Time of Dietary Energy and Nutrient Intake and Body Mass Index in Children: Compositional Data Analysis from the Childhood Obesity Project (CHOP) Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Dietary Assessment
2.3. Anthropometric Measurement (Outcome)
2.4. Statistical Analysis
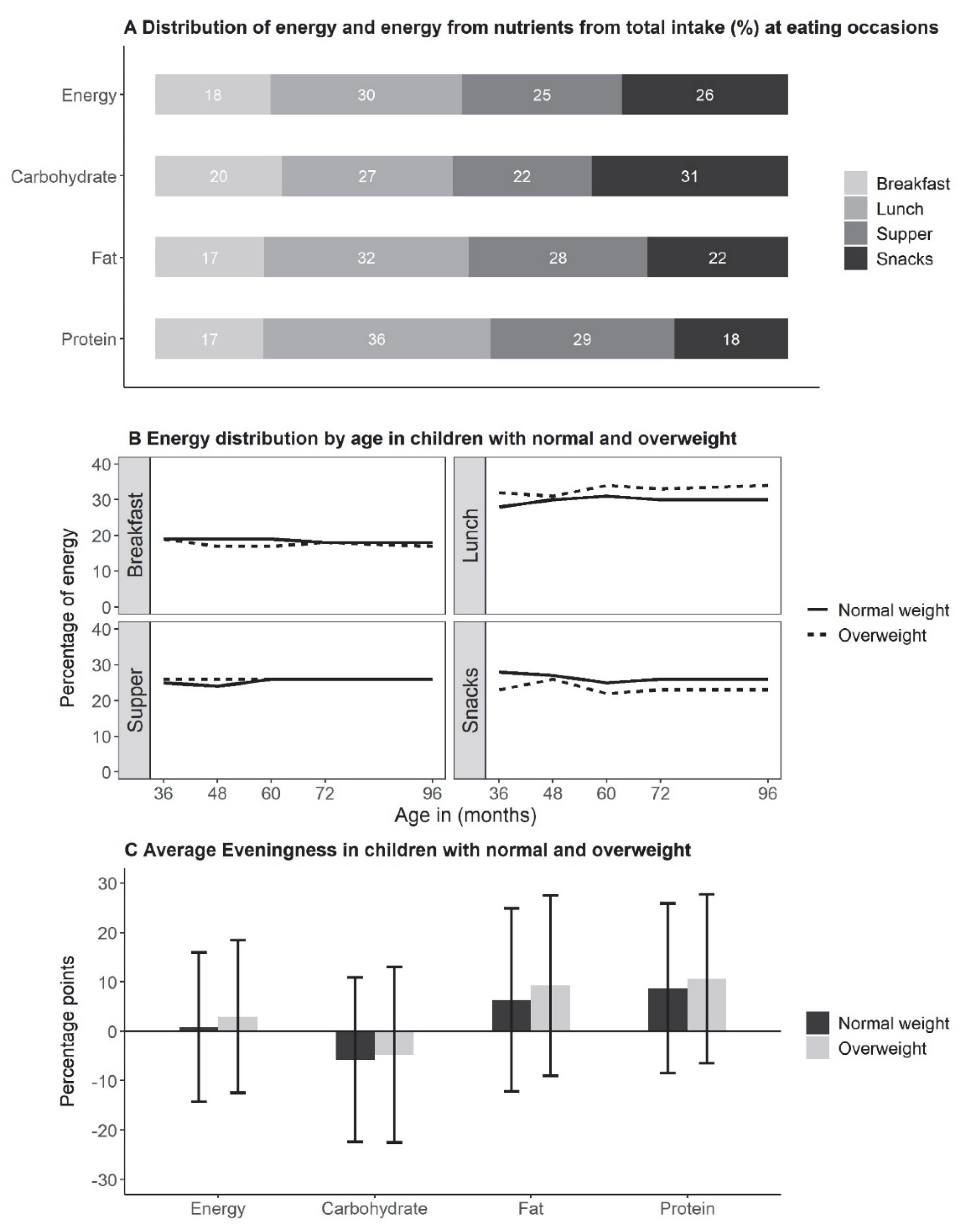
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
Appendix A
Compositional Data Analysis and Calculation of ILR-Coordinates
| Partition | Breakfast | Lunch | Supper | Snacks | Ratio |
|---|---|---|---|---|---|
| 1 | 1 | −1 | −1 | −1 | Breakfast: mean (lunch, supper, snacks) |
| 2 | 0 | 1 | −1 | −1 | Lunch: mean (supper, snacks) |
| 3 | 0 | 0 | 1 | −1 | Supper: Snacks |
- r and s, respectively, are the number of parts in the first (coded as 1) and second group (coded as −1) at each order of the partition;
- is the proportional intake (coded as 1);
- is the geometric mean of the components of y, for j = i + 1,…, D (geometric mean of the components coded as −1).
