Vitamin D and the Central Nervous System: Causative and Preventative Mechanisms in Brain Disorders
Abstract
1. Introduction
2. Vitamin D Signalling in the Brain, the Basics and Controversies
3. Vitamin D and Normal Brain Development
3.1. Vitamin D and Neurite Growth
3.2. Vitamin D and Neurotrophic Factors
3.3. Vitamin D Regulates the Development of Dopamine Neurons
4. Developmental Vitamin D Deficiency Effect on Brain Function and Behaviour
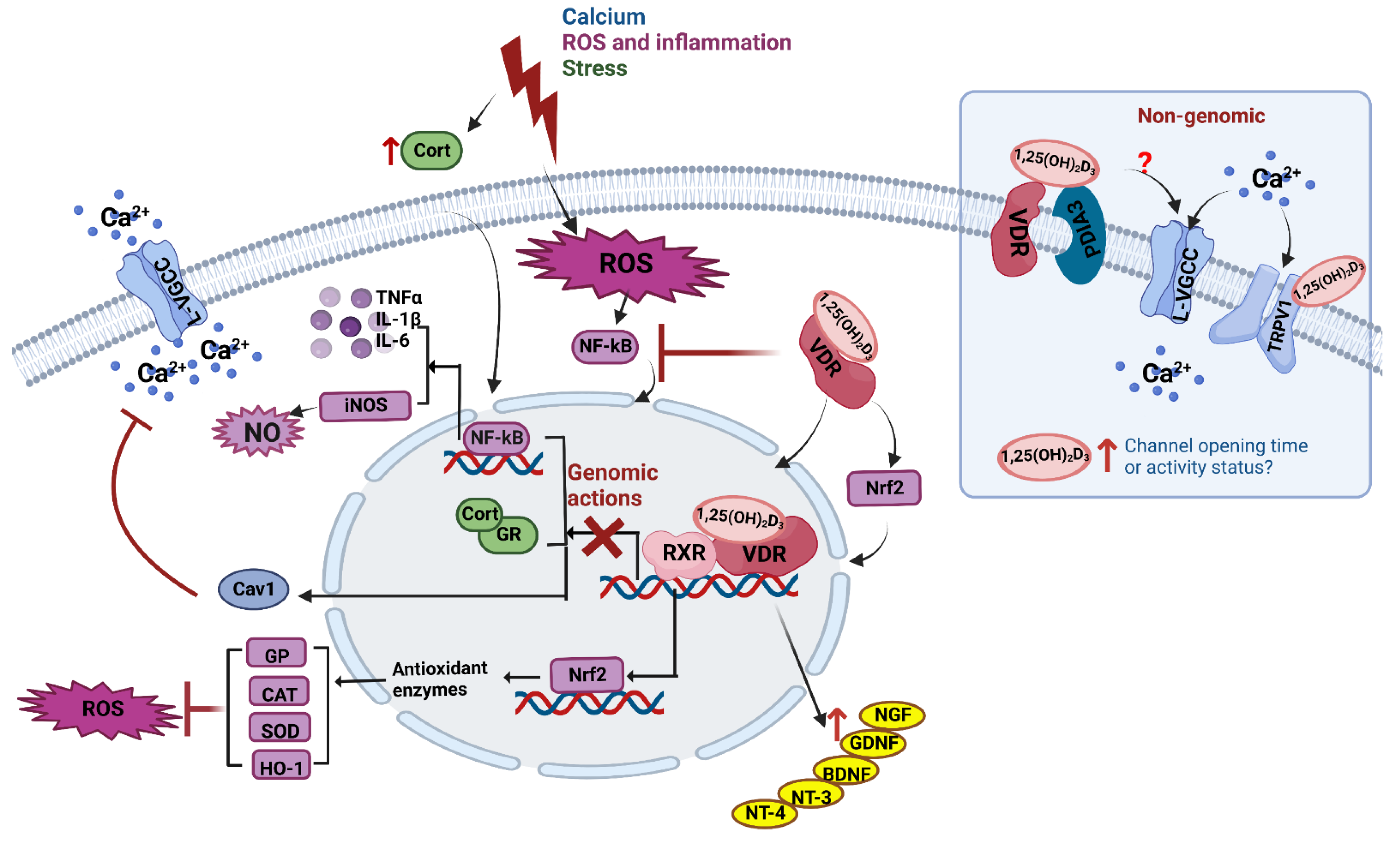
5. Vitamin D Is Neuroprotective in Neurons and Adult Brain
5.1. Calcium Regulation
5.2. ROS and Inflammation
5.3. Glucocorticoids and Stress
6. Hypervitaminosis D and Adverse CNS Outcomes
7. Conclusions and Future Challenges
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Evans, R.M. The nuclear receptor superfamily: A rosetta stone for physiology. Mol. Endocrinol. 2005, 19, 1429–1438. [Google Scholar] [CrossRef] [PubMed]
- McGrath, J.; Feron, F.; Eyles, D. Vitamin D: The neglected neurosteroid? Trends Neurosci. 2001, 24, 570–572. [Google Scholar] [CrossRef]
- Cui, X.; Gooch, H.; Petty, A.; McGrath, J.J.; Eyles, D. Vitamin D and the brain: Genomic and non-genomic actions. Mol. Cell. Endocrinol. 2017, 453, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Pertile, R.; Kiltschewskij, D.G.; Geaghan, M.; Barnett, M.; Cui, X.; Cairns, M.J.; Eyles, D.W. Developmental vitamin D-deficiency increases the expression of microRNAs involved in dopamine neuron development. Brain Res. 2022, 1789, 147953. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Vasileva, S.; Langguth, M.; Alexander, S.; Cui, X.; Whitehouse, A.; McGrath, J.J.; Eyles, D. Deveopmental vitamin D deficiency produces behavioral phenotypes of relevance to autism in an animal model. Nutrients 2019, 11, 1187. [Google Scholar] [CrossRef] [PubMed]
- Melcangi, R.C.; Panzica, G. Neuroactive steroids: An update of their roles in central and peripheral nervous system. Psychoneuroendocrinology 2009, 34, S1–S8. [Google Scholar] [CrossRef]
- Eyles, D.W.; Burne, T.H.; McGrath, J.J. Vitamin D, effects on brain development, adult brain function and the links between low levels of vitamin D and neuropsychiatric disease. Front. Neuroendocrinol. 2013, 34, 47–64. [Google Scholar] [CrossRef]
- McCann, J.C.; Ames, B.N. Is there convincing biological or behavioral evidence linking vitamin D deficiency to brain dysfunction? FASEB J. 2008, 22, 982–1001. [Google Scholar] [CrossRef]
- McGrath, J.J.; Eyles, D.W.; Pedersen, C.B.; Anderson, C.; Ko, P.; Burne, T.H.; Norgaard-Pedersen, B.; Hougaard, D.M.; Mortensen, P.B. Neonatal vitamin D status and risk of schizophrenia: A population-based case-control study. Arch. Gen. Psychiatry 2010, 67, 889–894. [Google Scholar] [CrossRef]
- Eyles, D.W.; Trzaskowski, M.; Vinkhuyzen, A.A.E.; Mattheisen, M.; Meier, S.; Gooch, H.; Anggono, V.; Cui, X.; Tan, M.C.; Burne, T.H.J.; et al. The association between neonatal vitamin D status and risk of schizophrenia. Sci. Rep. 2018, 8, 17692. [Google Scholar] [CrossRef]
- Vinkhuyzen, A.A.E.; Eyles, D.W.; Burne, T.H.J.; Blanken, L.M.E.; Kruithof, C.J.; Verhulst, F.; Jaddoe, V.W.; Tiemeier, H.; McGrath, J.J. Gestational vitamin D deficiency and autism-related traits: The Generation R study. Mol. Psychiatry 2016, 23, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Vinkhuyzen, A.; Eyles, D.; Burne, T.; Blanken, L.; Kruithof, C.; Verhulst, F.; White, T.; Jaddoe, V.W.; Tiemeier, H.; McGrath, J. Gestational Vitamin D Deficiency and Autism Spectrum Disorder. Br. J. Psychiatry Open 2017, 3, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.K.; Eyles, D.W.; Magnusson, C.; Newschaffer, C.J.; McGrath, J.J.; Kvaskoff, D.; Ko, P.; Dalman, C.; Karlsson, H.; Gardner, R.M. Developmental vitamin D and autism spectrum disorders: Findings from the Stockholm Youth Cohort. Mol. Psychiatry 2019, 26, 1578–1588. [Google Scholar] [CrossRef] [PubMed]
- Sourander, A.; Upadhyaya, S.; Surcel, H.M.; Hinkka-Yli-Salomaki, S.; Cheslack-Postava, K.; Silwal, S.; Sucksdorff, M.; McKeague, I.W.; Brown, A.S. Maternal Vitamin D Levels During Pregnancy and Offspring Autism Spectrum Disorder. Biol. Psychiatry 2021, 90, 790–797. [Google Scholar] [CrossRef] [PubMed]
- Strøm, M.; Halldorsson, T.; Hansen, S.; Granström, C.; Maslova, E.; Petersen, S.B.; Cohen, A.S.; Olsen, S.F. Vitamin D measured in maternal serum and offspring neurodevelopmental outcomes: A prospective study with long-term follow-up. Ann. Nutr. Metab. 2014, 64, 254–261. [Google Scholar] [CrossRef]
- Morales, E.; Julvez, J.; Torrent, M.; Ballester, F.; Rodríguez-Bernal, C.L.; Andiarena, A.; Vegas, O.; Castilla, A.M.; Rodriguez-Dehli, C.; Tardón, A.; et al. Vitamin D in Pregnancy and Attention Deficit Hyperactivity Disorder-like Symptoms in Childhood. Epidemiology 2015, 26, 458–465. [Google Scholar] [CrossRef]
- Arns, M.; van der Heijden, K.B.; Arnold, L.E.; Kenemans, J.L. Geographic variation in the prevalence of ateention-deficit/hyperactivity disorder: The sunny perspective. Biol. Psychiatry 2013, 74, 585–590. [Google Scholar] [CrossRef]
- Groves, N.; McGrath, J.; Burne, T. Adult vitamin D deficiency and adverse Brain Outcomes. In Vitamin D Vol 2 Health, Disease and Therapeutics, 4th ed.; Feldman, D., Ed.; Elsevier: London, UK, 2018; Volume 1, pp. 1147–1158. [Google Scholar]
- Cui, X.; McGrath, J.J.; Burne, T.H.J.; Eyles, D.W. Vitamin D and schizophrenia: 20 years on. Mol. Psychiatry 2021, 26, 2708–2720. [Google Scholar] [CrossRef]
- Holmoy, T.; Moen, S.M.; Gundersen, T.A.; Holick, M.F.; Fainardi, E.; Castellazzi, M.; Casetta, I. 25-hydroxyvitamin D in cerebrospinal fluid during relapse and remission of multiple sclerosis. Mult. Scler. 2009, 15, 1280–1285. [Google Scholar] [CrossRef]
- Balabanova, S.; Richter, H.P.; Antoniadis, G.; Homoki, J.; Kremmer, N.; Hanle, J.; Teller, W.M. 25-Hydroxyvitamin D, 24, 25-dihydroxyvitamin D and 1,25-dihydroxyvitamin D in human cerebrospinal fluid. Klin. Wochenschr. 1984, 62, 1086–1090. [Google Scholar] [CrossRef]
