Effect of p-Synephrine on Fat Oxidation Rate during Exercise of Increasing Intensity in Healthy Active Women
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Sample Size Calculation
2.3. Experimental Procedure
2.4. Pre-Experimental and Familiarization Trials
2.5. Experimental Trials
2.6. Urine p-Synephrine and 4-hydroxymandelic Acid Concentrations
2.7. Statistical Analysis
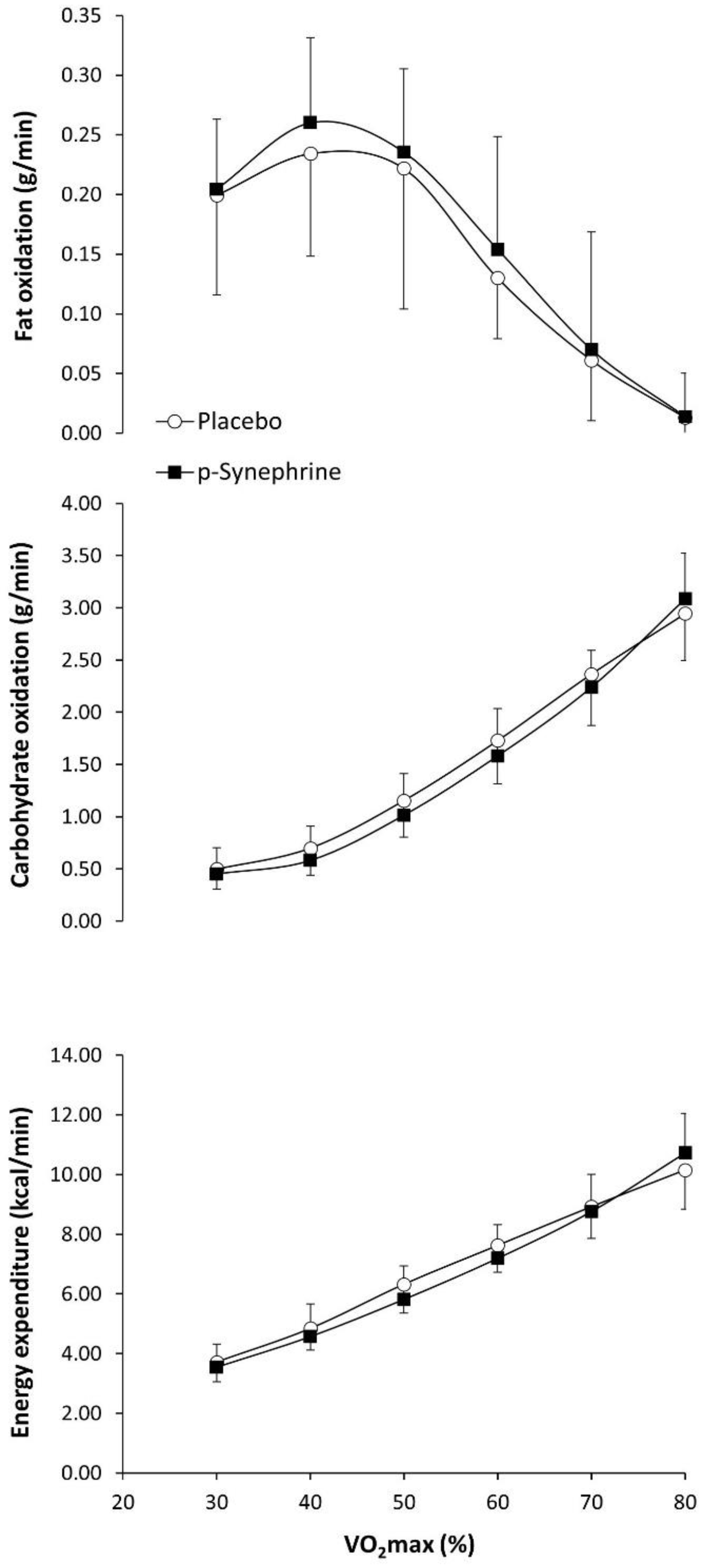
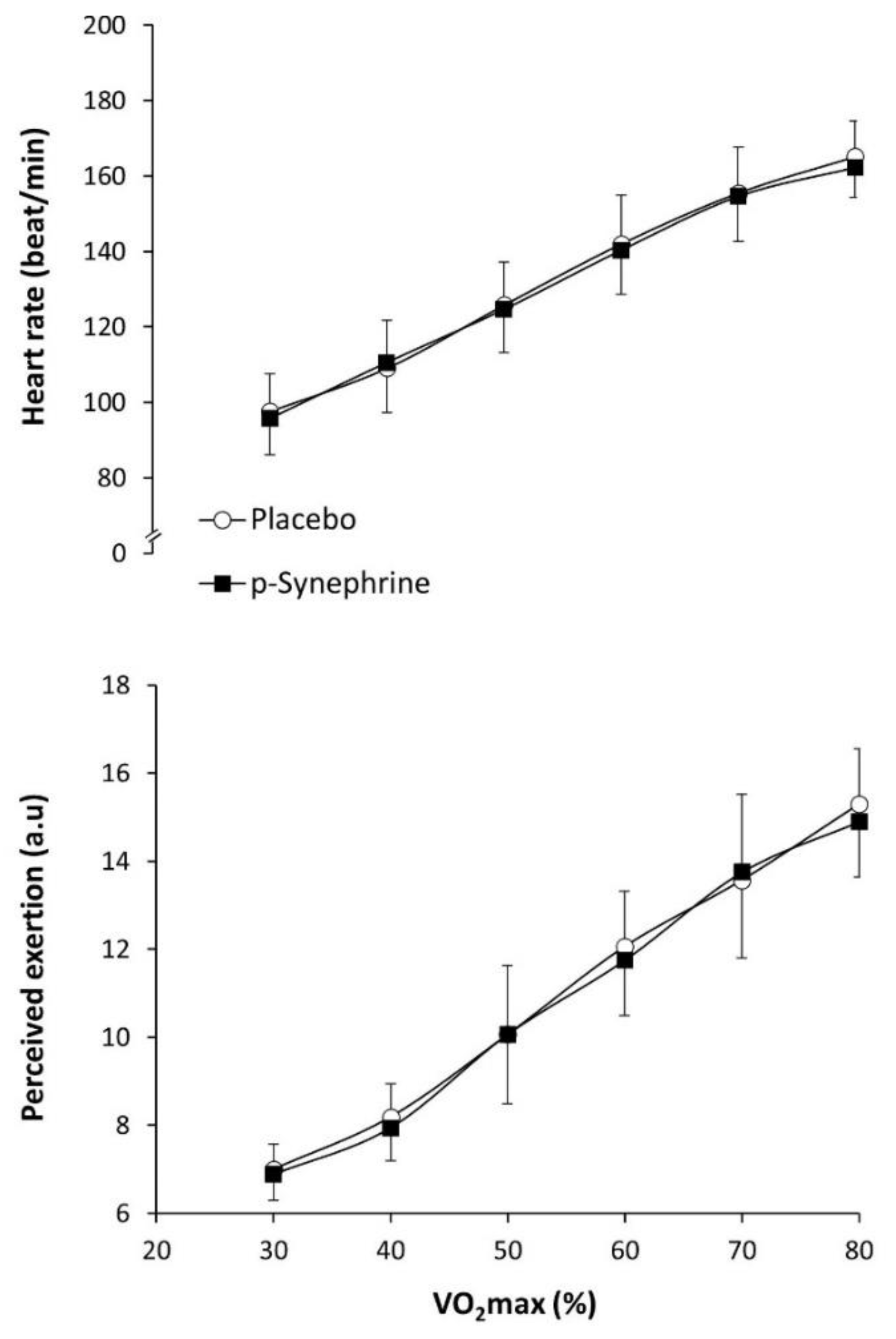
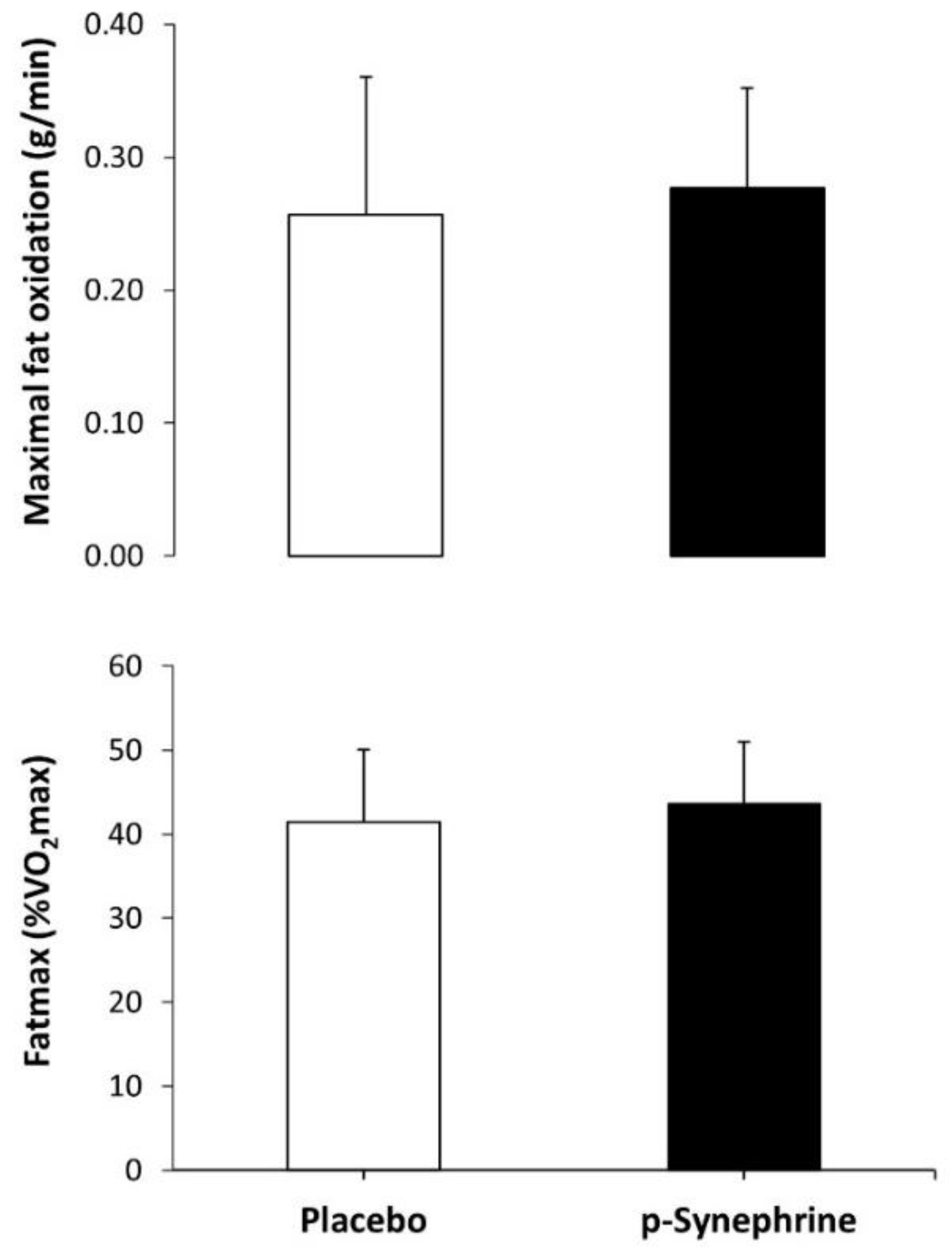
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dragull, K.; Breksa, A.P., 3rd; Cain, B. Synephrine content of juice from Satsuma mandarins (Citrus unshiu Marcovitch). J. Agric. Food Chem. 2008, 56, 8874–8878. [Google Scholar] [CrossRef] [PubMed]
- Rossato, L.G.; Costa, V.M.; Limberger, R.P.; Bastos Mde, L.; Remião, F. Synephrine: From trace concentrations to massive consumption in weight-loss. Food Chem. Toxicol. 2011, 49, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Avula, B.; Upparapalli, S.K.; Navarrete, A.; Khan, I.A. Simultaneous quantification of adrenergic amines and flavonoids in C. aurantium, various Citrus species, and dietary supplements by liquid chromatography. J. AOAC Int. 2005, 88, 1593–1606. [Google Scholar] [CrossRef] [PubMed]