References
- Lobstein, T.; Baur, L.; Uauy, R. Obesity in children and young people: A crisis in public health. Obes. Rev. 2004, 5 (Suppl. 1), 4–104. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Obesity: Preventing and Managing the Global Epidemic: Report of a WHO Consultation; WHO Technical Report Series; World Health Organization: Geneva, Switzerland, 2000; Volume 894. [Google Scholar]
- Emmett, P.M.; Jones, L.R.; Northstone, K. Dietary patterns in the Avon Longitudinal Study of Parents and Children. Nutr. Rev. 2015, 73 (Suppl. 3), 207–230. [Google Scholar] [CrossRef] [PubMed]
- Luque, V.; Escribano, J.; Closa-Monasterolo, R.; Zaragoza-Jordana, M.; Ferré, N.; Grote, V.; Koletzko, B.; Totzauer, M.; Verduci, E.; ReDionigi, A.; et al. Unhealthy Dietary Patterns Established in Infancy Track to Mid-Childhood: The EU Childhood Obesity Project. J. Nutr. 2018, 148, 752–759. [Google Scholar] [CrossRef]
- Flanagan, A.; Bechtold, D.A.; Pot, G.K.; Johnston, J.D. Chrono-nutrition: From molecular and neuronal mechanisms to human epidemiology and timed feeding patterns. J. Neurochem. 2021, 157, 53–72. [Google Scholar] [CrossRef] [PubMed]
- Covassin, N.; Singh, P.; Somers, V.K. Keeping Up With the Clock: Circadian Disruption and Obesity Risk. Hypertension 2016, 68, 1081–1090. [Google Scholar] [CrossRef] [PubMed]
- Almoosawi, S.; Vingeliene, S.; Karagounis, L.G.; Pot, G.K. Chrono-nutrition: A review of current evidence from observational studies on global trends in time-of-day of energy intake and its association with obesity. Proc. Nutr. Soc. 2016, 75, 487–500. [Google Scholar] [CrossRef] [PubMed]
- Almoosawi, S.; Vingeliene, S.; Gachon, F.; Voortman, T.; Palla, L.; Johnston, J.; Van Dam, R.M.; Darimont, C.; Karagounis, L.G. Chronotype: Implications for Epidemiologic Studies on Chrono-Nutrition and Cardiometabolic Health. Adv. Nutr. Int. Rev. J. 2019, 10, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Maffeis, C.; Provera, S.; Filippi, L.; Sidoti, G.; Schena, S.; Pinelli, L.; Tatò, L. Distribution of food intake as a risk factor for childhood obesity. Int. J. Obes. 2000, 24, 75–80. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vilela, S.; Oliveira, A.; Severo, M.; Lopes, C. Chrono-Nutrition: The Relationship between Time-of-Day Energy and Macronutrient Intake and Children’s Body Weight Status. J. Biol. Rhythm. 2019, 34, 332–342. [Google Scholar] [CrossRef] [PubMed]
- Thompson, O.M.; Ballew, C.; Resnicow, K.; Gillespie, C.; Must, A.; Bandini, L.G.; Cyr, H.; Dietz, W.H. Dietary pattern as a predictor of change in BMI z-score among girls. Int. J. Obes. 2006, 30, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Lioret, S.; Touvier, M.; Lafay, L.; Volatier, J.-L.; Maire, B. Are Eating Occasions and Their Energy Content Related to Child Overweight and Socioeconomic Status? Obesity 2008, 16, 2518–2523. [Google Scholar] [CrossRef] [PubMed]
