A Health Care Professional Delivered Low Carbohydrate Diet Program Reduces Body Weight, Haemoglobin A1c, Diabetes Medication Use and Cardiovascular Risk Markers—A Single-Arm Intervention Analysis
Abstract
1. Introduction
2. Methods
2.1. Study Setting, Participants, and Design
2.2. Intervention
- Medical Monitoring—Medical review with a Diversa Health General Medical Practitioner at a minimum frequency of every 3 months to review pathology results and make necessary medication adjustments.
- One-on-One Coaching Real-Time Support—Live progress review and consultation with a health coach at least monthly and up to weekly. Ongoing support and advice from a health coach via SMS, WhatsApp, Facebook and Email, as required.
- Online member Portal—Access to an online portal in which participants can access/track pathology results, share notes with a health coach and chat via a Live Chat function, and access education resources personally selected by the health coach.
- Community—Access to a range of health coach-led group webinars, with option to learn, connect and share resources with other members via a moderated Facebook community. Family members are provided access to a family-based webinar to support the members journey.
- Resources—Food guides including information of what to eat, meal planning ideas and shopping advice to tailor the dietary plan to individual dietary requirements and sensitivities. Recipes and information fact sheets personally selected by the members health coach.
2.3. Data Collection and Study Outcomes
2.4. Statistical Analysis
3. Results
3.1. Characteristics
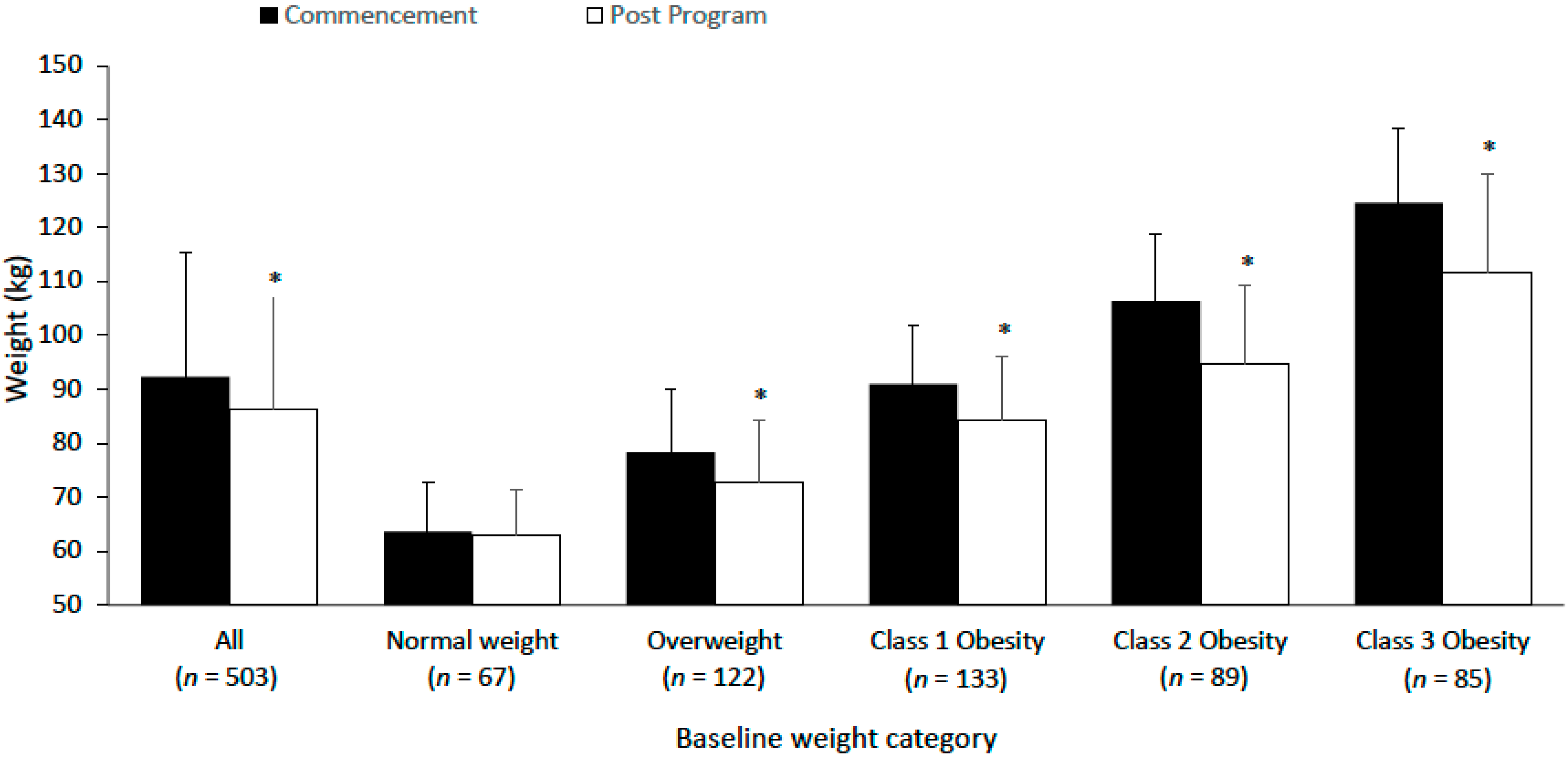
3.2. Body Weight
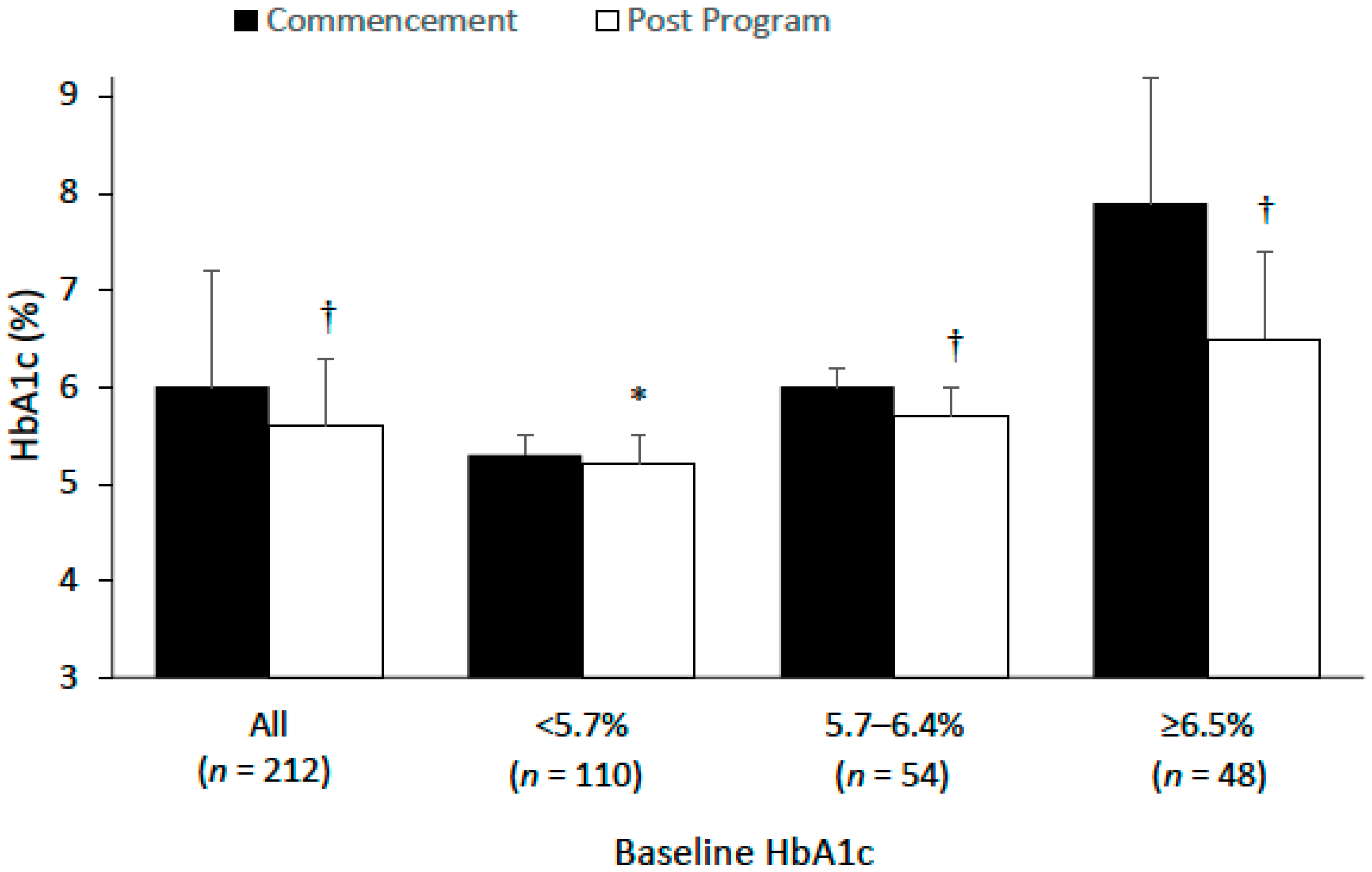
3.3. HbA1c and Diabetes Status
3.4. Metabolic Risk Factors
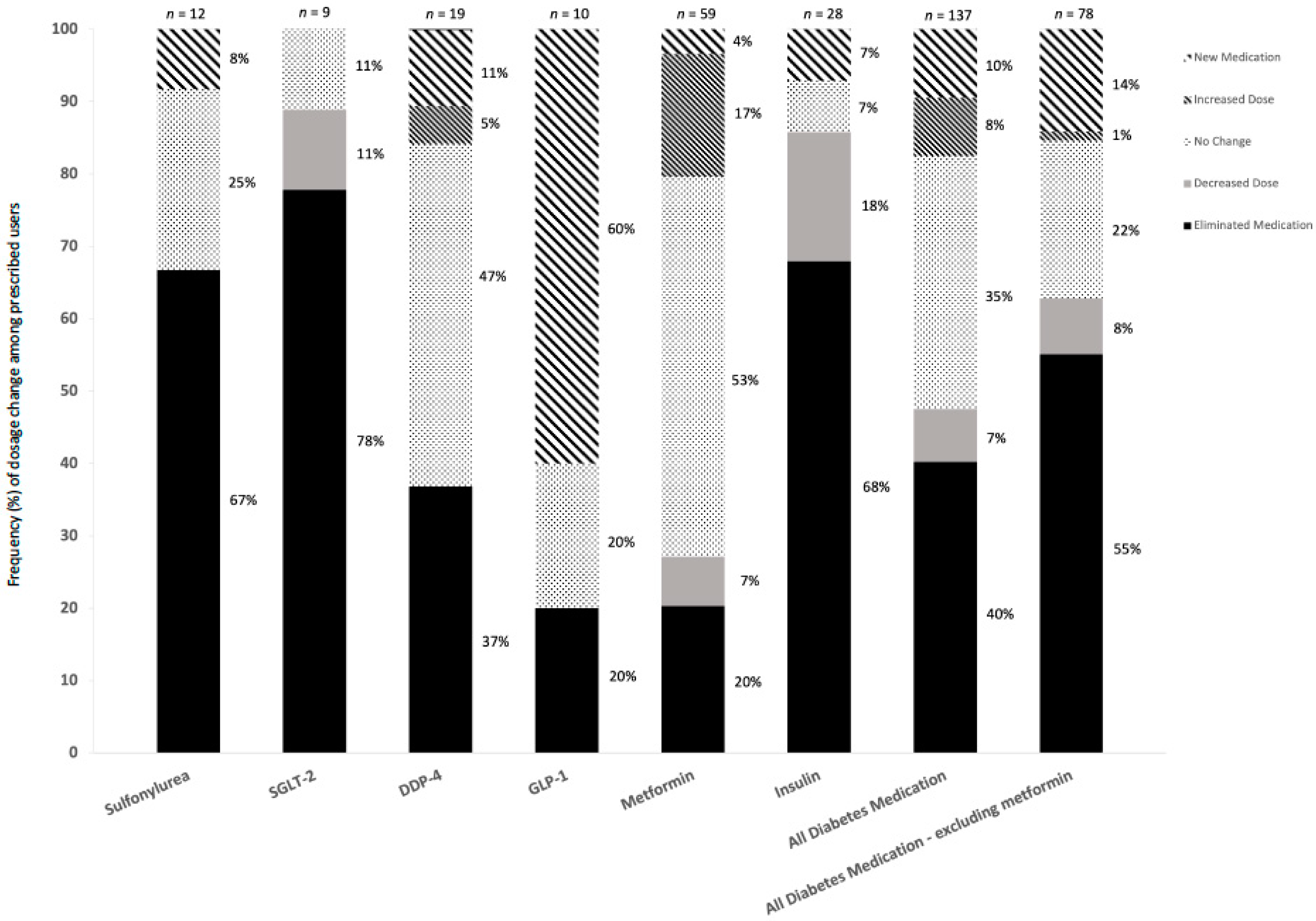
3.5. Medication
4. Discussion
4.1. Weight Loss
4.2. Blood Pressure
4.3. HbA1c
4.4. Medication Changes
4.5. Blood Lipids
4.6. Liver Function Markers
4.7. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- International Diabetes Federation. IDF Diabetes Atlas, 10th ed.; International Diabetes Federation: Brussels, Belgium, 2021. [Google Scholar]
- Australian Institute of Health and Welfare. Overweight and Obesity. Australian Government. Available online: https://www.aihw.gov.au/reports/australias-health/overweight-and-obesity (accessed on 26 August 2022).