- Sharpless, K.E.; Anderson, D.L.; Betz, J.M.; Butler, T.A.; Capar, S.G.; Cheng, J.; Fraser, C.A.; Gardner, G.; Gay, M.L.; Howell, D.W.; et al. Preparation and characterization of a suite of ephedra-containing standard reference materials. J. AOAC Int. 2006, 89, 1483–1495. [Google Scholar] [CrossRef] [PubMed]
- Pellati, F.; Benvenuti, S.; Melegari, M. High-performance liquid chromatography methods for the analysis of adrenergic amines and flavanones in Citrus aurantium L. var. amara. Phytochem. Anal. 2004, 15, 220–225. [Google Scholar] [CrossRef]
- Stohs, S.J.; Preuss, H.G.; Shara, M. A review of the human clinical studies involving Citrus aurantium (bitter orange) extract and its primary protoalkaloid p-synephrine. Int. J. Med. Sci. 2012, 9, 527–538. [Google Scholar] [CrossRef] [PubMed]
- Vieira-Brock, P.L.; Vaughan, B.M.; Vollmer, D.L. Thermogenic Blend Alone or in Combination with Whey Protein Supplement Stimulates Fat Metabolism and Improves Body Composition in Mice. Pharmacogn. Res. 2018, 10, 37–43. [Google Scholar] [CrossRef]
- Gutiérrez-Hellín, J.; Baltazar-Martins, G.; Rodríguez, I.; Lara, B.; Ruiz-Moreno, C.; Aguilar-Navarro, M.; Del Coso, J. p-Synephrine, the main protoalkaloid of Citrus aurantium, raises fat oxidation during exercise in elite cyclists. Eur. J. Sport Sci. 2021, 21, 1273–1282. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Hellín, J.; Del Coso, J. Effects of p-Synephrine and Caffeine Ingestion on Substrate Oxidation during Exercise. Med. Sci. Sports Exerc. 2018, 50, 1899–1906. [Google Scholar] [CrossRef]