- Kupek, E.; Lobo, A.S.; Leal, D.B.; Bellisle, F.; de Assis, M.A.A. Dietary patterns associated with overweight and obesity among Brazilian schoolchildren: An approach based on the time-of-day of eating events. Br. J. Nutr. 2016, 116, 1954–1965. [Google Scholar] [CrossRef] [PubMed]
- Diederichs, T.; Roßbach, S.; Herder, C.; Alexy, U.; Buyken, A.E. Relevance of Morning and Evening Energy and Macronutrient Intake during Childhood for Body Composition in Early Adolescence. Nutrients 2016, 8, 716. [Google Scholar] [CrossRef] [PubMed]
- Leite, M.L.C. Applying compositional data methodology to nutritional epidemiology. Stat. Methods Med Res. 2016, 25, 3057–3065. [Google Scholar] [CrossRef] [PubMed]
- Koletzko, B.; von Kries, R.; Closa, R.; Escribano, J.; Scaglioni, S.; Giovannini, M.; Beyer, J.; Demmelmair, H.; Gruszfeld, D.; Dobrzanska, A.; et al. Lower protein in infant formula is associated with lower weight up to age 2 y: A randomized clinical trial. Am. J. Clin. Nutr. 2009, 89, 1836–1845. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.; Luque, V.; Escribano, J.; Closa-Monasterolo, R.; Verduci, E.; ReDionigi, A.; Hoyos, J.; Langhendries, J.-P.; Gruszfeld, D.; Socha, P.; et al. Effect of Lower Versus Higher Protein Content in Infant Formula Through the First Year on Body Composition from 1 to 6 Years: Follow-Up of a Randomized Clinical Trial. Obesity 2018, 26, 1203–1210. [Google Scholar] [CrossRef]
- Gomes, D.; Luque, V.; Xhonneux, A.; Verduci, E.; Socha, P.; Koletzko, B.; Berger, U.; Grote, V. A simple method for identification of misreporting of energy intake from infancy to school age: Results from a longitudinal study. Clin. Nutr. 2018, 37, 1053–1060. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority. Guidance on the EU Menu methodology. EFSA J. 2014, 12, 3944. [Google Scholar] [CrossRef]
- De Onis, M.; Garza, C.; Victora, C.; Onyango, A.W.; Frongillo, E.A.; Martines, J. The who Multicentre Growth Reference Study: Planning, Study Design, and Methodology. Food Nutr. Bull. 2004, 25 (Suppl. 1), S15–S26. [Google Scholar] [CrossRef]
- De Onis, M.; Onyango, A.W.; Borghi, E.; Siyam, A.; Nishida, C.; Siekmann, J. Development of a WHO growth reference for school-aged children and adolescents. Bull. World Health Organ. 2007, 85, 660–667. [Google Scholar] [CrossRef] [PubMed]
- Cole, T.J.; Bellizzi, M.C.; Flegal, K.M.; Dietz, W.H. Establishing a standard definition for child overweight and obesity worldwide: International survey. BMJ 2000, 320, 1240–1243. [Google Scholar] [CrossRef] [PubMed]
- Leite, M.L.C. Compositional data analysis as an alternative paradigm for nutritional studies. Clin. Nutr. ESPEN 2019, 33, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Dumuid, D.; Stanford, T.E.; Martín-Fernández, J.A.; Pedišić, Ž.; Maher, C.; Lewis, L.K.; Hron, K.; Katzmarzyk, P.; Chaput, J.-P.; Fogelholm, M.; et al. Compositional data analysis for physical activity, sedentary time and sleep research. Stat. Methods Med. Res. 2017, 27, 3726–3738. [Google Scholar] [CrossRef] [PubMed]
