A Longitudinal Study of Exposure to Manganese and Incidence of Metabolic Syndrome
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Urinary Metal Assessment
2.3. Metabolic Syndrome Definition and Measurement
2.4. Covariate Measurement
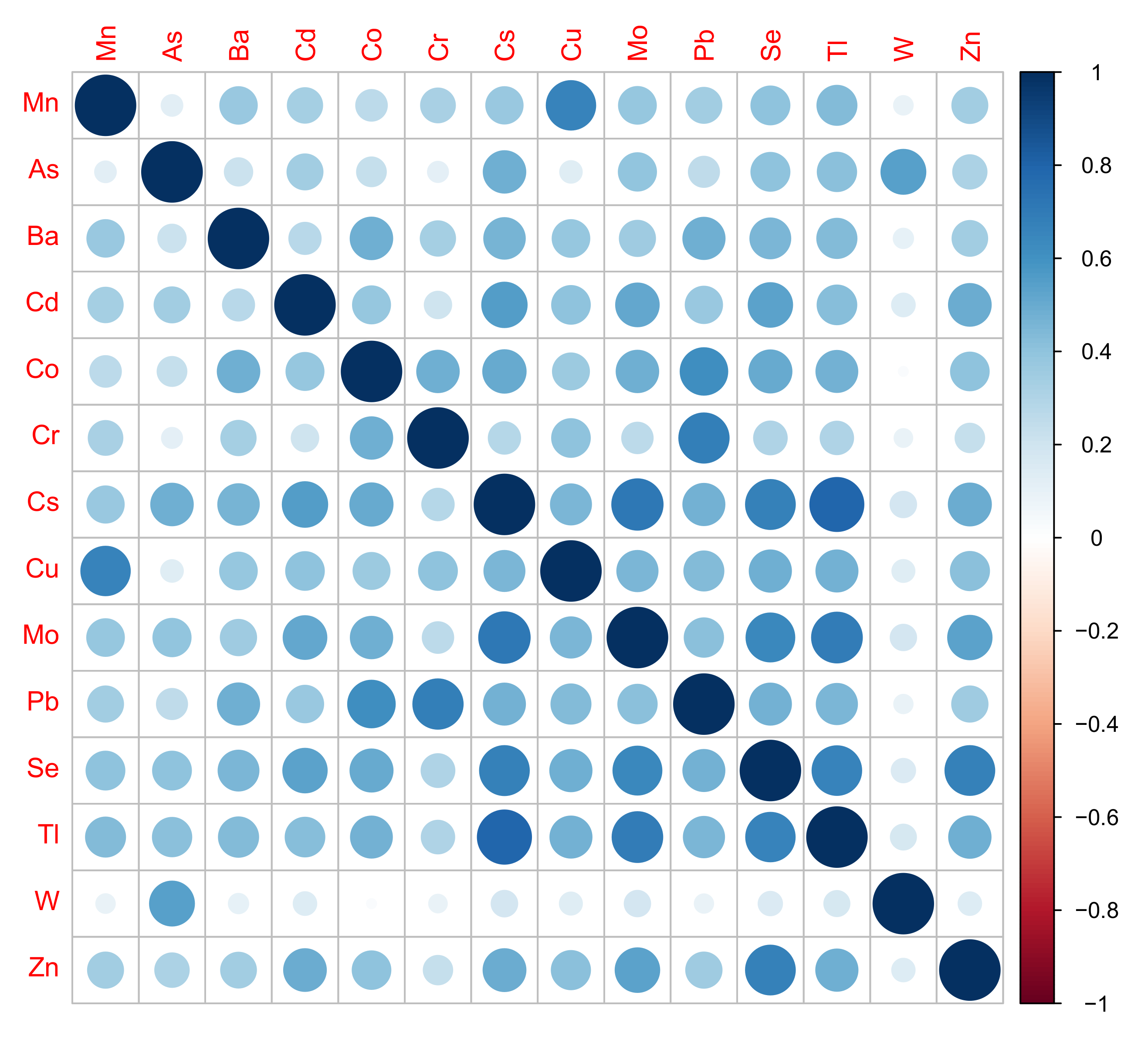
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Outcome Follow-up
3.3. Cross-sectional Analyses
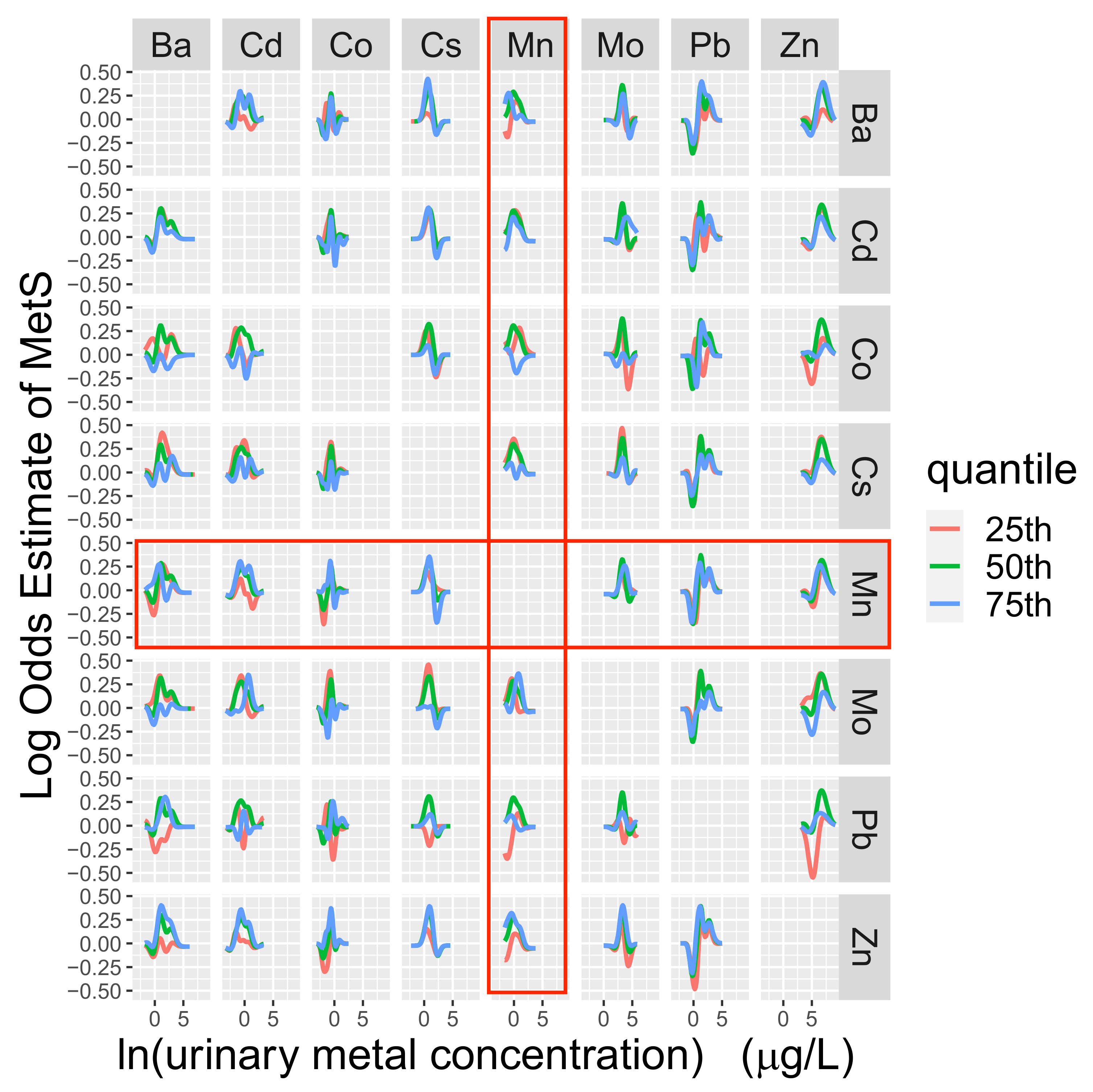
3.4. Longitudinal Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hirode, G.; Wong, R.J. Trends in the Prevalence of Metabolic Syndrome in the United States. 2011–2016. JAMA. 2020, 323, 2526–2528. [Google Scholar] [CrossRef]
- Saklayen, M.G. The Global Epidemic of the Metabolic Syndrome. Curr. Hypertens. Rep. 2018, 20, 1–8. [Google Scholar] [CrossRef]
- Samson, S.L.; Garber, A.J. Metabolic Syndrome. Endocrinol. Metab. Clin. North Am. 2014, 43, 1–23. [Google Scholar] [CrossRef]
- Rossi, J.L.S.; Barbalho, S.M.; de Araujo, R.R.; Bechara, M.D.; Sloan, K.P.; Sloan, L.A. Metabolic syndrome and cardiovascular diseases: Going beyond traditional risk factors. Diabetes/Metab. Res. Rev. 2022, 38, e3502. [Google Scholar] [CrossRef]
- Moore, J.X.; Chaudhary, N.; Akinyemiju, T. Metabolic Syndrome Prevalence by Race/Ethnicity and Sex in the United States, National Health and Nutrition Examination Survey, 1988–2012. Prev. Chronic Dis. 2017, 14, E24. [Google Scholar] [CrossRef]
- Trivedi, T.; Liu, J.; Probst, J.C.; Martin, A.B. The Metabolic Syndrome: Are Rural Residents at Increased Risk? J. Rural. Health 2013, 29, 188–197. [Google Scholar] [CrossRef]
- Bulka, C.M.; Persky, V.W.; Daviglus, M.L.; Durazo-Arvizu, R.A.; Argos, M. Multiple Metal Exposures and Metabolic Syndrome: A Cross-Sectional Analysis of the National Health and Nutrition Examination Survey 2011–2014. Environ. Res. 2019, 168, 397–405. [Google Scholar] [CrossRef]
- Planchart, A.; Green, A.; Hoyo, C.; Mattingly, C.J. Heavy Metal Exposure and Metabolic Syndrome: Evidence from Human and Model System Studies. Curr. Environ. Health Rep. 2018, 5, 110–124. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Services. Toxicological Profile for Manganese; Department of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry: Atlanta, GA, USA, 2012; p. 556. [Google Scholar]
