The Combined Use of Medium- and Short-Chain Fatty Acids Improves the Pregnancy Outcomes of Sows by Enhancing Ovarian Steroidogenesis and Endometrial Receptivity
Abstract
1. Introduction
2. Materials and Methods
2.1. Porcine Primary Cultured Ovarian Granulosa Cells
2.2. Cell Viability Assay
2.3. Sex Hormone and Blood Biochemistry Detection
2.4. Cell Culture
2.5. qPCR Analysis
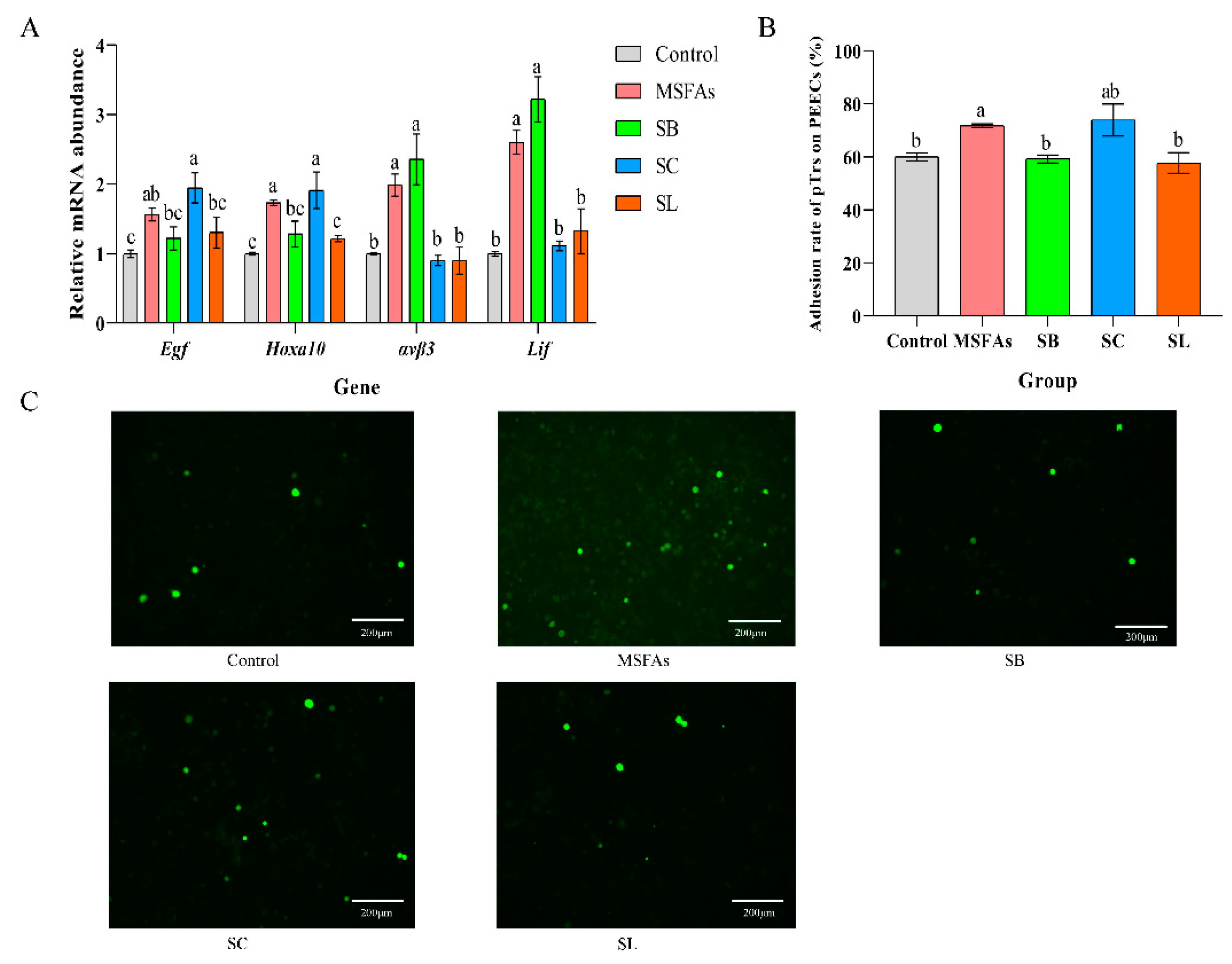
2.6. Adhesion Assay
2.7. Animals and Experimental Design
2.8. Bacterial DNA Extraction and 16S rRNA Gene Sequencing
2.9. Statistical Analysis
3. Results
3.1. MSFAs Can Increase the Expression Levels of Steroid Steroidogenesis Genes and Luteinizing Genes and Promote Estrogen Synthesis
3.2. MSFAs Can Improve the Receptivity of Pig Endometrial Cells
3.3. Effects of Dietary Supplementation with MSFAs on the Reproductive Performance of Sows
3.4. Effects of Dietary Supplementation of MSFAs on Serum Steroid Hormones and Serum Metabolites at Day 28 of Gestation
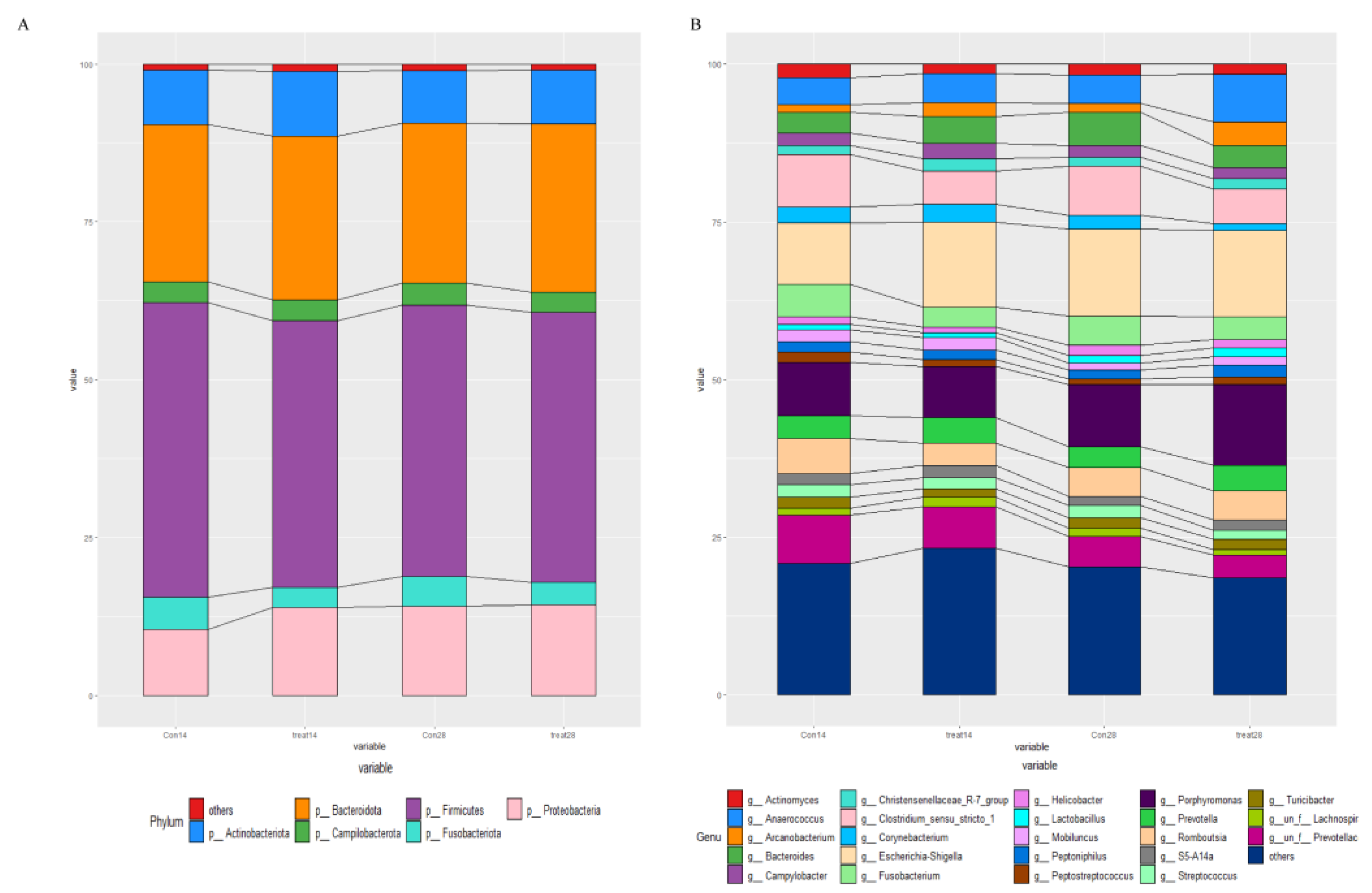
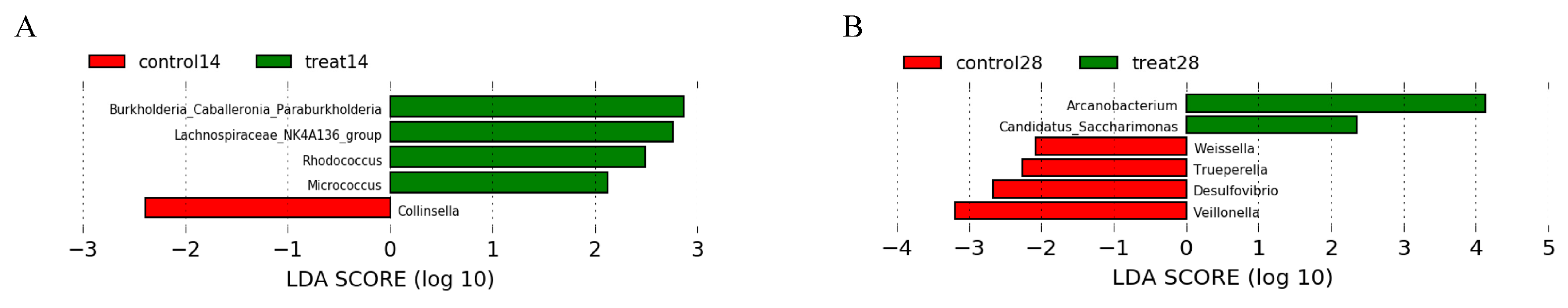
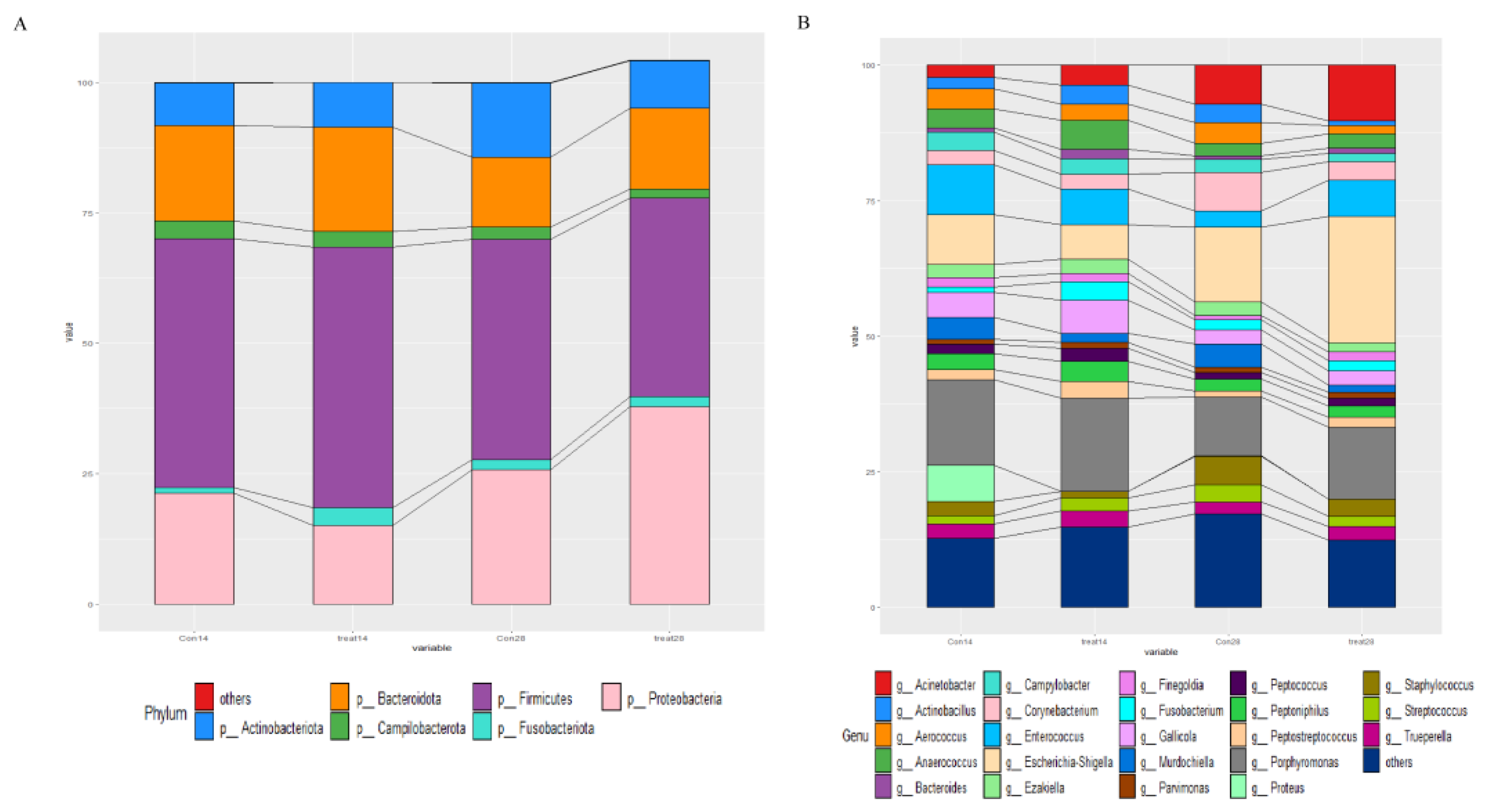
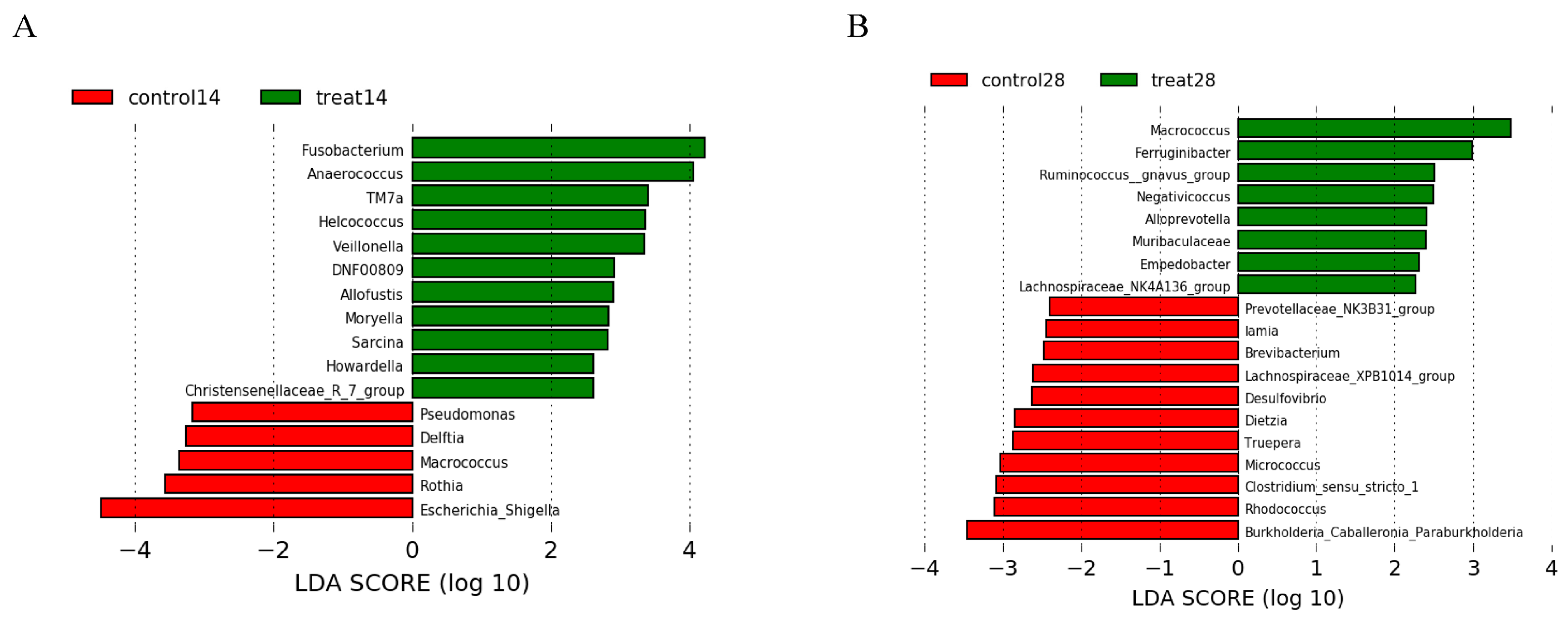
3.5. Effects of Dietary Supplementation with MSFAs on the Rectal Swab Microbial Diversity and Composition of Sows at 14 and 28 Days of Gestation
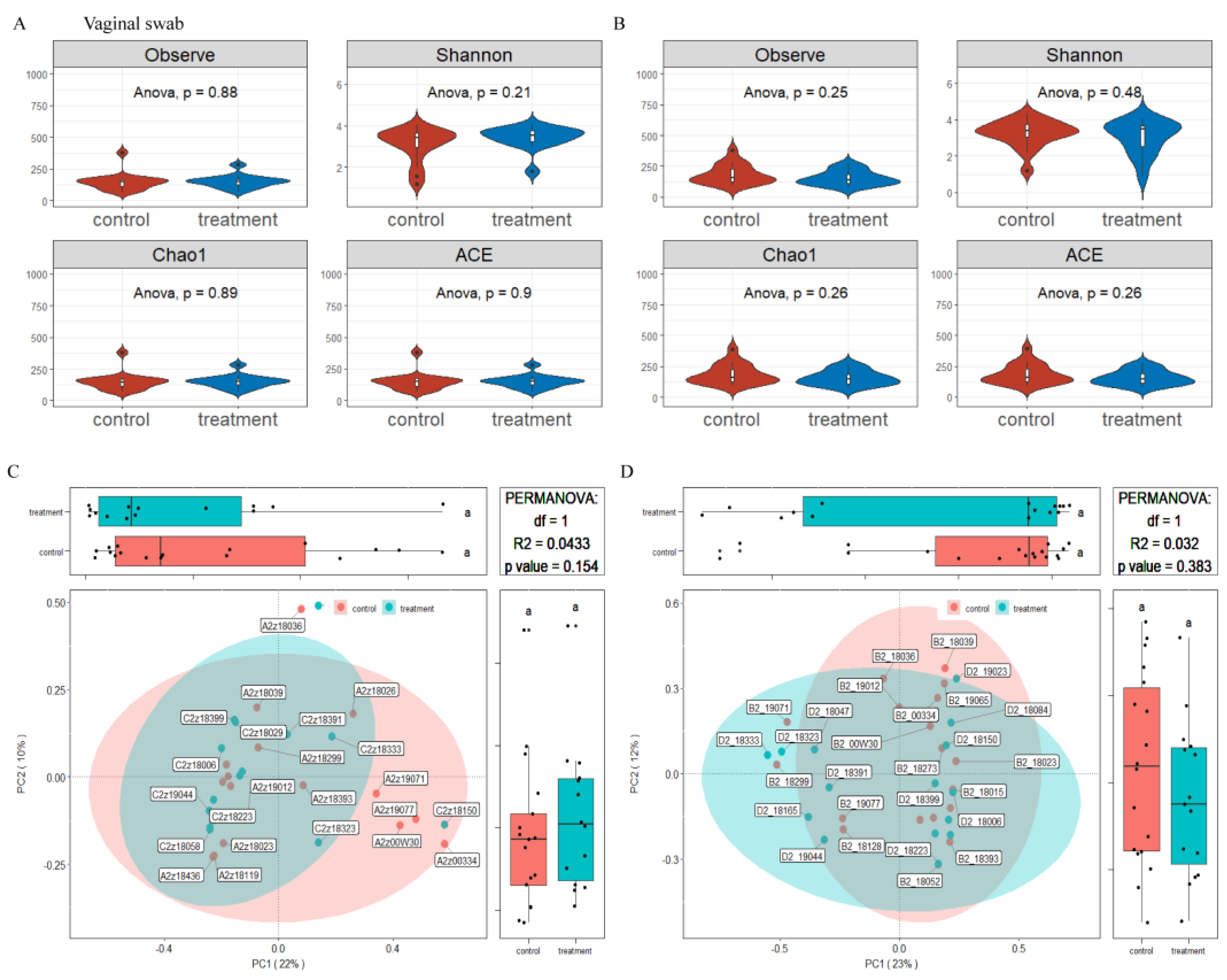
3.6. Effects of Dietary Supplementation with MSFAs on Vaginal Microbial Diversity and Composition of Sows at 14 and 28 Days of Gestation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goff, A.K. Embryonic Signals and Survival. Reprod. Domest. Anim. 2002, 37, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Eppig, J.J. Oocyte control of ovarian follicular development and function in mammals. Reproduction 2001, 122, 829–838. [Google Scholar] [CrossRef] [PubMed]
- Boland, M.; Lonergan, P.; O’Callaghan, D. Effect of nutrition on endocrine parameters, ovarian physiology, and oocyte and embryo development. Theriogenology 2001, 55, 1323–1340. [Google Scholar] [CrossRef]
- Fleming, R.; Lloyd, F.; Herbert, M.; Fenwick, J.; Griffiths, T.; Murdoch, A. Effects of profound suppression of luteinizing hormone during ovarian stimulation on follicular activity, oocyte and embryo function in cycles stimulated with purified follicle stimulating hormone. Hum. Reprod. 1998, 13, 1788–1792. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Conejero, J.A.; Simón, C.; Pellicer, A.; Horcajadas, J.A. Is ovarian stimulation detrimental to the endo-metrium? Reprod. BioMedicine Online 2007, 15, 45–50. [Google Scholar] [CrossRef]
- Young, S.L. Oestrogen and progesterone action on endometrium: A translational approach to understanding endometrial receptivity. Reprod. Biomed. Online 2013, 27, 497–505. [Google Scholar] [CrossRef]
- Governini, L.; Luongo, F.P.; Haxhiu, A.; Piomboni, P.; Luddi, A. Main actors behind the endometrial receptivity and successful implantation. Tissue Cell 2021, 73, 101656. [Google Scholar] [CrossRef]