- Chastin, S.F.; Palarea-Albaladejo, J.; Dontje, M.L.; Skelton, D.A. Combined Effects of Time Spent in Physical Activity, Sedentary Behaviors and Sleep on Obesity and Cardio-Metabolic Health Markers: A Novel Compositional Data Analysis Approach. PLoS ONE 2015, 10, e0139984. [Google Scholar] [CrossRef]
- Rasmussen, C.L.; Palarea-Albaladejo, J.; Johansson, M.S.; Crowley, P.; Stevens, M.L.; Gupta, N.; Karstad, K.; Holtermann, A. Zero problems with compositional data of physical behaviors: A comparison of three zero replacement methods. Int. J. Behav. Nutr. Phys. Act. 2020, 17, 126. [Google Scholar] [CrossRef]
- Jessri, M.; Lou, W.Y.; L’Abbé, M.R. Evaluation of different methods to handle misreporting in obesity research: Evidence from the Canadian national nutrition survey. Br. J. Nutr. 2016, 115, 147–159. [Google Scholar] [CrossRef]
- Diederichs, T.; Perrar, I.; Roßbach, S.; Alexy, U.; Buyken, A.E. In adolescence a higher ‘eveningness in energy intake’ is associated with higher total daily energy intake. Appetite 2018, 128, 159–166. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 20 February 2021).
- Blaine, R.E.; Kachurak, A.; Davison, K.K.; Klabunde, R.; Fisher, J.O. Food parenting and child snacking: A systematic review. Int. J. Behav. Nutr. Phys. Act. 2017, 14, 146. [Google Scholar] [CrossRef]
- Njike, V.Y.; Smith, T.M.; Shuval, O.; Shuval, K.; Edshteyn, I.; Kalantari, V.; Yaroch, A.L. Snack food, satiety, and weight. Adv. Nutr. 2016, 7, 866–878. [Google Scholar] [CrossRef]
- Marangoni, F.; Martini, D.; Scaglioni, S.; Sculati, M.; Donini, L.M.; Leonardi, F.; Agostoni, C.; Castelnuovo, G.; Ferrara, N.; Ghiselli, A.; et al. Snacking in nutrition and health. Int. J. Food Sci. Nutr. 2019, 70, 909–923. [Google Scholar] [CrossRef]
- Palla, L.; Almoosawi, S. Diurnal Patterns of Energy Intake Derived via Principal Component Analysis and Their Relationship with Adiposity Measures in Adolescents: Results from the National Diet and Nutrition Survey RP (2008–2012). Nutrients 2019, 11, 422. [Google Scholar] [CrossRef] [PubMed]
- Coulthard, J.D.; Pot, G.K. The timing of the evening meal: How is this associated with weight status in UK children? Br. J. Nutr. 2016, 115, 1616–1622. [Google Scholar] [CrossRef] [PubMed]
- Chiva, M. Cultural aspects of meals and meal frequency. Br. J. Nutr. 1997, 77 (Suppl. 1), S21–S28. [Google Scholar] [CrossRef]
- Jaeger, V.; Koletzko, B.; Luque, V.; Ferré, N.; Gruszfeld, D.; Gradowska, K.; Verduci, E.; Zuccotti, G.V.; Xhonneux, A.; Poncelet, P.; et al. Distribution of energy and macronutrient intakes across eating occasions in European children from 3 to 8 years of age: The EU Childhood Obesity Project Study. Eur. J. Nutr. 2022. [Google Scholar] [CrossRef]
