Oral Administration of Branched-Chain Amino Acids Attenuates Atherosclerosis by Inhibiting the Inflammatory Response and Regulating the Gut Microbiota in ApoE-Deficient Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Studies
2.2. Sample Collection
2.3. Histological Analysis
2.4. Serum Biochemical Assays
2.5. Assay of BCAAs in Serum
2.6. Enzyme-Linked Immunosorbent Assay (ELISA)
2.7. Western Blotting
2.8. 16S rRNA Gene Sequencing
2.9. Assessment of Bile Acids in Intestines
2.10. Statistical Analysis
3. Results
3.1. BCAA Supplementation Reduces Atherosclerotic Plaques Induced by a High-Fat Diet in ApoE−/− Mice
3.2. BCAA Supplementation Ameliorates Dyslipidemia Induced by a High-Fat Diet in ApoE−/− Mice
3.3. Effects of BCAA Supplementation on Serum Levels of BCAAs and BCAA Metabolic Enzymes in ApoE−/− Mice
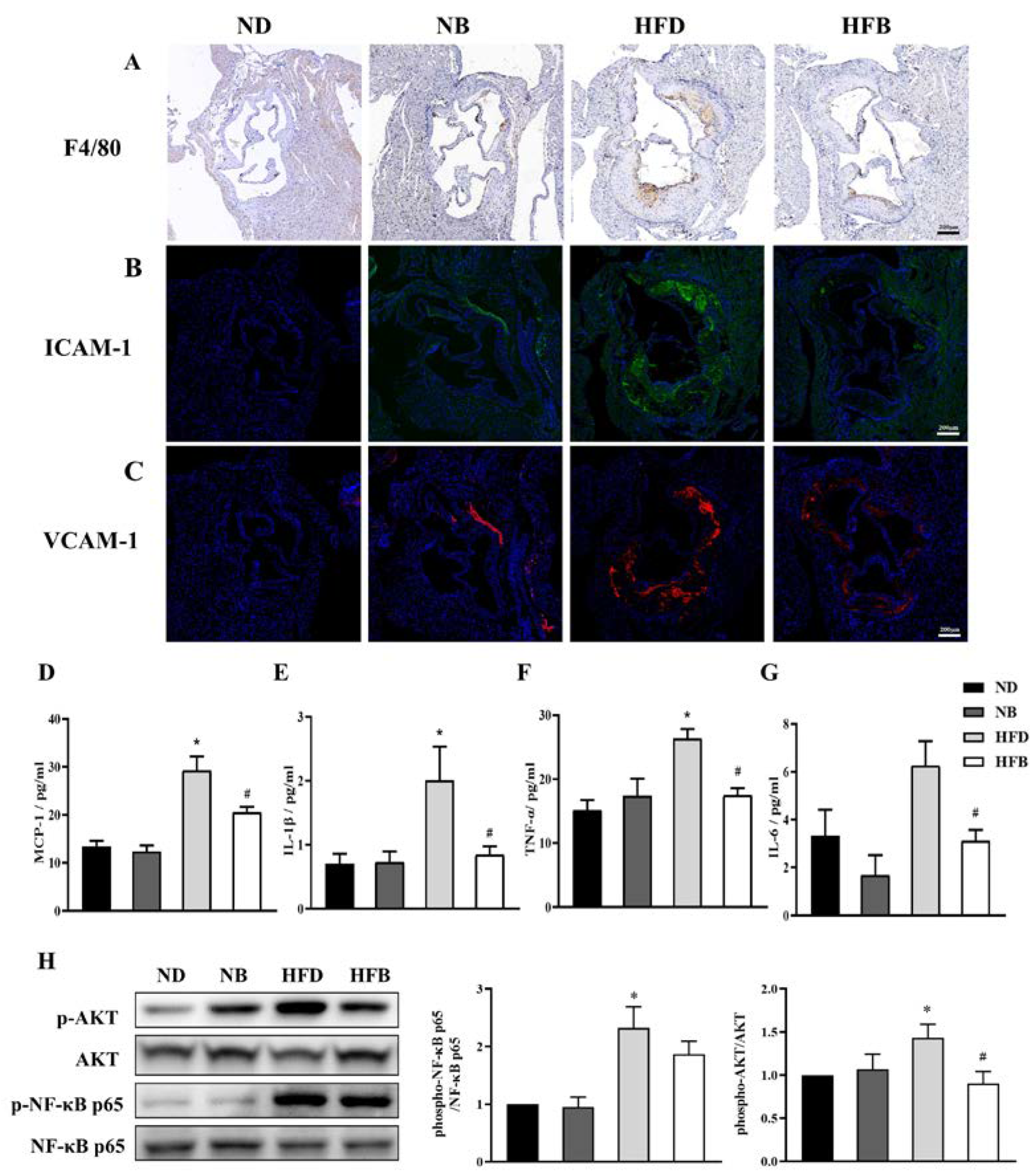
3.4. BCAA Supplementation Relieves Inflammation Induced by a High-Fat Diet in ApoE−/− Mice
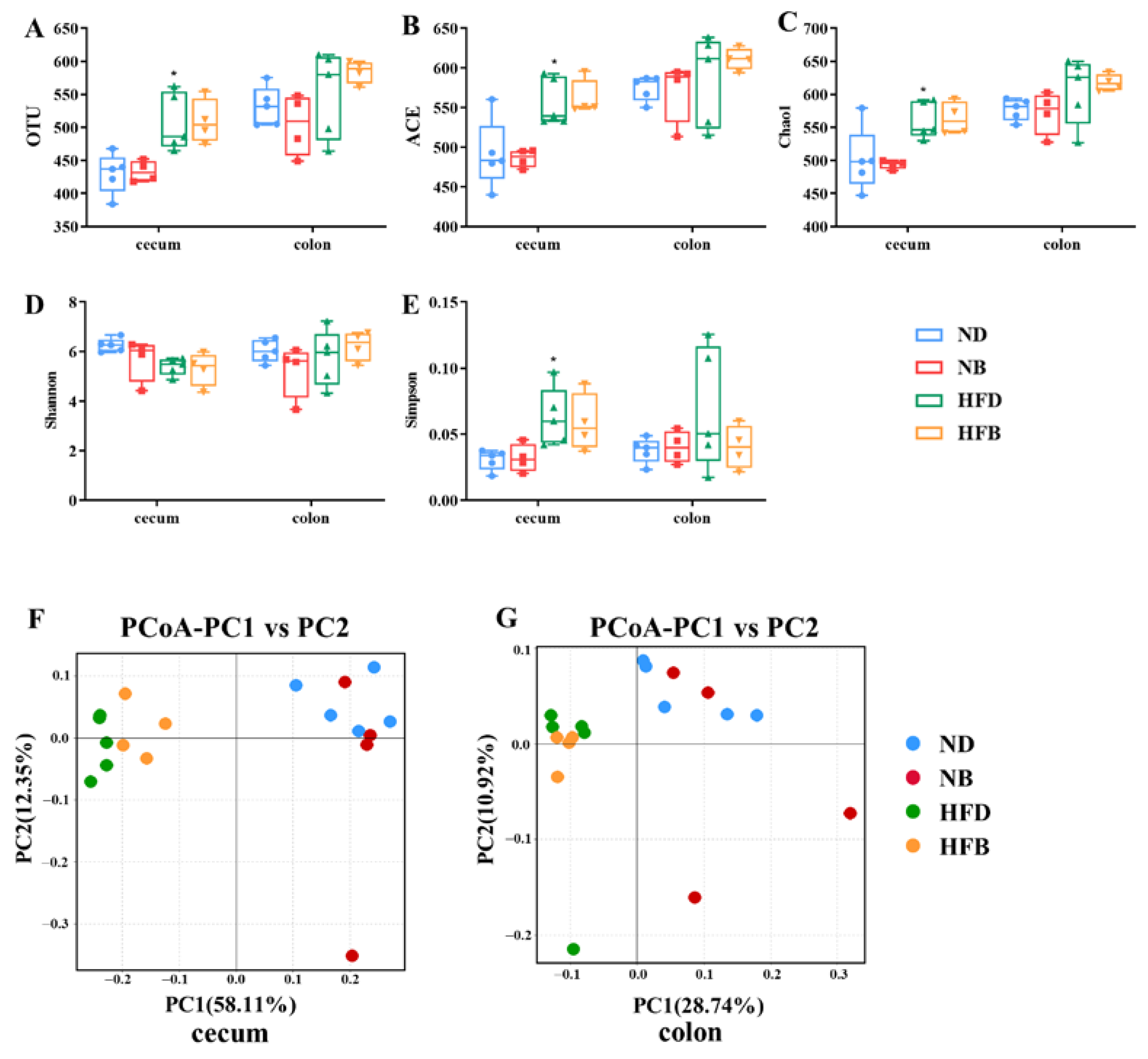
3.5. BCAA Supplementation Alters Intestinal Flora Diversity in ApoE−/− Mice
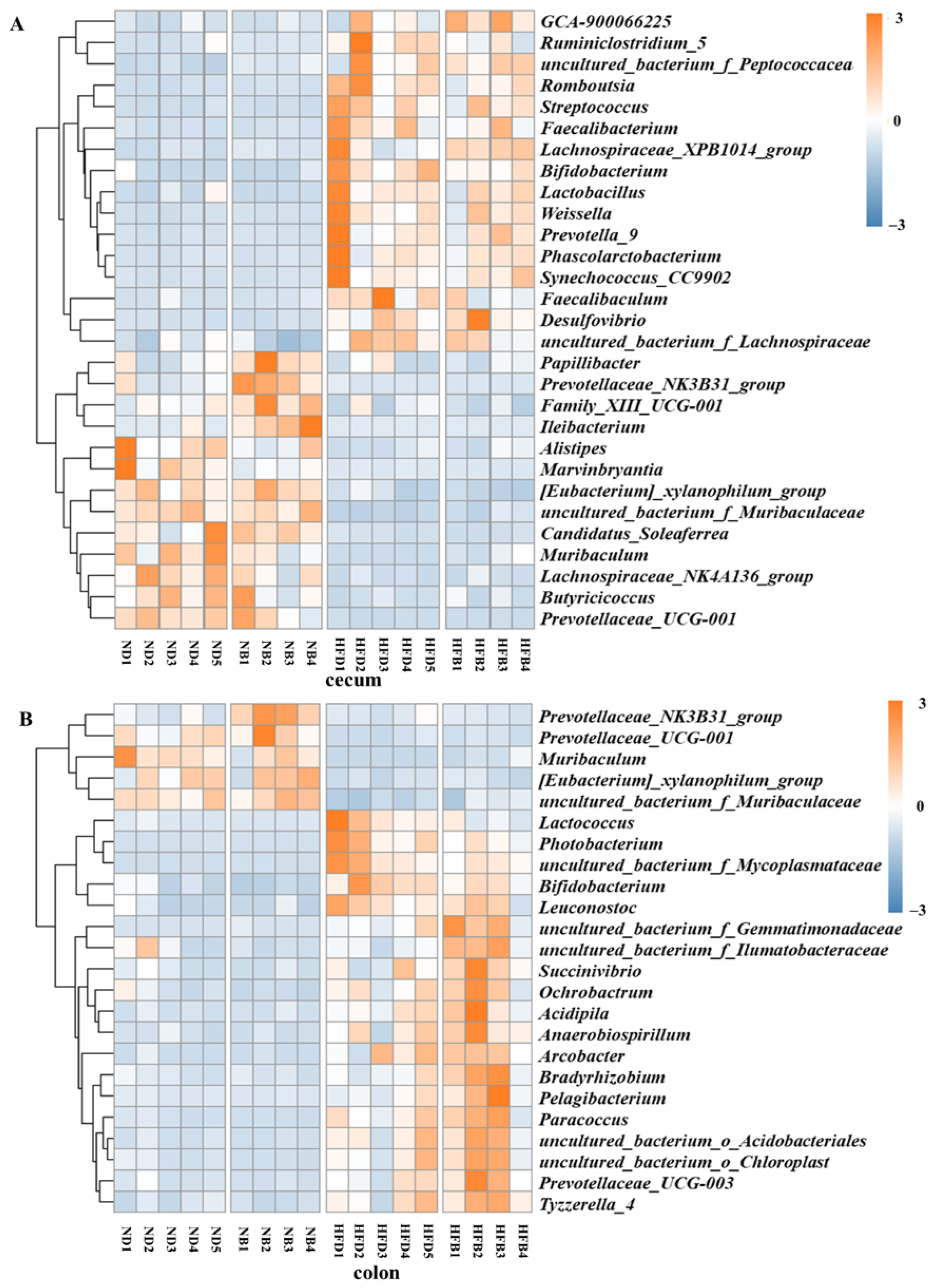
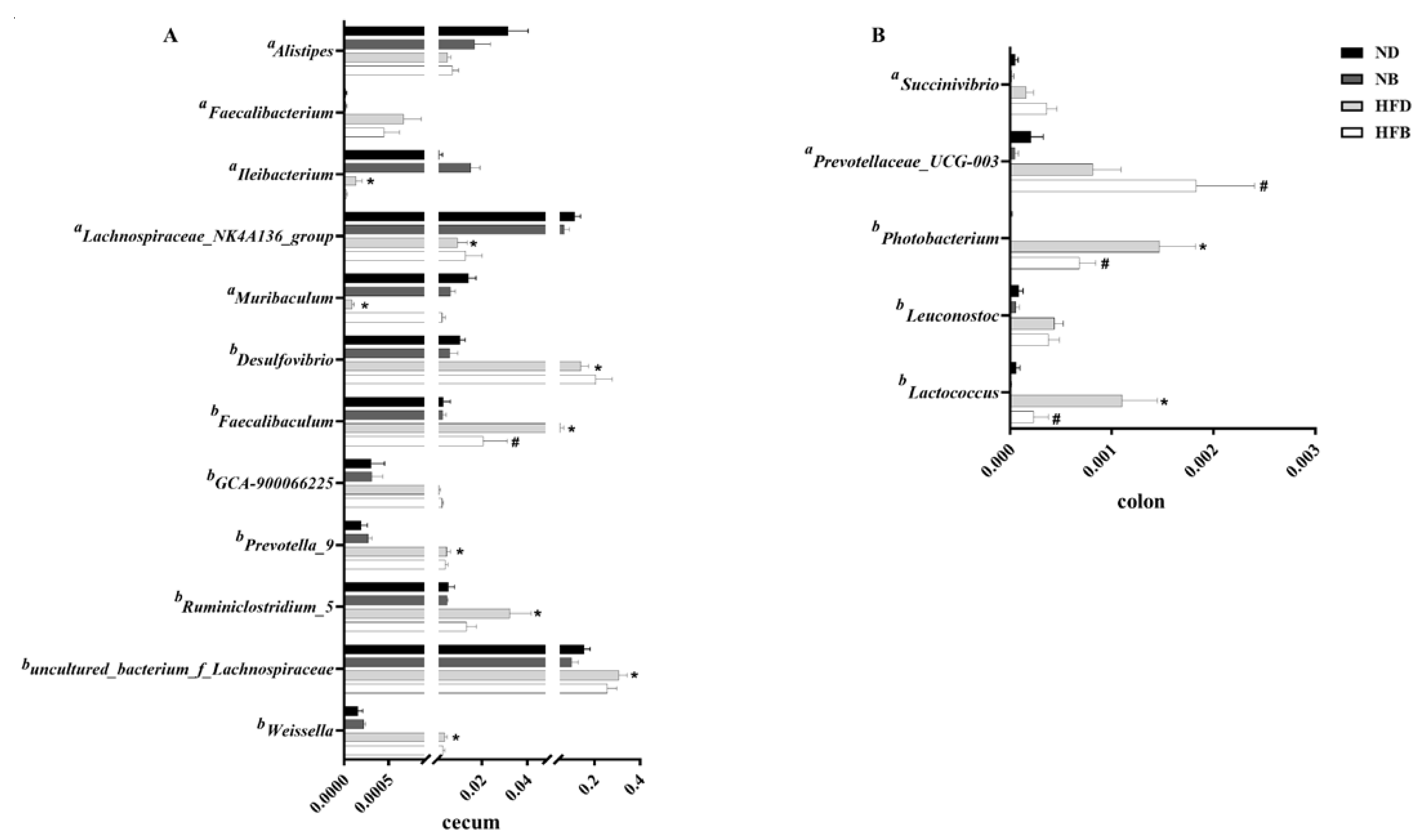
3.6. BCAA Supplementation Alters Gut Microbiota Composition in ApoE−/− Mice
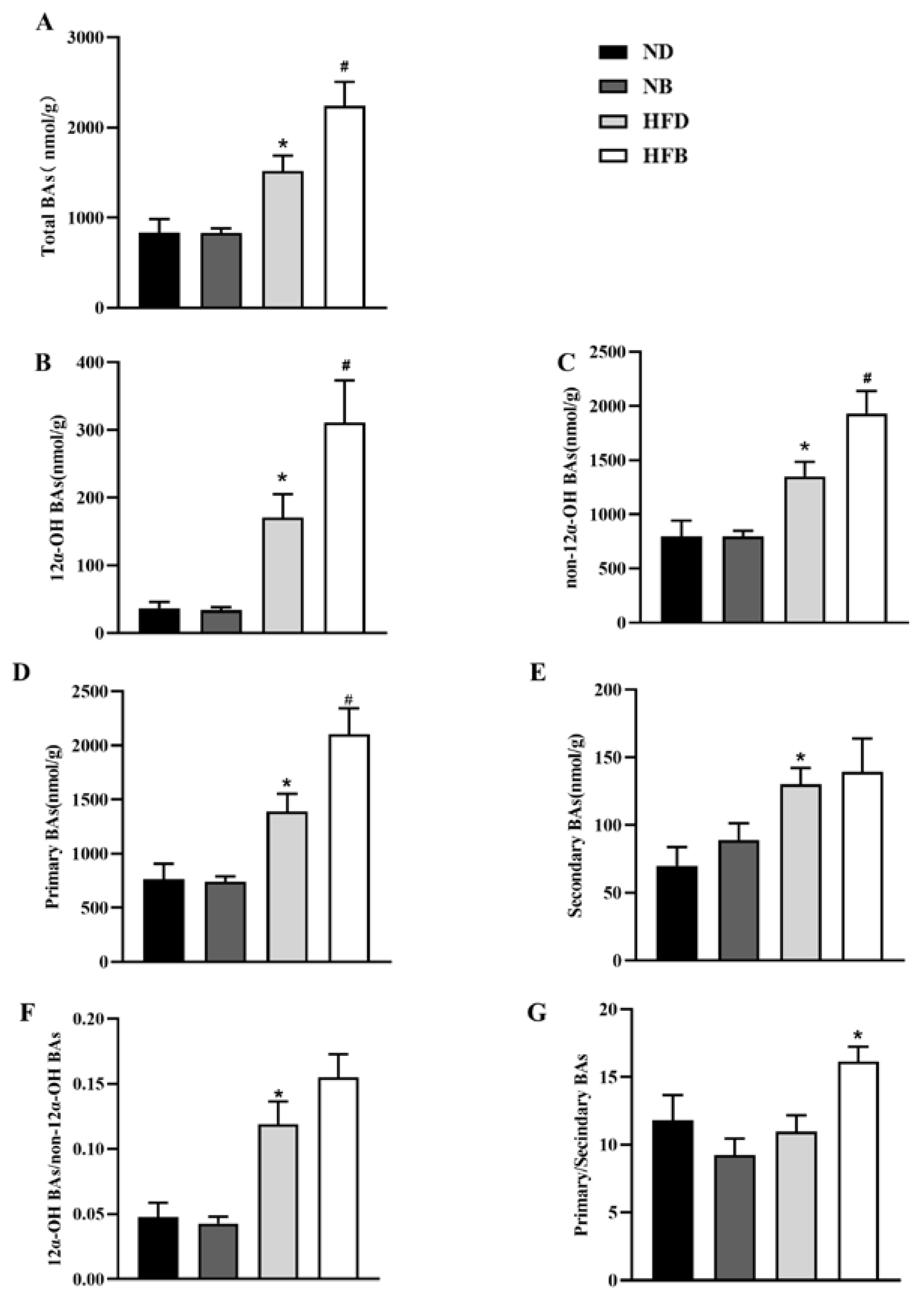
3.7. BCAA Supplementation Promotes Bile Acid Excretion in ApoE−/− Mice
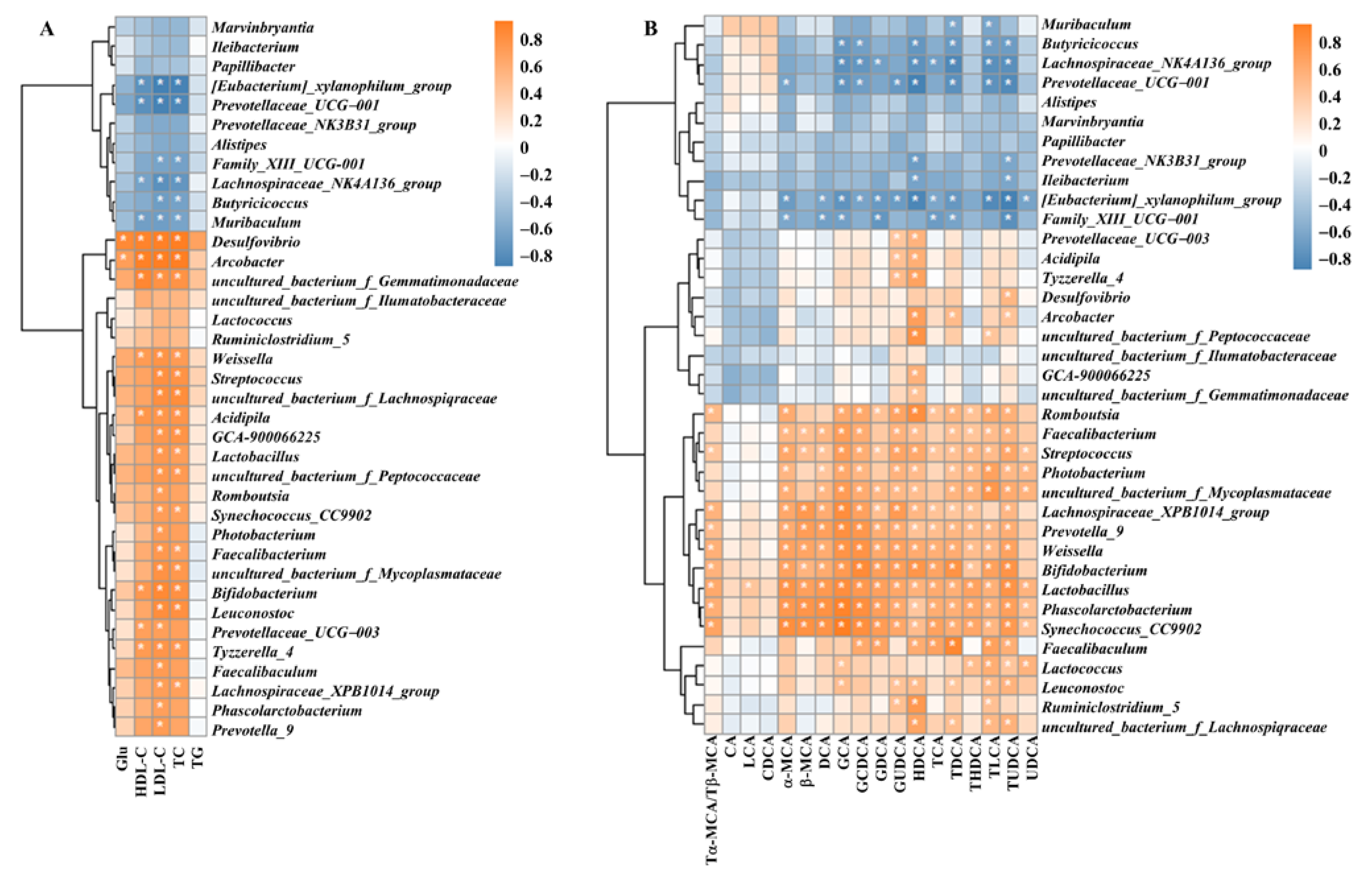
3.8. Correlation of Intestinal Flora with Blood Glucose, Lipid Indices and Bile Acids
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Libby, P.; Buring, J.E.; Badimon, L.; Hansson, G.K.; Deanfield, J.; Bittencourt, M.S.; Tokgözoğlu, L.; Lewis, E.F. Atherosclerosis. Nat. Rev. Dis. Prim. 2019, 5, 56. [Google Scholar] [CrossRef] [PubMed]
- Kobiyama, K.; Ley, K. Atherosclerosis: A chronic inflammatory disease with an autoimmune component. Circ. Res. 2018, 123, 1118. [Google Scholar] [CrossRef] [PubMed]
- Powell-Wiley, T.M.; Poirier, P.; Burke, L.E.; Després, J.-P.; Gordon-Larsen, P.; Lavie, C.J.; Lear, S.A.; Ndumele, C.E.; Neeland, I.J.; Sanders, P.; et al. Obesity and Cardiovascular Disease: A Scientific Statement From the American Heart Association. Circulation 2021, 143, e984–e1010. [Google Scholar] [CrossRef]
- Andersson, C.; Vasan, R.S. Epidemiology of cardiovascular disease in young individuals. Nat. Rev. Cardiol. 2018, 15, 230–240. [Google Scholar] [CrossRef]
- Benjamin, E.J.; Blaha, M.J.; Chiuve, S.E.; Cushman, M.; Das, S.R.; Deo, R.; De Ferranti, S.D.; Floyd, J.; Fornage, M.; Gillespie, C. Heart disease and stroke statistics-2016 update a report from the American Heart Association. Circulation 2017, 133, e38–e48. [Google Scholar]