- Peres, T.V.; Schettinger, M.R.C.; Chen, P.; Carvalho, F.; Avila, D.S.; Bowman, A.B.; Aschner, M.; Peres, T.V.; Schettinger, M.R.C.; Chen, P.; et al. Manganese-induced neurotoxicity: A review of its behavioral consequences and neuroprotective strategies. BMC Pharmacol. Toxicol. 2016, 17, 1–20. [Google Scholar] [CrossRef]
- Kim, H.; Harrison, F.E.; Aschner, M.; Bowman, A.B. Exposing the role of metals in neurological disorders: A focus on manganese. Trends Mol. Med. 2022, 28, 555–568. [Google Scholar] [CrossRef]
- Institute of Medicine (U.S.). Panel on Micronutrients. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc; National Academy Press: Washington, DC, USA, 2002. [Google Scholar]
- Li, L.; Yang, X. The Essential Element Manganese, Oxidative Stress, and Metabolic Diseases: Links and Interactions. Oxid. Med. Cell Longev. 2018, 2018, 1–11. [Google Scholar] [CrossRef]
- Choi, M.-K.; Bae, Y.-J. Relationship between Dietary Magnesium, Manganese, and Copper and Metabolic Syndrome Risk in Korean Adults: The Korea National Health and Nutrition Examination Survey (2007–2008). Biol. Trace Elem. Res. 2013, 156, 56–66. [Google Scholar] [CrossRef]
- Feng, X.; Li, L.; Huang, L.; Zhang, H.; Mo, Z.; Yang, X. Associations Between Serum Multiple Metals Exposures and Metabolic Syndrome: A Longitudinal Cohort Study. Biol. Trace Elem. Res. 2021, 199, 2444–2455. [Google Scholar] [CrossRef]
- Lo, K.; Yang, J.-L.; Chen, C.-L.; Liu, L.; Huang, Y.-Q.; Feng, Y.-Q.; Yang, A.-M. Associations between blood and urinary manganese with metabolic syndrome and its components: Cross-sectional analysis of National Health and Nutrition Examination Survey 2011–2016. Sci. Total Environ. 2021, 780, 146527. [Google Scholar] [CrossRef]
- Ma, J.; Zhou, Y.; Wang, D.; Guo, Y.; Wang, B.; Xu, Y.; Chen, W. Associations between essential metals exposure and metabolic syndrome (MetS): Exploring the mediating role of systemic inflammation in a general Chinese population. Environ. Int. 2020, 140, 105802. [Google Scholar] [CrossRef]
