The Protective Effects of Corn Oligopeptides on Acute Alcoholic Liver Disease by Inhibiting the Activation of Kupffer Cells NF-κB/AMPK Signal Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of CPs
2.2. Identification of Corn Oligopeptides
2.3. Animal Models
2.4. Evaluation of Hepatic Biomarkers
2.4.1. Enzyme Activities
2.4.2. ELISA for TNF-α, IL-1, and IL-6 in Mice Serum
2.4.3. Real-Time Quantitative PCR
2.4.4. Isolation and Culture of Murine Kupffer Cells (KCs)
2.4.5. Extraction of Proteins
2.5. Western Blot Analyses
2.6. Statistical Analyses
3. Results
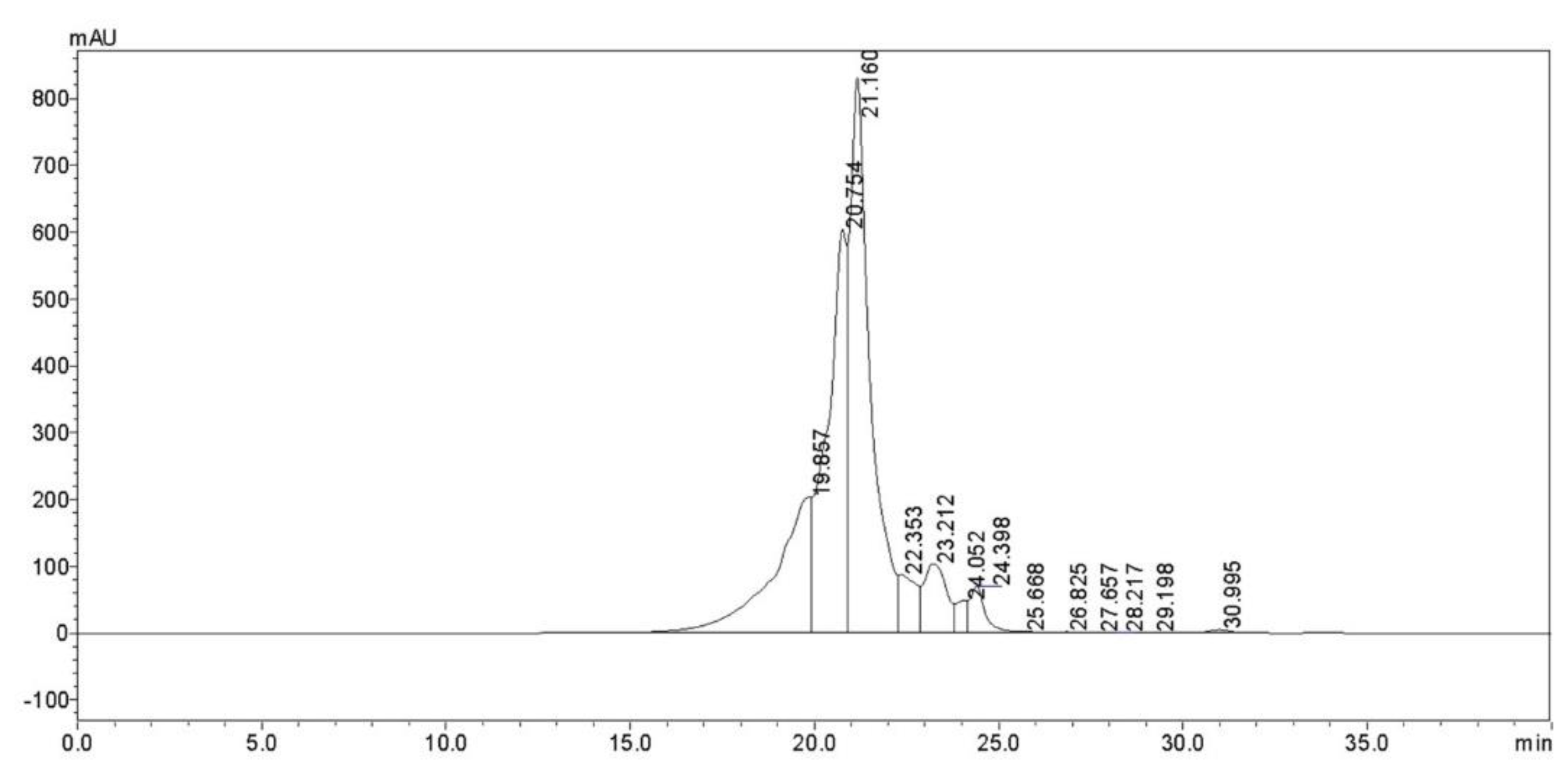
3.1. The Chemical Composition of CPs
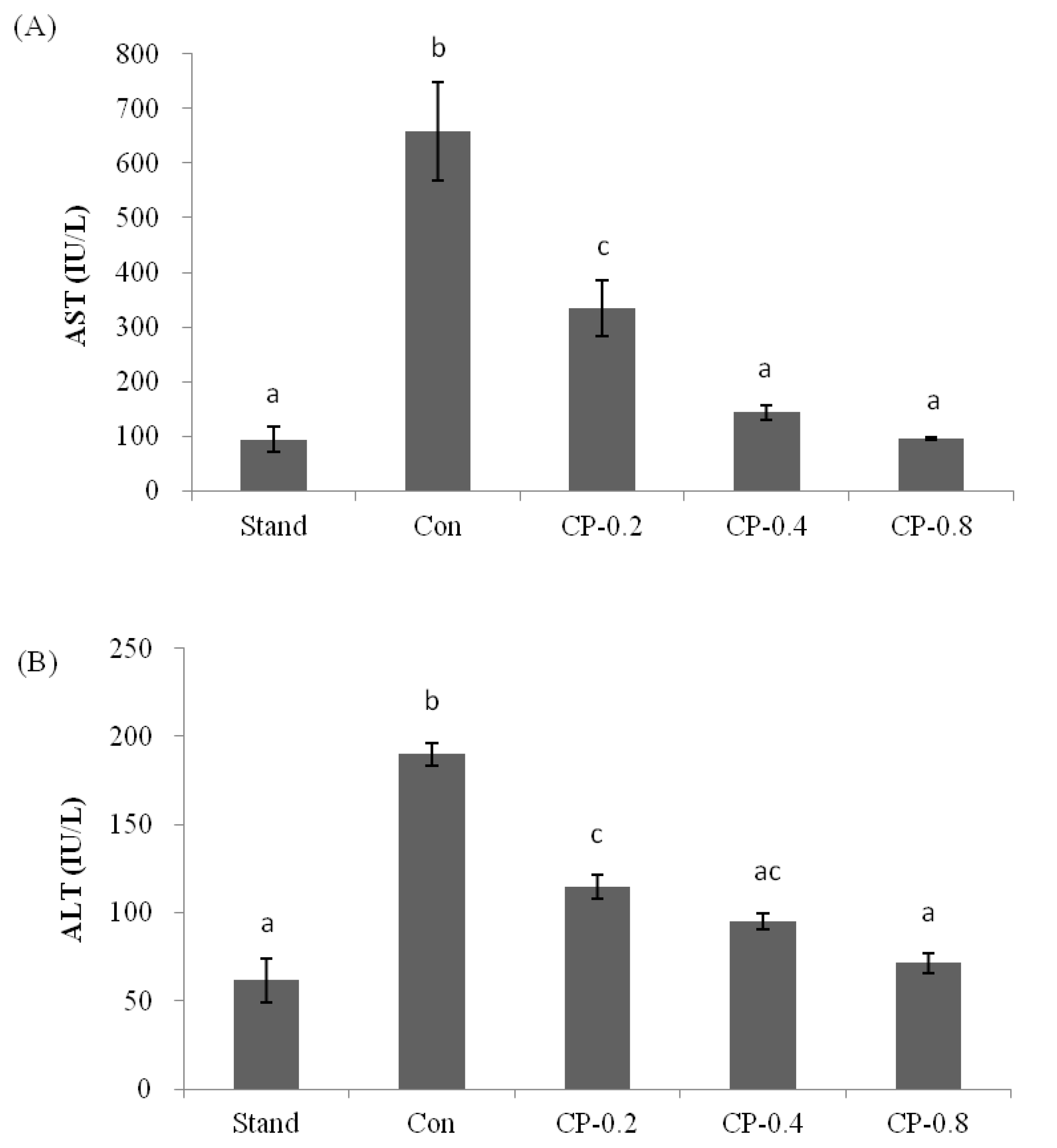
3.2. The Inhibition Effects of CPs on AST and ALT in Mice Serum
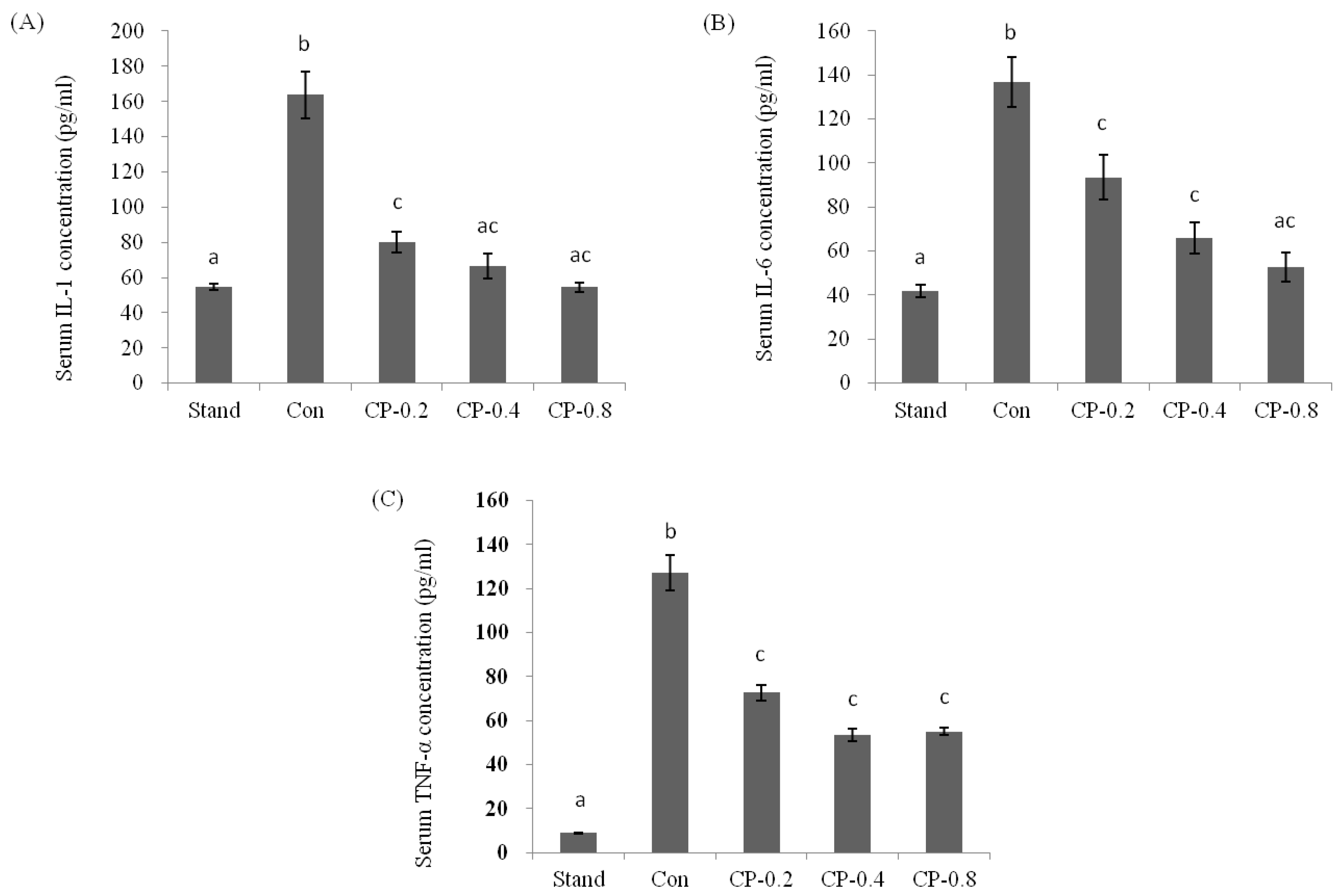
3.3. The Inhibition Effects of CPs on Inflammatory Factors in Mice Serum
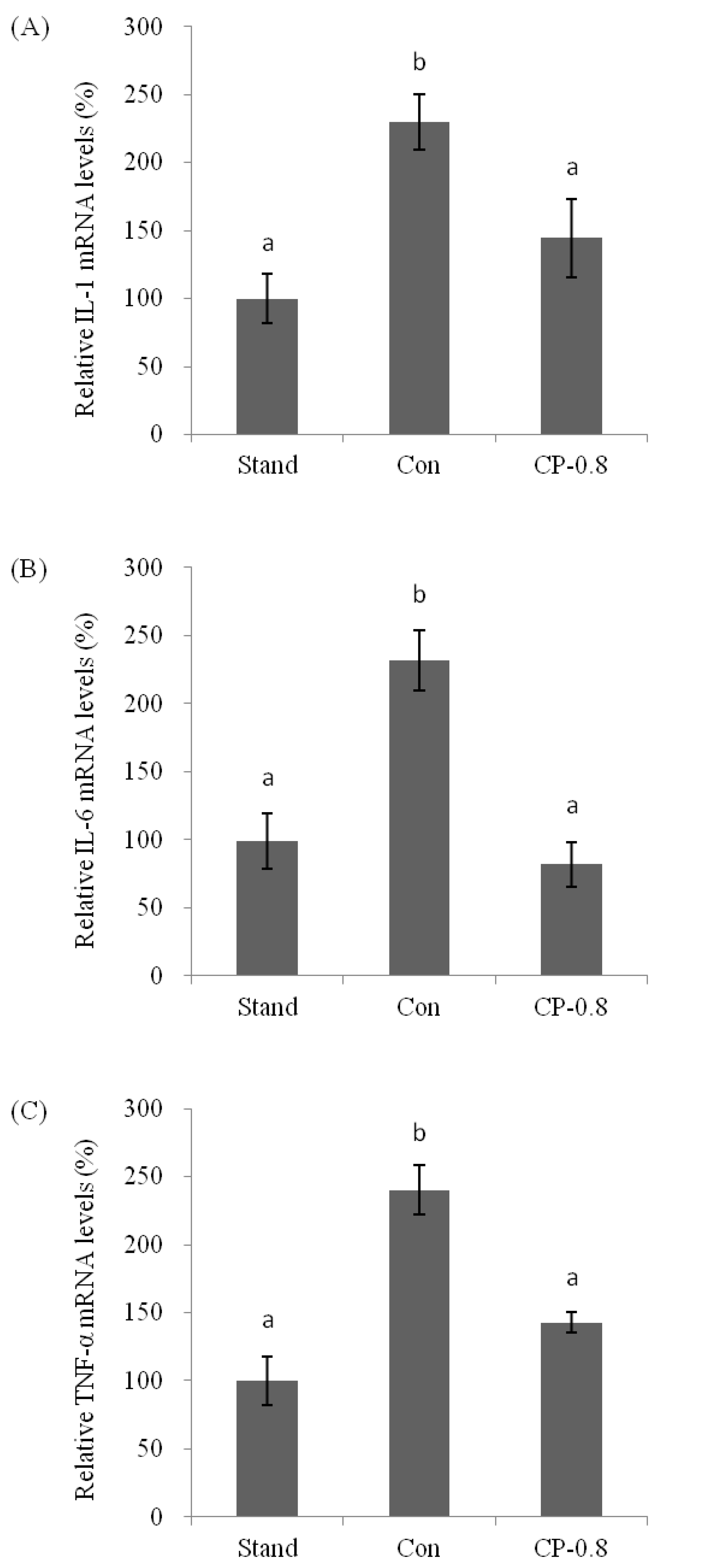
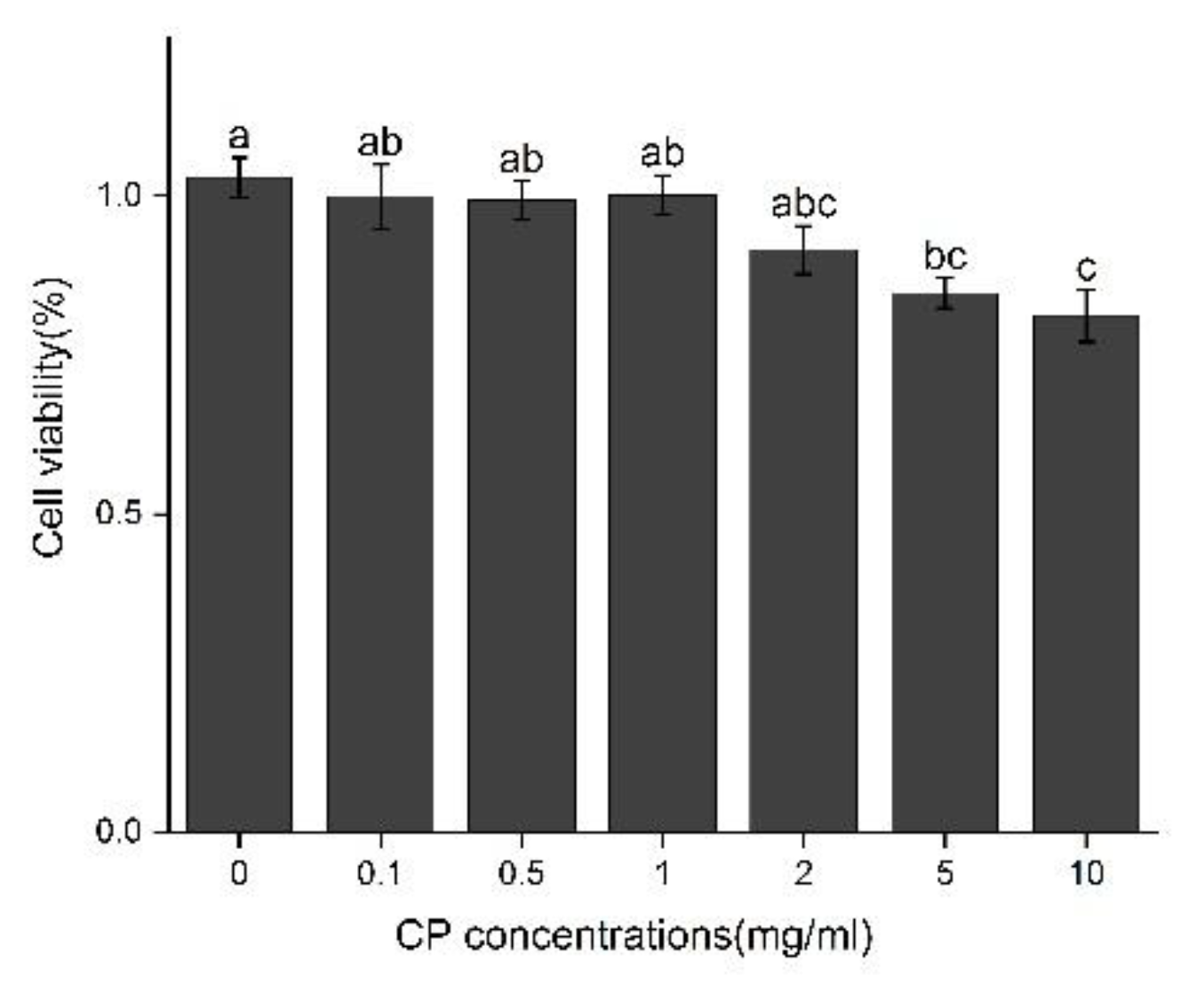
3.4. The Molecular Mechanism of Effects from CPs on LPS-Induced Kuffer Cells (KCs)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ma, Z.; Hou, T.; Shi, W.; Liu, W.; He, H. Inhibition of Hepatocyte Apoptosis: An Important Mechanism of Corn Peptides Attenuating Liver Injury Induced by Ethanol. Int. J. Mol. Sci. 2015, 16, 22062–22080. [Google Scholar] [CrossRef] [PubMed]