- Miller, Y.I.; Choi, S.-H.; Wiesner, P.; Fang, L.; Harkewicz, R.; Hartvigsen, K.; Boullier, A.; Gonen, A.; Diehl, C.J.; Que, X.; et al. Oxidation-Specific Epitopes Are Danger-Associated Molecular Patterns Recognized by Pattern Recognition Receptors of Innate Immunity. Circ. Res. 2011, 108, 235–248. [Google Scholar] [CrossRef] [PubMed]
- Navab, M.; Ananthramaiah, G.M.; Reddy, S.T.; Van Lenten, B.J.; Ansell, B.J.; Fonarow, G.C.; Vahabzadeh, K.; Hama, S.; Hough, G.; Kamranpour, N.; et al. Thematic review series: The Pathogenesis of Atherosclerosis The oxidation hypothesis of atherogenesis: The role of oxidized phospholipids and HDL. J. Lipid Res. 2004, 45, 993–1007. [Google Scholar] [CrossRef] [PubMed]
- Wolf, D.; Ley, K. Immunity and Inflammation in Atherosclerosis. Circ. Res. 2019, 124, 315–327. [Google Scholar] [CrossRef]
- Zhu, Y.; Xian, X.; Wang, Z.; Bi, Y.; Chen, Q.; Han, X.; Tang, D.; Chen, R. Research Progress on the Relationship between Atherosclerosis and Inflammation. Biomolecules 2018, 8, 80. [Google Scholar] [CrossRef]
- Geovanini, G.R.; Libby, P. Atherosclerosis and inflammation: Overview and updates. Clin. Sci. 2018, 132, 1243–1252. [Google Scholar] [CrossRef]
- Ranjit, N.; Diez-Roux, A.V.; Shea, S.; Cushman, M.; Seeman, T.; Jackson, S.A.; Ni, H. Psychosocial Factors and Inflammation in the Multi-Ethnic Study of Atherosclerosis. Arch. Intern. Med. 2007, 167, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yu, H.; Zhang, Y.; Zhao, Y. TLRs are important inflammatory factors in atherosclerosis and may be a therapeutic target. Med. Hypotheses 2008, 70, 314–316. [Google Scholar] [CrossRef]
- Fan, Y.; Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 2021, 19, 55. [Google Scholar] [CrossRef] [PubMed]
- Witkowski, M.; Weeks, T.L.; Hazen, S.L. Gut Microbiota and Cardiovascular Disease. Circ. Res. 2020, 127, 553–570. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Rodriguez, E.; Egea-Zorrilla, A.; Plaza-Díaz, J.; Aragón-Vela, J.; Muñoz-Quezada, S.; Tercedor-Sánchez, L.; Abadia-Molina, F. The Gut Microbiota and Its Implication in the Development of Atherosclerosis and Related Cardiovascular Diseases. Nutrients 2020, 12, 605. [Google Scholar] [CrossRef]
- Zhu, L.; Zhang, D.; Zhu, H.; Zhu, J.; Weng, S.; Dong, L.; Liu, T.; Hu, Y.; Shen, S. Berberine treatment increases Akkermansia in the gut and improves high-fat diet-induced atherosclerosis in Apoe−/− mice. Atherosclerosis 2018, 268, 117. [Google Scholar] [CrossRef]
- Gregory, J.C.; Buffa, J.A.; Org, E.; Wang, Z.; Levison, B.S.; Zhu, W.; Wagner, M.A.; Bennett, B.J.; Li, L.; DiDonato, J.A.; et al. Transmission of Atherosclerosis Susceptibility with Gut Microbial Transplantation. J. Biol. Chem. 2015, 290, 5647–5660. [Google Scholar] [CrossRef]