- World Health Organization. Global Report on Diabetes; World Health Organization: Geneva, Switzerland, 2016. Available online: https://www.who.int/publications/i/item/9789241565257 (accessed on 29 May 2022).
- WHO Consultation on Obesity (1999: Geneva, Switzerland) & World Health Organization. Obesity: Preventing and Managing the Global Epidemic: Report of a WHO Consultation. World Health Organization. 2000. Available online: https://apps.who.int/iris/handle/10665/42330 (accessed on 12 September 2022).
- Rodbard, H.W.; Blonde, L.; Braithwaite, S.S.; Brett, E.M.; Cobin, R.H.; Handelsman, Y.; Hellman, R.; Jellinger, P.S.; Jovanovic, L.G.; Levy, P.; et al. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the management of diabetes mellitus. Endocr. Pract. 2007, 13 (Suppl. S1), 1–68. [Google Scholar] [PubMed]
- Colagiuri, S.; Dickinson, S.; Girgis, S.; Colagiuri, R. National Evidence Based Guidelines for Blood Glucose Control in Type 2 Diabetes. Diabetes and the NHMRC. Canberra. 2009. Available online: https://www.diabetesaustralia.com.au/wp-content/uploads/National-Evidence-Based-Guideline-for-Blood-Glucose-Control-in-Type-2-Diabetes.pdf (accessed on 29 May 2022).
- National Health and Medical Research Council. Australian Dietary Guidelines; National Health and Medical Research Council: Canberra, Australia, 2013.
- Snorgaard, O.; Poulsen, G.M.; Andersen, H.K.; Astrup, A. Systematic review and meta-analysis of dietary carbohydrate restriction in patients with type 2 diabetes. BMJ Open Diabetes Res. Care 2017, 5, e000354. [Google Scholar] [CrossRef] [PubMed]
- Sainsbury, E.; Kizirian, N.V.; Partridge, S.R.; Gill, T.; Colagiuri, S.; Gibson, A.A. Effect of dietary carbohydrate restriction on glycemic control in adults with diabetes: A systematic review and meta-analysis. Diabetes Res. Clin. Pract. 2018, 139, 239–252. [Google Scholar] [CrossRef]
- Huntriss, R.; Campbell, M.; Bedwell, C. The interpretation and effect of a low-carbohydrate diet in the management of type 2 diabetes: A systematic review and meta-analysis of randomised controlled trials. Eur. J. Clin. Nutr. 2018, 72, 311–325. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Bai, H.; Wang, S.; Li, Z.; Wang, Q.; Chen, L. Efficacy of low carbohydrate diet for type 2 diabetes mellitus management: A systematic review and meta-analysis of randomized controlled trials. Diabetes Res. Clin. Pract. 2017, 131, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Ding, L.; Xiao, X. Comparing the Efficacy and Safety of Low-Carbohydrate Diets with Low-Fat Diets for Type 2 Diabetes Mellitus Patients: A systematic Review and Meta-Analysis of Randomized Clinical Trials. Int. J. Endocrinol. 2021, 2021, 8521756. [Google Scholar] [CrossRef]
- Goldenberg, J.Z.; Johnston, B.C. Low and very low carbohydrate diets for diabetes remission. BMJ 2021, 373, n262. [Google Scholar] [CrossRef]
- Faruqi, N.; Thomas, L.; Parker, S.; Harris-Roxas, B.; Taggart, J.; Spooner, C.; Wong, V.; Harris, M.F. Primary health care provider–focused interventions for improving outcomes for people with type 2 diabetes: A rapid review. Public Health Res. Pract. 2019, 29, e29121903. [Google Scholar] [CrossRef] [PubMed]
- Saslow, L.R.; Summers, C.; Aikens, J.E.; Unwin, D.J. Outcomes of a Digitally Delivered Low-Carbohydrate Type 2 Diabetes Self-Management Program: 1-Year Results of a Single-Arm Longitudinal Study. JMIR Diabetes 2018, 3, e12. [Google Scholar] [CrossRef]
- Saslow, L.R.; Mason, A.E.; Kim, S.; Goldman, V.; Ploutz-Snyder, R.; Bayandorian, H.; Daubenmier, J.; Hecht, F.M.; Moskowitz, J.T. An Online Intervention Comparing a Very Low-Carbohydrate Ketogenic Diet and Lifestyle Recommendations Versus a Plate Method Diet in Overweight Individuals With Type 2 Diabetes: A Randomized Controlled Trial. J. Med. Internet Res. 2017, 19, e36. [Google Scholar] [CrossRef]
- WHO Consultation on Obesity (1997: Geneva, Switzerland), World Health Organization. Division of Noncommunicable Diseases & World Health Organization. Programme of Nutrition, Family and Reproductive Health. Obesity: Preventing and managing the global epidemic: Report of a WHO Consultation on Obesity, Geneva, 3–5 June 1997. World Health Organization. 1998. Available online: https://apps.who.int/iris/handle/10665/63854 (accessed on 12 September 2022).