- O’Shea, R.S.; Dasarathy, S.; McCullough, A.J. Alcoholic liver disease. Hepatology 2010, 51, 307–328. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Wang, L.; Wang, Y.; Lin, D.; Liu, J. Hepatoprotective Effect of Albumin Peptides from Corn Germ Meal on Chronic Alcohol-Induced Liver Injury in Mice. J. Food Sci. 2017, 82, 2997–3004. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zhang, J.; Li, Y. Corn oligopeptides protect against early alcoholic liver injury in rats. Food Chem. Toxicol. 2012, 50, 2149–2154. [Google Scholar] [CrossRef]
- Mathurin, P.; Bataller, R. Trends in the management and burden of alcoholic liver disease. J. Hepatol. 2015, 62 (Suppl. 1), S38–S46. [Google Scholar] [CrossRef]
- Fan, J.G.; Farrell, G.C. Epidemiology of non-alcoholic fatty liver disease in China. J. Hepatol. 2009, 50, 204–210. [Google Scholar] [CrossRef]
- Fan, J.G. Epidemiology of alcoholic and nonalcoholic fatty liver disease in China. J. Gastroenterol. Hepatol. 2013, 28 (Suppl. 1), 11–17. [Google Scholar] [CrossRef]
- Ong, J.P.; Pitts, A.; Younossi, Z.M. Increased overall mortality and liver-related mortality in non-alcoholic fatty liver disease. J. Hepatol. 2008, 49, 608–612. [Google Scholar] [CrossRef]
- Targher, G.; Arcaro, G. Non-alcoholic fatty liver disease and increased risk of cardiovascular disease. Atherosclerosis 2007, 191, 235–240. [Google Scholar] [CrossRef]
- Lieber, C.S. Alcoholic liver disease: New insights in pathogenesis lead to new treatments. J. Hepatol. 2000, 32, 113–128. [Google Scholar] [CrossRef]
- Hao, F.; Cubero, F.J.; Ramadori, P.; Liao, L.; Haas, U.; Lambertz, D.; Nevzorova, Y.A. Inhibition of Caspase-8 does not protect from alcohol-induced liver apoptosis but alleviates alcoholic hepatic steatosis in mice. Cell Death Dis. 2017, 8, e3152. [Google Scholar] [CrossRef] [PubMed]