- Gutiérrez-Hellín, J.; Del Coso, J. Acute p-synephrine ingestion increases fat oxidation rate during exercise. Br. J. Clin. Pharmacol. 2016, 82, 362–368. [Google Scholar] [CrossRef]
- Gutiérrez-Hellín, J.; Ruiz-Moreno, C.; Del Coso, J. Acute p-synephrine ingestion increases whole-body fat oxidation during 1-h of cycling at Fatmax. Eur. J. Nutr. 2020, 59, 3341–3345. [Google Scholar] [CrossRef]
- Gutiérrez-Hellín, J.; Del Coso, J. Dose-Response Effects of p-Synephrine on Fat Oxidation Rate During Exercise of Increasing Intensity. Phytother. Res. 2018, 32, 370–374. [Google Scholar] [CrossRef]
- Achten, J.; Gleeson, M.; Jeukendrup, A.E. Determination of the exercise intensity that elicits maximal fat oxidation. Med. Sci. Sports Exerc. 2002, 34, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Maunder, E.; Plews, D.J.; Kilding, A.E. Contextualising Maximal Fat Oxidation During Exercise: Determinants and Normative Values. Front. Physiol. 2018, 9, 599. [Google Scholar] [CrossRef] [PubMed]
- Stohs, S.J. Safety, Efficacy, and Mechanistic Studies Regarding Citrus aurantium (Bitter Orange) Extract and p-Synephrine. Phytother. Res. 2017, 31, 1463–1474. [Google Scholar] [CrossRef]
