bestatin on T and B lymphocyte subsets and the concentration of cytokines released by Th1/Th2 lymphocytes in cyclophosphamide-treated mice. Cent. Eur. J. Immunol. 2013, 38, 42–53. [Google Scholar] [CrossRef]
- Ehrenstein, M.R.; Notley, C.A. The importance of natural IgM: Scavenger, protector and regulator. Nat. Rev. Immunol. 2010, 10, 778–786. [Google Scholar] [CrossRef]
- Nimmerjahn, F.; Ravetch, J.V. Antibody-mediated modulation of immune responses. Immunol. Rev. 2010, 236, 265–275. [Google Scholar] [CrossRef]
- Woof, J.M.; Kerr, M.A. The function of immunoglobulin A in immunity. J. Pathol. 2006, 208, 270–282. [Google Scholar] [CrossRef] [PubMed]
- Maestri, E.; Marmiroli, M.; Marmiroli, N. Bioactive peptides in plant-derived foodstuffs. J. Proteom. 2016, 147, 140–155. [Google Scholar] [CrossRef]
- Yu, F.; Zhang, Z.; Ye, S.; Hong, X.; Jin, H.; Huang, F.; Yang, Z.; Tang, Y.; Chen, Y.; Ding, G. Immunoenhancement effects of pentadecapeptide derived from Cyclina sinensis on immune-deficient mice induced by Cyclophosphamide. J. Funct. Foods 2019, 60, 103408. [Google Scholar] [CrossRef]
- Musso, N.; Caruso, G.; Bongiorno, D.; Grasso, M.; Bivona, D.A.; Campanile, F.; Caraci, F.; Stefani, S. Different Modulatory Effects of Four Methicillin-Resistant Staphylococcus aureus Clones on MG-63 Osteoblast-Like Cells. Biomolecules 2021, 11, 72. [Google Scholar] [CrossRef]
- Fujiwara, M.; Ozono, K. Cytokines and osteogenesis. Clin. Calcium 2014, 24, 845–851. [Google Scholar] [PubMed]
- Chan, C.C.; Caspi, R.; Mochizuki, M.; Diamantstein, T.; Gery, I.; Nussenblatt, R.B. Cyclosporine and dexamethasone inhibit T-lymphocyte MHC class II antigens and IL-2 receptor expression in experimental autoimmune uveitis. Immunol. Investig. 1987, 16, 319–331. [Google Scholar] [CrossRef] [PubMed]
- Ivashkiv, L.B. IFN gamma: Signalling, epigenetics and roles in immunity, metabolism, disease and cancer immunotherapy. Nat. Rev. Immunol. 2018, 18, 545–558. [Google Scholar] [CrossRef] [PubMed]
- Castillo, P.; Kolls, J.K. IL-10: A Paradigm for Counterregulatory Cytokines. J. Immunol. 2016, 197, 1529–1530. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Gong, L.; Liu, Y.; Zhou, Z.; Wan, C.; Xu, J.; Wu, Q.; Chen, L.; Lu, Y.; Chen, Y. Immunoenhancement effect of crude polysaccharides of Helvella leucopus on cyclophosphamide-induced immunosuppressive mice. J. Funct. Foods 2020, 69, 103942. [Google Scholar] [CrossRef]
- Jia, D.; Lu, W.; Wang, C.; Sun, S.; Cai, G.; Li, Y.; Wang, G.; Liu, Y.; Zhang, M.; Wang, D. Investigation on Immunomodulatory Activity of Calf Spleen Extractive Injection in Cyclophosphamide-induced Immunosuppressed Mice and Underlying Mechanisms. Scand. J. Immunol. 2016, 84, 20–27. [Google Scholar] [CrossRef]
- Xu, P.; Fang, X.; Shu, G.; Zhu, X.; Luo, Z.; Jiang, Q.; Gao, P.; Zhang, Y. Prokaryotic Expression of Gly-Gln Dipeptide and Its Bioactive Analysis: A Novel Method for Short Peptide Production. Agric. Sci. China 2010, 9, 736–744. [Google Scholar] [CrossRef]
- Yoo, J.-H.; Lee, Y.-S.; Ku, S.; Lee, H.-J. Phellinus baumii enhances the immune response in cyclophosphamide-induced immunosuppressed mice. Nutr. Res. 2020, 75, 15–31. [Google Scholar] [CrossRef]
- Kurelic, R.; Krieg, P.F.; Sonner, J.K.; Bhaiyan, G.; Ramos, G.C.; Frantz, S.; Friese, M.A.; Nikolaev, V.O. Upregulation of Phosphodiesterase 2A Augments T Cell Activation by Changing cGMP/cAMP Cross-Talk. Front. Pharmacol. 2021, 12, 748798. [Google Scholar] [CrossRef]
- Tripathi, P.; Tripathi, P.; Kashyap, L.; Singh, V.; Bogdan, C. The role of nitric oxide in inflammatory reactions. FEMS Immunol. Med. Microbiol. 2012, 66, 449. [Google Scholar] [CrossRef]