- Vaduganathan, M.; van Meijgaard, J.; Mehra, M.R.; Joseph, J.; O’Donnell, C.J.; Warraich, H.J. Prescription Fill Patterns for Commonly Used Drugs During the COVID-19 Pandemic in the United States. JAMA 2020, 323, 2524–2526. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.M.; Horiguchi, N.; Jeong, W.I.; Radaeva, S.; Gao, B. Molecular mechanisms of alcoholic liver disease: Innate immunity and cytokines. Alcohol. Clin. Exp. Res. 2011, 35, 787–793. [Google Scholar] [CrossRef]
- Seitz, H.K.; Bataller, R.; Cortez-Pinto, H.; Gao, B.; Gual, A.; Lackner, C.; Tsukamoto, H. Alcoholic liver disease. Nat. Rev. Dis. Primers 2018, 4, 16. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Sun, J.; He, H.; Guo, H.; Zhang, S. Hepatoprotective effects of Ganoderma lucidum peptides against D-galactosamine-induced liver injury in mice. J. Ethnopharmacol. 2008, 117, 415–419. [Google Scholar] [CrossRef]
- Xie, Q.; Guo, F.-F.; Zhou, W. Protective effects of cassia seed ethanol extract against carbon tetrachloride-induced liver injury in mice. Acta Biochim. Pol. 2012, 59, 265–270. [Google Scholar] [CrossRef]
- Liu, W.L.; Chen, X.W.; Li, H.; Zhang, J.; An, J.L.; Liu, X.Q. Anti-Inflammatory Function of Plant-Derived Bioactive Peptides: A Review. Foods 2022, 11, 2361. [Google Scholar] [CrossRef]
- Zhou, C.; Hu, J.; Ma, H.; Yagoub, A.E.; Yu, X.; Owusu, J.; Qin, X. Antioxidant peptides from corn gluten meal: Orthogonal design evaluation. Food Chem 2015, 187, 270–278. [Google Scholar] [CrossRef]