- Ahonena, L.; Maireb, F.B.R.; Savolainenc, S.; Koprac, J.; Vreekenb, R.J.; Hankemeierb, T.; Myöhänenc, T.; Kyllia, P.; Kostiainena, R. Analysis of oxysterols and vitamin D metabolites in mouse brain and cell line samples by ultra-high-performance liquid chromatography-atmospheric pressure photoionization–mass spectrometry. J. Chromatogr. A 2014, 1364, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; He, X.; Li, H.-D.; Deng, Y.; Yan, M.; Cai, H.-L.; Tang, M.-M.; Dang, R.-L.; Jiang, P. Simultaneous quantification of 25-Hydroxyvitamin D3 and 24,25-dihydroxyvitamin D3 in rats shows strong correlations between serum and brain tissue levels. Int. J. Endocrinol. 2015, 2015, 296531. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Dolnikowski, G.G.; Patterson, W.B.; Dawson-Hughes, B.; Zheng, T.; Morris, M.C.; Holland, T.M.; Booth, S.L. Determination of Vitamin D and Its Metabolites in Human Brain Using an Ultra-Pressure LC-Tandem Mass Spectra Method. Curr. Dev. Nutr. 2019, 3, nzz074. [Google Scholar] [CrossRef] [PubMed]
- Eyles, D.W.; Smith, S.; Kinobe, R.; Hewison, M.; McGrath, J.J. Distribution of the vitamin D receptor and 1 alpha-hydroxylase in human brain. J. Chem. Neuroanat. 2005, 29, 21–30. [Google Scholar] [CrossRef]
- Prufer, K.; Jirikowski, G.F. 1.25-Dihydroxyvitamin D3 receptor is partly colocalized with oxytocin immunoreactivity in neurons of the male rat hypothalamus. Cell. Mol. Biol. 1997, 43, 543–548. [Google Scholar]
- Clemens, T.L.; McGlade, S.A.; Garrett, K.P.; Horiuchi, N.; Hendy, G.N. Tissue-specific regulation of avian vitamin D-dependent calcium-binding protein 28-kDa mRNA by 1,25-dihydroxyvitamin D3. J. Biol. Chem. 1988, 263, 13112–13116. [Google Scholar] [CrossRef]
- Prufer, K.; Veenstra, T.D.; Jirikowski, G.F.; Kumar, R. Distribution of 1,25-dihydroxyvitamin D3 receptor immunoreactivity in the rat brain and spinal cord. J. Chem. Neuroanat. 1999, 16, 135–145. [Google Scholar] [CrossRef]
- Walbert, T.; Jirikowski, G.F.; Prufer, K. Distribution of 1,25-dihydroxyvitamin D3 receptor immunoreactivity in the limbic system of the rat. Horm. Metab. Res. 2001, 33, 525–531. [Google Scholar] [CrossRef]
- Craig, T.A.; Sommer, S.; Sussman, C.R.; Grande, J.P.; Kumar, R. Expression and regulation of the vitamin D receptor in the zebrafish, Danio rerio. J. Bone Miner. Res. 2008, 23, 1486–1496. [Google Scholar] [CrossRef]
- Wang, Y.; Becklund, B.R.; DeLuca, H.F. Identification of a highly specific and versatile vitamin D receptor antibody. Arch. Biochem. Biophys. 2010, 494, 166–177. [Google Scholar] [CrossRef]
- Wang, Y.; DeLuca, H.F. Is the vitamin D receptor found in muscle? Endocrinology 2011, 152, 354–363. [Google Scholar] [CrossRef] [PubMed]
- Eyles, D.W.; Liu, P.Y.; Josh, P.; Cui, X. Intracellular distribution of the vitamin D receptor in the brain: Comparison with classic target tissues and redistribution with development. Neuroscience 2014, 268, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Stumpf, W.E.; O’Brien, L.P. 1,25 (OH)2 vitamin D3 sites of action in the brain. An autoradiographic study. Histochemistry 1987, 87, 393–406. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Pelekanos, M.; Liu, P.Y.; Burne, T.H.J.; McGrath, J.J.; Eyles, D. The vitamin D receptor in dopamine neurons; its presence in human substantia nigra and its ortogenesis in rat midbrain. Neuroscience 2013, 236, 77–87. [Google Scholar] [CrossRef]
- Veenstra, T.D.; Prufer, K.; Koenigsberger, C.; Brimijoin, S.W.; Grande, J.P.; Kumar, R. 1,25-Dihydroxyvitamin D3 receptors in the central nervous system of the rat embryo. Brain Res. 1998, 804, 193–205. [Google Scholar] [CrossRef]
- Burkert, R.; McGrath, J.; Eyles, D. Vitamin D receptor expression in the embryonic rat brain. Neurosci. Res. Commun. 2003, 33, 63–71. [Google Scholar] [CrossRef]
- Erben, R.G.; Soegiarto, D.W.; Weber, K.; Zeitz, U.; Lieberherr, M.; Gniadecki, R.; Moller, G.; Adamski, J.; Balling, R. Deletion of deoxyribonucleic acid binding domain of the vitamin D receptor abrogates genomic and nongenomic functions of vitamin D. Mol. Endocrinol. 2002, 16, 1524–1537. [Google Scholar] [CrossRef]
- Cui, X.; McGrath, J.J.; Burne, T.H.; Mackay-Sim, A.; Eyles, D.W. Maternal vitamin D depletion alters neurogenesis in the developing rat brain. Int. J. Dev. Neurosci. 2007, 25, 227–232. [Google Scholar] [CrossRef]
- Liu, H.; He, Y.; Beck, J.; da Silva Teixeira, S.; Harrison, K.; Xu, Y.; Sisley, S. Defining vitamin D receptor expression in the brain using a novel VDR(Cre) mouse. J. Comp. Neurol. 2021, 529, 2362–2375. [Google Scholar] [CrossRef]
- Fu, G.K.; Lin, D.; Zhang, M.Y.; Bikle, D.D.; Shackleton, C.H.; Miller, W.L.; Portale, A.A. Cloning of human 25-hydroxyvitamin D-1 alpha-hydroxylase and mutations causing vitamin D-dependent rickets type 1. Mol. Endocrinol. 1997, 11, 1961–1970. [Google Scholar]
- Zehnder, D.; Bland, R.; Williams, M.C.; McNinch, R.W.; Howie, A.J.; Stewart, P.M.; Hewison, M. Extrarenal expression of 25-hydroxyvitamin d(3)-1 alpha-hydroxylase. J. Clin. Endocrinol. Metab. 2001, 86, 888–894. [Google Scholar] [PubMed]
- Landel, V.; Stephan, D.; Cui, X.; Eyles, D.; Feron, F. Differential expression of vitamin D-associated enzymes and receptors in brain cell subtypes. J. Steroid Biochem. Mol. Biol. 2018, 177, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Naveilhan, P.; Neveu, I.; Baudet, C.; Ohyama, K.Y.; Brachet, P.; Wion, D. Expression of 25(OH) vitamin D3 24-hydroxylase gene in glial cells. Neuroreport 1993, 5, 255–257. [Google Scholar] [CrossRef] [PubMed]
- Hendrix, I.; Anderson, P.; May, B.; Morris, H. Regulation of gene expression by the CYP27B1 promoter-study of a transgenic mouse model. J. Steroid Biochem. Mol. Biol. 2004, 89, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Eyles, D.W.; McGrath, J.J. Vitamin D brain development and function. In Vitamin D Vol 1 Biochemistry, Physiology and Diagnostics; Feldman, D., Ed.; Elsevier: London, UK, 2018; Volume 1, pp. 563–581. [Google Scholar]
- Eyles, D.W. Vitamin D: Brain and Behavior. JBMR Plus 2021, 5, e10419. [Google Scholar] [CrossRef]
- Brown, J.; Bianco, J.I.; McGrath, J.J.; Eyles, D.W. 1,25-dihydroxyvitamin D3 induces nerve growth factor, promotes neurite outgrowth and inhibits mitosis in embryonic rat hippocampal neurons. Neurosci. Lett. 2003, 343, 139–143. [Google Scholar] [CrossRef]
- Marini, F.; Bartoccini, E.; Cascianelli, G.; Voccoli, V.; Baviglia, M.G.; Magni, M.V.; Garcia-Gil, M.; Albi, E. Effect of 1alpha,25-dihydroxyvitamin D3 in embryonic hippocampal cells. Hippocampus 2010, 20, 696–705. [Google Scholar] [CrossRef]
- Horst, R.L.; Napoli, J.L.; Littledike, E.T. Discrimination in the metabolism of orally dosed ergocalciferol and cholecalciferol by the pig, rat and chick. Biochem. J. 1982, 204, 185–189. [Google Scholar] [CrossRef]