- Rhee, S.Y.; Hwang, Y.-C.; Woo, J.-T.; Sinn, D.H.; Chin, S.O.; Chon, S.; Kim, Y.S.Y.S. Blood lead is significantly associated with metabolic syndrome in Korean adults: An analysis based on the Korea National Health and Nutrition Examination Survey (KNHANES), 2008. Cardiovasc. Diabetol. 2013, 12, 9. [Google Scholar] [CrossRef]
- Wen, W.-L.; Wang, C.-W.; Wu, D.-W.; Chen, S.-C.; Hung, C.-H.; Kuo, C.-H. Associations of Heavy Metals with Metabolic Syndrome and Anthropometric Indices. Nutrients 2020, 12, 2666. [Google Scholar] [CrossRef]
- Zhou, B.; Su, X.; Su, D.; Zeng, F.; Wang, M.H.; Huang, L.; Huang, E.; Zhu, Y.; Zhao, D.; He, D.; et al. Dietary intake of manganese and the risk of the metabolic syndrome in a Chinese population. Br. J. Nutr. 2016, 116, 853–863. [Google Scholar] [CrossRef]
- Zhou, J.; Meng, X.; Deng, L.; Liu, N. Non-linear associations between metabolic syndrome and four typical heavy metals: Data from NHANES 2011–2018. Chemosphere 2022, 291, 132953. [Google Scholar] [CrossRef]
- Rotter, I.; Kosik-Bogacka, D.; Dołęgowska, B.; Safranow, K.; Lubkowska, A.; Laszczyńska, M. Relationship between the Concentrations of Heavy Metals and Bioelements in Aging Men with Metabolic Syndrome. Int. J. Environ. Res. Public Health 2015, 12, 3944–3961. [Google Scholar] [CrossRef]
- Huang, S.; Zhong, D.; Lv, Z.; Cheng, J.; Zou, X.; Wang, T.; Wen, Y.; Wang, C.; Yu, S.; Huang, H.; et al. Associations of multiple plasma metals with the risk of metabolic syndrome: A cross-sectional study in the mid-aged and older population of China. Ecotoxicol. Environ. Saf. 2022, 231, 113183. [Google Scholar] [CrossRef]
- Ngu, Y.J.; Skalny, A.V.; Tinkov, A.A.; Tsai, C.-S.; Chang, C.-C.; Chuang, Y.-K.; Nikolenko, V.N.; Zotkin, D.A.; Chiu, C.-F.; Chang, J.-S. Association Between Essential and Non-essential Metals, Body Composition, and Metabolic Syndrome in Adults. Biol. Trace Elem. Res. 2022, 1–13. [Google Scholar] [CrossRef]