- Wang, Y.; Song, X.; Feng, Y.; Cui, Q. Changes in peptidomes and Fischer ratios of corn-derived oligopeptides depending on enzyme hydrolysis approaches. Food Chem. 2019, 297, 124931. [Google Scholar] [CrossRef]
- Ortiz-Martinez, M.; Winkler, R.; Garcia-Lara, S. Preventive and therapeutic potential of peptides from cereals against cancer. J. Proteom. 2014, 111, 165–183. [Google Scholar] [CrossRef]
- Zhuang, H.; Tang, N.; Dong, S.T.; Sun, B.; Liu, J.B. Optimisation of antioxidant peptide preparation from corn gluten meal. J. Sci. Food Agric. 2013, 93, 3264–3270. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Guo, M.; Hua, Y.; Cao, D.; Zhang, C. Enzymatic preparation of immunomodulating hydrolysates from soy proteins. Bioresour. Technol. 2008, 99, 8873–8879. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Zhang, R.; Fang, L.; Qin, X.; Cai, M.; Gu, R.; Wang, Y. Hypoglycemic effects and biochemical mechanisms of Pea oligopeptide on high-fat diet and streptozotocin-induced diabetic mice. J. Food Biochem. 2019, 43, e13055. [Google Scholar] [CrossRef] [PubMed]
- Maemura, K.; Zheng, Q.; Wada, T.; Ozaki, M.; Takao, S.; Aikou, T.; Sun, Z. Reactive oxygen species are essential mediators in antigen presentation by Kupffer cells. Immunol. Cell Biol. 2005, 83, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.N.; Chen, J.; Gong, L.-L.; Su, R.-N.; Yang, R.; Yang, W.-W.; Lu, Y.-M. Structural characterization and in vitro hypoglyce-mic activity of a glucan from Euryale ferox Salisb. seeds. Carbohydr. Polym. 2019, 209, 363–371. [Google Scholar] [CrossRef]