- Wang, P.-X.; Deng, X.-R.; Zhang, C.-H.; Yuan, H.-J. Gut microbiota and metabolic syndrome. Chin. Med. J. 2020, 133, 808–816. [Google Scholar] [CrossRef]
- Molinaro, A.; Wahlström, A.; Marschall, H.U. Role of Bile Acids in Metabolic Control. Trends Endocrinol. Metab. TEM 2018, 29, 31. [Google Scholar] [CrossRef]
- Wahlström, A.; Sayin, S.I.; Marschall, H.-U.; Bäckhed, F. Intestinal Crosstalk between Bile Acids and Microbiota and Its Impact on Host Metabolism. Cell Metab. 2016, 24, 41–50. [Google Scholar] [CrossRef]
- Fuchs, C.D.; Trauner, M. Role of bile acids and their receptors in gastrointestinal and hepatic pathophysiology. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 432–450. [Google Scholar] [CrossRef] [PubMed]
- Fiorucci, S.; Distrutti, E. Bile Acid-Activated Receptors, Intestinal Microbiota, and the Treatment of Metabolic Disorders. Trends Mol. Med. 2015, 21, 702. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Liu, Z.; Yu, W.; Wen, S.; Wang, X.; Qi, X.; Hao, H.; Lu, Y.; Li, J.; Li, S.; et al. Effects of PPM1K rs1440581 and rs7678928 on serum branched-chain amino acid levels and risk of cardiovascular disease. Ann. Med. 2021, 53, 1317–1327. [Google Scholar] [CrossRef] [PubMed]
- Tobias, D.K.; Lawler, P.R.; Harada, P.H.; Demler, O.V.; Ridker, P.M.; Manson, J.E.; Cheng, S.; Mora, S. Circulating Branched-Chain Amino Acids and Incident Cardiovascular Disease in a Prospective Cohort of US Women. Circ. Genom. Precis. Med. 2018, 11, e002157. [Google Scholar] [CrossRef]
- Yang, P.; Hu, W.; Fu, Z.; Sun, L.; Zhou, Y.; Gong, Y.; Yang, T.; Zhou, H. The positive association of branched-chain amino acids and metabolic dyslipidemia in Chinese Han population. Lipids Health Dis. 2016, 15, 120. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Canela, M.; Toledo, E.; Clish, C.B.; Hruby, A.; Liang, L.; Salas-Salvadó, J.; Razquin, C.; Corella, D.; Estruch, R.; Ros, E.; et al. Plasma Branched-Chain Amino Acids and Incident Cardiovascular Disease in the PREDIMED Trial. Clin. Chem. 2016, 62, 582–592. [Google Scholar] [CrossRef]
- Grajeda-Iglesias, C.; Aviram, M. Specific Amino Acids Affect Cardiovascular Diseases and Atherogenesis via Protection against Macrophage Foam Cell Formation: Review Article. Rambam. Maimonides Med. J. 2018, 9, e0022. [Google Scholar] [CrossRef]
- Zhang, R.; Mu, H.; Li, Z.; Zeng, J.; Zhou, Q.; Li, H.; Wang, S.; Li, X.; Zhao, X.; Sun, L.; et al. Oral administration of branched-chain amino acids ameliorates high-fat diet-induced metabolic-associated fatty liver disease via gut microbiota-associated mechanisms. Front. Microbiol. 2022, 13, 920277. [Google Scholar] [CrossRef]