- Lu, H.-C.; Mackie, K. An Introduction to the Endogenous Cannabinoid System. Biol. Psychiatry 2016, 79, 516–525. [Google Scholar] [CrossRef]
- Weems, C.; Weems, Y.; Randel, R. Prostaglandins and reproduction in female farm animals. Vet. J. 2006, 171, 206–228. [Google Scholar] [CrossRef]
- Aoki, J. Mechanisms of lysophosphatidic acid production. Semin. Cell Dev. Biol. 2004, 15, 477–489. [Google Scholar] [CrossRef]
- Spiegel, S.; Milstien, S. Sphingosine-1-phosphate: An enigmatic signalling lipid. Nat. Rev. Mol. Cell Biol. 2003, 4, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Sumida, C. Fatty acids: Ancestral ligands and modern co-regulators of the steroid hormone receptor cell signalling pathway. Prostaglandins Leukot. Essent. Fat. Acids 1995, 52, 137–144. [Google Scholar] [CrossRef]
- Herrera, E.; Ortega-Senovilla, H. Maternal lipid metabolism during normal pregnancy and its implications to fetal development. Clin. Lipidol. 2010, 5, 899–911. [Google Scholar] [CrossRef]
- Sturmey, R.G.; Reis, A.; Leese, H.J.; McEvoy, T.G. Role of Fatty Acids in Energy Provision During Oocyte Maturation and Early Embryo Development. Reprod. Domest. Anim. 2009, 44 (Suppl. S3), 50–58. [Google Scholar] [CrossRef]
- Mennitti, L.V.; Oliveira, J.L.; Morais, C.A.; Estadella, D.; Oyama, L.M.; Nascimento, C.M.O.D.; Pisani, L.P. Type of fatty acids in maternal diets during pregnancy and/or lactation and metabolic consequences of the offspring. J. Nutr. Biochem. 2015, 26, 99–111. [Google Scholar] [CrossRef]
- Zhao, Z.-A.; Zhang, Z.-R.; Xu, X.; Deng, W.-B.; Li, M.; Leng, J.-Y.; Liang, X.-H.; Yang, Z.-M. Arachidonic acid regulation of the cytosolic phospholipase A2α/cyclooxygenase-2 pathway in mouse endometrial stromal cells. Fertil. Steril. 2012, 97, 1199–1205.e9. [Google Scholar] [CrossRef]
- Abolghasemi, A.; Dirandeh, E.; Pirsaraei, Z.A.; Shohreh, B. Dietary conjugated linoleic acid supplementation alters the expression of genes involved in the endocannabinoid system in the bovine endometrium and increases plasma progesterone concentrations. Theriogenology 2016, 86, 1453–1459. [Google Scholar] [CrossRef]
- Van Hoeck, V.; Sturmey, R.G.; Bermejo-Alvarez, P.; Rizos, D.; Gutierrez-Adan, A.; Leese, H.J.; Bols, P.E.J.; Leroy, J.L.M.R. Elevated Non-Esterified Fatty Acid Concentrations during Bovine Oocyte Maturation Compromise Early Embryo Physiology. PLoS ONE 2011, 6, e23183. [Google Scholar] [CrossRef]
- Jungheim, E.S.; Louden, E.D.; Chi, M.M.-Y.; Frolova, A.I.; Riley, J.K.; Moley, K.H. Preimplantation Exposure of Mouse Embryos to Palmitic Acid Results in Fetal Growth Restriction Followed by Catch-Up Growth in the Offspring. Biol. Reprod. 2011, 85, 678–683. [Google Scholar] [CrossRef]
- Hague, A.; Elder, D.J.E.; Hicks, D.J.; Paraskeva, C. Apoptosis in colorectal tumour cells: Induction by the short chain fatty acids butyrate, propionate and acetate and by the bile salt deoxycholate. Int. J. Cancer 1995, 60, 400–406. [Google Scholar] [CrossRef]
