Protein Intake and Oral Health in Older Adults—A Narrative Review
Abstract
1. Introduction
2. Methods
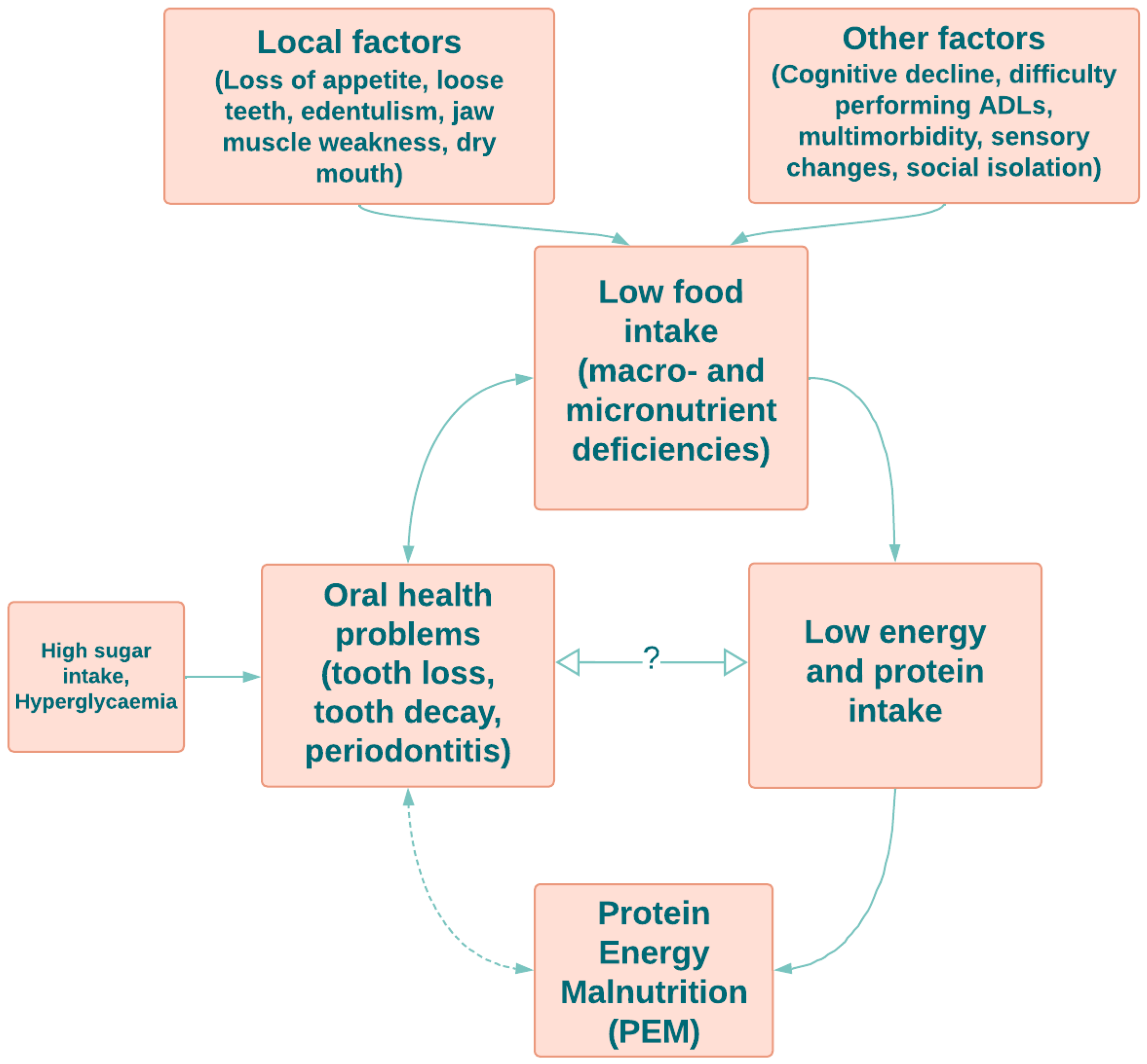
3. Factors Affecting Optimal Dietary Intake in Older Adults
3.1. Local Factors
3.2. Other Factors
4. Effect of Various Dietary Protein Sources on Oral Health
Dietary Amino Acid Composition and Its Effect on Oral Health
| Amino Acid | Effect on Oral Health | Material | References |
|---|---|---|---|
| Alanine | Alanine and histidine form citrulline. A higher concentration of citrulline in saliva is correlated with periodontitis. | Human | [105] |
| Arginine | Arginine improves calcium absorption by the formation of soluble complexes with calcium that maintain calcium in an absorbent form, which is important for enamel maturation. Higher concentration saliva in Stage III Grade C generalised periodontitis. | Human | [105] |
| L-Arginine | L-Arginine monohydrochloride in saliva inhibits bacterial coaggregation in the oral cavity by decreasing the viscosity of extracellular polymeric substances produced by bacteria and altering cellular metabolism resulting in biofilm dispersion and reducing antibiotic tolerance. | Human | [102] |
| Aspartic acid | Adult age estimation is based on aspartic acid racemisation in dentine. | Human | [106] |
| Cysteine | Toxic to oral Streptococci through inhibiting an enzymatic step in the valine-leucine biosynthetic pathway. | Human | [107] |
| Reduces bacterial biofilm adherence and biofilm biomass. | A multi-species plaque-derived biofilm model | [108] | |
| N-Acetyl-L-cysteine (from L-cysteine) | Reduces pain and hypersensitivity of teeth. Protects gingivae from white lesions and oral mucosal inflammation after using bleaching agents. | Human | [109] |
| As mouthwash, it treats and prevents gingivitis | Human | [110] | |
| Glutamic acid | Higher in Stage III Grade C generalised periodontitis. | Human | [105] |
| Glutamine | Topical administration to patients receiving stomatoxic chemotherapy resulted in 20% decrease in moderate and severe oral mucositis. | Human | [111] |
| Glycine | Glycine supplement reduced dental caries development by 65.7% through the changes in the fatty acid composition of the tooth and a reduction in growth rate (no effect on the retention of either calcium or phosphorus by dietary glycine). | Rodent (rat) | [112] |
| Glycine is an integral part of collagen that is an intrinsic component of the tooth structure. Reduced level of saliva glycine has been associated with collagen degradation. Hence, higher salivary glycine has been associated with reduced risk of dental caries and periodontitis through reduced collagen degradation and decreased collagenase activity, leading to less inflammation in gingiva. | Human | [113,114] | |
| Histidine † | Reduces the risk of dental caries. Lack of histidine and its derivatives in saliva results in chelation, i.e., formation of metal complexes with amino acids, leading to initial lesion and secondary to destruction of the organic matrix by the action of proteolytic bacteria. | Human | [103] |
| Isoleucine † | Found in carious dentine | Human | [115] |
| Leucine † | Repaired carious enamel. | Human | [116] |
| Leucine-rich amelogenin peptide regulates receptor activator of NF-kappa B ligand (RANKL) expression in cementoblast/periodontal ligament cells. | Rodent (mouse) | [117] | |
| Lysine † | Important for the integrity of dentally attached epithelium to act as a barrier to microbial products. | Lysine decarboxylas extracted on Eikenella corrodens bacterial cell surface | [118] |
| Methionine † | Methionine reduces the adverse effect of fluorides on soft tissue, and this has been found to be optimal for the prevention of the adverse effects of chronic fluoride intoxication together with vitamin E in drinking water. | Rodent (rat) | [119] |
| Phenylalanine † | May inhibit dental caries development. In bacteria, phenylalanine is converted to phenylpropionate or phenylacetate, resulting in alkali environment which is an essential factor in maintaining plaque pH homeostasis. | Human | [120] |
| Proline | Salivary proline-rich glycoprotein regulates the oral calcium homeostasis by controlling the supersaturated state of saliva with respect to calcium phosphate salts, countering the plaque acidity, formation of dental pellicle, and influencing the composition of plaque. | Human | [121] |
| Moreover, this prevents the adherence of oral microorganisms inhibiting their growth and neutralises acids from biofilms protecting from dental caries. | Human | [122] | |
| Serine and threonine † | Interact with host cytoplasmic phosphoproteins, facilitating internalisation of bacteria. | Primary cultures of human gingival epithelial cells | [123,124] |
| Tryptophan † | Tryptophan metabolites generated from oral supplementation of tryptophan promote regulatory T-cell (Treg) differentiation and suppress proinflammatory T-helper cell (Th)1 and Th17 phenotypes. | Rodent (mice) | [125] |
| Higher saliva tryptophan level was observed in Stage III Grade B generalised periodontitis. | Human | [105] | |
| Tyrosine | Potential biomarker of oral lichen planus (lower levels). Tyrosine is suggested to be involved in the antioxidative defence. | Human | [126] |
| Valine † | Detected in sound dentine compared to carious dentine. | Human | [115] |
| Homocysteine ‡ | Associated with high narrow palate, mandibular prognathia (protruding lower jaw), crowding and early eruption of teeth and short dental roots. | Human | [127] |