- Wang, M.; Yang, R.; Dong, J.; Zhang, T.; Wang, S.; Zhou, W.; Li, H.; Zhao, H.; Zhang, L.; Wang, S.; et al. Simultaneous quantification of cardiovascular disease related metabolic risk factors using liquid chromatography tandem mass spectrometry in human serum. J. Chromatogr. B 2016, 1009–1010, 144–151. [Google Scholar] [CrossRef]
- Zhao, Y.; Dai, X.-Y.; Zhou, Z.; Zhao, G.; Wang, X.; Xu, M.-J. Leucine supplementation via drinking water reduces atherosclerotic lesions in apoE null mice. Acta Pharmacol. Sin. 2016, 37, 196–203. [Google Scholar] [CrossRef]
- Nadkarni, S.K.; Bouma, B.E.; De Boer, J.; Tearney, G.J. Evaluation of collagen in atherosclerotic plaques: The use of two coherent laser-based imaging methods. Lasers Med. Sci. 2009, 24, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Yurdagul, A. Crosstalk Between Macrophages and Vascular Smooth Muscle Cells in Atherosclerotic Plaque Stability. Arter. Thromb. Vasc. Biol. 2022, 42, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Harman, J.L.; Jørgensen, H.F. The role of smooth muscle cells in plaque stability: Therapeutic targeting potential. Br. J. Pharmacol. 2019, 176, 3741–3753. [Google Scholar] [CrossRef] [PubMed]
- Ohayon, J.; Finet, G.; Le Floc’h, S.; Cloutier, G.; Gharib, A.; Heroux, J.; Pettigrew, R.I. Biomechanics of Atherosclerotic Coronary Plaque: Site, Stability and In Vivo Elasticity Modeling. Ann. Biomed. Eng. 2014, 42, 269–279. [Google Scholar] [CrossRef]
- Zhong, S.; Li, L.; Shen, X.; Li, Q.; Xu, W.; Wang, X.; Tao, Y.; Yin, H. An update on lipid oxidation and inflammation in cardiovascular diseases. Free. Radic. Biol. Med. 2019, 144, 266–278. [Google Scholar] [CrossRef] [PubMed]
- Ference, B.A.; Ginsberg, H.N.; Graham, I.; Ray, K.K.; Packard, C.J.; Bruckert, E.; Hegele, R.A.; Krauss, R.M.; Raal, F.J.; Schunkert, H.; et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur. Heart J. 2017, 38, 2459–2472. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, K.; Harada, S.; Takeuchi, A.; Kurihara, A.; Iida, M.; Fukai, K.; Kuwabara, K.; Kato, S.; Matsumoto, M.; Hirata, A.; et al. Association between dyslipidemia and plasma levels of branched-chain amino acids in the Japanese population without diabetes mellitus. J. Clin. Lipidol. 2019, 13, 932–939.e2. [Google Scholar] [CrossRef] [PubMed]
- Grajeda-Iglesias, C.; Rom, O.; Hamoud, S.; Volkova, N.; Hayek, T.; Abu-Saleh, N.; Aviram, M. Leucine supplementation attenuates macrophage foam-cell formation: Studies in humans, mice, and cultured macrophages. BioFactors 2018, 44, 245–262. [Google Scholar] [CrossRef]
- Manning, B.D.; Toker, A. AKT/PKB Signaling: Navigating the Network. Cell 2017, 169, 381–405. [Google Scholar] [CrossRef]