- Chabas, J.F.; Alluin, O.; Rao, G.; Garcia, S.; Lavaut, M.N.; Risso, J.J.; Legre, R.; Magalon, G.; Khrestchatisky, M.; Marqueste, T.; et al. Vitamin D2 potentiates axon regeneration. J. Neurotrauma 2008, 25, 1247–1256. [Google Scholar] [CrossRef]
- Ribeiro, M.C.; Moore, S.M.; Kishi, N.; Macklis, J.D.; MacDonald, J.L. Vitamin D Supplementation Rescues Aberrant NF-kappaB Pathway Activation and Partially Ameliorates Rett Syndrome Phenotypes in Mecp2 Mutant Mice. eNeuro 2020, 7. [Google Scholar] [CrossRef]
- Tague, S.E.; Smith, P.G. Vitamin D deficiency leads to sensory and sympathetic denervation of the rat synovium. Neuroscience 2014, 279, 77–93. [Google Scholar] [CrossRef] [PubMed]
- Feron, F.; Marqueste, T.; Bianco, J.; Gueye, Y.; Chabas, J.F.; Decherchi, P. Repairing the spinal cord with vitamin D: A promising strategy. Biol. Aujourdhui 2014, 208, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Neveu, I.; Naveilhan, P.; Jehan, F.; Baudet, C.; Wion, D.; De Luca, H.F.; Brachet, P. 1,25-dihydroxyvitamin D3 regulates the synthesis of nerve growth factor in primary cultures of glial cells. Brain Res. Mol. Brain Res. 1994, 24, 70–76. [Google Scholar] [CrossRef]
- Wion, D.; MacGrogan, D.; Neveu, I.; Jehan, F.; Houlgatte, R.; Brachet, P. 1,25-Dihydroxyvitamin D3 is a potent inducer of nerve growth factor synthesis. J. Neurosci. Res. 1991, 28, 110–114. [Google Scholar] [CrossRef]
- Neveu, I.; Naveilhan, P.; Baudet, C.; Brachet, P.; Metsis, M. 1,25-dihydroxyvitamin D3 regulates NT-3, NT-4 but not BDNF mRNA in astrocytes. Neuroreport 1994, 6, 124–126. [Google Scholar] [CrossRef]
- Dursun, E.; Gezen-Ak, D.; Yilmazer, S. A novel perspective for Alzheimer’s disease: Vitamin D receptor suppression by amyloid-beta and preventing the amyloid-beta induced alterations by vitamin D in cortical neurons. J. Alzheimers. Dis. 2011, 23, 207–219. [Google Scholar] [CrossRef]
- Gezen-Ak, D.; Dursun, E.; Yilmazer, S. The Effects of Vitamin D Receptor Silencing on the Expression of LVSCC-A1C and LVSCC-A1D and the Release of NGF in Cortical Neurons. PLoS ONE 2011, 6, e17553. [Google Scholar] [CrossRef]
- Saporito, M.S.; Wilcox, H.M.; Hartpence, K.C.; Lewis, M.E.; Vaught, J.L.; Carswell, S. Pharmacological induction of nerve growth factor mRNA in adult rat brain. Exp. Neurol. 1993, 123, 295–302. [Google Scholar] [CrossRef]
- Granholm, A.C.; Reyland, M.; Albeck, D.; Sanders, L.; Gerhardt, G.; Hoernig, G.; Shen, L.; Westphal, H.; Hoffer, B. Glial cell line-derived neurotrophic factor is essential for postnatal survival of midbrain dopamine neurons. J. Neurosci. 2000, 20, 3182–3190. [Google Scholar] [CrossRef]
- Oo, T.F.; Burke, R.E. The time course of developmental cell death in phenotypically defined dopaminergic neurons of the substantia nigra. Brain Res. Dev. Brain Res. 1997, 98, 191–196. [Google Scholar] [CrossRef]
- Shirazi, H.A.; Rasouli, J.; Ciric, B.; Rostami, A.; Zhang, G.X. 1,25-Dihydroxyvitamin D3 enhances neural stem cell proliferation and oligodendrocyte differentiation. Exp. Mol. Pathol. 2015, 98, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Orme, R.P.; Bhangal, M.S.; Fricker, R.A. Calcitriol imparts neuroprotection in vitro to midbrain dopaminergic neurons by upregulating GDNF expression. PLoS ONE 2013, 23, e62040. [Google Scholar] [CrossRef] [PubMed]
- Pertile, R.; Cui, X.; Hammond, L.A.; Eyles, D.W. Vitamin D regulation of GDNF/Ret signaling in dopaminergic neurons. FASEB J. 2018, 32, 819–828. [Google Scholar] [CrossRef] [PubMed]
- Eyles, D.; Brown, J.; Mackay-Sim, A.; McGrath, J.; Feron, F. Vitamin D3 and brain development. Neuroscience 2003, 118, 641–653. [Google Scholar] [CrossRef]
- Hawes, J.E.; Tesic, D.; Whitehouse, A.J.; Zosky, G.R.; Smith, J.T.; Wyrwoll, C.S. Maternal vitamin D deficiency alters fetal brain development in the BALB/c mouse. Behav. Brain Res. 2015, 286, 192–200. [Google Scholar] [CrossRef]
- Abdollahzadeh, M.; Panahpour, H.; Ghaheri, S.; Saadati, H. Calcitriol supplementation attenuates cisplatin-induced behavioral and cognitive impairments through up-regulation of BDNF in male rats. Brain Res. Bull. 2022, 181, 21–29. [Google Scholar] [CrossRef]
- Bakhtiari-Dovvombaygi, H.; Izadi, S.; Zare, M.; Asgari Hassanlouei, E.; Dinpanah, H.; Ahmadi-Soleimani, S.M.; Beheshti, F. Vitamin D3 administration prevents memory deficit and alteration of biochemical parameters induced by unpredictable chronic mild stress in rats. Sci. Rep. 2021, 11, 16271. [Google Scholar] [CrossRef]
- Mansouri, F.; Ghanbari, H.; Marefati, N.; Arab, Z.; Salmani, H.; Beheshti, F.; Hosseini, M. Protective effects of vitamin D on learning and memory deficit induced by scopolamine in male rats: The roles of brain-derived neurotrophic factor and oxidative stress. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2021, 394, 1451–1466. [Google Scholar] [CrossRef]
- Bayat, M.; Kohlmeier, K.A.; Haghani, M.; Haghighi, A.B.; Khalili, A.; Bayat, G.; Hooshmandi, E.; Shabani, M. Co-treatment of vitamin D supplementation with enriched environment improves synaptic plasticity and spatial learning and memory in aged rats. Psychopharmacology 2021, 238, 2297–2312. [Google Scholar] [CrossRef]
- Tan, X.; Gao, L.; Cai, X.; Zhang, M.; Huang, D.; Dang, Q.; Bao, L. Vitamin D3 alleviates cognitive impairment through regulating inflammatory stress in db/db mice. Food Sci. Nutr. 2021, 9, 4803–4814. [Google Scholar] [CrossRef]
- Manjari, S.K.V.; Maity, S.; Poornima, R.; Yau, S.Y.; Vaishali, K.; Stellwagen, D.; Komal, P. Restorative Action of Vitamin D3 on Motor Dysfunction Through Enhancement of Neurotrophins and Antioxidant Expression in the Striatum. Neuroscience 2022, 492, 67–81. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Pelekanos, M.; Burne, T.H.; McGrath, J.J.; Eyles, D.W. Maternal vitamin D deficiency alters the expression of genes involved in dopamine specification in the developing rat mesencephalon. Neurosci. Lett. 2010, 486, 220–223. [Google Scholar] [CrossRef] [PubMed]
- Luan, W.; Hammond, L.A.; Cotter, E.; Osborne, G.W.; Alexander, S.A.; Nink, V.; Cui, X.; Eyles, D.W. Developmental Vitamin D (DVD) Deficiency Reduces Nurr1 and TH Expression in Post-mitotic Dopamine Neurons in Rat Mesencephalon. Mol. Neurobiol. 2018, 55, 2243–2453. [Google Scholar] [CrossRef] [PubMed]
- Kesby, J.P.; Cui, X.; Ko, P.; McGrath, J.J.; Burne, T.H.; Eyles, D.W. Developmental vitamin D deficiency alters dopamine turnover in neonatal rat forebrain. Neurosci. Lett. 2009, 461, 155–158. [Google Scholar] [CrossRef] [PubMed]