5. Dietary Protein Intake in Older Adults
6. Protein–Energy Malnutrition and Oral Health in Older Adults
7. How Does Inadequate Protein Intake Affect Saliva and Oral Health?
7.1. Protein Intake and Tooth Decay
7.2. Protein Intake and Periodontitis
7.3. Protein Intake and Tooth Loss
7.4. Protein Intake and Oral Mucosal Lesions and Oral Cancers
8. Excessive Protein Intake and Oral Health
9. Discussion
10. Conclusions
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- AHMAC. Aboriginal and Torres Strait Islander Health Performance Framework 2017 Report; AHMAC: Canberra, Australia, 2017. Available online: https://www.niaa.gov.au/sites/default/files/publications/2017-health-performance-framework-report_1.pdf (accessed on 22 June 2022).
- Baiju, R. Oral Health and Quality of Life: Current Concepts. J. Clin. Diagn. Res. 2017, 11, ZE21–ZE26. [Google Scholar] [CrossRef] [PubMed]
- Sanders, A.E.; Slade, G.D.; Lim, S.; Reisine, S.T. Impact of oral disease on quality of life in the US and Australian populations. Community Dent. Oral Epidemiol. 2009, 37, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Roca, J.A.; Fuentes, D.M.; García, F.J.G.; Martínez-Beneyto, Y. Oral status of older people in medium to long-stay health and social care setting: A systematic review. BMC Geriatr. 2021, 21, 363. [Google Scholar] [CrossRef] [PubMed]
- Division of Oral Health, Facts about Older Adult Oral Health. 2021. Available online: https://www.cdc.gov/oralhealth/basics/adult-oral-health/adult_older.htm (accessed on 20 June 2022).
- Dye, B.; Thornton-Evans, G.; Li, X.; Iafolla, T. Dental caries and tooth loss in adults in the United States, 2011–2012. NCHS Data Brief 2015, 197. [Google Scholar] [PubMed]
- Dodington, D.W.; Young, H.E.; Beaudette, J.R.; Fritz, P.C.; Ward, W.E. Improved Healing after Non-Surgical Periodontal Therapy Is Associated with Higher Protein Intake in Patients Who Are Non-Smokers. Nutrients 2021, 13, 3722. [Google Scholar] [CrossRef]
- Eke, P.I.; Thornton-Evans, G.O.; Wei, L.; Borgnakke, W.; Dye, B.A.; Genco, R.J. Periodontitis in US Adults. J. Am. Dent. Assoc. 2018, 149, 576–588.e6. [Google Scholar] [CrossRef]
- González-Moles, M.; Ramos-García, P. State of Evidence on Oral Health Problems in Diabetic Patients: A Critical Review of the Literature. J. Clin. Med. 2021, 10, 5383. [Google Scholar] [CrossRef]
- Kotronia, E.; Brown, H.; Papacosta, A.O.; Lennon, L.T.; Weyant, R.J.; Whincup, P.H.; Wannamethee, S.G.; Ramsay, S.E. Oral health and all-cause, cardiovascular disease, and respiratory mortality in older people in the UK and USA. Sci. Rep. 2021, 11, 16452. [Google Scholar] [CrossRef]
- Deschamps-Lenhardt, S.; Martin-Cabezas, R.; Hannedouche, T.; Huck, O. Association between periodontitis and chronic kidney disease: Systematic review and meta-analysis. Oral Dis. 2019, 25, 385–402. [Google Scholar] [CrossRef]
- Rapp, L.; Maret, D.; Diemer, F.; Ferré, M.L. Dental Caries in Geriatric Dentistry: An Update for Clinicians. Int. J. Oral Dent. Health 2019, 5, 080. [Google Scholar] [CrossRef]
- Chan, A.K.Y.; Tamrakar, M.; Jiang, C.M.; Lo, E.C.M.; Leung, K.C.M.; Chu, C.H. A Systematic Review on Caries Status of Older Adults. Int. J. Environ. Res. Public Health 2021, 18, 10662. [Google Scholar] [CrossRef] [PubMed]
- Chapple, I.L.C.; Bouchard, P.; Cagetti, M.G.; Campus, G.; Carra, M.-C.; Cocco, F.; Nibali, L.; Hujoel, P.; Laine, M.L.; Lingström, P.; et al. Interaction of lifestyle, behaviour or systemic diseases with dental caries and periodontal diseases: Consensus report of group 2 of the joint EFP/ORCA workshop on the boundaries between caries and periodontal diseases. J. Clin. Periodontol. 2017, 44 (Suppl. S18), S39–S51. [Google Scholar] [CrossRef] [PubMed]
- Choowong, P.; Wali, J.A.; Nguyen, A.T.M.; Jayasinghe, T.N.; Eberhard, J. Macronutrient-induced modulation of periodontitis in rodents—A systematic review. Nutr. Rev. 2021, 80, 1160–1178. [Google Scholar] [CrossRef] [PubMed]
- Hujoel, P.P.; Lingström, P. Nutrition, dental caries and periodontal disease: A narrative review. J. Clin. Periodontol. 2017, 44, S79–S84. [Google Scholar] [CrossRef]
- Hwang, S.-Y.; Park, J.-E. The Relationship Between Periodontal Disease and Nutrient Intake in Korean Adults: The Korea National Health and Nutrition Examination Survey (KNHANES VII) from 2016–2018. Oral Health Prev. Dent. 2022, 20, 313–320. [Google Scholar] [CrossRef]
- Santonocito, S.; Polizzi, A.; Palazzo, G.; Indelicato, F.; Isola, G. Dietary Factors Affecting the Prevalence and Impact of Periodontal Disease. Clin. Cosmet. Investig. Dent. 2021, 13, 283–292. [Google Scholar] [CrossRef]
- Griffin, S.O.; Jones, J.A.; Brunson, D.; Griffin, P.M.; Bailey, W.D. Burden of Oral Disease Among Older Adults and Implications for Public Health Priorities. Am. J. Public Health 2012, 102, 411–418. [Google Scholar] [CrossRef]
- Lingström, P.; Simark Mattsson, C. Chapter 2: Oral Conditions. Monogr. Oral Sci. 2020, 28, 14–21. [Google Scholar] [CrossRef]
- Iorgulescu, G. Saliva between normal and pathological. Important factors in determining systemic and oral health. J. Med. Life 2010, 2, 303–307. [Google Scholar] [PubMed]
- McArthur, W.P. Oral Immunology. In Encyclopedia of Immunology; Elsevier: Amsterdam, The Netherlands, 1998; pp. 1888–1893. [Google Scholar] [CrossRef]
- Psoter, W.; Reid, B.; Katz, R. Malnutrition and Dental Caries: A Review of the Literature. Caries Res. 2005, 39, 441–447. [Google Scholar] [CrossRef]
- Llena-Puy, C. The rôle of saliva in maintaining oral health and as an aid to diagnosis. Med. Oral Patol. Oral Cir. Bucal 2006, 11, E449–E455. [Google Scholar] [PubMed]
- Toan, N.; Ahn, S.-G. Aging-Related Metabolic Dysfunction in the Salivary Gland: A Review of the Literature. Int. J. Mol. Sci. 2021, 22, 5835. [Google Scholar] [CrossRef] [PubMed]