- Ruiz-Moreno, C.; Del Coso, J.; Giráldez-Costas, V.; González-García, J.; Gutiérrez-Hellín, J. Effects of p-Synephrine during Exercise: A Brief Narrative Review. Nutrients 2021, 13, 233. [Google Scholar] [CrossRef]
- Ruíz-Moreno, C.; Gutiérrez-Hellín, J.; González-García, J.; GiráLdez-Costas, V.; Brito de Souza, D.; Del Coso, J. Effect of ambient temperature on fat oxidation during an incremental cycling exercise test. Eur. J. Sport Sci. 2021, 21, 1140–1147. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Maldonado, M.; Jurado-Fasoli, L.; Del Coso, J.; Ruiz, J.R.; Amaro-Gahete, F.J. Caffeine increases maximal fat oxidation during a graded exercise test: Is there a diurnal variation? J. Int. Soc. Sports Nutr. 2021, 18, 5. [Google Scholar] [CrossRef]
- Edvardsen, E.; Hem, E.; Anderssen, S.A. End criteria for reaching maximal oxygen uptake must be strict and adjusted to sex and age: A cross-sectional study. PLoS ONE 2014, 9, e85276. [Google Scholar] [CrossRef] [PubMed]
- McDermott, B.P.; Anderson, S.A.; Armstrong, L.E.; Casa, D.J.; Cheuvront, S.N.; Cooper, L.; Kenney, W.L.; O’Connor, F.G.; Roberts, W.O. National Athletic Trainers’ Association Position Statement: Fluid Replacement for the Physically Active. J. Athl. Train. 2017, 52, 877–895. [Google Scholar] [CrossRef]