- Pan, J.X. LncRNA H19 promotes atherosclerosis by regulating MAPK and NF-kB signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 322. [Google Scholar]
- Simion, V.; Zhou, H.; Pierce, J.B.; Yang, D.; Haemmig, S.; Tesmenitsky, Y.; Sukhova, G.; Stone, P.H.; Libby, P.; Feinberg, M.W. LncRNA VINAS regulates atherosclerosis by modulating NF-κB and MAPK signaling. JCI Insight 2020, 5, e140627. [Google Scholar] [CrossRef]
- Ben, J.; Jiang, B.; Wang, D.; Liu, Q.; Zhang, Y.; Qi, Y.; Tong, X.; Chen, L.; Liu, X.; Zhang, Y. Major vault protein suppresses obesity and atherosclerosis through inhibiting IKK-NF-κB signaling mediated inflammation. Nat. Commun. 2019, 10, 1801. [Google Scholar] [CrossRef]
- Lawrence, T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb. Perspect. Biol. 2009, 1, a001651. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Rane, M. Interleukin-6 Signaling and Anti-Interleukin-6 Therapeutics in Cardiovascular Disease. Circ. Res. 2021, 128, 1728–1746. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Lei, J.; Lei, H.; Ruan, X.; Liu, Q.; Chen, Y.; Huang, W. MicroRNA-101 overexpression by IL-6 and TNF-α inhibits cholesterol efflux by suppressing ATP-binding cassette transporter A1 expression. Exp. Cell Res. 2015, 336, 33–42. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, X.; Bian, F.; Wu, P.; Xing, S.; Xu, G.; Li, W.; Chi, J.; Ouyang, C.; Zheng, T. TNF-α promotes early atherosclerosis by increasing transcytosis of LDL across endothelial cells: Crosstalk between NF-κB and PPAR-γ. J. Mol. Cell. Cardiol. 2014, 72, 85. [Google Scholar] [CrossRef] [PubMed]
- Buckley, L.F.; Abbate, A. Interleukin-1 blockade in cardiovascular diseases: A clinical update. Eur. Heart J. 2018, 39, 2063–2069. [Google Scholar] [CrossRef] [PubMed]
- Abbate, A.; Van Tassell, B.W.; Biondi-Zoccai, G.G. Blocking interleukin-1 as a novel therapeutic strategy for secondary prevention of cardiovascular events. BioDrugs Clin. Immunother. Biopharm. Gene Ther. 2012, 26, 217. [Google Scholar] [CrossRef]
- Grebe, A.; Hoss, F.; Latz, E. NLRP3 Inflammasome and the IL-1 Pathway in Atherosclerosis. Circ. Res. 2018, 122, 1722–1740. [Google Scholar] [CrossRef] [PubMed]
- Galea, J.; Armstrong, J.; Gadsdon, P.; Holden, H.; Francis, S.E. Holt CM, Interleukin-1 beta in coronary arteries of patients with ischemic heart disease. Arterioscler. Thromb. Vasc. Biol. 1996, 16, 1000. [Google Scholar] [CrossRef]
- Aiello, R.J.; Bourassa, P.-A.K.; Lindsey, S.; Weng, W.; Natoli, E.; Rollins, B.J.; Milos, P.M. Monocyte Chemoattractant Protein-1 Accelerates Atherosclerosis in Apolipoprotein E-Deficient Mice. Arter. Thromb. Vasc. Biol. 1999, 19, 1518–1525. [Google Scholar] [CrossRef] [PubMed]