- Dodds, M.; Roland, S.; Edgar, M.; Thornhill, M. Saliva A review of its role in maintaining oral health and preventing dental disease. BDJ Team 2015, 2, 15123. [Google Scholar] [CrossRef]
- Kossioni, A.E. The Association of Poor Oral Health Parameters with Malnutrition in Older Adults: A Review Considering the Potential Implications for Cognitive Impairment. Nutrients 2018, 10, 1709. [Google Scholar] [CrossRef] [PubMed]
- Vach, K.; Woelber, J.P. (Eds.) Nutrition and Human Oral Health; MDPI: Basel, Switzerland, 2022. [Google Scholar] [CrossRef]
- Azzolino, D.; Passarelli, P.C.; De Angelis, P.; Piccirillo, G.B.; D’Addona, A.; Cesari, M. Poor Oral Health as a Determinant of Malnutrition and Sarcopenia. Nutrients 2019, 11, 2898. [Google Scholar] [CrossRef]
- Naka, O.; Anastassiadou, V.; Pissiotis, A. Association between functional tooth units and chewing ability in older adults: A systematic review. Gerodontology 2012, 31, 166–177. [Google Scholar] [CrossRef]
- O’Connor, J.-L.P.; Milledge, K.L.; O’Leary, F.; Cumming, R.; Eberhard, J.; Hirani, V. Poor dietary intake of nutrients and food groups are associated with increased risk of periodontal disease among community-dwelling older adults: A systematic literature review. Nutr. Rev. 2020, 78, 175–188. [Google Scholar] [CrossRef]
- Sheiham, A.; Steele, J.; Marcenes, W.; Lowe, C.; Finch, S.; Bates, C.; Prentice, A.; Walls, A. The Relationship among Dental Status, Nutrient Intake, and Nutritional Status in Older People. J. Dent. Res. 2001, 80, 408–413. [Google Scholar] [CrossRef]
- Iwasaki, M.; Hirano, H.; Ohara, Y.; Motokawa, K. The association of oral function with dietary intake and nutritional status among older adults: Latest evidence from epidemiological studies. Jpn. Dent. Sci. Rev. 2021, 57, 128–137. [Google Scholar] [CrossRef]
- Bauer, J.; Biolo, G.; Cederholm, T.; Cesari, M.; Cruz-Jentoft, A.J.; Morley, J.E.; Phillips, S.; Sieber, C.; Stehle, P.; Teta, D.; et al. Evidence-Based Recommendations for Optimal Dietary Protein Intake in Older People: A Position Paper From the PROT-AGE Study Group. J. Am. Med. Dir. Assoc. 2013, 14, 542–559. [Google Scholar] [CrossRef]
- Deutz, N.E.; Bauer, J.M.; Barazzoni, R.; Biolo, G.; Boirie, Y.; Bosy-Westphal, A.; Cederholm, T.; Cruz-Jentoft, A.; Krznariç, Z.; Nair, K.S.; et al. Protein intake and exercise for optimal muscle function with aging: Recommendations from the ESPEN Expert Group. Clin. Nutr. 2014, 33, 929–936. [Google Scholar] [CrossRef] [PubMed]
- Baum, J.I.; Kim, I.-Y.; Wolfe, R.R. Protein Consumption and the Elderly: What Is the Optimal Level of Intake? Nutrients 2016, 8, 359. [Google Scholar] [CrossRef] [PubMed]
- Bomfim, R.A.; de Souza, L.B.; Corrente, J.E. Tooth loss and its relationship with protein intake by elderly Brazilians—A structural equation modelling approach. Gerodontology 2017, 35, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Kotronia, E.; Brown, H.; Papacosta, A.O.; Lennon, L.T.; Weyant, R.J.; Whincup, P.H.; Wannamethee, S.G.; Ramsay, S.E. Poor oral health and the association with diet quality and intake in older people in two studies in the UK and USA. Br. J. Nutr. 2021, 126, 118–130. [Google Scholar] [CrossRef]
- Woelber, J.P.; Bremer, K.; Vach, K.; König, D.; Hellwig, E.; Ratka-Krüger, P.; Al-Ahmad, A.; Tennert, C. An oral health optimized diet can reduce gingival and periodontal inflammation in humans—A randomized controlled pilot study. BMC Oral Health 2016, 17, 28. [Google Scholar] [CrossRef]
- Morais, J.A.; Chevalier, S.; Gougeon, R. Protein turnover and requirements in the healthy and frail elderly. J. Nutr. Health Aging 2006, 10, 272–283. [Google Scholar] [PubMed]
- Yeung, S.S.Y.; Lee, J.S.W.; Kwok, T. A Nutritionally Complete Oral Nutritional Supplement Powder Improved Nutritional Outcomes in Free-Living Adults at Risk of Malnutrition: A Randomized Controlled Trial. Int. J. Environ. Res. Public Health 2022, 19, 11354. [Google Scholar] [CrossRef]
- Thomson, K.H.; Rice, S.; Arisa, O.; Johnson, E.; Tanner, L.; Marshall, C.; Sotire, T.; Richmond, C.; O’Keefe, H.; Mohammed, W.; et al. Effectiveness and cost-effectiveness of oral nutritional supplements in frail older people who are malnourished or at risk of malnutrition: A systematic review and meta-analysis. Lancet Health Longev. 2022, 3, e654–e666. [Google Scholar] [CrossRef]
- Algra, Y.; Haverkort, E.; Kok, W.; van Etten-Jamaludin, F.; van Schoot, L.; Hollaar, V.; Naumann, E.; de van der Schueren, M.; Jerković-Ćosić, K. The Association between Malnutrition and Oral Health in Older People: A Systematic Review. Nutrients 2021, 13, 3584. [Google Scholar] [CrossRef]
- Coelho-Júnior, H.J.; Rodrigues, B.; Uchida, M.; Marzetti, E. Low Protein Intake Is Associated with Frailty in Older Adults: A Systematic Review and Meta-Analysis of Observational Studies. Nutrients 2018, 10, 1334. [Google Scholar] [CrossRef]
- Nishi, K.; Kanouchi, H.; Tanaka, A.; Nakamura, M.; Hamada, T.; Mishima, Y.; Goto, Y.; Kume, K.; Beppu, M.; Hijioka, H.; et al. Relationship between Oral Hypofunction, and Protein Intake: A Cross-Sectional Study in Local Community-Dwelling Adults. Nutrients 2021, 13, 4377. [Google Scholar] [CrossRef] [PubMed]
- Thomson, W.M. Epidemiology of oral health conditions in older people. Gerodontology 2014, 31, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.; Wallace, J.; Doshi, M.; Gadanya, M.; Ben Yahya, I.; Roseman, J.; Srisilapanan, P. Oral health for healthy ageing. Lancet Health Longev. 2021, 2, e521–e527. [Google Scholar] [CrossRef]
- Ramsay, S.E.; Whincup, P.H.; Watt, R.G.; Tsakos, G.; Papacosta, A.O.; Lennon, L.T.; Wannamethee, S.G. Burden of poor oral health in older age: Findings from a population-based study of older British men. BMJ Open 2015, 5, e009476. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, L.J.; Barbagallo, M. Nutritional prevention of cognitive decline and dementia. Acta Biomed. 2018, 89, 276–290. [Google Scholar] [CrossRef]