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2021. Diabetes Care 2021, 44 (Suppl. S1), S15–S33. [Google Scholar] [CrossRef] [PubMed]
- Riddle, M.C.; Cefalu, W.T.; Evans, P.H.; Gerstein, H.C.; Nauck, M.A.; Oh, W.K.; Rothberg, A.E.; le Roux, C.W.; Rubino, F.; Schauer, P.; et al. Consensus Report: Definition and interpretation of remission in type 2 diabetes. Diabetes Care 2021, 44, 2438–2444. [Google Scholar] [CrossRef] [PubMed]
- Ryan, D.H.; Yockey, S.R. Weight Loss and Improvement in Comorbidity: Differences at 5%, 10%, 15%, and Over. Curr. Obes. Rep. 2017, 6, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Arauz-Pacheco, C.; Parrott, M.A.; Raskin, P.; American Diabetes Association. Treatment of hypertension in adults with diabetes. Diabetes Care 2003, 26 (Suppl. S1), S80–S82. [Google Scholar] [PubMed]
- Lind, M.; Oden, A.; Fahlen, M.; Eliasson, B. A systematic review of HbA1c variables used in the study of diabetic complications. Diabetes Metab. Syndr. 2008, 2, 282–293. [Google Scholar] [CrossRef]
- Lind, M.; Oden, A.; Fahlen, M.; Eliasson, B. The shape of the metabolic memory of HbA1c: Re-analysing the DCCT with respect to time-dependent effects. Diabetologia 2010, 53, 1093–1098. [Google Scholar] [CrossRef]
- Marston, N.A.; Giugliano, R.P.; Im, K.; Silverman, M.G.; O’Donoghue, M.L.; Wiviott, S.D.; Ference, B.A.; Sabatine, M.S. Association Between Triglyceride Lowering and Reduction of Cardiovascular Risk Across Multiple Lipid-Lowering Therapeutic Classes: A Systematic Review and Meta-Regression Analysis of Randomized Controlled Trials. Circulation 2019, 140, 1308–1317. [Google Scholar] [CrossRef]
- Boden, W.E. High-density lipoprotein cholesterol as an independent risk factor in cardiovascular disease: Assessing the data from Framingham to the Veterans Affairs High--Density Lipoprotein Intervention Trial. Am. J. Cardiol. 2000, 86, 19–22. [Google Scholar] [CrossRef]
- Finley, C.E.; Barlow, C.E.; Greenway, F.L.; Rock, C.L.; Rolls, B.J.; Blair, S.N. Retention rates and weight loss in a commercial weight loss program. Int. J. Obes. 2007, 31, 292–298. [Google Scholar] [CrossRef]
- Hwang, K.O.; Ning, J.; Trickey, A.W.; Sciamanna, C.N. Website usage and weight loss in a free commercial online weight loss program: Retrospective cohort study. J. Med. Internet Res. 2013, 15, e11. [Google Scholar] [CrossRef]
- Thomas, J.G.; Raynor, H.A.; Bond, D.S.; Luke, A.K.; Cardoso, C.C.; Foster, G.D.; Wing, R.R. Weight loss in Weight Watchers Online with and without an activity tracking device compared to control: A randomized trial. Obesity 2017, 25, 1014–1021. [Google Scholar] [CrossRef] [PubMed]
- Postrach, E.; Aspalter, R.; Elbelt, U.; Koller, M.; Longin, R.; Schulzke, J.D.; Valentini, L. Determinants of successful weight loss after using a commercial web-based weight reduction program for six months: Cohort study. J. Med. Internet Res. 2013, 15, e219. [Google Scholar] [CrossRef] [PubMed]
- Wing, R.R.; Lang, W.; Wadden, T.A.; Safford, M.; Knowler, W.C.; Bertoni, A.G.; Hill, J.O.; Brancati, F.L.; Peters, A.; Wagenknecht, L.; et al. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care 2011, 34, 1481–1486. [Google Scholar] [CrossRef] [PubMed]
- Aune, D.; Sen, A.; Prasad, M.; Norat, T.; Janszky, I.; Tonstad, S.; Romundstad, P.; Vatten, L.J. BMI and all cause mortality: Systematic review and non-linear dose-response meta-analysis of 230 cohort studies with 3.74 million deaths among 30.3 million participants. BMJ 2016, 353, i2156. [Google Scholar] [CrossRef]
- Global BMI Mortality Collaboration; Di Angelantonio, E.; Bhupathiraju, S.; Wormser, D.; Gao, P.; Kaptoge, S.; de Gonzalez, A.B.; Cairns, B.; Huxley, R.; Jackson, C.; et al. Body-mass index and all-cause mortality: Individual-participant-data meta-analysis of 239 prospective studies in four continents. Lancet 2016, 388, 776–786. [Google Scholar] [CrossRef]
- Reboldi, G.; Gentile, G.; Angeli, F.; Ambrosio, G.; Mancia, G.; Verdecchia, P. Effects of intensive blood pressure reduction on myocardial infarction and stroke in diabetes: A meta-analysis in 73,913 patients. J. Hypertens. 2011, 29, 1253–1269. [Google Scholar] [CrossRef]
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2019. Diabetes Care 2019, 42 (Suppl. S1), S13–S28. [Google Scholar] [CrossRef]
- Goldstein, T.; Kark, J.D.; Berry, E.M.; Adler, B.; Ziv, E.; Raz, I. The effect of a low carbohydrate energy-unrestricted diet on weight loss in obese type 2 diabetes patients—A randomized controlled trial. e-SPEN 2011, 6, e178–e186. [Google Scholar] [CrossRef]