- Jiao, J.; Han, S.-F.; Zhang, W.; Xu, J.-Y.; Tong, X.; Yin, X.-B.; Yuan, L.-X.; Qin, L.-Q. Chronic leucine supplementation improves lipid metabolism in C57BL/6J mice fed with a high-fat/cholesterol diet. Food Nutr. Res. 2016, 60, 31304. [Google Scholar] [CrossRef] [PubMed]
- Önal, B.; Özen, D.; Demir, B.; Ak, D.G.; Dursun, E.; Demir, C.; Akkan, A.G.; Özyazgan, S. The Anti-Inflammatory Effects of Anacardic Acid on a TNF-α—Induced Human Saphenous Vein Endothelial Cell Culture Model. Curr. Pharm. Biotechnol. 2020, 21, 710. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Ha, S.J.; Park, J.; Kim, Y.H.; Lee, N.H.; Hong, Y.-S.; Song, K.-M. Arctium lappa root extract containing L-arginine prevents TNF-α-induced early atherosclerosis in vitro and in vivo. Nutr. Res. 2020, 77, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Jiang, H.; Li, L.; Chen, F.; Liu, Y.; Zhou, M.; Wang, J.; Jiang, J.; Li, X.; Fan, X.; et al. Branched-Chain Amino Acid Catabolism Promotes Thrombosis Risk by Enhancing Tropomodulin-3 Propionylation in Platelets. Circulation 2020, 142, 49. [Google Scholar] [CrossRef]
- Holeček, M. Branched-chain amino acids in health and disease: Metabolism, alterations in blood plasma, and as supplements. Nutr. Metab. 2018, 15, 33. [Google Scholar] [CrossRef]
- Tobias, D.K.; Clish, C.; Mora, S.; Li, J.; Liang, L.; Hu, F.B.; Manson, J.E.; Zhang, C. Dietary Intakes and Circulating Concentrations of Branched-Chain Amino Acids in Relation to Incident Type 2 Diabetes Risk Among High-Risk Women with a History of Gestational Diabetes Mellitus. Clin. Chem. 2018, 64, 1203–1210. [Google Scholar] [CrossRef]
- Tung, Y.-C.; Chou, R.-F.; Nagabhushanam, K.; Ho, C.-T.; Pan, M.-H. 3′-Hydroxydaidzein Improves Obesity Through the Induced Browning of Beige Adipose and Modulation of Gut Microbiota in Mice with Obesity Induced by a High-Fat Diet. J. Agric. Food Chem. 2020, 68, 14513–14522. [Google Scholar] [CrossRef]
- Fusco, V.; Quero, G.M.; Cho, G.-S.; Kabisch, J.; Meske, D.; Neve, H.; Bockelmann, W.; Franz, C.M.A.P. The genus Weissella: Taxonomy, ecology and biotechnological potential. Front. Microbiol. 2015, 6, 155. [Google Scholar] [CrossRef]
- Hiraishi, K.; Zhao, F.; Kurahara, L.-H.; Li, X.; Yamashita, T.; Hashimoto, T.; Matsuda, Y.; Sun, Z.; Zhang, H.; Hirano, K. Lactulose Modulates the Structure of Gut Microbiota and Alleviates Colitis-Associated Tumorigenesis. Nutrients 2022, 14, 649. [Google Scholar] [CrossRef]