- Fuchs, J.; Gaertner, B.; Prütz, F. Limitations in activities of daily living and support needs—Analysis of GEDA 2019/2020-EHIS. J. Health Monit. 2022, 7, 6–25. [Google Scholar] [CrossRef]
- Kitamura, M.; Izawa, K.P.; Yaekura, M.; Mimura, Y.; Ikeda, Y.; Nagashima, H.; Brubaker, P.H. Relationship among Activities of Daily Living, Nutritional Status, and 90 Day Readmission in Elderly Patients with Heart Failure. Int. J. Environ. Res. Public Health 2019, 16, 5068. [Google Scholar] [CrossRef]
- Locher, J.L.; Ritchie, C.S.; Roth, D.L.; Baker, P.S.; Bodner, E.V.; Allman, R.M. Social isolation, support, and capital and nutritional risk in an older sample: Ethnic and gender differences. Soc. Sci. Med. 2005, 60, 747–761. [Google Scholar] [CrossRef]
- Barrington, G.; Khan, S.; Kent, K.; Brennan, D.S.; Crocombe, L.A.; Bettiol, S. Obesity, dietary sugar and dental caries in Australian adults. Int. Dent. J. 2019, 69, 383–391. [Google Scholar] [CrossRef]
- Raju, K.; Taylor, G.W.; Tahir, P.; Hyde, S. Association of tooth loss with morbidity and mortality by diabetes status in older adults: A systematic review. BMC Endocr. Disord. 2021, 21, 205. [Google Scholar] [CrossRef]
- Norman, K.; Haß, U.; Pirlich, M. Malnutrition in Older Adults—Recent Advances and Remaining Challenges. Nutrients 2021, 13, 2764. [Google Scholar] [CrossRef]
- Mathewson, S.L.; Azevedo, P.S.; Gordon, A.L.; Phillips, B.E.; Greig, C.A. Overcoming protein-energy malnutrition in older adults in the residential care setting: A narrative review of causes and interventions. Ageing Res. Rev. 2021, 70, 101401. [Google Scholar] [CrossRef]
- Chapman, I.M. Endocrinology of anorexia of ageing. Best Pract. Res. Clin. Endocrinol. Metab. 2004, 18, 437–452. [Google Scholar] [CrossRef]
- van den Beld, A.W.; Kaufman, J.-M.; Zillikens, M.C.; Lamberts, S.W.J.; Egan, J.M.; Van Der Lely, A.J. The physiology of endocrine systems with ageing. Lancet Diabetes Endocrinol. 2018, 6, 647–658. [Google Scholar] [CrossRef]
- Landi, F.; Calvani, R.; Tosato, M.; Martone, A.M.; Ortolani, E.; Savera, G.; Sisto, A.; Marzetti, E. Anorexia of Aging: Risk Factors, Consequences, and Potential Treatments. Nutrients 2016, 8, 69. [Google Scholar] [CrossRef]
- Do, L.; Luzzi, L.; Oral Health Status. Australia’s Oral Health: National Study of Adult Oral Health 2017–18; ARCPOH: Adelaide, Australia, 2019; Available online: https://www.adelaide.edu.au/arcpoh/national-study/report/Australias_Oral_Health_2017-18.pdf (accessed on 15 July 2022).
- Savoca, M.R.; Arcury, T.A.; Leng, X.; Chen, H.; Bell, R.A.; Anderson, A.M.; Kohrman, T.; Frazier, R.J.; Gilbert, G.H.; Quandt, S.A. Severe tooth loss in older adults as a key indicator of compromised dietary quality. Public Health Nutr. 2009, 13, 466–474. [Google Scholar] [CrossRef]
- Idowu, A.T.; Handelman, S.L.; Graser, G.N. Effect of denture stability, retention, and tooth form on masticatory function in the elderly. Gerodontics 1987, 3, 161–164. [Google Scholar] [PubMed]
- Drewnowski, A.; Shultz, J.M. Impact of aging on eating behaviors, food choices, nutrition, and health status. J. Nutr. Health Aging 2001, 5, 75–79. [Google Scholar] [PubMed]
- Cunha-Cruz, J.; Scott, J.; Rothen, M.; Mancl, L.; Lawhorn, T.; Brossel, K.; Berg, J. Salivary characteristics and dental caries. J. Am. Dent. Assoc. 2013, 144, e31–e40. [Google Scholar] [CrossRef]
- Villa, A.; Connell, C.L.; Abati, S. Diagnosis and management of xerostomia and hyposalivation. Ther. Clin. Risk Manag. 2014, 11, 45–51. [Google Scholar] [CrossRef]
- Wolff, A.; Joshi, R.K.; Ekström, J.; Aframian, D.; Pedersen, A.M.L.; Proctor, G.; Narayana, N.; Villa, A.; Sia, Y.W.; Aliko, A.; et al. A Guide to Medications Inducing Salivary Gland Dysfunction, Xerostomia, and Subjective Sialorrhea: A Systematic Review Sponsored by the World Workshop on Oral Medicine VI. Drugs R D 2016, 17, 1–28. [Google Scholar] [CrossRef]
- Turner, M.D.; Ship, J.A. Dry Mouth and Its Effects on the Oral Health of Elderly People. J. Am. Dent. Assoc. 2007, 138, S15–S20. [Google Scholar] [CrossRef]
- Quandt, S.A.; Savoca, M.R.; Leng, X.; Chen, H.; Bell, R.A.; Gilbert, G.H.; Anderson, A.M.; Kohrman, T.; Arcury, T.A. Dry Mouth and Dietary Quality in Older Adults in North Carolina. J. Am. Geriatr. Soc. 2011, 59, 439–445. [Google Scholar] [CrossRef]
- Van Der Linden, C.M.J. Polypharmacy in Older People. Ned Tijdschr Voor Dermatol. En Venereol 2017, 27, 479–481. [Google Scholar]
- Miranda-Rius, J.; Brunet-Llobet, L.; Lahor-Soler, E.; Farré, M. Salivary Secretory Disorders, Inducing Drugs, and Clinical Management. Int. J. Med. Sci. 2015, 12, 811–824. [Google Scholar] [CrossRef]
- Jyrkkä, J.; Mursu, J.; Enlund, H.; Lönnroos, E. Polypharmacy and nutritional status in elderly people. Curr. Opin. Clin. Nutr. Metab. Care 2012, 15, 1–6. [Google Scholar] [CrossRef]
- Kose, E.; Wakabayashi, H.; Yasuno, N. Polypharmacy and Malnutrition Management of Elderly Perioperative Patients with Cancer: A Systematic Review. Nutrients 2021, 13, 1961. [Google Scholar] [CrossRef]
- Kok, W.E.; Haverkort, E.B.; Algra, Y.A.; Mollema, J.; Hollaar, V.R.Y.; Naumann, E.; de van der Schueren, M.A.E.; Kerkovic-Cosica, K. The association between polypharmacy and malnutrition(risk) in older people: A systematic review. Clin. Nutr. ESPEN 2022, 49, 163–171. [Google Scholar] [CrossRef]
- Walston, J.D. Sarcopenia in older adults. Curr. Opin. Rheumatol. 2012, 24, 623–627. [Google Scholar] [CrossRef]
- Wakabayashi, H. Presbyphagia and Sarcopenic Dysphagia: Association between Aging, Sarcopenia, and Deglutition Disorders. J. Frailty Aging 2014, 3, 97–103. [Google Scholar] [CrossRef]
- Mathieu, M.-E.; Reid, R.E.R.; King, N.A. Sensory Profile of Adults with Reduced Food Intake and the Potential Roles of Nutrition and Physical Activity Interventions. Adv. Nutr. Int. Rev. J. 2019, 10, 1120–1125. [Google Scholar] [CrossRef]