- Mayer, S.B.; Jeffreys, A.S.; Olsen, M.K.; McDuffie, J.R.; Feinglos, M.N.; Yancy, W.S., Jr. Two diets with different haemoglobin A1c and antiglycaemic medication effects despite similar weight loss in type 2 diabetes. Diabetes Obes. Metab. 2014, 16, 90–93. [Google Scholar] [CrossRef]
- Samaha, F.F.; Iqbal, N.; Seshadri, P.; Chicano, K.L.; Daily, D.A.; McGrory, J.; Williams, T.; Williams, M.; Gracely, E.J.; Stern, L. A low-carbohydrate as compared with a low-fat diet in severe obesity. N. Engl. J. Med. 2003, 348, 2074–2081. [Google Scholar] [CrossRef]
- Yancy, W.S., Jr.; Foy, M.; Chalecki, A.M.; Vernon, M.C.; Westman, E.C. A low-carbohydrate, ketogenic diet to treat type 2 diabetes. Nutr. Metab. 2005, 2, 34. [Google Scholar] [CrossRef] [PubMed]
- Westman, E.C.; Yancy, W.S., Jr.; Mavropoulos, J.C.; Marquart, M.; McDuffie, J.R. The effect of a low-carbohydrate, ketogenic diet versus a low-glycemic index diet on glycemic control in type 2 diabetes mellitus. Nutr. Metab. 2008, 5, 36. [Google Scholar] [CrossRef] [PubMed]
- Tay, J.; Luscombe-Marsh, N.D.; Thompson, C.H.; Noakes, M.; Buckley, J.D.; Wittert, G.A.; Yancy, W.S., Jr.; Brinkworth, G.D. Comparison of low- and high-carbohydrate diets for type 2 diabetes management: A randomized trial. Am. J. Clin. Nutr. 2015, 102, 780–790. [Google Scholar] [CrossRef] [PubMed]
- Tay, J.; Thompson, C.H.; Luscombe-Marsh, N.D.; Wycherley, T.P.; Noakes, M.; Buckley, J.D.; Wittert, G.A.; Yancy, W.S., Jr.; Brinkworth, G.D. Effects of an energy-restricted low-carbohydrate, high unsaturated fat/low saturated fat diet versus a high-carbohydrate, low-fat diet in type 2 diabetes: A 2-year randomized clinical trial. Diabetes Obes. Metab. 2018, 20, 858–871. [Google Scholar] [CrossRef]
- Yamada, Y.; Uchida, J.; Izumi, H.; Tsukamoto, Y.; Inoue, G.; Watanabe, Y.; Irie, J.; Yamada, S. A non-calorie-restricted low-carbohydrate diet is effective as an alternative therapy for patients with type 2 diabetes. Intern. Med. 2014, 53, 13–19. [Google Scholar] [CrossRef]
- Lenters-Westra, E.; Schindhelm, R.K.; Bilo, H.J.; Groenier, K.H.; Slingerland, R.J. Differences in interpretation of haemoglobin A1c values among diabetes care professionals. Neth. J. Med. 2014, 72, 462–466. [Google Scholar]
- Hallberg, S.J.; McKenzie, A.L.; Williams, P.T.; Bhanpuri, N.H.; Peters, A.L.; Campbell, W.W.; Hazbun, T.L.; Volk, B.M.; McCarter, J.P.; Phinney, S.D.; et al. Effectiveness and Safety of a Novel Care Model for the Management of Type 2 Diabetes at 1 Year: An Open-Label, Non-Randomized, Controlled Study. Diabetes Ther. 2018, 9, 583–612. [Google Scholar] [CrossRef]
- Perreault, L.; Pan, Q.; Mather, K.J.; Watson, K.E.; Hamman, R.F.; Kahn, S.E.; Diabetes Prevention Program Research Group. Effect of regression from prediabetes to normal glucose regulation on long-term reduction in diabetes risk: Results from the Diabetes Prevention Program Outcomes Study. Lancet 2012, 379, 2243–2251. [Google Scholar] [CrossRef]
- Klonoff, D.C. United Kingdom prospective diabetes study follow-up studies establish a legacy effect of therapy for hyperglycemia but not hypertension. J. Diabetes Sci. Technol. 2008, 2, 922–924. [Google Scholar] [CrossRef]
- Gummesson, A.; Nyman, E.; Knutsson, M.; Karpefors, M. Effect of weight reduction on glycated haemoglobin in weight loss trials in patients with type 2 diabetes. Diabetes Obes. Metab. 2017, 19, 1295–1305. [Google Scholar] [CrossRef]
- Gannon, M.C.; Nuttall, F.Q. Effect of a high-protein, low-carbohydrate diet on blood glucose control in people with type 2 diabetes. Diabetes 2004, 53, 2375–2382. [Google Scholar] [CrossRef] [PubMed]
- Action to Control Cardiovascular Risk in Diabetes Study Group; Gerstein, H.C.; Miller, M.E.; Byington, R.P.; Goff, D.C., Jr.; Bigger, J.T.; Buse, J.B.; Cushman, W.C.; Genuth, S.; Ismail-Beigi, F.; et al. Effects of intensive glucose lowering in type 2 diabetes. N. Engl. J. Med. 2008, 358, 2545–2559. [Google Scholar]
- Henry, R.R.; Gumbiner, B.; Ditzler, T.; Wallace, P.; Lyon, R.; Glauber, H.S. Intensive conventional insulin therapy for type II diabetes. Metabolic effects during a 6-mo outpatient trial. Diabetes Care 1993, 16, 21–31. [Google Scholar] [PubMed]
- Isomaa, B.O.; Almgren, P.; Tuomi, T.; Forsen, B.; Lahti, K.; Nissen, M.; Taskinen, M.R.; Groop, L. Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care 2001, 24, 683–689. [Google Scholar] [CrossRef] [PubMed]
- Yancy, W.S.; Jr Olsen, M.K.; Guyton, J.R.; Bakst, R.P.; Westman, E.C. A low-carbohydrate, ketogenic diet versus a low-fat diet to treat obesity and hyperlipidemia: A randomized, controlled trial. Ann. Intern. Med. 2004, 140, 769–777. [Google Scholar] [CrossRef]