- Ma, Z.L.; Hou, T.; Shi, W.; Liu, W.W.; Ibrahim, S.A.; He, H. Purification and identification of corn peptides that facilitate alcohol metabolism by semi-preparative high-performance liquid chromatography and nano liquid chromatography with electrospray ionization tandem mass spectrometry. J. Sep. Sci. 2016, 39, 4234–4242. [Google Scholar] [CrossRef]
- Díaz-Gómez, J.L.; Castorena-Torres, F.; Preciado-Ortiz, R.E.; García-Lara, S. Anti-cancer activity of maize bioactive peptides. Front. Chem. 2017, 5, 44. [Google Scholar] [CrossRef]
- Liang, Q.; Chalamaiah, M.; Ren, X.; Ma, H.; Wu, J. Identification of new anti-inflammatory peptides from zein hydrolysate after simulated gastrointestinal digestion and transport in caco-2 cells. J. Agric. Food Chem. 2018, 66, 1114–1120. [Google Scholar] [CrossRef]
- Kany, S.; Vollrath, J.T.; Relja, B. Cytokines in Inflammatory Disease. Int. J. Mol. Sci. 2019, 20, 6008. [Google Scholar] [CrossRef]
- Dunn, W.; Shah, V.H. Pathogenesis of Alcoholic Liver Disease. Clin. Liver Dis. 2016, 20, 445–456. [Google Scholar] [CrossRef]
- Farooq, M.O.; Bataller, R. Pathogenesis and Management of Alcoholic Liver Disease. Dig. Dis. 2016, 34, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Stickel, F.; Datz, C.; Hampe, J.; Bataller, R. Pathophysiology and Management of Alcoholic Liver Disease: Update 2016. Gut Liver 2017, 11, 173–188. [Google Scholar] [CrossRef] [PubMed]