- Yaffe, K.; Middleton, L.E.; Lui, L.-Y.; Spira, A.P.; Stone, K.L.; Racine, C.A.; Ensrud, K.E.; Kramer, J.H. Mild Cognitive Impairment, Dementia, and Their Subtypes in Oldest Old Women. Arch. Neurol. 2011, 68, 631–636. [Google Scholar] [CrossRef]
- Chen, X.; Xie, X.-J.; Yu, L. The pathway from cognitive impairment to caries in older adults. J. Am. Dent. Assoc. 2018, 149, 967–975. [Google Scholar] [CrossRef]
- Jockusch, J.; Hopfenmüller, W.; Nitschke, I. Influence of cognitive impairment and dementia on oral health and the utilization of dental services. BMC Oral Health 2021, 21, 399. [Google Scholar] [CrossRef]
- Tada, A.; Miura, H. Association between mastication and cognitive status: A systematic review. Arch. Gerontol. Geriatr. 2017, 70, 44–53. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, F.; Wang, Z.; Qian, X.; Ji, Y.; Gong, L.; Ge, S.; Yan, F. Poor oral health conditions and cognitive decline: Studies in humans and rats. PLoS ONE 2020, 15, e0234659. [Google Scholar] [CrossRef]
- Lee, K.H.; Jung, E.S.; Choi, Y.Y. Association of oral health and activities of daily living with cognitive impairment. Gerodontology 2019, 37, 38–45. [Google Scholar] [CrossRef]
- Ortega, R.M.; Requejo, A.M.; Andres, P.; López-Sobaler, A.M.; Quintas, M.E.; Redondo, M.R.; Navia, B.; Rivas, T. Dietary intake and cognitive function in a group of elderly people. Am. J. Clin. Nutr. 1997, 66, 803–809. [Google Scholar] [CrossRef]
- Klimova, B.; Dziuba, S.; Cierniak-Emerych, A. The Effect of Healthy Diet on Cognitive Performance Among Healthy Seniors—A Mini Review. Front. Hum. Neurosci. 2020, 14, 32. [Google Scholar] [CrossRef]
- McEvoy, C.T.; Leng, Y.; Peeters, G.M.; Kaup, A.R.; Allen, I.E.; Yaffe, K. Interventions involving a major dietary component improve cognitive function in cognitively healthy adults: A systematic review and meta-analysis. Nutr. Res. 2019, 66, 1–12. [Google Scholar] [CrossRef]
- Okubo, H.; Inagaki, H.; Gondo, Y.; Kamide, K.; Ikebe, K.; Masui, Y.; Arai, Y.; Ishizaki, T.; Sasaki, S.; Nakagawa, T.; et al. Association between dietary patterns and cognitive function among 70-year-old Japanese elderly: A cross-sectional analysis of the SONIC study. Nutr. J. 2017, 16, 56. [Google Scholar] [CrossRef] [PubMed]
- Kioka, K.; Aikawa, Y.; Wakasugi, Y.; Narukawa, T.; Fukuyasu, T.; Ohtsuki, M.; Yamashita, T.; Sasai, N.; Omi, N. Soy protein intake increased bone mineral density under non-energy deficiency conditions but decreased it under energy deficiency conditions in young female rats. Nutr. Res. 2022, 106, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.W.; Kang, J.-H. Relationship between skeletal bone mineral density and subjective masticatory difficulty. BMC Oral Health 2022, 22, 136. [Google Scholar] [CrossRef] [PubMed]
- Takeda, M.; Matsuda, Y.; Ikebuchi, K.; Takeda, M.; Abe, T.; Tominaga, K.; Isomura, M.; Nabika, T.; Kanno, T. Relationship between Oral Health Status and Bone Mineral Density in Community-Dwelling Elderly Individuals: A Cross-Sectional Study. Healthcare 2021, 9, 432. [Google Scholar] [CrossRef]
- Tungare, S.; Paranjpe, A.G. Diet and Nutrition to Prevent Dental Problems; StatPearls: Treasure Island, FL, USA, 2022. [PubMed]
- Krall, E.A.; Wehler, C.; Garcia, R.; Harris, S.S.; Dawson-Hughes, B. Calcium and vitamin D supplements reduce tooth loss in the elderly. Am. J. Med. 2001, 111, 452–456. [Google Scholar] [CrossRef]
- Gondivkar, S.M.; Gadbail, A.R.; Gondivkar, R.S.; Sarode, S.C.; Sarode, G.S.; Patil, S.; Awan, K.H. Nutrition and oral health. Disease-A-Month 2018, 65, 147–154. [Google Scholar] [CrossRef]
- Lee, K.; Kim, J. Dairy Food Consumption is Inversely Associated with the Prevalence of Periodontal Disease in Korean Adults. Nutrients 2019, 11, 1035. [Google Scholar] [CrossRef]
- Lee, Y.; Yoon, Y.; Choi, K.-H. Probiotics-Mediated Bioconversion and Periodontitis. Korean J. Food Sci. Anim. Resour. 2021, 41, 905–922. [Google Scholar] [CrossRef]
- Kato, K.; Toba, Y.; Matsuyama, H.; Yamamura, J.-I.; Matsuoka, Y.; Kawakami, H.; Itabashi, A.; Kumegawa, M.; Aoe, S.; Takada, Y. Milk basic protein enhances the bone strength in overectimised rats. J. Food Biochem. 2000, 24, 467–476. [Google Scholar] [CrossRef]
- Seto, H.; Toba, Y.; Takada, Y.; Kawakami, H.; Ohba, H.; Hama, H.; Horibe, M.; Nagata, T. Milk basic protein increases alveolar bone formation in rat experimental periodontitis. J. Periodontal Res. 2006, 42, 85–89. [Google Scholar] [CrossRef]
- Aimutis, W.R. Bioactive Properties of Milk Proteins with Particular Focus on Anticariogenesis. J. Nutr. 2004, 134, 989S–995S. [Google Scholar] [CrossRef] [PubMed]
- Berlutti, F.; Pilloni, A.; Pietropaoli, M.; Polimeni, A.; Valenti, P. Lactoferrin and oral diseases: Current status and perspective in periodontitis. Ann. Stomatol. 2011, 2, 10–18. [Google Scholar] [PubMed]
- Reema, S.D.; Lahiri, P.K.; Roy, S.S. Review of casein phosphopeptides-amorphous calcium phosphate. Chin. J. Dent. Res. Off. J. Sci. Sect. Chin. Stomatol. Assoc. 2014, 1, 7–14. [Google Scholar]
- Sionov, R.V.; Tsavdaridou, D.; Aqawi, M.; Zaks, B.; Steinberg, D.; Shalish, M. Tooth mousse containing casein phosphopeptide-amorphous calcium phosphate prevents biofilm formation of Streptococcus mutans. BMC Oral Health 2021, 21, 136. [Google Scholar] [CrossRef]
- Lopez, M.J.; Mohiuddin, S.S. Biochemistry, Essential Amino Acids; StatPearls: Treasure Island, FL, USA, 2022. [PubMed]
- Kolderman, E.; Bettampadi, D.; Samarian, D.; Dowd, S.E.; Foxman, B.; Jakubovics, N.S.; Rickard, A.H. L-Arginine Destabilizes Oral Multi-Species Biofilm Communities Developed in Human Saliva. PLoS ONE 2015, 10, e0121835. [Google Scholar] [CrossRef]
- Vranić, L.; Granić, P.; Rajić, Z. Basic amino acid in the pathogenesis of caries. Acta Stomatol. Croat. 1991, 25, 71–76. [Google Scholar]