- SSzajewska, H.; Ruszczyński, M. Systematic Review Demonstrating that Breakfast Consumption Influences Body Weight Outcomes in Children and Adolescents in Europe. Crit. Rev. Food Sci. Nutr. 2010, 50, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Coulthard, J.D.; Palla, L.; Pot, G.K. Breakfast consumption and nutrient intakes in 4–18-year-olds: UK National Diet and Nutrition Survey Rolling Programme (2008–2012). Br. J. Nutr. 2017, 118, 280–290. [Google Scholar] [CrossRef] [PubMed]
- Fayet-Moore, F.; McConnell, A.; Tuck, K.; Petocz, P. Breakfast and Breakfast Cereal Choice and Its Impact on Nutrient and Sugar Intakes and Anthropometric Measures among a Nationally Representative Sample of Australian Children and Adolescents. Nutrients 2017, 9, 1045. [Google Scholar] [CrossRef] [PubMed]
- Roßbach, S.; Diederichs, T.; Nöthlings, U.; Buyken, A.E.; Alexy, U. Relevance of chronotype for eating patterns in adolescents. Chronobiol. Int. 2018, 35, 336–347. [Google Scholar] [CrossRef]
- Roenneberg, T.; Kuehnle, T.; Juda, M.; Kantermann, T.; Allebrandt, K.; Gordijn, M.; Merrow, M. Epidemiology of the human circadian clock. Sleep Med. Rev. 2007, 11, 429–438. [Google Scholar] [CrossRef]
- Patterson, F.; Malone, S.K.; Lozano, A.; Grandner, M.; Hanlon, A.L. Smoking, Screen-Based Sedentary Behavior, and Diet Associated with Habitual Sleep Duration and Chronotype: Data from the UK Biobank. Ann. Behav. Med. 2016, 50, 715–726. [Google Scholar] [CrossRef]
- Poorolajal, J.; Sahraei, F.; Mohamdadi, Y.; Doosti-Irani, A.; Moradi, L. Behavioral factors influencing childhood obesity: A systematic review and meta-analysis. Obes. Res. Clin. Pract. 2020, 14, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Madjd, A.; Taylor, M.A.; Delavari, A.; Malekzadeh, R.; Macdonald, I.A.; Farshchi, H.R. Effects of consuming later evening meal v. earlier evening meal on weight loss during a weight loss diet: A randomised clinical trial. Br. J. Nutr. 2020, 126, 632–640. [Google Scholar] [CrossRef] [PubMed]
- Garaulet, M.; Gómez-Abellán, P.; Alburquerque-Béjar, J.J.; Lee, Y.C.; Ordovás, J.M.; Scheer, F.A. Timing of food intake predicts weight loss effectiveness. Int. J. Obes. 2013, 37, 604–611. [Google Scholar] [CrossRef] [PubMed]
- Morgan, L.M.; Shi, J.-W.; Hampton, S.M.; Frost, G. Effect of meal timing and glycaemic index on glucose control and insulin secretion in healthy volunteers. Br. J. Nutr. 2012, 108, 1286–1291. [Google Scholar] [CrossRef] [PubMed]
- Van Cauter, E.; Shapiro, E.T.; Tillil, H.; Polonsky, K.S. Circadian modulation of glucose and insulin responses to meals: Relationship to cortisol rhythm. Am. J. Physiol. Metab. 1992, 262 Pt 1, E467–E475. [Google Scholar] [CrossRef] [PubMed]
- Romon, M.; Edme, J.L.; Boulenguez, C.; Lescroart, J.L.; Frimat, P. Circadian variation of diet-induced thermogenesis. Am. J. Clin. Nutr. 1993, 57, 476–480. [Google Scholar] [CrossRef]