- Luan, W.; Hammond, L.A.; Vuillermot, S.; Meyer, U.; Eyles, D.W. Maternal vitamin D prevents abnormal dopaminergic development and function in a mouse model of prenatal immune activation. Sci. Rep. 2018, 8, 9741. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Pertile, R.; Liu, P.; Eyles, D.W. Vitamin D regulates tyrosine hydroxylase expression: N-cadherin a possible mediator. Neuroscience 2015, 304, 90–100. [Google Scholar] [CrossRef]
- Pertile, R.A.N.; Cui, X.; Eyles, D.W. Vitamin D signalling and the differentiation of developing dopamine systems. Neuroscience 2016, 333, 193–203. [Google Scholar] [CrossRef]
- Yates, N.J.; Tesic, D.; Feindel, K.W.; Smith, J.T.; Clarke, M.W.; Wale, C.; Crew, R.C.; Wharfe, M.; Whitehouse, A.J.; Wyrwoll, C.S. Vitamin D is crucial for maternal care and offspring social behaviour in rats. J. Endocrinol. 2018, 237, 73–85. [Google Scholar] [CrossRef]
- Ali, A.A.; Cui, X.; Pertile, R.A.N.; Li, X.; Medley, G.; Alexander, S.A.; Whitehouse, A.J.O.; McGrath, J.J.; Eyles, D.W. Developmental vitamin D deficiency increases foetal exposure to testosterone. Mol. Autism. 2020, 11, 96. [Google Scholar] [CrossRef]
- Xu, X.J.; Shou, X.J.; Li, J.; Jia, M.X.; Zhang, J.S.; Guo, Y.; Wei, Q.Y.; Zhang, X.T.; Han, S.P.; Zhang, R.; et al. Mothers of autistic children: Lower plasma levels of oxytocin and Arg-vasopressin and a higher level of testosterone. PLoS ONE 2013, 8, e74849. [Google Scholar] [CrossRef]
- Burne, T.H.; Becker, A.; Brown, J.; Eyles, D.W.; Mackay-Sim, A.; McGrath, J.J. Transient prenatal Vitamin D deficiency is associated with hyperlocomotion in adult rats. Behav. Brain Res. 2004, 154, 549–555. [Google Scholar] [CrossRef]
- Kesby, J.P.; Burne, T.H.; McGrath, J.J.; Eyles, D.W. Developmental vitamin D deficiency alters MK 801-induced hyperlocomotion in the adult rat: An animal model of schizophrenia. Biol. Psychiatry 2006, 60, 591–596. [Google Scholar] [CrossRef]
- Kesby, J.P.; O’Loan, J.C.; Alexander, S.; Deng, C.; Huang, X.F.; McGrath, J.J.; Eyles, D.W.; Burne, T.H. Developmental vitamin D deficiency alters MK-801-induced behaviours in adult offspring. Psychopharmacology 2012, 220, 455–463. [Google Scholar] [CrossRef]
- O’Loan, J.; Eyles, D.W.; Kesby, J.; Ko, P.; McGrath, J.J.; Burne, T.H. Vitamin D deficiency during various stages of pregnancy in the rat; its impact on development and behaviour in adult offspring. Psychoneuroendocrinology 2007, 32, 227–234. [Google Scholar] [CrossRef]
- Kesby, J.P.; Cui, X.; O’Loan, J.; McGrath, J.J.; Burne, T.H.; Eyles, D.W. Developmental vitamin D deficiency alters dopamine-mediated behaviors and dopamine transporter function in adult female rats. Psychopharmacology 2010, 208, 159–168. [Google Scholar] [CrossRef]
- Becker, A.; Grecksch, G. Pharmacological treatment to augment hole board habituation in prenatal Vitamin D-deficient rats. Behav. Brain Res. 2006, 166, 177–183. [Google Scholar] [CrossRef]
- Grecksch, G.; Ruthrich, H.; Hollt, V.; Becker, A. Transient prenatal vitamin D deficiency is associated with changes of synaptic plasticity in the dentate gyrus in adult rats. Psychoneuroendocrinology 2009, 34, S258–S264. [Google Scholar] [CrossRef]
- Becker, A.; Eyles, D.W.; McGrath, J.J.; Grecksch, G. Transient prenatal vitamin D deficiency is associated with subtle alterations in learning and memory functions in adult rats. Behav. Brain Res. 2005, 161, 306–312. [Google Scholar] [CrossRef]
- Turner, K.M.; Young, J.W.; McGrath, J.J.; Eyles, D.W.; Burne, T.H.J. Cognitive performance and response inhibition in developmentally vitamin D (DVD)-deficient rats. Behav. Brain Res. 2013, 242, 47–53. [Google Scholar] [CrossRef]
- Overeem, K.; Alexander, S.; Burne, T.H.J.; Ko, P.; Eyles, D.W. Developmental Vitamin D Deficiency in the Rat Impairs Recognition Memory, but Has No Effect on Social Approach or Hedonia. Nutrients 2019, 11, 2713. [Google Scholar] [CrossRef]
- Vuillermot, S.; Luan, W.; Meyer, U.; Eyles, D. Vitamin D treatment during pregnancy prevents autism-related phenotypes in a mouse model of maternal immune activation. Mol. Autism. 2017, 8, 9. [Google Scholar] [CrossRef]
- Kazemi, F.; Babri, S.; Keyhanmehr, P.; Farid-Habibi, M.; Rad, S.N.; Farajdokht, F. Maternal vitamin D supplementation and treadmill exercise attenuated vitamin D deficiency-induced anxiety-and depressive-like behaviors in adult male offspring rats. Nutr. Neurosci. 2022, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Schoenrock, S.A.; Tarantino, L.M. Deveopmental vitamin D deficiency and schizophrenia: The role of animal models. Genes Brain Behav. 2016, 15, 45–61. [Google Scholar] [CrossRef] [PubMed]
- Harms, L.H.; Turner, K.M.; Eyles, D.W.; Young, J.W.; McGrath, J.J.; BUrne, T.H.J. Attentional processing in C57BL/6J mice exposed to developmental vitamin D deficiency. PLoS ONE 2012, 7, e35896. [Google Scholar]
- Harms, L.R.; Eyles, D.W.; McGrath, J.J.; Mackay-Sim, A.; Burne, T.H. Developmental vitamin D deficiency alters adult behaviour in 129/SvJ and C57BL/6J mice. Behav. Brain Res. 2008, 187, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Harms, L.H.; Cowin, G.; Eyles, D.W.; Kurniawan, N.; McGrath, J.J.; Burne, T.H.J. Neuroanatomy and psychomimetic-induced locomotion in C57BL/6J and 129/X1SvJ mice exposed to developmental vitamin D deficiency. Behav. Brain Res. 2012, 230, 125–131. [Google Scholar] [CrossRef]
- Hart, P.H.; Lucas, R.M.; Walsh, J.P.; Zosky, G.R.; Whitehouse, A.J.; Zhu, K.; Allen, K.L.; Kusel, M.M.; Anderson, D.; Mountain, J.A. Vitamin D in fetal development: Findings from a birth cohort study. Pediatrics 2015, 135, e167–e173. [Google Scholar] [CrossRef]
- Voltas, N.; Canals, J.; Hernandez-Martinez, C.; Serrat, N.; Basora, J.; Arija, V. Effect of Vitamin D Status during Pregnancy on Infant Neurodevelopment: The ECLIPSES Study. Nutrients 2020, 12, 3196. [Google Scholar] [CrossRef]
- Mutua, A.M.; Mogire, R.M.; Elliott, A.M.; Williams, T.N.; Webb, E.L.; Abubakar, A.; Atkinson, S.H. Effects of vitamin D deficiency on neurobehavioural outcomes in children: A systematic review. Wellcome Open Res. 2020, 5, 28. [Google Scholar] [CrossRef]
- Arrhenius, B.; Upadhyaya, S.; Hinkka-Yli-Salomaki, S.; Brown, A.S.; Cheslack-Postava, K.; Ohman, H.; Sourander, A. Prenatal Vitamin D Levels in Maternal Sera and Offspring Specific Learning Disorders. Nutrients 2021, 13, 3321. [Google Scholar] [CrossRef]