- Tan, J.; McKenzie, C.; Potamitis, M.; Thorburn, A.N.; Mackay, C.R.; Macia, L. The Role of Short-Chain Fatty Acids in Health and Disease. Adv. Immunol. 2014, 121, 91–119. [Google Scholar] [CrossRef] [PubMed]
- Liberato, M.V.; Nascimento, A.S.; Ayers, S.D.; Lin, J.Z.; Cvoro, A.; Silveira, R.L.; Martínez, L.; Souza, P.C.T.; Saidemberg, D.; Deng, T.; et al. Medium Chain Fatty Acids Are Selective Peroxisome Proliferator Activated Receptor (PPAR) γ Activators and Pan-PPAR Partial Agonists. PLoS ONE 2012, 7, e36297. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Fang, Z.-F.; Che, L.-Q.; Xu, S.-Y.; Wu, D.; Wu, C.-M.; Wu, X.-Q. Use of Sodium Butyrate as an Alternative to Dietary Fiber: Effects on the Embryonic Development and Anti-Oxidative Capacity of Rats. PLoS ONE 2014, 9, e97838. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Takanashi, K.; Hamatani, T.; Hirayama, A.; Akutsu, H.; Fukunaga, T.; Ogawa, S.; Sugawara, K.; Shinoda, K.; Soga, T.; et al. A medium-chain fatty acid as an alternative energy source in mouse preimplantation development. Sci. Rep. 2012, 2, 930. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Cai, S.; Wang, S.; Zeng, X.; Ye, C.; Chen, M.; Zeng, X.; Qiao, S. Maternal short and medium chain fatty acids supply during early pregnancy improves embryo survival through enhancing progesterone synthesis in rats. J. Nutr. Biochem. 2019, 69, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.-Y.; Choi, H.-J.; Chung, T.-W.; Choi, J.-Y.; Kim, H.S.; Jung, Y.-S.; Lee, S.-O.; Ha, K.-T. Water-extracted Perilla frutescens increases endometrial receptivity though leukemia inhibitory factor-dependent expression of integrins. J. Pharmacol. Sci. 2016, 131, 259–266. [Google Scholar] [CrossRef]
- Ollion, J.; Cochennec, J.; Loll, F.; Escudé, C.; Boudier, T. TANGO: A generic tool for high-throughput 3D image analysis for studying nuclear organization. Bioinformatics 2013, 29, 1840–1841. [Google Scholar] [CrossRef]
- Magoč, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef]
- Stackebrandt, E.; Goebel, B.M. Taxonomic Note: A Place for DNA-DNA Reassociation and 16S rRNA Sequence Analysis in the Present Species Definition in Bacteriology. Int. J. Syst. Evol. Microbiol. 1994, 44, 846–849. [Google Scholar] [CrossRef]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef] [PubMed]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef] [PubMed]
- Norwitz, E.R.; Schust, D.J.; Fisher, S.J. Implantation and the Survival of Early Pregnancy. N. Engl. J. Med. 2001, 345, 1400–1408. [Google Scholar] [CrossRef] [PubMed]
- Bazer, F.W.; Spencer, T.E.; Johnson, G.A.; Burghardt, R.C.; Wu, G. Comparative aspects of implantation. Reproduction 2009, 138, 195–209. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Wang, M.; Guo, H.; Jia, Y.; Yang, X.; Zhao, R. Effects of sodium butyrate supplementation on reproductive performance and colostrum composition in gilts. Animal 2016, 10, 1722–1727. [Google Scholar] [CrossRef]