- Stern, L.; Iqbal, N.; Seshadri, P.; Chicano, K.L.; Daily, D.A.; McGrory, J.; Williams, M.; Gracely, E.J.; Samaha, F.F. The effects of low-carbohydrate versus conventional weight loss diets in severely obese adults: One-year follow-up of a randomized trial. Ann. Intern. Med. 2004, 140, 778–785. [Google Scholar]
- Dyson, P.A.; Beatty, S.; Matthews, D.R. A low-carbohydrate diet is more effective in reducing body weight than healthy eating in both diabetic and non-diabetic subjects. Diabet Med. 2007, 24, 1430–1435. [Google Scholar]
- Iqbal, N.; Vetter, M.L.; Moore, R.H.; Chittams, J.L.; Dalton-Bakes, C.V.; Dowd, M.; Williams-Smith, C.; Cardillo, S.; Wadden, T.A. Effects of a Low-intensity Intervention That Prescribed a Low-carbohydrate vs. a Low-fat Diet in Obese, Diabetic Participants. Obes. (Silver Spring) 2009, 18, 1733–1738. [Google Scholar]
- Krauss, R.M. All low-density lipoprotein particles are not created equal. Arter. Thromb. Vasc. Biol. 2014, 34, 959–961. [Google Scholar]
- Athinarayanan, S.J.; Adams, R.N.; Hallberg, S.J.; McKenzie, A.L.; Bhanpuri, N.H.; Campbell, W.W.; Volek, J.S.; Phinney, S.D.; McCarter, J.P. Long-Term Effects of a Novel Continuous Remote Care Intervention Including Nutritional Ketosis for the Management of Type 2 Diabetes: A 2-Year Non-randomized Clinical Trial. Front. Endocrinol. 2019, 10, 348. [Google Scholar]
- Krauss, R.M.; Blanche, P.J.; Rawlings, R.S.; Fernstrom, H.S.; Williams, P.T. Separate effects of reduced carbohydrate intake and weight loss on atherogenic dyslipidemia. Am. J. Clin. Nutr. 2006, 83, 1025–1031. [Google Scholar] [CrossRef] [PubMed]
- Bell, D.S.; Al Badarin, F.; O’Keefe, J.H., Jr. Therapies for diabetic dyslipidaemia. Diabetes Obes. Metab. 2011, 13, 313–325. [Google Scholar] [CrossRef] [PubMed]
- Sniderman, A.D.; Lamarche, B.; Contois, J.H.; de Graaf, J. Discordance analysis and the Gordian Knot of LDL and non-HDL cholesterol versus apoB. Curr. Opin. Lipidol. 2014, 25, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Pouwels, S.; Sakran, N.; Graham, Y.; Leal, A.; Pintar, T.; Yang, W.; Kassir, R.; Singhal, R.; Mahawar, K.; Ramnarain, D. Non-alcoholic fatty liver disease (NAFLD): A review of pathophysiology, clinical management and effects of weight loss. BMC Endocr. Disord. 2022, 22, 63. [Google Scholar] [CrossRef]
- Koutoukidis, D.A.; Koshiaris, C.; Henry, J.A.; Noreik, M.; Morris, E.; Manoharan, I.; Tudor, K.; Bodenham, E.; Dunnigan, A.; Jebb, S.A.; et al. The effect of the magnitude of weight loss on non-alcoholic fatty liver disease: A systematic review and meta-analysis. Metabolism 2021, 115, 154455. [Google Scholar] [CrossRef]
- Koutoukidis, D.A.; Astbury, N.M.; Tudor, K.E.; Morris, E.; Henry, J.A.; Noreik, M.; Jebb, S.A.; Aveyard, P. Association of Weight Loss Interventions With Changes in Biomarkers of Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-analysis. JAMA Intern. Med. 2019, 179, 1262–1271. [Google Scholar] [CrossRef]
- Park, E.O.; Bae, E.J.; Park, B.H.; Chae, S.W. The Associations between Liver Enzymes and Cardiovascular Risk Factors in Adults with Mild Dyslipidemia. J. Clin. Med. 2020, 9, 1147. [Google Scholar] [CrossRef]
- Shang, H.-S.; Yang, B.-H.; Perng, C.-L.; Tang, S.-H.; Lin, C.-M.; Chang, J.-B. Correlation between Liver Function Tests and Metabolic Syndrome in Hepatitis-free Elderly. J. Med. Sci. 2015, 35, 182–189. [Google Scholar]
- Lallukka, S.; Yki-Jarvinen, H. Non-alcoholic fatty liver disease and risk of type 2 diabetes. Best Pract. Res. Clin. Endocrinol. Metab. 2016, 30, 385–395. [Google Scholar] [CrossRef]
- Hill-Briggs, F.; Adler, N.E.; Berkowitz, S.A.; Chin, M.H.; Gary-Webb, T.L.; Navas-Acien, A.; Thornton, P.L.; Haire-Joshu, D. Social Determinants of Health and Diabetes: A Scientific Review. Diabetes Care 2020, 44, 258–279. [Google Scholar] [CrossRef]
- Freedman, M.; King, J.; Kennedy, E. Popular diets: A scientific review. Obes. Res. 2001, 9, 1S–40S. [Google Scholar] [CrossRef] [PubMed]
| Mealtime | Day 1 Total Daily Intake Excluding Snacks: 24 g Carbohydrate, 161 g Fat, 150 g Protein | Day 2 Total Daily Intake Excluding Snacks: 17 g Carbohydrate, 232 g Fat, 84 g Protein |
|---|---|---|
| Breakfast | Bacon, Spinach and Mushroom omelette:
| Coconut and Chia Pudding:
|
| Lunch | Bacon, Spinach and Mushroom omelette:
| Pork with Blue Cheese Sauce
|
| Dinner | Creaming baked Atlantic salmon on buttery cauliflower mash:
| Bacon, Lettuce, Tomato (BLT) Creamy Salad
|
| Snacks (optional) | 2 hardboiled eggs | 30 g prosciutto |
| Variable | Initial Consultation—All Participants | Initial Consultation—Participants with Pre/Post Program Data | Final Visit | Change | p Value | Duration from Initial Consultation to Last Recorded Observation—Days | ||