- Li, Y.; Guo, H.; Wu, M.; Liu, M. Serum and dietary antioxidant status is associated with lower prevalence of the metabolic syndrome in a study in Shanghai, China. Asia Pac. J. Clin. Nutr. 2013, 22, 60–68. [Google Scholar] [CrossRef]
- Ghaedrahmat, Z.; Cheraghian, B.; Jaafarzadeh, N.; Takdastan, A.; Shahbazian, H.B.; Ahmadi, M. Relationship between urinary heavy metals with metabolic syndrome and its components in population from Hoveyzeh cohort study: A case-control study in Iran. J. Trace Elem. Med. Biol. 2021, 66, 126757. [Google Scholar] [CrossRef]
- Zhang, W.; Du, J.; Li, H.; Yang, Y.; Cai, C.; Gao, Q.; Xing, Y.; Shao, B.; Li, G. Multiple-element exposure and metabolic syndrome in Chinese adults: A case-control study based on the Beijing population health cohort. Environ. Int. 2020, 143, 105959. [Google Scholar] [CrossRef]
- Wong, M.M.H.; Chan, K.Y.; Lo, K. Manganese Exposure and Metabolic Syndrome: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 825. [Google Scholar] [CrossRef]
- San Luis Valley Statistical Profile. San Luis Valley Council of Governments. 2015. Available online: https://www.fs.usda.gov/nfs/11558/www/nepa/103623_FSPLT3_4298484.pdf (accessed on 1 January 2021).
- Hamman, R.F.; Marshall, J.A.; Baxter, J.; Kahn, L.B.; Mayer, E.J.; Orleans, M.; Murphy, J.R.; Lezott, D.C. Methods and Prevalence of Non-Insulin-Dependent Diabetes Mellitus in a Biethnic Colorado Population. Am. J. Epidemiol. 1989, 129, 295–311. [Google Scholar] [CrossRef]
- Riseberg, E.; James, K.A.; Woodin, M.; Melamed, R.; Alderete, T.; Corlin, L. Multipollutant, longitudinal analysis of the association between urinary tungsten and incident diabetes in a rural population. Environ. Epidemiol. 2021, 5, e173. [Google Scholar] [CrossRef]
- Hokanson, J.E.; Kamboh, M.I.; Scarboro, S.; Eckel, R.H.; Hamman, R.F. Effects of the Hepatic Lipase Gene and Physical Activity on Coronary Heart Disease Risk. Am. J. Epidemiol. 2003, 158, 836–843. [Google Scholar] [CrossRef]
- Caldwell, K. Laboratory Procedure Manual. Available online: https://www.cdc.gov/nchs/data/nhanes/nhanes_11_12/uhm_g_met_heavy_metals.pdf (accessed on 11 September 2022).