- Vieira, E.; Watanabe, P.; Andrade, T.; Araújo, G.; Silva, B.; Pinheiro, R.; Mendonça, I. Dietary supplementation of sodium butyrate for mixed-parity sows during lactation. Livest. Sci. 2020, 232, 103915. [Google Scholar] [CrossRef]
- Zhai, J.; Liu, J.; Cheng, X.; Li, S.; Hong, Y.; Sun, K.; Chen, Z.-J.; Du, Y.; Li, W. Zinc finger gene 217 (ZNF217) Promoted Ovarian Hyperstimulation Syndrome (OHSS) through Regulating E2 Synthesis and Inhibiting Thrombospondin-1 (TSP-1). Sci. Rep. 2017, 7, 3245. [Google Scholar] [CrossRef]
- Kezele, P.; Skinner, M.K. Regulation of Ovarian Primordial Follicle Assembly and Development by Estrogen and Progesterone: Endocrine Model of Follicle Assembly. Endocrinology 2003, 144, 3329–3337. [Google Scholar] [CrossRef]
- Richards, J.S.; Pangas, S.A. The ovary: Basic biology and clinical implications. J. Clin. Investig. 2010, 120, 963–972. [Google Scholar] [CrossRef]
- Greco, T.L.; Payne, A.H. Ontogeny of expression of the genes for steroidogenic enzymes P450 side-chain cleavage, 3 beta-hydroxysteroid dehydrogenase, P450 17 alpha-hydroxylase/C17-20 lyase, and P450 aromatase in fetal mouse gonads. Endocrinology 1994, 135, 262–268. [Google Scholar] [CrossRef]
- Li, Q.; Du, X.; Pan, Z.; Zhang, L.; Li, Q. The transcription factor SMAD4 and miR-10b contribute to E2 release and cell apoptosis in ovarian granulosa cells by targeting CYP19A1. Mol. Cell Endocrinol. 2018, 476, 84–95. [Google Scholar] [CrossRef] [PubMed]
- Simoni, M.; Gromoll, J.; Nieschlag, E. The Follicle-Stimulating Hormone Receptor: Biochemistry, Molecular Biology, Physiology, and Pathophysiology. Endocr. Rev. 1997, 18, 739–773. [Google Scholar] [CrossRef]
- Richards, J.S. Maturation of ovarian follicles: Actions and interactions of pituitary and ovarian hormones on follicular cell differentiation. Physiol. Rev. 1980, 60, 51–89. [Google Scholar] [CrossRef] [PubMed]
- Hsueh, A.J.W.; Adashi, E.Y.; Jones, P.B.C.; Welsh, J.T.H. Hormonal Regulation of the Differentiation of Cultured Ovarian Granulosa Cells. Endocr. Rev. 1984, 5, 76–127. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Yu, Y.; Li, Y.; Wang, S.; Zhang, R.; Guo, Y.; Li, Y.; Yan, Y.; Sun, Y.P. Human chorionic gonadotropin-induced amphiregulin stimulates aromatase expression in human granulosa-lutein cells: A mechanism for estradiol production in the luteal phase. Hum. Reprod. 2019, 34, 2018–2026. [Google Scholar] [CrossRef] [PubMed]
- Simón, C.; Moreno, C.; Remohí, J.; Pellicer, A. Cytokines and embryo implantation. J. Reprod. Immunol. 1998, 39, 117–131. [Google Scholar] [CrossRef]
- Ledee-Bataille, N.; Laprée-Delage, G.; Taupin, J.-L.; Dubanchet, S.; Frydman, R.; Chaouat, G. Concentration of leukaemia inhibitory factor (LIF) in uterine flushing fluid is highly predictive of embryo implantation. Hum. Reprod. 2002, 17, 213–218. [Google Scholar] [CrossRef]
- Bagot, C.N.; Kliman, H.J.; Taylor, H.S. Maternal Hoxa10 is required for pinopod formation in the development of mouse uterine receptivity to embryo implantation. Dev. Dyn. 2001, 222, 538–544. [Google Scholar] [CrossRef]