- Wicklow, B.; Gallo, S.; Majnemer, A.; Vanstone, C.; Comeau, K.; Jones, G.; L’Abbe, M.; Khamessan, A.; Sharma, A.; Weiler, H.; et al. Impact of Vitamin D Supplementation on Gross Motor Development of Healthy Term Infants: A Randomized Dose-Response Trial. Phys. Occup. Ther. Pediatr. 2016, 36, 330–342. [Google Scholar] [CrossRef] [PubMed]
- Whitehouse, A.J.; Holt, B.J.; Serralha, M.; Holt, P.G.; Kusel, M.M.; Hart, P.H. Maternal serum vitamin D levels during pregnancy and offspring neurocognitive development. Pediatrics 2012, 129, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Hanieh, S.; Ha, T.T.; Simpson, J.A.; Thuy, T.T.; Khuong, N.C.; Thoang, D.D.; Tran, T.D.; Tuan, T.; Fisher, J.; Biggs, B.A. Maternal vitamin D status and infant outcomes in rural Vietnam: A prospective cohort study. PLoS ONE 2014, 9, e99005. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, R.J.; Niu, Q.; Eyles, D.W.; Hansen, R.L.; Iosif, A.M. Neonatal vitamin D status in relation to autism spectrum disorder and developmental delay in the CHARGE case-control study. Autism. Res. 2019, 12, 976–988. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Vicente, M.; Sunyer, J.; Lertxundi, N.; Gonzalez, L.; Rodriguez-Dehli, C.; Espada Saenz-Torre, M.; Vrijheid, M.; Tardon, A.; Llop, S.; Torrent, M.; et al. Maternal circulating Vitamin D3 levels during pregnancy and behaviour across childhood. Sci. Rep. 2019, 9, 14792. [Google Scholar] [CrossRef]
- Sass, L.; Vinding, R.K.; Stokholm, J.; Bjarnadottir, E.; Noergaard, S.; Thorsen, J.; Sunde, R.B.; McGrath, J.; Bonnelykke, K.; Chawes, B.; et al. High-Dose Vitamin D Supplementation in Pregnancy and Neurodevelopment in Childhood: A Prespecified Secondary Analysis of a Randomized Clinical Trial. JAMA Netw. Open 2020, 3, e2026018. [Google Scholar] [CrossRef]
- Li, B.; Xu, Y.; Zhang, X.; Zhang, L.; Wu, Y.; Wang, X.; Zhu, C. The effect of vitamin D supplementation in treatment of children with autism spectrum disorder: A systematic review and meta-analysis of randomized controlled trials. Nutr. Neurosci. 2022, 25, 835–845. [Google Scholar] [CrossRef]
- Dehbokri, N.; Noorazar, G.; Ghaffari, A.; Mehdizadeh, G.; Sarbakhsh, P.; Ghaffary, S. Effect of vitamin D treatment in children with attention-deficit hyperactivity disorder. World J. Pediatr. 2019, 15, 78–84. [Google Scholar] [CrossRef]
- Elshorbagy, H.H.; Barseem, N.F.; Abdelghani, W.E.; Suliman, H.A.I.; Al-Shokary, A.H.; Abdulsamea, S.E.; Elsadek, A.E.; Abdel Maksoud, Y.H.; Nour El Din, D. Impact of Vitamin D Supplementation on Attention-Deficit Hyperactivity Disorder in Children. Ann. Pharmacother. 2018, 52, 623–631. [Google Scholar] [CrossRef]
- Miller, M.C.; Pan, X.; Eugene Arnold, L.; Mulligan, A.; Connor, S.; Bergman, R.; deBeus, R.; Roley-Roberts, M.E. Vitamin D levels in children with attention deficit hyperactivity disorder: Association with seasonal and geographical variation, supplementation, inattention severity, and theta:beta ratio. Biol. Psychol. 2021, 162, 108099. [Google Scholar] [CrossRef]
- Mohammadpour, N.; Jazayeri, S.; Tehrani-Doost, M.; Djalali, M.; Hosseini, M.; Effatpanah, M.; Davari-Ashtiani, R.; Karami, E. Effect of vitamin D supplementation as adjunctive therapy to methylphenidate on ADHD symptoms: A randomized, double blind, placebo-controlled trial. Nutr. Neurosci. 2018, 21, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Wolraich, M.L.; Cao, A.H.; Jia, F.Y.; Liu, B.; Zhu, L.; Liu, Y.; Li, X.; Li, C.; Peng, B.; et al. Adjuvant effects of vitamin A and vitamin D supplementation on treatment of children with attention-deficit/hyperactivity disorder: A study protocol for a randomised, double-blinded, placebo-controlled, multicentric trial in China. BMJ Open 2021, 11, e050541. [Google Scholar] [CrossRef] [PubMed]
- Pilz, S.; Trummer, C.; Theiler-Schwetz, V.; Grubler, M.R.; Verheyen, N.D.; Odler, B.; Karras, S.N.; Zittermann, A.; Marz, W. Critical Appraisal of Large Vitamin D Randomized Controlled Trials. Nutrients 2022, 14, 303. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Yoshioka, M.; Hashimoto, M.; Murakami, M.; Noya, M.; Takahashi, D.; Urashima, M. Randomized, double-blind, placebo-controlled trial of vitamin D supplementation in Parkinson disease. Am. J. Clin. Nutr. 2013, 97, 1004–1013. [Google Scholar] [CrossRef]
- Kang, J.H.; Vyas, C.M.; Okereke, O.I.; Ogata, S.; Albert, M.; Lee, I.M.; D’Agostino, D.; Buring, J.E.; Cook, N.R.; Grodstein, F.; et al. Effect of vitamin D on cognitive decline: Results from two ancillary studies of the VITAL randomized trial. Sci. Rep. 2021, 11, 23253. [Google Scholar] [CrossRef]
- Feige, J.; Moser, T.; Bieler, L.; Schwenker, K.; Hauer, L.; Sellner, J. Vitamin D Supplementation in Multiple Sclerosis: A Critical Analysis of Potentials and Threats. Nutrients 2020, 12, 783. [Google Scholar] [CrossRef]
- Lieberherr, M. Effects of vitamin D3 metabolites on cytosolic free calcium in confluent mouse osteoblasts. J. Biol. Chem. 1987, 262, 13168–13173. [Google Scholar] [CrossRef]
- Caffrey, J.M.; Farach-Carson, M.C. Vitamin D3 metabolites modulate dihydropyridine-sensitive calcium currents in clonal rat osteosarcoma cells. J. Biol. Chem. 1989, 264, 20265–20274. [Google Scholar] [CrossRef]
- Ibi, M.; Sawada, H.; Nakanishi, M.; Kume, T.; Katsuki, H.; Kaneko, S.; Shimohama, S.; Akaike, A. Protective effects of 1 alpha,25-(OH)(2)D-3 against the neurotoxicity of glutamate and reactive oxygen species in mesencephalic culture. Neuropharmacology 2001, 40, 761–771. [Google Scholar] [CrossRef]
- Brewer, L.D.; Thibault, V.; Chen, K.C.; Langub, M.C.; Landfield, P.W.; Porter, N.M. Vitamin D hormone confers neuroprotection in parallel with downregulation of L-type calcium channel expression in hippocampal neurons. J. Neurosci. 2001, 21, 98–108. [Google Scholar] [CrossRef]
- Gezen-Ak, D.; Dursun, E.; Yilmazer, S. Vitamin D inquiry in hippocampal neurons: Consequences of vitamin D-VDR pathway disruption on calcium channel and the vitamin D requirement. Neurol. Sci. 2013, 34, 1453–1458. [Google Scholar] [CrossRef] [PubMed]
- Zanatta, L.; Goulart, P.B.; Gonçalves, R.; Pierozan, P.; Winkelmann-Duarte, E.C.; Woehl, V.M.; Pessoa-Pureur, R.; Silva, F.R.; Zamoner, A. 1α,25-dihydroxyvitamin D(3) mechanism of action: Modulation of L-type calcium channels leading to calcium uptake and intermediate filament phosphorylation in cerebral cortex of young rats. Biochim. Biophys. Acta 2012, 1823, 1708–1719. [Google Scholar] [CrossRef] [PubMed]