- Morris, C.J.; Garcia, J.I.; Myers, S.; Yang, J.N.; Trienekens, N.; Scheer, F.A. The Human Circadian System Has a Dominating Role in Causing the Morning/Evening Difference in Diet-Induced Thermogenesis. Obesity 2015, 23, 2053–2058. [Google Scholar] [CrossRef]
- Hebert, J.R.; Clemow, L.; Pbert, L.; Ockene, I.S.; Ockene, J.K. Social desirability bias in dietary self-report may compromise the validity of dietary intake measures. Int. J. Epidemiol. 1995, 24, 389–398. [Google Scholar] [CrossRef]
- Xiao, Q.; Garaulet, M.; Scheer, F.A. Meal timing and obesity: Interactions with macronutrient intake and chronotype. Int. J. Obes. 2019, 43, 1701–1711. [Google Scholar] [CrossRef]
- Pawlowsky-Glahn, V.; Egozcue, J.J.; Tolosana-Delgado, R. Modeling and Analysis of Compositional Data; John Wiley & Sons: Chichester, UK, 2015. [Google Scholar]
- Dumuid, D.; Pedišić, Z.; Palarea-Albaladejo, J.; Martín-Fernández, J.A.; Hron, K.; Olds, T. Compositional Data Analysis in Time-Use Epidemiology: What, Why, How. Int. J. Environ. Res. Public Health 2020, 17, 2220. [Google Scholar] [CrossRef]
- Willett, W.C.; Howe, G.R.; Kushi, L.H. Adjustment for total energy intake in epidemiologic studies. Am. J. Clin. Nutr. 1997, 65 (Suppl. S4), 1220S–1228S, discussion 1229S–1231S. [Google Scholar] [CrossRef] [PubMed]
- Egozcue, J.J.; Pawlowsky-Glahn, V. Groups of Parts and Their Balances in Compositional Data Analysis. Math. Geol. 2005, 37, 795–828. [Google Scholar] [CrossRef]
- Hron, K.; Filzmoser, P.; Thompson, K. Linear regression with compositional explanatory variables. J. Appl. Stat. 2012, 39, 1115–1128. [Google Scholar] [CrossRef]

| Partition | Breakfast | Lunch | Supper | Snacks |
|---|---|---|---|---|
| 1 | Breakfast: mean (lunch, supper, snacks) | Lunch: mean (supper, snacks, breakfast) | Supper: mean (snacks, breakfast, lunch) | Snacks: mean (breakfast, lunch, supper) |
| 2 | Lunch: mean (supper, snacks) | Supper: mean (snacks, breakfast) | Snacks: mean (breakfast, lunch) | Breakfast: mean (lunch, supper) |
| 3 | Supper: Snacks | Snacks: Breakfast | Breakfast: Lunch | Lunch: Supper |
| Age in Years | 3 (n = 541) | 4 (n = 516) | 5 (n = 476) | 6 (n = 515) | 8 (n = 439) | Overall (N = 729) * |
|---|---|---|---|---|---|---|
| BMI (kg/m2) | 16.0 ± 1.3 | 15.9 ± 1.4 | 15.9 ± 1.7 | 16.0 ± 2.0 | 16.9 ± 2.7 | 16.1 ± 1.9 |
| zBMI | 0.3 ± 1.0 | 0.4 ± 1.0 | 0.4 ± 1.0 | 0.4 ± 1.2 | 0.4 ± 1.2 | 0.4 ± 1.1 |
| Overweight **, N *** (%) | 52 (9.6) | 62 (12) | 68 (14.3) | 85 (16.5) | 99 (22.6) | 366 (14.7) |
| Energy (kcal/day) | 1202 ± 237 | 1307 ± 235 | 1374 ± 249 | 1454 ± 250 | 1568 ± 285 | 1374 ± 279 |
| kcal/kg/day | 82.5 ± 17.7 | 78.2 ± 16.0 | 72.0 ± 15.7 | 67.2 ± 14.0 | 56.6 ± 13.5 | 71.9 ± 17.9 |
| Carbohydrate (%E) | 50.4 ± 7.7 | 50.0 ± 7.0 | 50.2 ± 7.2 | 50.3 ± 6.9 | 49.0 ± 7.0 | 50.0 ± 7.2 |