- Nikas, G.; Develioglu, O.H.; Toner, J.P.; Jones, H.W. Endometrial pinopodes indicate a shift in the window of receptivity in IVF cycles. Hum. Reprod. 1999, 14, 787–792. [Google Scholar] [CrossRef]
- Nardo, L.; Sabatini, L.; Rai, R.; Nardo, F. Pinopode expression during human implantation. Eur. J. Obstet. Gynecol. Reprod. Biol. 2002, 101, 104–108. [Google Scholar] [CrossRef]
- Cavagna, M.; Mantese, J. Biomarkers of Endometrial Receptivity—A Review. Placenta 2003, 24, S39–S47. [Google Scholar] [CrossRef]
- Aghajanova, L.; Stavreus-Evers, A.; Nikas, Y.; Hovatta, O.; Landgren, B.M. Coexpression of pinopodes and leukemia inhibitory factor, as well as its receptor, in human endometrium. Fertil. Steril. 2003, 79 (Suppl. S1), 808–814. [Google Scholar] [CrossRef]
- Lessey, B.A.; Damjanovich, L.; Coutifaris, C.; Castelbaum, A.; Albelda, S.M.; Buck, C.A. Integrin adhesion molecules in the human endometrium. Correlation with the normal and abnormal menstrual cycle. J. Clin. Investig. 1992, 90, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Somkuti, S.G.; Yuan, L.; Fritz, M.A.; Lessey, B.A. Epidermal Growth Factor and Sex Steroids Dynamically Regulate a Marker of Endometrial Receptivity in Ishikawa Cells. J. Clin. Endocrinol. Metab. 1997, 82, 2192–2197. [Google Scholar] [CrossRef] [PubMed]
- Arici, A.; Engin, O.; Attar, E.; Olive, D.L. Modulation of leukemia inhibitory factor gene expression and protein biosynthesis in human endometrium. J. Clin. Endocrinol. Metab. 1995, 80, 1908–1915. [Google Scholar] [CrossRef]
- Ye, Q.; Zeng, X.; Wang, S.; Zeng, X.; Yang, G.; Ye, C.; Cai, S.; Chen, M.; Li, S.; Qiao, S. Butyrate drives the acetylation of histone H3K9 to activate steroidogenesis through PPARγ and PGC1α pathways in ovarian granulosa cells. FASEB J. 2021, 35, e21316. [Google Scholar] [CrossRef]
- Heil, B.A.; Paccamonti, D.L.; Sones, J.L. Role for the mammalian female reproductive tract microbiome in pregnancy out-comes. Physiol. Genom. 2019, 51, 390–399. [Google Scholar] [CrossRef]
- Qi, X.; Yun, C.; Pang, Y.; Qiao, J. The impact of the gut microbiota on the reproductive and metabolic endocrine system. Gut Microbes 2021, 13, 1894070. [Google Scholar] [CrossRef]
- Brown, R.G.; Al-Memar, M.; Marchesi, J.R.; Lee, Y.S.; Smith, A.; Chan, D.; Lewis, H.; Kindinger, L.; Terzidou, V.; Bourne, T.; et al. Establishment of vaginal microbiota composition in early pregnancy and its association with subsequent preterm prelabor rupture of the fetal membranes. Transl. Res. 2019, 207, 30–43. [Google Scholar] [CrossRef]
- Al-Memar, M.; Bobdiwala, S.; Fourie, H.; Mannino, R.; Lee, Y.; Smith, A.; Marchesi, J.; Timmerman, D.; Bourne, T.; Bennett, P.; et al. The association between vaginal bacterial composition and miscarriage: A nested case–control study. BJOG Int. J. Obstet. Gynaecol. 2020, 127, 264–274. [Google Scholar] [CrossRef]