- She, X.; Wang, F.; Ma, J.; Chen, X.; Ren, D.; Lu, J. In vitro antioxidant and protective effects of corn peptides on ethanol-induced damage in HepG2 cells. Food Agric. Immunol. 2016, 27, 99–110. [Google Scholar] [CrossRef]
- Tan, H.K.; Yates, E.; Lilly, K.; Dhanda, A.D. Oxidative stress in alcohol-related liver disease. World J. Hepatol. 2020, 12, 332–349. [Google Scholar] [CrossRef] [PubMed]
- Gluud, L.L.; Dam, G.; Borre, M.; Les, I.; Cordoba, J.; Marchesini, G.; Aagaard, N.K.; Risum, N.; Vilstrup, H. Oral branched-chain amino acids have a beneficial effect on manifestations of hepatic encephalopathy in a systematic review with meta-analyses of randomized controlled trials. J. Nutr. 2013, 143, 1263–1268. [Google Scholar] [CrossRef] [PubMed]
- Tedesco, L.; Corsetti, G.; Ruocco, C.; Ragni, M.; Rossi, F.; Carruba, M.O.; Valerio, A.; Nisoli, E. A specific amino acid formula prevents alcoholic liver disease in rodents. Am. J. Physiol. Gastrointest. Liver Physiol. 2018, 314, G566–G582. [Google Scholar] [CrossRef]
- Luo, W.; Xu, Q.; Wang, Q.; Wu, H.; Hua, J. Effect of modulation of PPAR-gamma activity on Kupffer cells M1/M2 polarization in the development of non-alcoholic fatty liver disease. Sci. Rep. 2017, 7, 44612. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; He, K.; Li, J.; Liu, Z.; Gong, J. The role of Kupffer cells in hepatic diseases. Mol. Immunol. 2017, 85, 222–229. [Google Scholar] [CrossRef]
- Zimmermann, H.W.; Trautwein, C.; Tacke, F. Functional role of monocytes and macrophages for the inflammatory response in acute liver injury. Front. Physiol. 2012, 3, 56. [Google Scholar] [CrossRef]