- Lechner, M.; Lirk, P.; Rieder, J. Inducible nitric oxide synthase (iNOS) in tumor biology: The two sides of the same coin. Semin. Cancer Biol. 2005, 15, 277–289. [Google Scholar] [CrossRef] [PubMed]
- Xue, Q.; Yan, Y.; Zhang, R.; Xiong, H. Regulation of iNOS on Immune Cells and Its Role in Diseases. Int. J. Mol. Sci. 2018, 19, 3805. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Xu, Y.; Lei, M.; Shi, J.; Gong, Q. Icariside II, a PDE5 inhibitor from Epimedium brevicornum, promotes neuron-like pheochromocytoma PC12 cell proliferation via activating NO/cGMP/PKG pathway. Neurochem. Int. 2018, 112, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, M.U.W.B.; Vendramini, P.; Kubo, C.A.; Soreiro-Pereira, P.V.; de Albuquerque, R.S.; Antunes, E.; Condino-Neto, A. BAY 41-2272 inhibits human T lymphocyte functions. Int. Immunopharmacol. 2019, 77, 105976. [Google Scholar] [CrossRef]
- Liopeta, K.; Boubali, S.; Virgilio, L.; Thyphronitis, G.; Mavrothalassitis, G.; Dimitracopoulos, G.; Paliogianni, F. cAMP regulates IL-10 production by normal human T lymphocytes at multiple levels: A potential role for MEF2. Mol. Immunol. 2009, 46, 345–354. [Google Scholar] [CrossRef]
- Zuo, L.; Shi, L.; Yan, F. The reciprocal interaction of sympathetic nervous system and cAMP-PKA-NF-kB pathway in immune suppression after experimental stroke. Neurosci. Lett. 2016, 627, 205–210. [Google Scholar] [CrossRef]




| Normal | CTX | Levamisole | CBH | HD | |
|---|---|---|---|---|---|
| Initial weight (g) | 36.30 ± 0.78 a | 34.57 ± 1.35 b | 36.04 ± 1.21 a | 35.86 ± 1.43 ab | 35.85 ± 1.84 ab |
| Final weight (g) | 38.49 ± 1.31 a | 36.91 ± 1.85 a | 37.69 ± 1.51 a | 36.87 ± 2.31 a | 37.16 ± 2.45 a |
| Weight gain (g) | 2.19 ± 0.99 a | 2.34 ± 0.90 a | 1.65 ± 1.02 a | 1.01 ± 1.75 a | 1.31 ± 1.04 a |
| WBC (K/μL) | 7.72 ± 2.80 a | 1.46 ± 0.67 b | 2.50 ± 0.46 c | 3.04 ± 0.56 c | 2.70 ± 0.43 c |
| NEU (K/μL) | 2.01 ± 1.13 a | 0.50 ± 0.34 b | 0.80 ± 0.17 bc | 1.08 ± 0.34 ac | 0.76 ± 0.17 bc |
| LYM (K/μL) | 5.21 ± 1.92 a | 0.79 ± 0.34 b | 1.32 ± 0.27 c | 1.80 ± 0.46 c | 1.65 ± 0.36 c |
| RBC (M/μL) | 10.18 ± 1.76 ac | 11.69 ± 1.80 b | 11.22 ± 1.14 ab | 10.28 ± 0.54 ac | 9.68 ± 1.07 c |
| HGB (g/dL) | 16.20 ± 2.97 ac | 18.66 ± 2.52 b | 17.58 ± 1.74 ab | 16.29 ± 0.80 ac | 15.19 ± 1.42 c |
| PLT (K/μL) | 1104.90 ± 166.68 a | 712.10 ± 223.60 b | 855.50 ± 235.86 bc | 939.60 ± 215.84 ac | 1029.22 ± 154.29 ac |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Wang, X.; Li, H.; Chen, C.; Liu, X. Immunomodulatory Effects of Chicken Broth and Histidine Dipeptides on the Cyclophosphamide-Induced Immunosuppression Mouse Model. Nutrients 2022, 14, 4491. https://doi.org/10.3390/nu14214491
Zhang J, Wang X, Li H, Chen C, Liu X. Immunomodulatory Effects of Chicken Broth and Histidine Dipeptides on the Cyclophosphamide-Induced Immunosuppression Mouse Model. Nutrients. 2022; 14(21):4491. https://doi.org/10.3390/nu14214491
Chicago/Turabian StyleZhang, Jian, Xixi Wang, He Li, Cunshe Chen, and Xinqi Liu. 2022. "Immunomodulatory Effects of Chicken Broth and Histidine Dipeptides on the Cyclophosphamide-Induced Immunosuppression Mouse Model" Nutrients 14, no. 21: 4491. https://doi.org/10.3390/nu14214491
APA StyleZhang, J., Wang, X., Li, H., Chen, C., & Liu, X. (2022). Immunomodulatory Effects of Chicken Broth and Histidine Dipeptides on the Cyclophosphamide-Induced Immunosuppression Mouse Model. Nutrients, 14(21), 4491. https://doi.org/10.3390/nu14214491