- Nunez, Z.R.; Meliker, J.R.; Meeker, J.D.; Slotnick, M.J.; Nriagu, J.O.; Rivera, N.Z. Urinary arsenic species, toenail arsenic, and arsenic intake estimates in a Michigan population with low levels of arsenic in drinking water. J. Expo. Sci. Environ. Epidemiol. 2012, 22, 182–190. [Google Scholar] [CrossRef]
- James, K.A.; Meliker, J.R.; Marshall, J.A.; Hokanson, J.E.; Zerbe, G.O.; Byers, T.E. Validation of estimates of past exposure to arsenic in drinking water using historical urinary arsenic concentrations. J. Expo. Sci. Environ. Epidemiol. 2013, 23, 450–454. [Google Scholar] [CrossRef] [PubMed]
- Srikanthan, P.; Seeman, T.E.; Karlamangla, A.S. Waist-Hip-Ratio as a Predictor of All-Cause Mortality in High-Functioning Older Adults. Ann. Epidemiol. 2009, 19, 724–731. [Google Scholar] [CrossRef] [PubMed]
- Beckman Instruments. Glucose Analyzer 2 Operating Manual. 1988. Available online: https://scholar.google.com/scholar_lookup?title=Glucose+Analyzer+2+Operating+Manual&publication_year=1988& (accessed on 4 May 2021).
- Riseberg, E.; Melamed, R.D.; James, K.A.; Alderete, T.L.; Corlin, L. Development and application of an evidence-based directed acyclic graph to evaluate the associations between metal mixtures and cardiometabolic outcomes. medRxiv 2022. [Google Scholar] [CrossRef]
- Marshall, J.A.; Weiss, N.S.; Hamman, R.F. The role of dietary fiber in the etiology of non-insulin-dependent diabetes mellitus: The San Luis Valley Diabetes Study. Ann. Epidemiol. 1993, 3, 18–26. [Google Scholar] [CrossRef]
- Delanghe, J.R.; Speeckaert, M.M. Creatinine determination according to Jaffe—What does it stand for? NDT Plus 2011, 4, 83–86. [Google Scholar] [CrossRef] [PubMed]
- Bobb, J.F.; Valeri, L.; Henn, B.C.; Christiani, D.C.; Wright, R.; Mazumdar, M.; Godleski, J.J.; Coull, B.A. Bayesian kernel machine regression for estimating the health effects of multi-pollutant mixtures. Biostatistics 2015, 16, 493–508. [Google Scholar] [CrossRef]
- Bobb, J.F.; Henn, B.C.; Valeri, L.; Coull, B.A. Statistical software for analyzing the health effects of multiple concurrent exposures via Bayesian kernel machine regression. Environ. Health 2018, 17, 67. [Google Scholar] [CrossRef]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Gray, B. Cmprsk: Subdistribution Analysis of Competing Risks. Available online: https://CRAN.R-project.org/package=cmprsk (accessed on 25 October 2021).
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S. lme4: Linear Mixed-Effects Models using “Eigen” and S4. Available online: https://CRAN.R-project.org/package=lme4 (accessed on 25 October 2021).
- Wei, T.; Simko, V.; Levy, M.; Xie, Y.; Jin, Y.; Zemla, J.; Freidank, M.; Cai, J.; Protivinsky, T. corrplot: Visualization of a Correlation Matrix. Available online: https://CRAN.R-project.org/package=corrplot (accessed on 25 October 2021).
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B.; Jensen, S.P. lmerTest: Tests in Linear Mixed Effects Models. Available online: https://CRAN.R-project.org/package=lmerTest (accessed on 8 June 2022).