- Kai, L.; Zong, X.; Jiang, Q.; Lu, Z.; Wang, F.; Wang, Y.; Wang, T.; Jin, M. Protective effects of polysaccharides from Atractylodes macrocephalae Koidz. against dextran sulfate sodium induced intestinal mucosal injury on mice. Int. J. Biol. Macromol. 2022, 195, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Goel, R.; Kumar, A.; Qi, Y.; Lobaton, G.; Hosaka, K.; Mohammed, M.; Handberg, E.; Richards, E.M.; Pepine, C.J.; et al. Imbalance of gut microbiome and intestinal epithelial barrier dysfunction in patients with high blood pressure. Clin. Sci. 2018, 132, 701–718. [Google Scholar] [CrossRef] [PubMed]
- Verstreken, I.; Laleman, W.; Wauters, G.; Verhaegen, J. Desulfovibrio desulfuricans Bacteremia in an Immunocompromised Host with a Liver Graft and Ulcerative Colitis. J. Clin. Microbiol. 2012, 50, 199–201. [Google Scholar] [CrossRef]
- Duan, J.; Huang, Y.; Tan, X.; Chai, T.; Wu, J.; Zhang, H.; Li, Y.; Hu, X.; Zheng, P.; Ji, P.; et al. Characterization of gut microbiome in mice model of depression with divergent response to escitalopram treatment. Transl. Psychiatry 2021, 11, 303. [Google Scholar] [CrossRef] [PubMed]
- Hull, M.A. Nutritional prevention of colorectal cancer. Proc. Nutr. Soc. 2021, 80, 59. [Google Scholar] [CrossRef] [PubMed]
- Buhman, K.K.; Furumoto, E.J.; Donkin, S.S.; Story, J.A. Dietary Psyllium Increases Fecal Bile Acid Excretion, Total Steroid Excretion and Bile Acid Biosynthesis in Rats. J. Nutr. 1998, 128, 1199–1203. [Google Scholar] [CrossRef] [PubMed]
- Bertaggia, E.; Jensen, K.K.; Castro-Perez, J.; Xu, Y.; Di Paolo, G.; Chan, R.B.; Wang, L.; Haeusler, R.A. Cyp8b1 ablation prevents Western diet-induced weight gain and hepatic steatosis because of impaired fat absorption. Am. J. Physiol. Metab. 2017, 313, E121–E133. [Google Scholar] [CrossRef]
- Li, P.; Ruan, X.; Yang, L.; Kiesewetter, K.; Zhao, Y.; Luo, H.; Chen, Y.; Gucek, M.; Zhu, J.; Cao, H. A Liver-Enriched Long Non-Coding RNA, lncLSTR, Regulates Systemic Lipid Metabolism in Mice. Cell Metab. 2015, 21, 455–467. [Google Scholar] [CrossRef]
- Haeusler, R.A.; Astiarraga, B.; Camastra, S.; Accili, D.; Ferrannini, E. Human Insulin Resistance Is Associated With Increased Plasma Levels of 12α-Hydroxylated Bile Acids. Diabetes 2013, 62, 4184–4191. [Google Scholar] [CrossRef]
- Zeng, H.; Umar, S.; Rust, B.; Lazarova, D.; Bordonaro, M. Secondary Bile Acids and Short Chain Fatty Acids in the Colon: A Focus on Colonic Microbiome, Cell Proliferation, Inflammation, and Cancer. Int. J. Mol. Sci. 2019, 20, 1214. [Google Scholar] [CrossRef]
- Zeng, S.-L.; Li, S.-Z.; Xiao, P.-T.; Cai, Y.-Y.; Chu, C.; Chen, B.-Z.; Li, P.; Li, J.; Liu, E.-H. Citrus polymethoxyflavones attenuate metabolic syndrome by regulating gut microbiome and amino acid metabolism. Sci. Adv. 2020, 6, eaax6208. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.-L.; Lee, S.; Luong, T.T.; Nguyen, C.T.; Park, S.-S.; Pyo, S.; Rhee, D.-K. Effect of decreased BCAA synthesis through disruption of ilvC gene on the virulence of Streptococcus pneumoniae. Arch. Pharmacal Res. 2017, 40, 921–932. [Google Scholar] [CrossRef]