- Borg, G. Psychophysical scaling with applications in physical work and the perception of exertion. Scand. J. Work Environ. Health 1990, 16 (Suppl. 1), 55–58. [Google Scholar] [CrossRef]
- Achten, J.; Venables, M.C.; Jeukendrup, A.E. Fat oxidation rates are higher during running compared with cycling over a wide range of intensities. Metabolism 2003, 52, 747–752. [Google Scholar] [CrossRef]
- Brouwer, E. On simple formulae for calculating the heat expenditure and the quantities of carbohydrate and fat oxidized in metabolism of men and animals, from gaseous exchange (Oxygen intake and carbonic acid output) and urine-N. Acta Physiol. Pharmacol. Neerl. 1957, 6, 795–802. [Google Scholar] [PubMed]
- Frayn, K.N. Calculation of substrate oxidation rates in vivo from gaseous exchange. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1983, 55, 628–634. [Google Scholar] [CrossRef] [PubMed]
- Salinero, J.J.; Lara, B.; Abian-Vicen, J.; Gonzalez-Millán, C.; Areces, F.; Gallo-Salazar, C.; Ruiz-Vicente, D.; Del Coso, J. The use of energy drinks in sport: Perceived ergogenicity and side effects in male and female athletes. Br. J. Nutr. 2014, 112, 1494–1502. [Google Scholar] [CrossRef]
- Hopkins, W.G. A Scale of Magnitudes for Effect Statistics: A New View of Statistics. 2002. Available online: http://www.sportsci.org/resource/stats/effectmag.html (accessed on 31 July 2022).