- Kindinger, L.M.; MacIntyre, D.A.; Lee, Y.S.; Marchesi, J.R.; Smith, A.; McDonald, J.A.K.; Terzidou, V.; Cook, J.R.; Lees, C.; Israfil-Bayli, F.; et al. Relationship between vaginal microbial dysbiosis, inflammation, and pregnancy outcomes in cervical cerclage. Sci. Transl. Med. 2016, 8, 350ra102. [Google Scholar] [CrossRef] [PubMed]
- Dou, X.; Gao, N.; Yan, D.; Shan, A. Sodium Butyrate Alleviates Mouse Colitis by Regulating Gut Microbiota Dysbiosis. Animals 2020, 10, 1154. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Xiao, Z.; An, W.; Dong, Y.; Zhang, B. Dietary sodium butyrate improves intestinal development and function by modulating the microbial community in broilers. PLoS ONE 2018, 13, e0197762. [Google Scholar] [CrossRef]
- Huang, C.; Song, P.; Fan, P.; Hou, C.; Thacker, P.; Ma, X. Dietary Sodium Butyrate Decreases Postweaning Diarrhea by Mod-ulating Intestinal Permeability and Changing the Bacterial Communities in Weaned Piglets. J. Nutr. 2015, 145, 2774–2780. [Google Scholar] [CrossRef]
- Zhang, T.; Ding, C.; Zhao, M.; Dai, X.; Yang, J.; Li, Y.; Gu, L.; Wei, Y.; Gong, J.; Zhu, W.; et al. Sodium Butyrate Reduces Colitogenic Immunoglobulin A-Coated Bacteria and Modifies the Composition of Microbiota in IL-10 Deficient Mice. Nutrients 2016, 8, 728. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Wang, Y.; Jiang, P.; Yao, H.; Zhao, C.; Hu, X.; Cao, Y.; Zhang, N.; Fu, Y.; Shen, H. Sodium butyrate alleviates lipopolysaccharide-induced endometritis in mice through inhibiting inflammatory response. Microb. Pathog. 2019, 137, 103792. [Google Scholar] [CrossRef]
- Upadhyaya, I.; Upadhyay, A.; Yin, H.-B.; Nair, M.S.; Bhattaram, V.K.; Karumathil, D.; Kollanoor-Johny, A.; Khan, M.I.; Darre, M.J.; Curtis, P.A.; et al. Reducing Colonization and Eggborne Transmission of Salmonella Enteritidis in Layer Chickens by In-Feed Supplementation of Caprylic Acid. Foodborne Pathog. Dis. 2015, 12, 591–597. [Google Scholar] [CrossRef]
- Santos, F.S.D.L.; Hume, M.; Venkitanarayanan, K.; Donoghue, A.M.; Hanning, I.; Slavik, M.F.; Aguiar, V.F.; Metcalf, J.H.; Reyes-Herrera, I.; Blore, P.J.; et al. Caprylic Acid Reduces Enteric Campylobacter Colonization in Market-Aged Broiler Chickens but Does Not Appear To Alter Cecal Microbial Populations. J. Food Prot. 2010, 73, 251–257. [Google Scholar] [CrossRef]
- Reich, W.J.; Nechtow, M.J.; Kurzon, A.M.; Subotnik, N.; Reich, J.B. The treatment of monilial vaginitis with caprylic acid. Am. J. Obstet. Gynecol. 1953, 65, 180–185. [Google Scholar] [CrossRef]
- Kabara, J.J.; Swieczkowski, D.M.; Conley, A.J.; Truant, J.P. Fatty Acids and Derivatives as Antimicrobial Agents. Antimicrob. Agents Chemother. 1972, 2, 23–28. [Google Scholar] [CrossRef]
- Rouse, M.S.; Rotger, M.; Piper, K.E.; Steckelberg, J.M.; Scholz, M.; Andrews, J.; Patel, R. In Vitro and In Vivo Evaluations of the Activities of Lauric Acid Monoester Formulations against Staphylococcus aureus. Antimicrob. Agents Chemother. 2005, 49, 3187–3191. [Google Scholar] [CrossRef] [PubMed]