- Chen, M.-L.; Yi, L.; Zhang, Y.; Zhou, X.; Ran, L.; Yang, J.; Zhu, J.-D.; Zhang, Q.-Y.; Mi, M.-T. Resveratrol Attenuates Trimethylamine-N-Oxide (TMAO)-Induced Atherosclerosis by Regulating TMAO Synthesis and Bile Acid Metabolism via Remodeling of the Gut Microbiota. mBio 2016, 7, e02210-15. [Google Scholar] [CrossRef]
- Liang, J.; Kou, S.; Chen, C.; Raza, S.H.A.; Wang, S.; Ma, X.; Zhang, W.-J.; Nie, C. Effects of Clostridium butyricum on growth performance, metabonomics and intestinal microbial differences of weaned piglets. BMC Microbiol. 2021, 21, 85. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Gómez, J.G.; López-Bonilla, A.; Trejo-Tapia, G.; Ávila-Reyes, S.V.; Jiménez-Aparicio, A.R.; Hernández-Sánchez, H. In Vitro Bile Salt Hydrolase (BSH) Activity Screening of Different Probiotic Microorganisms. Foods 2021, 10, 674. [Google Scholar] [CrossRef]
- Tanaka, H.; Doesburg, K.; Iwasaki, T.; Mierau, I. Screening of Lactic Acid Bacteria for Bile Salt Hydrolase Activity. J. Dairy Sci. 1999, 82, 2530–2535. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Zhang, R.; Mu, H.; Zhang, W.; Zeng, J.; Li, H.; Wang, S.; Zhao, X.; Chen, W.; Dong, J.; et al. Oral Administration of Branched-Chain Amino Acids Attenuates Atherosclerosis by Inhibiting the Inflammatory Response and Regulating the Gut Microbiota in ApoE-Deficient Mice. Nutrients 2022, 14, 5065. https://doi.org/10.3390/nu14235065
Li Z, Zhang R, Mu H, Zhang W, Zeng J, Li H, Wang S, Zhao X, Chen W, Dong J, et al. Oral Administration of Branched-Chain Amino Acids Attenuates Atherosclerosis by Inhibiting the Inflammatory Response and Regulating the Gut Microbiota in ApoE-Deficient Mice. Nutrients. 2022; 14(23):5065. https://doi.org/10.3390/nu14235065
Chicago/Turabian StyleLi, Ziyun, Ranran Zhang, Hongna Mu, Wenduo Zhang, Jie Zeng, Hongxia Li, Siming Wang, Xianghui Zhao, Wenxiang Chen, Jun Dong, and et al. 2022. "Oral Administration of Branched-Chain Amino Acids Attenuates Atherosclerosis by Inhibiting the Inflammatory Response and Regulating the Gut Microbiota in ApoE-Deficient Mice" Nutrients 14, no. 23: 5065. https://doi.org/10.3390/nu14235065
APA StyleLi, Z., Zhang, R., Mu, H., Zhang, W., Zeng, J., Li, H., Wang, S., Zhao, X., Chen, W., Dong, J., & Yang, R. (2022). Oral Administration of Branched-Chain Amino Acids Attenuates Atherosclerosis by Inhibiting the Inflammatory Response and Regulating the Gut Microbiota in ApoE-Deficient Mice. Nutrients, 14(23), 5065. https://doi.org/10.3390/nu14235065