- Solon-Biet, S.M.; Cogger, V.C.; Pulpitel, T.; Wahl, D.; Clark, X.; Bagley, E.E.; Gregoriou, G.C.; Senior, A.M.; Wang, Q.-P.; Brandon, A.E.; et al. Branched-chain amino acids impact health and lifespan indirectly via amino acid balance and appetite control. Nat. Metab. 2019, 1, 532–545. [Google Scholar] [CrossRef]
- Balci, N.; Kurgan, Ş.; Çekici, A.; Çakır, T.; Serdar, M.A. Free amino acid composition of saliva in patients with healthy periodontium and periodontitis. Clin. Oral Investig. 2021, 25, 4175–4183. [Google Scholar] [CrossRef]
- Sirin, N.; Matzenauer, C.; Reckert, A.; Ritz-Timme, S. Age estimation based on aspartic acid racemization in dentine: What about caries-affected teeth? Int. J. Leg. Med. 2018, 132, 623–628. [Google Scholar] [CrossRef]
- Cowman, R.A.; Baron, S.S.; Fitzgerald, R.J. Cysteine toxicity for oral streptococci and effect of branched-chain amino acids. Infect. Immun. 1983, 39, 1107–1113. [Google Scholar] [CrossRef]
- Rasmussen, K.; Nikrad, J.; Reilly, C.; Li, Y.; Jones, R. N-Acetyl-l-cysteine effects on multi-species oral biofilm formation and bacterial ecology. Lett. Appl. Microbiol. 2015, 62, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Kaur, K.; Paranjpe, A.; Lee, E.; Wasilewski, M.; Sung, D.; Han, D.; Sung, E.C.; Jewett, A. N-acetyl cysteine prevents pain and hypersensitivity of bleaching agents without affecting their aesthetic appeal; evidence from in vitro to animal studies and to human clinical trials. Transl. Med. Commun. 2019, 4, 19. [Google Scholar] [CrossRef]
- Al-Kamel, A.; Al-Hajj, W.A.; Halboub, E.; Abdulrab, S.; Al-Tahami, K.; Al-Hebshi, N.N. N-acetyl cysteine versus chlorhexidine mouthwashes in prevention and treatment of experimental gingivitis: A randomized, triple-blind, placebo-controlled clinical trial. Clin. Oral Investig. 2019, 23, 3833–3842. [Google Scholar] [CrossRef] [PubMed]
- Peterson, D.E.; Petit, R.G. Phase III study: AES-14 in chemotherapy patients at risk for mucositis [Abstract 2917]. Proc. Am. Soc. Clin. Oncol. 2003, 22, 725. [Google Scholar]
- Das, S.K.; Harris, R.S. Effect of Dietary Supplementation of Glycine on Caries Development and Lipids in Rat Molars. J. Dent. Res. 1975, 54, 987–992. [Google Scholar] [CrossRef] [PubMed]
- Fonteles, C.S.; Guerra, M.H.; Ribeiro, T.R.; Mendonça, D.N.; de Carvalho, C.B.; Monteiro, A.J.; Toyama, D.O.; Toyama, M.H.; Fonteles, M.C. Association of free amino acids with caries experience and mutans streptococci levels in whole saliva of children with early childhood caries. Arch. Oral Biol. 2009, 54, 80–85. [Google Scholar] [CrossRef]
- Syrjänen, S.; Piironen, P.; Markkanen, H. Free amino-acid content of wax-stimulated human whole saliva as related to periodontal disease. Arch. Oral Biol. 1987, 32, 607–610. [Google Scholar] [CrossRef]
- Lin, Y.H. Changes of dentin matrices during carious process. Tsurumi Shigaku. Tsurumi Univ. Dent. J. 1989, 15, 249–266. [Google Scholar] [PubMed]
- Mukherjee, K.; Ruan, Q.; Liberman, D.; White, S.N.; Moradian-Oldak, J. Repairing human tooth enamel with leucine-rich amelogenin peptide–chitosan hydrogel. J. Mater. Res. 2016, 31, 556–563. [Google Scholar] [CrossRef]
- Haruyama, N.; Yamaza, T.; Suzuki, S.; Hall, B.; Cho, A.; Gibson, C.W.; Kulkarni, A.B. Leucine rich amelogenin peptide prevents ovariectomy-induced bone loss in mice. PLoS ONE 2021, 16, e0259966. [Google Scholar] [CrossRef]
- Lohinai, Z.; Keremi, B.; Szöko, E.; Tábi, T.; Szabo, C.; Tulassay, Z.; Dicesare, J.C.; Davis, C.A.; Collins, L.M.; Levine, M. Biofilm Lysine Decarboxylase, a New Therapeutic Target for Periodontal Inflammation. J. Periodontol. 2015, 86, 1176–1184. [Google Scholar] [CrossRef] [PubMed]
- Błaszczyk, I.; Birkner, E.; Gutowska, I.; Romuk, E.; Chlubek, D. Influence of Methionine and Vitamin E on Fluoride Concentration in Bones and Teeth of Rats Exposed to Sodium Fluoride in Drinking Water. Biol. Trace Elem. Res. 2011, 146, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Tian, J.; Xu, H.; Wang, G.; Zhou, Q.; Qin, M. Carbon source utilization patterns in dental plaque and microbial responses to sucrose, lactose, and phenylalanine consumption in severe early childhood caries. J. Oral Microbiol. 2020, 12, 1782696. [Google Scholar] [CrossRef] [PubMed]
- Pateel, D.G.S.; Gunjal, S.; Fong, L.F.; Hanapi, N.S.M. Association of Salivary Statherin, Calcium, and Proline-Rich Proteins on Oral Hygiene: A Cross-Sectional Study. Int. J. Dent. 2021, 2021, 1982083. [Google Scholar] [CrossRef] [PubMed]
- Zakhary, G.; Clark, R.; Bidichandani, S.; Owen, W.; Slayton, R.; Levine, M. Acidic Proline-rich Protein Db and Caries in Young Children. J. Dent. Res. 2007, 86, 1176–1180. [Google Scholar] [CrossRef]
- Oda, D.; Watson, E. Human oral epithelial cell culture I. Improved conditions for reproducible culture in serum-free medium. Vitr. Cell. Dev. Biol. 1990, 26, 589–595. [Google Scholar] [CrossRef]
- Ren, L.; Shen, D.; Liu, C.; Ding, Y. Protein Tyrosine and Serine/Threonine Phosphorylation in Oral Bacterial Dysbiosis and Bacteria-Host Interaction. Front. Cell. Infect. Microbiol. 2022, 11, 814659. [Google Scholar] [CrossRef]
- Lanz, T.V.; Becker, S.; Mohapatra, S.R.; Opitz, C.A.; Wick, W.; Platten, M. Suppression of Th1 differentiation by tryptophan supplementation in vivo. Amino Acids 2017, 49, 1169–1175. [Google Scholar] [CrossRef]
- Darczuk, D.; Krzyściak, W.; Bystrowska, B.; Kęsek, B.; Kościelniak, D.; Chomyszyn-Gajewska, M.; Kaczmarzyk, T. The Relationship between the Concentration of Salivary Tyrosine and Antioxidants in Patients with Oral Lichen Planus. Oxidative Med. Cell. Longev. 2019, 2019, 5801570. [Google Scholar] [CrossRef]
- Björksved, M.; Arnrup, K. Homocystinuria and oral health. A report of 14 cases. Swed. Dent. J. 2012, 36, 101–108. [Google Scholar] [PubMed]
- Ministry of Health Australia. Nutrient Reference Values for Australia and New Zealand. 2014. Available online: https://www.nrv.gov.au/nutrients/protein (accessed on 10 August 2022).