- Wild, L.E.; Walters, M.; Powell, A.; James, K.A.; Corlin, L.; Alderete, T.L. County-Level Social Vulnerability Is Positively Associated with Cardiometabolic Disease in Colorado. Int. J. Environ. Res. Public Health 2022, 19, 2202. [Google Scholar] [CrossRef]
- Paschal, D.C.; Ting, B.G.; Morrow, J.C.; Pirkle, J.L.; Jackson, R.J.; Sampson, E.J.; Miller, D.T.; Caldwell, K.L. Trace Metals in Urine of United States Residents: Reference Range Concentrations. Environ. Res. 1998, 76, 53–59. [Google Scholar] [CrossRef]
- Yang, J.; Yang, A.; Cheng, N.; Huang, W.; Huang, P.; Liu, N.; Bai, Y. Sex-specific associations of blood and urinary manganese levels with glucose levels, insulin resistance and kidney function in US adults: National health and nutrition examination survey 2011–2016. Chemosphere 2020, 258, 126940. [Google Scholar] [CrossRef]
- US Department of Agriculture. Agricultural Research Service Food Data Central. Available online: https://fdc.nal.usda.gov/ (accessed on 17 November 2021).
- Satija, A.; Bhupathiraju, S.N.; Rimm, E.B.; Spiegelman, D.; Chiuve, S.; Borgi, L.; Willett, W.C.; Manson, J.E.; Sun, Q.; Hu, F.B. Plant-Based Dietary Patterns and Incidence of Type 2 Diabetes in US Men and Women: Results from Three Prospective Cohort Studies. PLoS Med. 2016, 13, e1002039. [Google Scholar] [CrossRef]
- Winham, D.M.; Hutchins, A.M.; Thompson, S.V. Glycemic Response to Black Beans and Chickpeas as Part of a Rice Meal: A Randomized Cross-Over Trial. Nutrients 2017, 9, 1095. [Google Scholar] [CrossRef] [PubMed]
- Marventano, S.; Vetrani, C.; Vitale, M.; Godos, J.; Riccardi, G.; Grosso, G. Whole Grain Intake and Glycaemic Control in Healthy Subjects: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2017, 9, 769. [Google Scholar] [CrossRef] [PubMed]
- Mazidi, M.; Vatanparast, H.; Katsiki, N.; Banach, M. The impact of nuts consumption on glucose/insulin homeostasis and inflammation markers mediated by adiposity factors among American adults. Oncotarget 2018, 9, 31173–31186. [Google Scholar] [CrossRef][Green Version]
- Ando, K.; Fujita, T. Metabolic syndrome and oxidative stress. Free. Radic. Biol. Med. 2009, 47, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Andrade, V.M.; Mateus, M.L.; Batoreu, M.C.; Aschner, M.; Dos Santos, A.P.M. Lead, arsenic and manganese metal mixture exposures: Focus on biomarkers of effect. Biol. Trace Elem. Res. 2015, 166, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Aschner, J.L.; Aschner, M. Nutritional aspects of manganese homeostasis. Mol. Asp. Med. 2005, 26, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Parhofer, K.G. The Treatment of Disorders of Lipid Metabolism. Dtsch. Arztebl. Int. 2016, 113, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Baly, D.L.; Curry, D.L.; Keen, C.L.; Hurley, L.S. Effect of manganese deficiency on insulin secretion and carbohydrate homeostasis in rats. J. Nutr. 1984, 114, 1438–1446. [Google Scholar] [CrossRef]