| g/day | 149.2 ± 44.3 | 160.1 ± 35.8 | 171.2 ± 43.5 | 183.0 ± 43.3 | 192.2 ± 49.0 | 170.3 ± 45.8 |
| g/kg/day | 10.3 ± 3.4 | 9.6 ± 2.4 | 9.0 ± 2.6 | 8.5 ± 2.3 | 6.9 ± 2.1 | 8.9 ± 2.8 |
| Protein (%E) | 15.3 ± 3.0 | 15.0 ± 2.9 | 14.9 ± 2.9 | 14.9 ± 2.6 | 15.2 ± 2.8 | 15.0 ± 2.8 |
| g/day | 44.9 ± 11.6 | 48.0 ± 12.3 | 50.5 ± 12.8 | 54.0 ± 12.5 | 59.5 ± 14.6 | 51.1 ± 13.6 |
| g/kg/day | 3.1 ± 0.8 | 2.9 ± 0.8 | 2.6 ± 0.7 | 2.5 ± 0.6 | 2.1 ± 0.6 | 2.7 ± 0.8 |
| Total fat (%E) | 34.3 ± 6.2 | 34.9 ± 5.8 | 34.9 ± 5.7 | 34.8 ± 5.6 | 35.8 ± 5.8 | 34.9 ± 5.8 |
| g/day | 45.0 ± 12.4 | 50.1 ± 13.1 | 52.8 ± 13.4 | 56.3 ± 13.6 | 62.7 ± 16.6 | 53.0 ± 15.0 |
| g/kg/day | 3.1 ± 0.9 | 3.0 ± 0.8 | 2.8 ± 0.8 | 2.6 ± 0.7 | 2.3 ± 0.7 | 2.8 ± 0.8 |
| ILR * | Energy | Carbohydrate | Protein | Fat | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | SE | p-Value | β | SE | p-Value | β | SE | p-Value | β | SE | p-Value | |
| Breakfast | −0.02 | 0.02 | 0.429 | −0.01 | 0.02 | 0.493 | 0.00 | 0.02 | 0.957 | −0.02 | 0.02 | 0.192 |
| Lunch | 0.00 | 0.03 | 0.945 | 0.01 | 0.02 | 0.747 | 0.00 | 0.02 | 0.942 | 0.00 | 0.02 | 0.825 |
| Supper | 0.01 | 0.03 | 0.616 | −0.00 | 0.02 | 0.864 | −0.01 | 0.02 | 0.577 | 0.03 | 0.02 | 0.123 |
| Snacks | 0.01 | 0.02 | 0.677 | 0.01 | 0.02 | 0.580 | 0.01 | 0.02 | 0.472 | −0.01 | 0.01 | 0.380 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaeger, V.; Koletzko, B.; Luque, V.; Gispert-Llauradó, M.; Gruszfeld, D.; Socha, P.; Verduci, E.; Zuccotti, G.V.; Etienne, L.; Grote, V. Time of Dietary Energy and Nutrient Intake and Body Mass Index in Children: Compositional Data Analysis from the Childhood Obesity Project (CHOP) Trial. Nutrients 2022, 14, 4356. https://doi.org/10.3390/nu14204356
Jaeger V, Koletzko B, Luque V, Gispert-Llauradó M, Gruszfeld D, Socha P, Verduci E, Zuccotti GV, Etienne L, Grote V. Time of Dietary Energy and Nutrient Intake and Body Mass Index in Children: Compositional Data Analysis from the Childhood Obesity Project (CHOP) Trial. Nutrients. 2022; 14(20):4356. https://doi.org/10.3390/nu14204356
Chicago/Turabian StyleJaeger, Vanessa, Berthold Koletzko, Veronica Luque, Mariona Gispert-Llauradó, Dariusz Gruszfeld, Piotr Socha, Elvira Verduci, Gian Vincenzo Zuccotti, Louise Etienne, and Veit Grote. 2022. "Time of Dietary Energy and Nutrient Intake and Body Mass Index in Children: Compositional Data Analysis from the Childhood Obesity Project (CHOP) Trial" Nutrients 14, no. 20: 4356. https://doi.org/10.3390/nu14204356
APA StyleJaeger, V., Koletzko, B., Luque, V., Gispert-Llauradó, M., Gruszfeld, D., Socha, P., Verduci, E., Zuccotti, G. V., Etienne, L., & Grote, V. (2022). Time of Dietary Energy and Nutrient Intake and Body Mass Index in Children: Compositional Data Analysis from the Childhood Obesity Project (CHOP) Trial. Nutrients, 14(20), 4356. https://doi.org/10.3390/nu14204356