- Tacke, F. Targeting hepatic macrophages to treat liver diseases. J. Hepatol. 2017, 66, 1300–1312. [Google Scholar] [CrossRef]
- Wu, T.; Liu, T.; Zhang, L.; Xing, L.J.; Zheng, P.Y.; Ji, G. Chinese medicinal formula, Qinggan Huoxue Recipe protects rats from alcoholic liver disease via the lipopolysaccharide-Kupffer cell signal conduction pathway. Exp. Med. 2014, 8, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Zhang, R.; Wu, Y.; Wu, C.; Jia, X.; Dong, L.; Liu, L.; Chen, Y.; Bai, Y.; Zhang, M. Rice Bran Phenolic Extract Protects against Alcoholic Liver Injury in Mice by Alleviating Intestinal Microbiota Dysbiosis, Barrier Dysfunction, and Liver Inflammation Mediated by the Endotoxin–TLR4–NF-κB Pathway. J. Agric. Food Chem. 2020, 68, 1237–1247. [Google Scholar] [CrossRef] [PubMed]


| Gene | Primer Sequences (5′-3′) | Product Length |
|---|---|---|
| TNF-α | forward: CATCTTCTCAAAATTCGAGTGACAA | 447 bp |
| reverse: TGGGAGTAGACAAGGTACAACCC | ||
| IL-1 | forward: CTTCATCTTTGAAGAAGAGCCC | 418 bp |
| reverse: CTCTGCAGACTCAAACTCCAC | ||
| IL-6 | forward: TTCACAAGTCCGGACAGGAG | 488 bp |
| reverse: TGGTCTTGGTCCTTAGCCAC 3 | ||
| GAPDH | forward: GAAGGTGAAGGTCGGAGTCA | 402 bp |
| reverse: TTCACACCCATGACGAACAT |
| Corn Oligopetides | |
|---|---|
| Moisture (%) | 3.78 ± 0.11 |
| Ash (%) | 3.90 ± 0.13 |
| Protein (%) | 83.60 ± 1.21 |
| Peptide (%) | 79.23 ± 1.19 |
| Amino acid composition (%) | |
| Ala | 8.17 |
| Pro | 6.52 |
| Val | 2.72 |
| Met | 1.97 |
| Ile | 1.99 |
| Leu | 16.73 |
| Phe | 4.33 |
| Trp | 0.23 |
| Asp | 4.73 |
| Ser | 4.14 |