- Kim, C.; Halter, J.B. Endogenous Sex Hormones, Metabolic Syndrome, and Diabetes in Men and Women. Curr. Cardiol. Rep. 2014, 16, 467. [Google Scholar] [CrossRef]
- Greger, J.L.; Davis, C.D.; Suttie, J.W.; Lyle, B.J. Intake, serum concentrations, and urinary excretion of manganese by adult males. Am. J. Clin. Nutr. 1990, 51, 457–461. [Google Scholar] [CrossRef] [PubMed]
| All Participants | Mn ≤ 0.63 | Mn > 0.63 | p-Value b | |
|---|---|---|---|---|
| Total | 1478 (100.0) | 743 (50.0) | 735 (50.0) | - |
| Sex | 0.04 * | |||
| Males | 723 (48.9) | 344 (46.3) | 379 (51.6) | |
| Females | 755 (51.1) | 399 (53.7) | 356 (48.4) | |
| Age (years) | 55.1 (45.3, 63.6) | 54.2 (44.5, 62.5) | 56.5 (45.9, 64.1) | 0.01 * |
| Ethnicity | 0.02 * | |||
| Hispanic | 696 (47.1) | 328 (44.1) | 368 (50.1) | |
| Non-Hispanic | 782 (52.9) | 415 (55.9) | 367 (49.9) | |
| Total Household Income | 0.84 | |||
| <USD 10,000 | 442 (29.9) | 217 (29.2) | 225 (30.6) | |
| USD 10,000–24,999 | 541 (36.6) | 274 (36.9) | 267 (36.3) | |
| ≥USD 25,000 | 495 (33.5) | 252 (33.9) | 243 (33.1) | |
| Smoking Status | <0.01 * | |||
| Never (<100 cigarettes in lifetime) | 645 (43.6) | 251 (33.8) | 394 (53.6) | |
| Current (≥100 cigarettes and currently smokes) | 361 (24.4) | 216 (29.1) | 145 (19.7) | |
| Former (≥100 cigarettes and currently does not smoke) | 472 (31.9) | 276 (37.1) | 196 (26.7) | |
| Caloric intake (kcal/day) | 1463 (1114, 1838) | 1448 (1108, 1829) | 1472 (1130, 1847) | 0.76 |
| Metabolic syndrome prevalence c | 831 (56.2) | 414 (55.7) | 417 (56.7) | 0.69 |
| Obesity | 1307 (88.4) | 640 (86.1) | 667 (90.7) | 0.01 * |
| Low values of high-density lipoprotein | 645 (43.6) | 306 (41.2) | 339 (46.1) | 0.06 |
| High triglycerides | 715 (48.4) | 359 (48.3) | 356 (48.4) | 0.96 |
| High fasting glucose | 738 (49.9) | 393 (52.9) | 345 (46.9) | 0.02 * |
| High blood pressure | 648 (43.8) | 333 (44.8) | 315 (42.9) | 0.45 |
| Quartile 2 0.33–0.63 µg/L | Quartile 3 0.63–1.13 µg/L | Quartile 4 1.13–42.5 µg/L | p-Value for T-rend | |
|---|---|---|---|---|
| Crude model c (n = 608) | 1.39 (0.95, 2.02) | 1.11 (0.74, 1.67) | 1.30 (0.88, 1.92) | 0.41 |
| Males (n = 254) | 1.45 (0.80, 2.63) | 1.00 (0.56, 1.79) | 1.21 (0.71, 2.07) | 0.76 |
| Females (n = 354) | 1.39 (0.85, 2.30) | 1.20 (0.69, 2.10) | 1.39 (0.80, 2.42) | 0.41 |
| Adjusted model d (n = 608) | 1.42 (0.97, 2.08) | 1.11 (0.74, 1.68) | 1.26 (0.84, 1.89) | 0.52 |
| Males (n = 254) | 1.51 (0.81, 2.81) | 0.97 (0.53, 1.80) | 1.25 (0.70, 2.21) | 0.71 |
| Females (n = 354) | 1.40 (0.84, 2.31) | 1.20 (0.68, 2.10) | 1.38 (0.77, 2.47) | 0.43 |
| Quartile 2 0.33–0.63 µg/L | Quartile 3 0.63–1.13 µg/L | Quartile 4 1.13–42.5 µg/L | p-Value for Trend | |
|---|---|---|---|---|
| Waist–hip ratio; n = 1477 | 0.000 (−0.006, 0.005) | −0.001 (−0.007, 0.004) | 0.000 (−0.006, 0.006) | 0.96 |
| Males | 0.003 (−0.004, 0.01) | 0.000 (−0.007, 0.008) | 0.000 (−0.007, 0.007) | 0.92 |
| Females | −0.003 (−0.011, 0.006) | −0.002 (−0.011, 0.007) | 0.001 (−0.009, 0.011) | 0.79 |