- Lämmler, C.; Hartwigk, H. Actinomyces pyogenes und Arcanobacterium haemolyticum. Handb. Der Bakteriellen Infekt. Bei Tieren 1995, 2, 3. [Google Scholar]
- Ashrafi Tamai, I.; Mohammadzadeh, A.; Zahraei Salehi, T.; Mahmoodi, P. Genomic characterisation, detection of genes encoding virulence factors and evaluation of antibiotic resistance of Trueperella pyogenes isolated from cattle with clinical metritis. Antonie Van Leeuwenhoek 2018, 111, 2441–2453. [Google Scholar] [CrossRef]
- Alssahen, M.; Hassan, A.A.; Wickhorst, J.-P.; Sammra, O.; Lämmler, C.; Glaeser, S.P.; Kämpfer, P.; Timke, M.; Prenger-Berninghoff, E.; Abdulmawjood, A. Epidemiological analysis of Trueperella abortisuis isolated from cases of pig abortion of a single farm. Folia Microbiol. 2020, 65, 491–496. [Google Scholar] [CrossRef]
- Wang, J.; Li, C.; Nesengani, L.; Gong, Y.; Zhang, S.; Lu, W. Characterization of vaginal microbiota of endometritis and healthy sows using high-throughput pyrosequencing of 16S rRNA gene. Microb. Pathog. 2017, 111, 325–330. [Google Scholar] [CrossRef] [PubMed]


| Genes | Forward (5′–3′) | Reverse (5′–3′) | Product Size (bp) |
|---|---|---|---|
| Cyp11a1 | GAGCAGGGAGAGTAGCAGTG | ACCAGGAGAGGGGATCTCAC | 196 |
| Cyp19a1 | CCACATGGAAACCACCCATCT | TGCACAAACTCTTGGCCTCC | 180 |
| Star | CTGGAAGTCCCTCAAAGACCAA | GGGCTGAGCTTTAACACCTGG | 177 |
| Pparγ | TAGATGACAGCGACCTGGCGA | AGCAGCTTAGCAAAGAGCTGG | 172 |
| Fshr | CTCGGTTCCTTATGTGTAATC | TCAATGGCATAGTTGTGGTA | 115 |
| Lhr | ACCTGCCAACAAAAGAGCAG | GTCTCCTTGCTGTGCTTTCAC | 81 |
| Egf | GGATTTGCCCTGACCCTACT | TCTCTGTGCTGACATCGCTC | 199 |
| Hoxa10 | TTTCACTTGTCCCGCTCTCC | CGCCTTTGGAATTGCCTTGA | 178 |
| ανβ3 | CTGTTTGCCCATGTTTGGCT | TCCAGCCAATCTTCTCGTCA | 167 |
| Lif | CGCCCTCTTTATTCTCTACTACACA | TCACAGCACCAGGATTGAGG | 215 |
| β-actin | TGCGGGACATCAAGGAGAAG | AGTTGAAGGTGGTCTCGTGG | 217 |
| Items | Estrus Phase | Pregnancy Phase |
|---|---|---|
| Ingredients (%) | ||
| Corn | 61.94 | 61.71 |
| Soybean meal | 15.50 | 17.50 |
| Wheat bran | - | 8.10 |
| Puffed soybeans | 5.00 | - |
| Beet meal | 6.30 | 8.00 |
| Fish meal | 3.00 | - |
| Glucose | 3.00 | - |
| Soybean oil | 1.30 | 0.80 |
| Dicalcium phosphate | 1.53 | 1.31 |
| Limestone | 0.82 | 1.18 |
| Vitamin-mineral premix 1 | 0.50 | 0.50 |
| Salt | 0.40 | 0.35 |
| L-Lysine HCl | 0.29 | 0.27 |
| Choline Chloride (50%) | 0.15 | 0.15 |
| L-Threonine (98.5%) | 0.11 | 0.09 |
| L-Valine | 0.09 | 0.02 |
| DL-Methionine | 0.05 | 0.02 |
| L-Tryptophan | 0.02 | - |
| Dry matter (%) | 88.06 | 88.09 |
| Digestible energy(kcal/kg) | 3355.00 | 3084.00 |
| Metabolizable energy(kcal/kg) | 3149.00 | 2920.00 |
| Crude protein (%) | 16.51 | 12.50 |
| Ether extract (%) | 5.26 | 3.83 |
| Crude fiber (%) | 3.50 | 4.78 |
| Calcium (%) | 0.90 | 0.85 |
| Available phosphorus (%) | 0.47 | 0.38 |
| Digestible lysine (%) | 0.95 | 0.62 |
| Digestible methionine (%) | 0.30 | 0.20 |
| Digestible arginine (%) | 0.92 | 0.63 |
| Items | Control | Treatment | p Value |
|---|---|---|---|