- Gooch, H.; Cui, X.; Anggono, V.; Trzaskowski, M.; Tan, M.C.; Eyles, D.W.; Burne, T.H.J.; Jang, S.E.; Mattheisen, M.; Hougaard, D.M.; et al. 1,25-Dihydroxyvitamin D modulates L-type voltage-gated calcium channels in a subset of neurons in the developing mouse prefrontal cortex. Transl. Psychiatry 2019, 9, 281. [Google Scholar] [CrossRef]
- Schizophrenia Working Group of the Psychiatric Genomics Consortium. Biological insights from 108 schizophrenia-associated genetic loci. Nature 2014, 511, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Long, W.; Fatehi, M.; Soni, S.; Panigrahi, R.; Philippaert, K.; Yu, Y.; Kelly, R.; Boonen, B.; Barr, A.; Golec, D.; et al. Vitamin D is an endogenous partial agonist of the transient receptor potential vanilloid 1 channel. J. Physiol. 2020, 598, 4321–4338. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef]
- Almeida Moreira Leal, L.K.; Lima, L.A.; Alexandre de Aquino, P.E.; Costa de Sousa, J.A.; Jatai Gadelha, C.V.; Felicio Calou, I.B.; Pereira Lopes, M.J.; Viana Lima, F.A.; Tavares Neves, K.R.; Matos de Andrade, G.; et al. Vitamin D (VD3) antioxidative and anti-inflammatory activities: Peripheral and central effects. Eur. J. Pharmacol. 2020, 879, 173099. [Google Scholar] [CrossRef]
- Uberti, F.; Morsanuto, V.; Bardelli, C.; Molinari, C. Protective effects of 1α,25-Dihydroxyvitamin D3 on cultured neural cells exposed to catalytic iron. Physiol. Rep. 2016, 4, e12769. [Google Scholar] [CrossRef]
- Chen, K.B.; Lin, A.M.; Chiu, T.H. Systemic vitamin D3 attenuated oxidative injuries in the locus coeruleus of rat brain. Ann. N. Y. Acad. Sci. 2003, 993, 313–324; discussion 345–349. [Google Scholar] [CrossRef]
- Lin, A.M.; Fan, S.F.; Yang, D.M.; Hsu, L.L.; Yang, C.H. Zinc-induced apoptosis in substantia nigra of rat brain: Neuroprotection by vitamin D3. Free Radic. Biol. Med. 2003, 34, 1416–1425. [Google Scholar] [CrossRef]
- Kasatkina, L.A.; Tarasenko, A.S.; Krupko, O.O.; Kuchmerovska, T.M.; Lisakovska, O.O.; Trikash, I.O. Vitamin D deficiency induces the excitation/inhibition brain imbalance and the proinflammatory shift. Int. J. Biochem. Cell Biol. 2020, 119, 105665. [Google Scholar] [CrossRef] [PubMed]
- Garcion, E.; Nataf, S.; Berod, A.; Darcy, F.; Brachet, P. 1,25-Dihydroxyvitamin D3 inhibits the expression of inducible nitric oxide synthase in rat central nervous system during experimental allergic encephalomyelitis. Brain Res. Mol. Brain Res. 1997, 45, 255–267. [Google Scholar] [CrossRef]
- Garcion, E.; Sindji, L.; Montero-Menei, C.; Andre, C.; Brachet, P.; Darcy, F. Expression of inducible nitric oxide synthase during rat brain inflammation: Regulation by 1,25-dihydroxyvitamin D3. Glia 1998, 22, 282–294. [Google Scholar] [CrossRef]
- Lefebvre d’Hellencourt, C.; Montero-Menei, C.N.; Bernard, R.; Couez, D. Vitamin D3 inhibits proinflammatory cytokines and nitric oxide production by the EOC13 microglial cell line. J. Neurosci. Res. 2003, 71, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Hur, J.; Lee, P.H.; Kim, M.J.; Cho, Y.-W. Regulatory effect of 25-hydroxyvitamin D3 on nitric oxide production in activated microglia. Korean J. Physiol. Pharmacol. 2014, 18, 397–402. [Google Scholar] [CrossRef]
- Huang, Y.; Ho, Y.; Lai, C.; Chiu, C.; Wang, Y. 1,25-dihydroxyvitamin D3 attenuates endotoxin-induced production of inflammatory mediators by inhibiting MAPK activation in primary cortical neuron-glia cultures. J. Neuroinflammation 2015, 12, 147. [Google Scholar] [CrossRef]
- Loginova, M.; Mishchenko, T.; Savyuk, M.; Guseva, S.; Gavrish, M.; Krivonosov, M.; Ivanchenko, M.; Fedotova, J.; Vedunova, M. Double-Edged Sword of Vitamin D3 Effects on Primary Neuronal Cultures in Hypoxic States. Int. J. Mol. Sci. 2021, 22, 5417. [Google Scholar] [CrossRef]
- Ali, A.; Cui, X.; Alexander, S.; Eyles, D. The placental immune response is dysregulated developmentally vitamin D deficient rats: Relevance to autism. J. Steroid. Biochem. Mol. Biol. 2018, 180, 73–80. [Google Scholar] [CrossRef]
- Alessio, N.; Belardo, C.; Trotta, M.C.; Paino, S.; Boccella, S.; Gargano, F.; Pieretti, G.; Ricciardi, F.; Marabese, I.; Luongo, L.; et al. Vitamin D Deficiency Induces Chronic Pain and Microglial Phenotypic Changes in Mice. Int. J. Mol. Sci. 2021, 22, 3604. [Google Scholar] [CrossRef]
- Rastegar-Moghaddam, S.H.; Hosseini, M.; Alipour, F.; Rajabian, A.; Ebrahimzadeh Bideskan, A. The effects of vitamin D on learning and memory of hypothyroid juvenile rats and brain tissue acetylcholinesterase activity and oxidative stress indicators. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2022, 395, 337–351. [Google Scholar] [CrossRef]
- Mokhtari-Zaer, A.; Hosseini, M.; Salmani, H.; Arab, Z.; Zareian, P. Vitamin D3 attenuates lipopolysaccharide-induced cognitive impairment in rats by inhibiting inflammation and oxidative stress. Life Sci. 2020, 253, 117703. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.L.; Yu, X.J.; Zhao, J.Q.; Du, Y.; Xia, W.J.; Su, Q.; Du, M.M.; Yang, Q.; Qi, J.; Li, Y.; et al. Calcitriol ameliorated autonomic dysfunction and hypertension by down-regulating inflammation and oxidative stress in the paraventricular nucleus of SHR. Toxicol. Appl. Pharmacol. 2020, 394, 114950. [Google Scholar] [CrossRef] [PubMed]
- Khassafi, N.; Zahraei, Z.; Vahidinia, Z.; Karimian, M.; Azami Tameh, A. Calcitriol Pretreatment Attenuates Glutamate Neurotoxicity by Regulating NMDAR and CYP46A1 Gene Expression in Rats Subjected to Transient Middle Cerebral Artery Occlusion. J. Neuropathol. Exp. Neurol. 2022, 81, 252–259. [Google Scholar] [CrossRef]
- Vahidinia, Z.; Khassafi, N.; Tameh, A.A.; Karimian, M.; Zare-Dehghanani, Z.; Moradi, F.; Joghataei, M.T. Calcitriol Ameliorates Brain Injury in the Rat Model of Cerebral Ischemia-Reperfusion Through Nrf2/HO-1 Signalling Axis: An in Silico and in Vivo Study. J. Stroke Cerebrovasc. Dis. 2022, 31, 106331. [Google Scholar] [CrossRef] [PubMed]
- Filomeni, G.; De Zio, D.; Cecconi, F. Oxidative stress and autophagy: The clash between damage and metabolic needs. Cell Death Differ. 2015, 22, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Bhowmick, S.; D’Mello, V.; Caruso, D.; Abdul-Muneer, P.M. Traumatic brain injury-induced downregulation of Nrf2 activates inflammatory response and apoptotic cell death. J. Mol. Med. 2019, 97, 1627–1641. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Wang, C.; Jin, F.; Yang, M.; Kong, L.; Han, W.; Jiang, P. Calcitriol confers neuroprotective effects in traumatic brain injury by activating Nrf2 signaling through an autophagy-mediated mechanism. Mol. Med. 2021, 27, 118. [Google Scholar] [CrossRef]