- Kim, I.-Y.; Schutzler, S.; Schrader, A.; Spencer, H.; Kortebein, P.; Deutz, N.E.P.; Wolfe, R.R.; Ferrando, A.A. Quantity of dietary protein intake, but not pattern of intake, affects net protein balance primarily through differences in protein synthesis in older adults. Am. J. Physiol.-Endocrinol. Metab. 2015, 308, E21–E28. [Google Scholar] [CrossRef] [PubMed]
- Krok-Schoen, J.L.; Price, A.A.; Luo, M.; Kelly, O.J.; Taylor, C.A. Low Dietary Protein Intakes and Associated Dietary Patterns and Functional Limitations in an Aging Population: A NHANES Analysis. J. Nutr. Health Aging 2019, 23, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Mendonça, N.; Granic, A.; Mathers, J.C.; Hill, T.R.; Siervo, M.; Adamson, A.; Jagger, C. Prevalence and determinants of low protein intake in very old adults: Insights from the Newcastle 85+ Study. Eur. J. Nutr. 2018, 57, 2713–2722. [Google Scholar] [CrossRef] [PubMed]
- Volpi, E.; Campbell, W.W.; Dwyer, J.; Johnson, M.A.; Jensen, G.L.; Morley, J.E.; Wolfe, R.R. Is the Optimal Level of Protein Intake for Older Adults Greater Than the Recommended Dietary Allowance? J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2013, 68, 677–681. [Google Scholar] [CrossRef] [PubMed]
- Scholes, G. Protein-energy malnutrition in older Australians: A narrative review of the prevalence, causes and consequences of malnutrition, and strategies for prevention. Health Promot. J. Aust. 2021, 33, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Swinburn, B.A.; Caterson, I.; Seidell, J.C.; James, W.P. Diet, nutrition and the prevention of excess weight gain and obesity. Public Health Nutr. 2004, 7, 123–146. [Google Scholar] [CrossRef] [PubMed]
- WHO. Diet, Nutrition and the Prevention of Chronic Diseases; World Health Organization Technical Report Series; WHO: Geneva, Switzerland, 2003; Volume 916. [PubMed]
- Wysokiński, A.; Sobów, T.; Kłoszewska, I.; Kostka, T. Mechanisms of the anorexia of aging—A review. AGE 2015, 37, 81. [Google Scholar] [CrossRef]
- Sheetal, A. Malnutrition and its Oral Outcome—A Review. J. Clin. Diagn. Res. 2013, 7, 178–180. [Google Scholar] [CrossRef]
- Kiesswetter, E.; Hengeveld, L.M.; Keijser, B.J.; Volkert, D.; Visser, M. Oral health determinants of incident malnutrition in community-dwelling older adults. J. Dent. 2019, 85, 73–80. [Google Scholar] [CrossRef]
- Psoter, W.J.; Spielman, A.L.; Gebrian, B.; Jean, R.S.; Katz, R.V. Effect of childhood malnutrition on salivary flow and pH. Arch. Oral Biol. 2008, 53, 231–237. [Google Scholar] [CrossRef]
- Cuthbertson, D.; Smith, K.; Babraj, J.; Leese, G.; Waddell, T.; Atherton, P.; Wackerhage, H.; Taylor, P.M.; Rennie, M.J. Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. FASEB J. 2004, 19, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Eggersdorfer, M.; Akobundu, U.; Bailey, R.L.; Shlisky, J.; Beaudreault, A.R.; Bergeron, G.; Blancato, R.B.; Blumberg, J.B.; Bourassa, M.W.; Gomes, F.; et al. Hidden Hunger: Solutions for America’s Aging Populations. Nutrients 2018, 10, 1210. [Google Scholar] [CrossRef]
- Rahman, N.; Walls, A. Chapter 12: Nutrient Deficiencies and Oral Health. Monogr. Oral Sci. 2020, 28, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, A.M.L.; Soerensen, C.; Proctor, G.; Carpenter, G. Salivary functions in mastication, taste and textural perception, swallowing and initial digestion. Oral Dis. 2018, 24, 1399–1416. [Google Scholar] [CrossRef]
- Huo, L.; Zhang, K.; Ling, J.; Peng, Z.; Huang, X.; Liu, H.; Gu, L. Antimicrobial and DNA-binding activities of the peptide fragments of human lactoferrin and histatin 5 against Streptococcus mutans. Arch. Oral Biol. 2011, 56, 869–876. [Google Scholar] [CrossRef] [PubMed]
- Louro, T.; Simões, C.; Lima, W.; Carreira, L.; Castelo, P.M.; Luis, H.; Moreira, P.; Lamy, E. Salivary Protein Profile and Food Intake: A Dietary Pattern Analysis. J. Nutr. Metab. 2021, 2021, 6629951. [Google Scholar] [CrossRef] [PubMed]
- Etzel, K.R. Role of salivary glands in nutrition. In Biology of the Salivary Glands; Dobrosielski-Vergona, K., Ed.; 1992; Volume 461, pp. 129–152. [Google Scholar]
- Fábián, T.K.; Hermann, P.; Beck, A.; Fejérdy, P.; Fábián, G. Salivary Defense Proteins: Their Network and Role in Innate and Acquired Oral Immunity. Int. J. Mol. Sci. 2012, 13, 4295–4320. [Google Scholar] [CrossRef]
- Blostein, F.A.; Jansen, E.C.; Jones, A.D.; Marshall, T.A.; Foxman, B. Dietary patterns associated with dental caries in adults in the United States. Community Dent. Oral Epidemiol. 2020, 48, 119–129. [Google Scholar] [CrossRef]
- Touger-Decker, R.; Van Loveren, C. Sugars and dental caries. Am. J. Clin. Nutr. 2003, 78, 881S–892S. [Google Scholar] [CrossRef]
- Price, W.A. Nutrition And Physical Degeneration, 8th ed.; Price Pottenger Nutrition: Houston, TX, USA, 2009. [Google Scholar]
- Menaker, L.; Navia, J.M. Effect of Undernutrition During the Perinatal Period on Caries Development in the Rat: II. Caries Susceptibility in Underfed Rats Supplemented with Protein or Caloric Additions During the Suckling Period. J. Dent. Res. 1973, 52, 680–687. [Google Scholar] [CrossRef]
- Aponte-Merced, L.; Navia, J. Pre-eruptive protein-energy malnutrition and acid solubility of rat molar enamel surfaces. Arch. Oral Biol. 1980, 25, 701–705. [Google Scholar] [CrossRef]
- Adegboye, A.R.A.; Christensen, L.B.; Holm-Pedersen, P.; Avlund, K.; Boucher, B.J.; Heitmann, B.L. Intake of Dairy Products in Relation to Periodontitis in Older Danish Adults. Nutrients 2012, 4, 1219–1229. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, M.; Yoshihara, A.; Ogawa, H.; Sato, M.; Muramatsu, K.; Watanabe, R.; Ansai, T.; Miyazaki, H. Longitudinal association of dentition status with dietary intake in Japanese adults aged 75 to 80 years. J. Oral Rehabil. 2016, 43, 737–744. [Google Scholar] [CrossRef]
- Cheruvathoor, D.D.; Thomas, V.; Kumar, N.R.; Jose, M. High prevalence of oral mucosal lesions in elderly: Call for revolutionizing geriatric dental care strategies. J. Fam. Med. Prim. Care 2020, 9, 4375–4380. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, A.M.L.; Nauntofte, B.; Smidt, D.; Torpet, L.A. Oral mucosal lesions in older people: Relation to salivary secretion, systemic diseases and medications. Oral Dis. 2015, 21, 721–729. [Google Scholar] [CrossRef]
- Jainkittivong, A.; Aneksuk, V.; Langlais, R.P. Oral mucosal conditions in elderly dental patients. Oral Dis. 2002, 8, 218–223. [Google Scholar] [CrossRef]
- American Cancer Society. 2022 Estimates. 2022. Available online: https://cancerstatisticscenter.cancer.org/#!/ (accessed on 1 September 2022).