|---|---|---|---|---|---|---|---|---|
| N (%) | Mean (SD) | N (%) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | ||
| Initial Consultation | 511 | 218.1 (207.5) | ||||||
| Age (years) | 455 | 57.1 (13.7) | - | - | - | - | - | - |
| Gender | - | - | - | - | - | - | - | - |
| Female | 296 (57.9) | - | - | - | - | - | - | - |
| Male | 145 (28.4) | - | - | - | - | - | - | - |
| Not reported | 70 (13.7) | - | - | - | - | - | - | - |
| Weight (kg) | 506 | 92.2 (23.0) | 503 | 92.3 (23.0) | 86.3 (21.1) | −6.0 (6.6) | <0.001 | 216.2 (203.2) |
| BMI (kg/m2) | 499 | 32.8 (7.6) | 496 | 32.9 (7.6) | 30.7 (6.9) | −2.2 (2.4) | <0.001 | 217.6 (203.8) |
| Weight (kg) by weight category | 499 | 496 | ||||||
| Underweight BMI: <18.5 kg/m2 | 0 (0) | N/A | 0 (0) | N/A | N/A | N/A | N/A | N/A |
| Normal Weight BMI: 18.5–24.9 kg/m2 | 69 (13.8) | 63.5 (8.4) | 67 (13.5) | 63.5 (8.4) | 62.6 (8.2) | −0.9 (2.8) | 0.443 | 252.9 (260.9) |
| Overweight Weight BMI: 25–29.9 kg/m2 | 122 (24.4) | 77.9 (10.7) | 122 (24.6) | 77.9 (10.7) | 73.4 (10.5) | −4.5 (4.3) | 0.010 | 208.6 (185.7) |
| Class 1 Obesity BMI: 30–34.9 kg/m2 | 134 (26.9) | 91.0 (11.3) | 133 (26.8) | 91.0 (11.3) | 85.0 (12.2) | −6.0 (5.5) | <0.001 | 233.7 (211.4) |
| Class 2 Obesity BMI: 35–39.9 kg/m2 | 89 (17.8) | 105.0 (11.6) | 89 (17.9) | 105.0 (11.6) | 96.3 (12.7) | −8.7 (7.0) | <0.001 | 205.9 (191.5) |
| Class 3 Obesity BMI: 40+ kg/m2 | 85 (17.0) | 125.6 (17.9) | 85 (17.1) | 125.6 (17.9) | 115.6 (19.2) | −10.0 (8.9) | <0.001 | 189.6 (175.0) |
| Weight status classification | 499 | 496 | ||||||
| Underweight BMI: <18.5 kg/m2 | 0 (0) | - | 0 (0) | - | 1 (0.2) | - | - | - |
| Normal Weight BMI: 18.5–24.9 kg/m2 | 69 (13.8) | - | 67 (13.5) | - | 108 (21.8) | - | - | - |
| Overweight Weight BMI: 25–29.9 kg/m2 | 122 (24.4 | - | 122 (24.6) | - | 143 (28.8) | - | - | - |
| Class 1 Obesity BMI: 30–34.9 kg/m2 | 134 (26.9) | - | 133 (26.8) | - | 129 (26.0) | - | - | - |
| Class 2 Obesity BMI: 35–39.9 kg/m2 | 89 (17.8) | - | 89 (17.9) | - | 64 (12.9) | - | - | - |
| Class 3 Obesity BMI: 40+ kg/m2 | 85 (17.0) | - | 85 (17.1) | - | 51 (10.3) | - | - | - |
| Waist Circumference | 424 | 104.2 (17.4) | 397 | 104.8 (17.2) | 99.1 (15.9) | −5.7 (6.9) | <0.001 | 210.6 (194.9) |
| Systolic Blood Pressure (mmHg) | 304 | 138.2 (20.6) | 256 | 139.6 (20.6) | 131.0 (18.7) | −8.6 (18.2) | <0.001 | 225.4 (211.9) |
| Diastolic Blood Pressure (mmHg) | 304 | 84.6 (12.7) | 256 | 84.9 (13.1) | 81.2 ± 13.1 | −3.7 ± 12.1 | <0.001 | 225.4 ± 211.9 |
| Variable | Initial Consultation—All Participants | Initial Consultation—Participants with Pre/Post Program Data | Final Visit | Change | p Value | Duration from Initial Consultation to Last Recorded Observation—Days | ||
|---|---|---|---|---|---|---|---|---|
| N (%) | Mean (SD) | N (%) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | ||
| Initial Consultation | 279 | 258.7 (206.8) | ||||||
| Age (years) | 274 | 59.4 (13.0) | - | - | - | - | - | - |
| Gender | - | - | - | - | - | - | ||
| Female | 176 (63.1) | |||||||
| Male | 91 (32.6) | |||||||
| Not reported | 12 (4.3) | |||||||
| Weight (kg) | 277 | 90.6 (22.1) | 277 | 90.6 (22.1) | 83.4 (19.8) | −7.2 (7.3) | <0.001 | 256.0 (201.6) |
| BMI (kg/m2) | 274 | 32.1 (7.1) | 274 | 32.1 (7.1) | 29.5 (6.3) | −2.6 (2.7) | <0.001 | 258.4 (201.5) |
| Weight (kg) by weight category | 274 | 274 | ||||||
| Underweight BMI: <18.5 kg/m2 | 0 (0) | N/A | 0 (0) | N/A | N/A | N/A | N/A | N/A |
| Normal Weight BMI: 18.5–24.9 kg/m2 | 37 (13.5) | 63.7 (9.1) | 37 (13.5) | 63.7 (9.1) | 62.9 (8.5) | −0.8 (2.6) | 0.071 | 263.4 (242.9) |
| Overweight Weight BMI: 25–29.9 kg/m2 | 74 (27.0) | 78.3 (11.6) | 74 (27.0) | 78.3 (11.6) | 72.7 (11.5) | −5.6 (4.6) | <0.001 | 245.4 (162.1) |
| Class 1 Obesity BMI: 30–34.9 kg/m2 | 84 (30.7) | 91.0 (11.0) | 84 (30.7) | 91.0 (11.0) | 84.2 (12.0) | −6.8 (6.0) | <0.001 | 280.2 (228.6) |
| Class 2 Obesity BMI: 35–39.9 kg/m2 | 41 (15.0) | 106.4 (12.3) | 41 (15.0) | 106.4 (12.3) | 94.9 (14.3) | −11.5 (8.0) | <0.001 | 225.5 (168.9) |
| Class 3 Obesity BMI: 40+ kg/m2 | 38 (13.9) | 124.6 (18.3) | 38 (13.9) | 124.6 (18.3) | 111.7 (19.5) | −12.9 (9.9) | <0.001 | 245.4 (162.1) |
| Weight (kg) by glycaemic control classification | 259 | 259 | ||||||
| Normoglycemia HbA1c: <5.7% | 158 (61.0) | 91.7 (22.7) | 158 (61.0) | 91.7 (22.7) | 84.6 (20.2) | −7.2 (7.3) | <0.001 | 251.7 (206.5) |
| Pre-diabetes HbA1c: 5.7–6.4 % | 55 (21.2) | 87.9 (21.1) | 55 (21.2) | 87.9 (21.1) | 81.4 (19.4) | −6.5 (6.0) | <0.001 | 261.8 (171.3) |