- Hosseinirad, H.; Shahrestanaki, J.K.; Moosazadeh Moghaddam, M.; Mousazadeh, A.; Yadegari, P.; Afsharzadeh, N. Protective Effect of Vitamin D3 Against Pb-Induced Neurotoxicity by Regulating the Nrf2 and NF-kappaB Pathways. Neurotox. Res. 2021, 39, 687–696. [Google Scholar] [CrossRef]
- Jiang, H.; Zhang, S. Therapeutic effect of acute and chronic use of different doses of vitamin D3 on seizure responses and cognitive impairments induced by pentylenetetrazole in immature male rats. Iran. J. Basic Med. Sci. 2022, 25, 84–95. [Google Scholar] [CrossRef]
- Bayo-Olugbami, A.; Nafiu, A.B.; Amin, A.; Ogundele, O.M.; Lee, C.C.; Owoyele, B.V. Vitamin D attenuated 6-OHDA-induced behavioural deficits, dopamine dysmetabolism, oxidative stress, and neuro-inflammation in mice. Nutr. Neurosci. 2022, 25, 823–834. [Google Scholar] [CrossRef]
- Lima, L.A.R.; Lopes, M.J.P.; Costa, R.O.; Lima, F.A.V.; Neves, K.R.T.; Calou, I.B.F.; Andrade, G.M.; Viana, G.S.B. Vitamin D protects dopaminergic neurons against neuroinflammation and oxidative stress in hemiparkinsonian rats. J. Neuroinflammation 2018, 15, 249. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Wu, J.; Wan, F.; Kou, L.; Yin, S.; Sun, Y.; Li, Y.; Zhou, Q.; Wang, T. Calcitriol Alleviates MPP(+)- and MPTP-Induced Parthanatos Through the VDR/PARP1 Pathway in the Model of Parkinson’s Disease. Front. Aging Neurosci. 2021, 13, 657095. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.G.; Pang, Z.Q.; Wu, T.Y.; Zhang, Y.H.; Xuan, W.Q.; Wang, Z.; Yu, X.; Li, Y.C.; Guo, C.; Wang, Z.Y. Vitamin D deficiency exacerbates Alzheimer-like pathologies by reducing antioxidant capacity. Free Radic. Biol. Med. 2020, 161, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Mehrabadi, S.; Sadr, S.S. Administration of Vitamin D3 and E supplements reduces neuronal loss and oxidative stress in a model of rats with Alzheimer’s disease. Neurol. Res. 2020, 42, 862–868. [Google Scholar] [CrossRef] [PubMed]
- Grimm, M.O.W.; Lauer, A.A.; Grosgen, S.; Thiel, A.; Lehmann, J.; Winkler, J.; Janitschke, D.; Herr, C.; Beisswenger, C.; Bals, R.; et al. Profiling of Alzheimer’s disease related genes in mild to moderate vitamin D hypovitaminosis. J. Nutr. Biochem. 2019, 67, 123–137. [Google Scholar] [CrossRef]
- Lin, F.Y.; Lin, Y.F.; Lin, Y.S.; Yang, C.M.; Wang, C.C.; Hsiao, Y.H. Relative D3 vitamin deficiency and consequent cognitive impairment in an animal model of Alzheimer’s disease: Potential involvement of collapsin response mediator protein-2. Neuropharmacology 2020, 164, 107910. [Google Scholar] [CrossRef]
- Lin, C.I.; Chang, Y.C.; Kao, N.J.; Lee, W.J.; Cross, T.W.; Lin, S.H. 1,25(OH)2D3 Alleviates Abeta(25-35)-Induced Tau Hyperphosphorylation, Excessive Reactive Oxygen Species, and Apoptosis Through Interplay with Glial Cell Line-Derived Neurotrophic Factor Signaling in SH-SY5Y Cells. Int. J. Mol. Sci. 2020, 21, 4215. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, L.R.C.; Mimura, L.A.N.; Fraga-Silva, T.F.C.; Ishikawa, L.L.W.; Fernandes, A.A.H.; Zorzella-Pezavento, S.F.G.; Sartori, A. Calcitriol Prevents Neuroinflammation and Reduces Blood-Brain Barrier Disruption and Local Macrophage/Microglia Activation. Front. Pharmacol. 2020, 11, 161. [Google Scholar] [CrossRef]
- Shirazi, H.A.; Rasouli, J.; Ciric, B.; Wei, D.; Rostami, A.; Zhang, G.X. 1,25-Dihydroxyvitamin D3 suppressed experimental autoimmune encephalomyelitis through both immunomodulation and oligodendrocyte maturation. Exp. Mol. Pathol. 2017, 102, 515–521. [Google Scholar] [CrossRef]
- Nystad, A.E.; Wergeland, S.; Aksnes, L.; Myhr, K.M.; Bo, L.; Torkildsen, O. Effect of high-dose 1.25 dihydroxyvitamin D3 on remyelination in the cuprizone model. APMIS 2014, 122, 1178–1186. [Google Scholar] [CrossRef]
- Paintlia, M.K.; Singh, I.; Singh, A.K. Effect of vitamin D3 intake on the onset of disease in a murine model of human Krabbe disease. J. Neurosci. Res. 2015, 93, 28–42. [Google Scholar] [CrossRef] [PubMed]
- Sapolsky, R.M. Stress, Glucocorticoids, and Damage to the Nervous System: The Current State of Confusion. Stress 1996, 1, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.L.; Cone, C.M.; Morey-Holton, E.; Feldman, D. Glucocorticoid regulation of 1,25(OH)2-vitamin D3 receptors in cultured mouse bone cells. J. Biol. Chem. 1982, 257, 13564–13569. [Google Scholar] [CrossRef]
- Chen, T.L.; Cone, C.M.; Morey-Holton, E.; Feldman, D. 1 alpha,25-dihydroxyvitamin D3 receptors in cultured rat osteoblast-like cells. Glucocorticoid treatment increases receptor content. J. Biol. Chem. 1983, 258, 4350–4355. [Google Scholar] [CrossRef]
- Neveu, I.; Barbot, N.; Jehan, F.; Wion, D.; Brachet, P. Antagonistic effects of dexamethasone and 1,25-dihydroxyvitamin D3 on the synthesis of nerve growth factor. Mol. Cell. Endocrinol. 1991, 78, R1–R6. [Google Scholar] [CrossRef]
- Neveu, I.; Jehan, F.; Wion, D. Alteration in the levels of 1,25-(OH)2D3 and corticosterone found in experimental diabetes reduces nerve growth factor (NGF) gene expression in vitro. Life Sci. 1992, 50, 1769–1772. [Google Scholar] [CrossRef]
- Lundqvist, J.; Norlin, M.; Wikvall, K. 1alpha,25-Dihydroxyvitamin D3 affects hormone production and expression of steroidogenic enzymes in human adrenocortical NCI-H295R cells. Biochim. Biophys. Acta 2010, 1801, 1056–1062. [Google Scholar] [CrossRef]
- Obradovic, D.; Gronemeyer, H.; Lutz, B.; Rein, T. Cross-talk of vitamin D and glucocorticoids in hippocampal cells. J. Neurochem. 2006, 96, 500–509. [Google Scholar] [CrossRef]
- Jiang, P.; Xue, Y.; Li, H.D.; Liu, Y.P.; Cai, H.L.; Tang, M.M.; Zhang, L.H. Dysregulation of vitamin D metabolism in the brain and myocardium of rats following prolonged exposure to dexamethasone. Psychopharmacology 2014, 231, 3345–3351. [Google Scholar] [CrossRef] [PubMed]
- Camargo, A.; Dalmagro, A.P.; Platt, N.; Rosado, A.F.; Neis, V.B.; Zeni, A.L.B.; Kaster, M.P.; Rodrigues, A.L.S. Cholecalciferol abolishes depressive-like behavior and hippocampal glucocorticoid receptor impairment induced by chronic corticosterone administration in mice. Pharmacol. Biochem. Behav. 2020, 196, 172971. [Google Scholar] [CrossRef]