| Number of sows | 26 | 26 | - |
| Parity | 3.19 ± 0.19 | 2.85 ± 0.13 | 0.14 |
| Weight after weaning (kg) | 238.65 ± 4.66 | 240.92 ± 4.15 | 0.72 |
| Weight at breeding (kg) | 233.31 ± 4.78 | 236.46 ± 4.03 | 0.62 |
| Weight at day 28 of gestation (kg) | 233.46 ± 4.19 | 235.96 ± 3.35 | 0.65 |
| Backfat thickness at breeding (mm) | 14.77 ± 0.46 | 15.08 ± 0.52 | 0.67 |
| Backfat thickness at day 28 of gestation (mm) | 15.19 ± 0.55 | 16.12 ± 0.58 | 0.27 |
| Items | Control | Treatment | p Value |
|---|---|---|---|
| Number of sows, n | 26 | 26 | -- |
| Total piglets born per litter, n/litter | 14.6 ± 0.45 | 15.6 ± 0.51 | 0.08 |
| Total piglets born alive per litter, n/litter | 13.2 ± 0.35 | 14.6 ± 0.45 | 0.009 |
| Litter birth weight of all piglets born alive (kg) | 18.1 ± 0.64 | 19.3 ± 0.63 | 0.09 |
| Weight of piglets alive (kg) | 1.38 ± 0.04 | 1.34 ± 0.04 | 0.25 |
| Male alive piglets, n | 7.08 ± 0.29 | 7.62 ± 0.29 | 0.20 |
| Female alive piglets, n | 6.19 ± 0.28 | 6.73 ± 0.32 | 0.22 |
| Weak, n | 1.69 ± 0.26 | 1.77 ± 0.30 | 0.43 |
| Stillborn, n | 1.27 ± 0.28 | 0.96 ± 0.17 | 0.18 |
| Deformed, n | 0.12 ± 0.06 | 0.12 ± 0.06 | 0.5 |
| Mummfied, n | 0.15 ± 0.12 | 0.04 ± 0.04 | 0.18 |
| Items | Control | Treatment | p Value |
|---|---|---|---|
| Estradiol (pg/mL) | 42.60 ± 6.68 | 84.20 ± 14.00 | 0.04 |
| Progesterone (pg/mL) | 748.00 ± 76.60 | 654.00 ± 98.50 | 0.52 |
| Total cholesterol (mmol/L) | 1.47 ± 0.06 | 1.32 ± 0.08 | 0.21 |
| HDL (mmol/L) | 0.64 ± 0.02 | 0.56 ± 0.04 | 0.17 |
| LDL (mmol/L) | 0.74 ± 0.04 | 0.67 ± 0.05 | 0.33 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, X.; Li, S.; Ye, Q.; Cai, S.; Quan, S.; Liu, L.; Zhang, S.; Chen, F.; Cai, C.; Wang, F.; et al. The Combined Use of Medium- and Short-Chain Fatty Acids Improves the Pregnancy Outcomes of Sows by Enhancing Ovarian Steroidogenesis and Endometrial Receptivity. Nutrients 2022, 14, 4405. https://doi.org/10.3390/nu14204405
Zeng X, Li S, Ye Q, Cai S, Quan S, Liu L, Zhang S, Chen F, Cai C, Wang F, et al. The Combined Use of Medium- and Short-Chain Fatty Acids Improves the Pregnancy Outcomes of Sows by Enhancing Ovarian Steroidogenesis and Endometrial Receptivity. Nutrients. 2022; 14(20):4405. https://doi.org/10.3390/nu14204405
Chicago/Turabian StyleZeng, Xiangzhou, Siyu Li, Qianhong Ye, Shuang Cai, Shuang Quan, Lu Liu, Shihai Zhang, Fang Chen, Chuanjiang Cai, Fenglai Wang, and et al. 2022. "The Combined Use of Medium- and Short-Chain Fatty Acids Improves the Pregnancy Outcomes of Sows by Enhancing Ovarian Steroidogenesis and Endometrial Receptivity" Nutrients 14, no. 20: 4405. https://doi.org/10.3390/nu14204405
APA StyleZeng, X., Li, S., Ye, Q., Cai, S., Quan, S., Liu, L., Zhang, S., Chen, F., Cai, C., Wang, F., Qiao, S., & Zeng, X. (2022). The Combined Use of Medium- and Short-Chain Fatty Acids Improves the Pregnancy Outcomes of Sows by Enhancing Ovarian Steroidogenesis and Endometrial Receptivity. Nutrients, 14(20), 4405. https://doi.org/10.3390/nu14204405