| Diabetes HbA1c: ≥6.5% | 46 (17.8) | 89.2 ± 22.0 | 46 (17.8) | 89.2 (22.0) | 81.6 (19.4) | −7.6 (7.6) | <0.001 | 269.1 (178.4) |
| Weight status classification | 274 | 274 | ||||||
| Underweight BMI: <18.5 kg/m2 | 0 (0) | - | 0 (0) | - | 1 (0.4) | - | - | - |
| Normal Weight BMI: 18.5–24.9 kg/m2 | 37 (13.5) | - | 37 (13.5) | - | 70 (25.5) | - | - | - |
| Overweight BMI: 25–29.9 kg/m2 | 74 (27.0) | - | 74 (27.0) | - | 87 (31.8) | - | - | - |
| Class 1 Obesity BMI: 30–34.9 kg/m2 | 84 (30.7) | - | 84 (30.7) | - | 70 (25.5) | - | - | - |
| Class 2 Obesity BMI: 35–39.9 kg/m2 | 41 (15.0) | - | 41 (15.0) | - | 29 (10.6) | - | - | - |
| Class 3 Obesity BMI: 40+ kg/m2 | 38 (13.9) | - | 38 (13.9) | - | 17 (6.2) | - | - | - |
| Waist Circumference | 228 | 103.9 (16.4) | 220 | 104.5 (16.1) | 97.7 (14.6) | −6.8 (7.2) | <0.001 | 246.3 (191.9) |
| Systolic Blood Pressure (mmHg) | 186 | 140.2 (21.5) | 167 | 141.0 (21.1) | 131.6 (19.3) | −9.3 (19.6) | <0.001 | 234.0 (185.9) |
| Diastolic Blood Pressure (mmHg) | 186 | 84.7 (13.6) | 167 | 85.0 (13.8) | 81.0 (13.3) | −4.0 (12.3) | <0.001 | 234.0 (185.9) |
| HbA1c | 261 | 5.9 (1.2) | 212 | 6.0 (1.2) | 5.6 (0.7) | 0.4 (0.8) | <0.001 | 254.2 (200.1) |
| HbA1c by glycaemic control classification | 261 | 212 | ||||||
| Normoglycemia HbA1c: <5.7% | 158 (60.5) | 5.2 ±0.3 | 110 (51.9) | 5.3 (0.2) | 5.2 (0.3) | −0.1 (0.2) | 0.010 | 252.6 (212.0) |
| Pre-diabetes HbA1c: 5.7–6.4 % | 55 (21.1) | 6.0 (0.2) | 54 (25.5) | 6.0 (0.2) | 5.7 (0.3) | −0.3 (0.3) | <0.001 | 242.4 (169.3) |
| Diabetes HbA1c: ≥6.5% | 48 (18.4) | 7.9 (1.3) | 48 (22.6) | 7.9 (1.3) | 6.5 (0.9) | −1.4 (1.3) | <0.001 | 271.4 (206.9) |
| Fasting Glucose (mmol/L) | 211 | 6.3 (3.5) | 186 | 6.1 (1.9) | 5.9 (1.3) | −0.3 (1.3) | 0.009 | 246.0 (202.8) |
| Insulin (mU/L) | 234 | 12.5 (8.5) | 214 | 12.6 (8.5) | 10.7 (7.1) | −1.9 (6.8) | <0.001 | 241.0 (191.0) |
| Triglycerides (mmol/L) | 258 | 1.4 (0.8) | 237 | 1.4 (0.9) | 1.2 (0.5) | −0.2 (0.7) | <0.001 | 243.5 (192.3) |
| Total Cholesterol (mmol/L) | 255 | 5.5 (1.5) | 231 | 5.5 (1.3) | 5.6 (1.8) | 0.2 (1.1) | 0.013 | 240.7 (188.7) |
| HDL-Cholesterol (mmol/L) | 255 | 1.4 (0.4) | 230 | 1.4 (0.4) | 1.5 (0.4) | 0.10 (0.22) | <0.001 | 241.2 (188.9) |
| LDL Cholesterol (mmol/L) | 250 | 3.45 ± 1.36 | 226 | 3.42 ± 1.22 | 3.60 ± 1.63 | 0.18 ± 1.02 | 0.009 | 241.5 ± 190.1 |
| Liver Function | ||||||||
| ALT (U/L) | 267 | 28.8 (27.7) | 250 | 29.1 (28.5) | 22.7 (11.7) | −6.4 (27.1) | <0.001 | 253.9 (205.2) |
| AST (U/L) | 262 | 24.0 (12.6) | 245 | 24.0 (12.7) | 20.5 (7.1) | −3.5 (12.6) | <0.001 | 256.8 (206.3) |
| GTT (U/L) | 224 | 27.0 (23.0) | 201 | 26.4 (19.0) | 20.9 (12.4) | −5.6 (11.5) | <0.001 | 265.1 (216.3) |
| Number of Participants, n | Number of Medications Prescribed, n | |||
|---|---|---|---|---|
| Prescribed Initial Consultation | Prescribed Post Program | Prescribed Initial Consultation | Prescribed Post Program | |
| Sulfonylurea | 11 | 4 | 11 | 4 |
| SGLT-2 | 9 | 2 | 9 | 2 |
| DDP-4 | 17 | 12 | 17 | 12 |
| GLP-1 | 4 | 8 | 4 | 8 |
| Metformin | 56 | 47 | 57 | 47 |
| Insulin | 17 | 7 | 26 | 9 |
| Diabetes medication—excluding metformin | 42 | 26 | 67 | 35 |
| All diabetes medication—including metformin | 63 | 51 | 124 | 82 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brinkworth, G.D.; Wycherley, T.P.; Taylor, P.J.; Thompson, C.H. A Health Care Professional Delivered Low Carbohydrate Diet Program Reduces Body Weight, Haemoglobin A1c, Diabetes Medication Use and Cardiovascular Risk Markers—A Single-Arm Intervention Analysis. Nutrients 2022, 14, 4406. https://doi.org/10.3390/nu14204406
Brinkworth GD, Wycherley TP, Taylor PJ, Thompson CH. A Health Care Professional Delivered Low Carbohydrate Diet Program Reduces Body Weight, Haemoglobin A1c, Diabetes Medication Use and Cardiovascular Risk Markers—A Single-Arm Intervention Analysis. Nutrients. 2022; 14(20):4406. https://doi.org/10.3390/nu14204406
Chicago/Turabian StyleBrinkworth, Grant D., Thomas P. Wycherley, Pennie J. Taylor, and Campbell H. Thompson. 2022. "A Health Care Professional Delivered Low Carbohydrate Diet Program Reduces Body Weight, Haemoglobin A1c, Diabetes Medication Use and Cardiovascular Risk Markers—A Single-Arm Intervention Analysis" Nutrients 14, no. 20: 4406. https://doi.org/10.3390/nu14204406
APA StyleBrinkworth, G. D., Wycherley, T. P., Taylor, P. J., & Thompson, C. H. (2022). A Health Care Professional Delivered Low Carbohydrate Diet Program Reduces Body Weight, Haemoglobin A1c, Diabetes Medication Use and Cardiovascular Risk Markers—A Single-Arm Intervention Analysis. Nutrients, 14(20), 4406. https://doi.org/10.3390/nu14204406