- Camargo, A.; Dalmagro, A.P.; Rikel, L.; da Silva, E.B.; Simao da Silva, K.A.B.; Zeni, A.L.B. Cholecalciferol counteracts depressive-like behavior and oxidative stress induced by repeated corticosterone treatment in mice. Eur. J. Pharmacol. 2018, 833, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Koshkina, A.; Dudnichenko, T.; Baranenko, D.; Fedotova, J.; Drago, F. Effects of Vitamin D3 in Long-Term Ovariectomized Rats Subjected to Chronic Unpredictable Mild Stress: BDNF, NT-3, and NT-4 Implications. Nutrients 2019, 11, 1726. [Google Scholar] [CrossRef] [PubMed]
- Sedaghat, K.; Yousefian, Z.; Vafaei, A.A.; Rashidy-Pour, A.; Parsaei, H.; Khaleghian, A.; Choobdar, S. Mesolimbic dopamine system and its modulation by vitamin D in a chronic mild stress model of depression in the rat. Behav. Brain Res. 2019, 356, 156–169. [Google Scholar] [CrossRef] [PubMed]
- Sedaghat, K.; Naderian, R.; Pakdel, R.; Bandegi, A.R.; Ghods, Z. Regulatory effect of vitamin D on pro-inflammatory cytokines and anti-oxidative enzymes dysregulations due to chronic mild stress in the rat hippocampus and prefrontal cortical area. Mol. Biol. Rep. 2021, 48, 7865–7873. [Google Scholar] [CrossRef]
- Neis, V.B.; Werle, I.; Moretti, M.; Rosa, P.B.; Camargo, A.; de, O. Dalsenter, Y.; Platt, N.; Rosado, A.F.; Engel, W.D.; de Almeida, G.R.L.; et al. Involvement of serotonergic neurotransmission in the antidepressant-like effect elicited by cholecalciferol in the chronic unpredictable stress model in mice. Metab. Brain Dis. 2022, 37, 1597–1608. [Google Scholar] [CrossRef]
- Kaneko, I.; Sabir, M.S.; Dussik, C.M.; Whitfield, G.K.; Karrys, A.; Hsieh, J.C.; Haussler, M.R.; Meyer, M.B.; Pike, J.W.; Jurutka, P.W. 1,25-Dihydroxyvitamin D regulates expression of the tryptophan hydroxylase 2 and leptin genes: Implication for behavioral influences of vitamin D. FASEB J. 2015, 29, 4023–4035. [Google Scholar] [CrossRef]
- Da Silva Souza, S.V.; da Rosa, P.B.; Neis, V.B.; Moreira, J.D.; Rodrigues, A.L.S.; Moretti, M. Effects of cholecalciferol on behavior and production of reactive oxygen species in female mice subjected to corticosterone-induced model of depression. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2020, 393, 111–120. [Google Scholar] [CrossRef]
- Eyles, D.W.; Rogers, F.; Buller, K.; McGrath, J.J.; Ko, P.; French, K.; Burne, T.H. Developmental vitamin D (DVD) deficiency in the rat alters adult behaviour independently of HPA function. Psychoneuroendocrinology 2006, 31, 958–964. [Google Scholar] [CrossRef]
- Tesic, D.; Hawes, J.E.; Zosky, G.R.; Wyrwoll, C.S. Vitamin D Deficiency in BALB/c Mouse Pregnancy Increases Placental Transfer of Glucocorticoids. Endocrinology 2015, 156, 3673–3679. [Google Scholar] [CrossRef]
- Meaney, M.J. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annu. Rev. Neurosci. 2001, 24, 1161–1192. [Google Scholar] [CrossRef]
- Rajabi-Naeeni, M.; Dolatian, M.; Qorbani, M.; Vaezi, A.A. Effect of omega-3 and vitamin D co-supplementation on psychological distress in reproductive-aged women with pre-diabetes and hypovitaminosis D: A randomized controlled trial. Brain Behav. 2021, 11, e2342. [Google Scholar] [CrossRef] [PubMed]
- Tebben, P.J.; Singh, R.J.; Kumar, R. Vitamin D-Mediated Hypercalcemia: Mechanisms, Diagnosis, and Treatment. Endocr. Rev. 2016, 37, 521–547. [Google Scholar] [CrossRef] [PubMed]
- Tuohimaa, P.; Keisala, T.; Minasyan, A.; Cachat, J.; Kalueff, A. Vitamin D, nervous system and aging. Psychoneuroendocrinology 2009, 34, S278–S286. [Google Scholar] [CrossRef] [PubMed]
- Lima, G.O.; Menezes da Silva, A.L.; Azevedo, J.E.C.; Nascimento, C.P.; Vieira, L.R.; Hamoy, A.O.; Oliveira Ferreira, L.; Bahia, V.; Muto, N.A.; Lopes, D.C.F.; et al. 100 YEARS of VITAMIN D: Supraphysiological doses of vitamin D changes brainwave activity patterns in rats. Endocr. Connect. 2022, 11, e210457. [Google Scholar] [CrossRef] [PubMed]
- Lam, V.; Takechi, R.; Mano, J. Vitamin D, Cerebrocapillary Integrity and Cognition in Murine Model of Accelerated Ageing. Alzheimer’s Dement. 2017, 13, 1304. [Google Scholar] [CrossRef]
- Razzaque, M.S.; Sitara, D.; Taguchi, T.; St-Arnaud, R.; Lanske, B. Premature aging-like phenotype in fibroblast growth factor 23 null mice is a vitamin D-mediated process. FASEB J. 2006, 20, 720–722. [Google Scholar] [CrossRef]
- Momen, N.C.; Plana-Ripoll, O.; Agerbo, E.; Benros, M.E.; Borglum, A.D.; Christensen, M.K.; Dalsgaard, S.; Degenhardt, L.; de Jonge, P.; Debost, J.P.G.; et al. Association between Mental Disorders and Subsequent Medical Conditions. N. Engl. J. Med. 2020, 382, 1721–1731. [Google Scholar] [CrossRef]
- Revez, J.A.; Lin, T.; Qiao, Z.; Xue, A.; Holtz, Y.; Zhu, Z.; Zeng, J.; Wang, H.; Sidorenko, J.; Kemper, K.E.; et al. Genome-wide association study identifies 143 loci associated with 25 hydroxyvitamin D concentration. Nat. Commun. 2020, 11, 1647. [Google Scholar] [CrossRef]
- Wu, D.M.; Wen, X.; Han, X.R.; Wang, S.; Wang, Y.J.; Shen, M.; Fan, S.H.; Zhuang, J.; Li, M.Q.; Hu, B.; et al. Relationship between Neonatal Vitamin D at Birth and Risk of Autism Spectrum Disorders: The NBSIB Study. J. Bone Miner. Res. 2018, 33, 458–466. [Google Scholar] [CrossRef]
- Windham, G.C.; Pearl, M.; Anderson, M.C.; Poon, V.; Eyles, D.; Jones, K.L.; Lyall, K.; Kharrazi, M.; Croen, L.A. Newborn vitamin D levels in relation to autism spectrum disorders and intellectual disability: A case-control study in california. Autism. Res. 2019, 12, 989–998. [Google Scholar] [CrossRef]
- Saraf, R.; Morton, S.M.B.; Camargo, C.A.J.; Grant, C.C. Global summary of maternal and newborn vitamin D status—A systematic review. Matern. Child Nutr. 2016, 12, 647–668. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, X.; Eyles, D.W. Vitamin D and the Central Nervous System: Causative and Preventative Mechanisms in Brain Disorders. Nutrients 2022, 14, 4353. https://doi.org/10.3390/nu14204353
Cui X, Eyles DW. Vitamin D and the Central Nervous System: Causative and Preventative Mechanisms in Brain Disorders. Nutrients. 2022; 14(20):4353. https://doi.org/10.3390/nu14204353
Chicago/Turabian StyleCui, Xiaoying, and Darryl W. Eyles. 2022. "Vitamin D and the Central Nervous System: Causative and Preventative Mechanisms in Brain Disorders" Nutrients 14, no. 20: 4353. https://doi.org/10.3390/nu14204353
APA StyleCui, X., & Eyles, D. W. (2022). Vitamin D and the Central Nervous System: Causative and Preventative Mechanisms in Brain Disorders. Nutrients, 14(20), 4353. https://doi.org/10.3390/nu14204353