- Gutiérrez-Hellín, J.; Salinero, J.J.; Abían-Vicen, J.; Areces, F.; Lara, B.; Gallo, C.; Puente, C.; Del Coso, J. Acute consumption of p-synephrine does not enhance performance in sprint athletes. Appl. Physiol. Nutr. Metab. 2016, 41, 63–69. [Google Scholar] [CrossRef]
- Ratamess, N.A.; Bush, J.A.; Kang, J.; Kraemer, W.J.; Stohs, S.J.; Nocera, V.G.; Leise, M.D.; Diamond, K.B.; Campbell, S.C.; Miller, H.B.; et al. The Effects of Supplementation with p-Synephrine Alone and in Combination with Caffeine on Metabolic, Lipolytic, and Cardiovascular Responses during Resistance Exercise. J. Am. Coll. Nutr. 2016, 35, 657–669. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, M.R.; Bracht, L.; de Sá-Nakanishi, A.B.; Corrêa, R.C.G.; Comar, J.F.; Peralta, R.M.; Bracht, A. Actions of p-synephrine on hepatic enzyme activities linked to carbohydrate metabolism and ATP levels in vivo and in the perfused rat liver. Cell Biochem. Funct. 2018, 36, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Uchida, Y.; Izumizaki, M. The use of wearable devices for predicting biphasic basal body temperature to estimate the date of ovulation in women. J. Therm. Biol. 2022, 108, 103290. [Google Scholar] [CrossRef] [PubMed]
- Pokora, I.; Wolowski, L.; Wyderka, P. The effect of a single dose of the Thermo Speed Extreme (Olimp) thermogenic supplement on circulatory functions and body temperatures at rest in male and female subjects. Balt. J. Health Phys. Act. 2019, 11, 11–25. [Google Scholar] [CrossRef]
- Gougeon, R.; Harrigan, K.; Tremblay, J.F.; Hedrei, P.; Lamarche, M.; Morais, J.A. Increase in the thermic effect of food in women by adrenergic amines extracted from Citrus aurantium. Obes. Res. 2005, 13, 1187–1194. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, G.C.; Arbo, M.D.; Lorensi, A.L.; Bemvenuti Jacques, A.n.L.; Nunes do Nascimento, S.; Mariotti, K.d.C.; Garcia, S.C.; Dallegrave, E.; Leal, M.B.; Limberger, R.P. Gender differences in biochemical markers and oxidative stress of rats after 28 days oral exposure to a mixture used for weight loss containing p-synephrine, ephedrine, salicin, and caffeine. Braz. J. Pharm. Sci. 2016, 52, 59–68. [Google Scholar] [CrossRef]
- Deshmukh, N.S.; Stohs, S.J.; Magar, C.C.; Kale, A.; Sowmya, B. Bitter orange (Citrus aurantium L.) extract subchronic 90-day safety study in rats. Toxicol. Rep. 2017, 4, 598–613. [Google Scholar] [CrossRef] [PubMed]
- Boisseau, N.; Isacco, L. Substrate metabolism during exercise: Sexual dimorphism and women’s specificities. Eur. J. Sport Sci. 2022, 22, 672–683. [Google Scholar] [CrossRef] [PubMed]
| Variable (Units) | Mean ± SD | Range |
|---|---|---|
| Age (year) | 26.9 ± 8.7 | 18.0–40.0 |
| Body mass (kg) | 62.3 ± 9.0 | 47.9–83.5 |
| Body height (cm) | 166.7 ± 6.7 | 162.0–175.0 |
| Fat mass (%) | 24.3 ± 5.5 | 15.0–39.2 |
| VO2max (mL/kg/min) | 39.7 ± 6.5 | 30.4–50.2 |