| Glu | 22.72 |
| His | 1.03 |
| Gly | 1.18 |
| Arg | 1.35 |
| Thr | 2.22 |
| Cys | 2.17 |
| Tyr | 4.29 |
| Lys | 0.25 |
| Molecular Weight (u) | Over 10,000 | 3000–10,000 | 1000–3000 | 150–1000 | Below 150 |
|---|---|---|---|---|---|
| Distributions (%) | 0.0000 | 0.1513 | 3.3431 | 77.5960 | 18.9096 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, Y.; Li, M.; Feng, Z.; Zhang, D.; Sun, M.; Wang, Y.; Chen, X. The Protective Effects of Corn Oligopeptides on Acute Alcoholic Liver Disease by Inhibiting the Activation of Kupffer Cells NF-κB/AMPK Signal Pathway. Nutrients 2022, 14, 4194. https://doi.org/10.3390/nu14194194
Wei Y, Li M, Feng Z, Zhang D, Sun M, Wang Y, Chen X. The Protective Effects of Corn Oligopeptides on Acute Alcoholic Liver Disease by Inhibiting the Activation of Kupffer Cells NF-κB/AMPK Signal Pathway. Nutrients. 2022; 14(19):4194. https://doi.org/10.3390/nu14194194
Chicago/Turabian StyleWei, Ying, Mingliang Li, Zhiyuan Feng, Di Zhang, Meiling Sun, Yong Wang, and Xiangning Chen. 2022. "The Protective Effects of Corn Oligopeptides on Acute Alcoholic Liver Disease by Inhibiting the Activation of Kupffer Cells NF-κB/AMPK Signal Pathway" Nutrients 14, no. 19: 4194. https://doi.org/10.3390/nu14194194
APA StyleWei, Y., Li, M., Feng, Z., Zhang, D., Sun, M., Wang, Y., & Chen, X. (2022). The Protective Effects of Corn Oligopeptides on Acute Alcoholic Liver Disease by Inhibiting the Activation of Kupffer Cells NF-κB/AMPK Signal Pathway. Nutrients, 14(19), 4194. https://doi.org/10.3390/nu14194194