- Buclin, T.; Cosma, M.; Appenzeller, M.; Jacquet, A.-F.; Décosterd, L.A.; Biollaz, J.; Burckhardt, P. Diet Acids and Alkalis Influence Calcium Retention in Bone. Osteoporos. Int. 2001, 12, 493–499. [Google Scholar] [CrossRef]
- Backman, T. Acid-Base Balance, Dentinogenesis and Dental Caries: Experimental Studies in Rats. Institute of Dentistry. 1999. Available online: http://jultika.oulu.fi/files/isbn9514253620.pdf (accessed on 15 August 2022).
- Sandhyarani, B.; Huddar, D.; Patil, A.; Sankeshwari, B. The dental management of troublesome twos: Renal tubular acidosis and rampant caries. BMJ Case Rep. 2013, 2013, bcr-2013-009224. [Google Scholar] [CrossRef] [PubMed]
- Adeva, M.M.; Souto, G. Diet-induced metabolic acidosis. Clin. Nutr. 2011, 30, 416–421. [Google Scholar] [CrossRef] [PubMed]
- Remer, T.; Manz, F. Estimation of the renal net acid excretion by adults consuming diets containing variable amounts of protein. Am. J. Clin. Nutr. 1994, 59, 1356–1361. [Google Scholar] [CrossRef]
- Kim, H.; Lee, S.; Choue, R. Metabolic responses to high protein diet in Korean elite bodybuilders with high-intensity resistance exercise. J. Int. Soc. Sports Nutr. 2011, 8, 10. [Google Scholar] [CrossRef] [PubMed]
- Woelber, J.P.; Vach, K. The Emerging Field of Nutritional Dentistry. Nutrients 2022, 14, 2076. [Google Scholar] [CrossRef] [PubMed]
- Antoniadou, M.; Varzakas, T. Breaking the vicious circle of diet, malnutrition and oral health for the independent elderly. Crit. Rev. Food Sci. Nutr. 2020, 61, 3233–3255. [Google Scholar] [CrossRef]
- Hengeveld, L.M.; Boer, J.M.; Gaudreau, P.; Heymans, M.W.; Jagger, C.; Mendonça, N.; Ocké, M.C.; Presse, N.; Sette, S.; Simonsick, E.M.; et al. Prevalence of protein intake below recommended in community-dwelling older adults: A meta-analysis across cohorts from the PROMISS consortium. J. Cachex-Sarcopenia Muscle 2020, 11, 1212–1222. [Google Scholar] [CrossRef]
- Martinon, P.; Fraticelli, L.; Giboreau, A.; Dussart, C.; Bourgeois, D.; Carrouel, F. Nutrition as a Key Modifiable Factor for Periodontitis and Main Chronic Diseases. J. Clin. Med. 2021, 10, 197. [Google Scholar] [CrossRef] [PubMed]
- Chawla, T.; Glickman, I. Protein deprivation and the periodontal structures of the albino rat. Oral Surg. Oral Med. Oral Pathol. 1951, 4, 578–602. [Google Scholar] [CrossRef]
- Stahl, S.; Sandler, H.C.; Cahn, L. The effects of protein deprivation upon the oral tissues of the rat and particularly upon periodontal structures under irritation. Oral Surg. Oral Med. Oral Pathol. 1955, 8, 760–768. [Google Scholar] [CrossRef]
- Adegboye, A.R.; Boucher, B.J.; Kongstad, J.; Fiehn, N.-E.; Christensen, L.B.; Heitmann, B.L. Calcium, vitamin D, casein and whey protein intakes and periodontitis among Danish adults. Public Health Nutr. 2015, 19, 503–510. [Google Scholar] [CrossRef]
- Eberhard, J.; Ruiz, K.; Tan, J.; Jayasinghe, T.N.; Khan, S.; Eroglu, E.; Adler, C.; Simpson, S.J.; Le Couteur, D.G.; Raubenheimer, D.; et al. A randomized clinical trial to investigate the effect of dietary protein sources on periodontal health. J. Clin. Periodontol. 2021, 49, 388–400. [Google Scholar] [CrossRef]
- Staufenbiel, I.; Weinspach, K.; Forster, G.; Geurtsen, W.; Günay, H. Periodontal conditions in vegetarians: A clinical study. Eur. J. Clin. Nutr. 2013, 67, 836–840. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jayasinghe, T.N.; Harrass, S.; Erdrich, S.; King, S.; Eberhard, J. Protein Intake and Oral Health in Older Adults—A Narrative Review. Nutrients 2022, 14, 4478. https://doi.org/10.3390/nu14214478
Jayasinghe TN, Harrass S, Erdrich S, King S, Eberhard J. Protein Intake and Oral Health in Older Adults—A Narrative Review. Nutrients. 2022; 14(21):4478. https://doi.org/10.3390/nu14214478
Chicago/Turabian StyleJayasinghe, Thilini N., Sanaa Harrass, Sharon Erdrich, Shalinie King, and Joerg Eberhard. 2022. "Protein Intake and Oral Health in Older Adults—A Narrative Review" Nutrients 14, no. 21: 4478. https://doi.org/10.3390/nu14214478
APA StyleJayasinghe, T. N., Harrass, S., Erdrich, S., King, S., & Eberhard, J. (2022). Protein Intake and Oral Health in Older Adults—A Narrative Review. Nutrients, 14(21), 4478. https://doi.org/10.3390/nu14214478