| Maximal heart rate (beat/min) | 179 ± 7 | 170–197 |
| Maximal wattage in the VO2max test (W) | 216 ± 37 | 170–300 |
| Variable (Units) | Placebo | p-Synephrine | d | p Value |
|---|---|---|---|---|
| Heart rate (beat/min) | 54 ± 9 | 58 ± 12 | 1.12 | 0.111 |
| Systolic blood pressure (mmHg) | 108.8 ± 6.1 | 108.8 ± 6.6 | 0.00 | 0.994 |
| Diastolic blood pressure (mmHg) | 67.9 ± 5.2 | 68.5 ± 7.5 | 0.08 | 0.751 |
| Tympanic temperature (°C) | 36.1 ± 0.5 | 36.4 ± 0.4 * | 0.87 | 0.033 |
| Urine specific gravity | 1.012 ± 0.061 | 1.010 ± 0.073 | 0.23 | 0.450 |
| Variable (Units) | Placebo | p-Synephrine | p Value |
|---|---|---|---|
| Nervousness (a.u.) | 1.2 ± 0.6 | 1.9 ± 1.8 | 0.236 |
| Vigour (a.u.) | 1.4 ± 0.7 | 2.1 ± 2.3 | 0.558 |
| Irritability (a.u.) | 1.3 ± 0.7 | 2.1 ± 1.3 * | 0.043 |
| Muscle pain (a.u.) | 2.1 ± 1.7 | 2.2 ± 1.3 | 0.681 |
| Headache (a.u.) | 2.7 ± 3.0 | 3.9 ± 2.8 | 0.347 |
| Gastrointestinal distress (a.u.) | 1.2 ± 0.6 | 2.1 ± 1.8 * | 0.044 |
| Diuresis (a.u.) | 1.8 ± 0.3 | 1.8 ± 1.1 | 0.829 |
| Insomnia (a.u.) | 2.9 ± 2.5 | 2.5 ± 2.6 | 0.574 |
| Sleep quality (a.u) | 7.2 ± 1.9 | 6.1 ± 2.4 | 0.092 |
| Variable (Units) | Placebo | p-Synephrine | d | p Value |
|---|---|---|---|---|
| Urine p-synephrine concentration (µg/L) | 84.21 ± 37.46 | 30,432.4 ± 4257.9 * | 0.71 | 0.006 |
| Urine 4-hydroxymandelic acid concentration (µg/L) | 0.07 ± 0.05 | 2.96 ± 5.28 * | 0.55 | 0.028 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gutiérrez-Hellín, J.; Aguilar-Navarro, M.; Ruiz-Moreno, C.; Muñoz, A.; Amaro-Gahete, F.J.; Posada-Ayala, M.; López-Samanes, Á.; Del Coso, J.; Varillas-Delgado, D. Effect of p-Synephrine on Fat Oxidation Rate during Exercise of Increasing Intensity in Healthy Active Women. Nutrients 2022, 14, 4352. https://doi.org/10.3390/nu14204352
Gutiérrez-Hellín J, Aguilar-Navarro M, Ruiz-Moreno C, Muñoz A, Amaro-Gahete FJ, Posada-Ayala M, López-Samanes Á, Del Coso J, Varillas-Delgado D. Effect of p-Synephrine on Fat Oxidation Rate during Exercise of Increasing Intensity in Healthy Active Women. Nutrients. 2022; 14(20):4352. https://doi.org/10.3390/nu14204352
Chicago/Turabian StyleGutiérrez-Hellín, Jorge, Millán Aguilar-Navarro, Carlos Ruiz-Moreno, Alejandro Muñoz, Francisco J. Amaro-Gahete, María Posada-Ayala, Álvaro López-Samanes, Juan Del Coso, and David Varillas-Delgado. 2022. "Effect of p-Synephrine on Fat Oxidation Rate during Exercise of Increasing Intensity in Healthy Active Women" Nutrients 14, no. 20: 4352. https://doi.org/10.3390/nu14204352
APA StyleGutiérrez-Hellín, J., Aguilar-Navarro, M., Ruiz-Moreno, C., Muñoz, A., Amaro-Gahete, F. J., Posada-Ayala, M., López-Samanes, Á., Del Coso, J., & Varillas-Delgado, D. (2022). Effect of p-Synephrine on Fat Oxidation Rate during Exercise of Increasing Intensity in Healthy Active Women. Nutrients, 14(20), 4352. https://doi.org/10.3390/nu14204352