| Body mass index (kg/m2); n = 1477 | −0.14 (−0.82, 0.54) | −0.04 (−0.74, 0.67) | 0.17 (−0.55, 0.90) | 0.61 |
| Males | 0.00 (−0.84, 0.84) | −0.60 (−1.44, 0.25) | −0.16 (−1.00, 0.68) | 0.51 |
| Females | −0.10 (−1.13, 0.94) | 0.49 (−0.62, 1.60) | 0.62 (−0.56, 1.80) | 0.21 |
| High-density lipoprotein (mg/dL); n = 1476 | −1.01 (−2.74, 0.73) | −1.02 (−2.83, 0.78) | −0.18 (−2.03, 1.67) | 0.87 |
| Males | −1.02 (−3.33, 1.29) | −0.01 (−2.34, 2.31) | −0.14 (−2.45, 2.16) | 0.91 |
| Females | −1.64 (−4.17, 0.90) | −2.00 (−4.71, 0.72) | −0.56 (−3.45, 2.33) | 0.65 |
| Triglycerides (mg/dL); n = 1477 | −12.5 (−29.9, 4.9) | −10.1 (−28.1, 7.9) | −17.8 (−36.2, 0.7) | 0.09 |
| Males | −1.3 (−21.8, 19.2) | −7.2 (−27.7, 13.4) | −13.9 (−34.2, 6.5) | 0.16 |
| Females | −17.3 (−45.8, 11.2) | −13.8 (−44.3, 16.8) | −18.6 (−51.1, 14.0) | 0.33 |
| Fasting glucose (mg/dL); n = 1475 | 1.0 (−6.2, 8.2) | −9.4 (−16.9, −1.9) | −12.6 (−20.3, −4.9) | <0.01 * |
| Males | 4.4 (−5.7, 14.6) | −14.9 (−25.1, −4.6) | −15.3 (−25.5, −5.2) | <0.01 * |
| Females | 0.3 (−9.9, 10.4) | −4.8 (−15.7, 6.1) | −10.9 (−22.5, 0.6) | 0.048 * |
| Systolic blood pressure (mmHg); n = 1477 | −2.1 (−4.4, 0.2) | −0.8 (−3.2, 1.6) | −2.2 (−4.7, 0.2) | 0.17 |
| Males | −1.0 (−4.1, 2.2) | −3.1 (−6.3, 0.1) | −2.7 (−5.8, 0.5) | 0.06 |
| Females | −3.2 (−6.5, 0.2) | 0.8 (−2.7, 4.4) | −2.2 (−6.0, 1.6) | 0.71 |
| Diastolic blood pressure (mmHg); n = 1477 | −1.2 (−2.3, 0.0) | −1.3 (−2.5, −0.1) | −0.8 (−2.0, 0.4) | 0.22 |
| Males | −1.0 (−2.7, 0.7) | −2.3 (−4.0, −0.6) | −1.2 (−2.9, 0.5) | 0.11 |
| Females | −1.4 (−2.9, 0.2) | −0.4 (−2.0, 1.2) | −0.4 (−2.2, 1.3) | 0.91 |
| Interacting Metal Model | Mn Quartile 2 0.33–0.63 µg/L | Mn Quartile 3 0.63–1.13 µg/L | Mn Quartile 4 1.13–42.5 µg/L | p-Value for Mn Trend |
|---|---|---|---|---|
| Barium | 1.44 (0.78, 2.68) | 1.25 (0.57, 2.73) | 1.00 (0.33, 3.08) | 0.71 |
| Cadmium | 1.16 (0.64, 2.09) | 1.14 (0.48, 2.71) | 1.04 (0.39, 2.72) | 0.80 |
| Cobalt | 1.56 (0.84, 2.90) | 1.15 (0.53, 2.52) | 1.99 (0.89, 4.45) | 0.17 |
| Cesium | 1.80 (0.97, 3.35) | 1.74 (0.74, 4.07) | 1.05 (0.46, 2.41) | 0.65 |
| Molybdenum | 1.69 (0.90, 3.17) | 1.93 (0.89, 4.18) | 1.22 (0.52, 2.88) | 0.37 |
| Lead | 2.45 (1.27, 4.72) | 1.97 (0.94, 4.10) | 3.36 (1.11, 10.17) | 0.01 * |
| Zinc | 1.09 (0.59, 2.01) | 1.50 (0.79, 2.83) | 1.18 (0.41, 3.42) | 0.37 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riseberg, E.; Chui, K.; James, K.A.; Melamed, R.; Alderete, T.L.; Corlin, L. A Longitudinal Study of Exposure to Manganese and Incidence of Metabolic Syndrome. Nutrients 2022, 14, 4271. https://doi.org/10.3390/nu14204271
Riseberg E, Chui K, James KA, Melamed R, Alderete TL, Corlin L. A Longitudinal Study of Exposure to Manganese and Incidence of Metabolic Syndrome. Nutrients. 2022; 14(20):4271. https://doi.org/10.3390/nu14204271
Chicago/Turabian StyleRiseberg, Emily, Kenneth Chui, Katherine A. James, Rachel Melamed, Tanya L. Alderete, and Laura Corlin. 2022. "A Longitudinal Study of Exposure to Manganese and Incidence of Metabolic Syndrome" Nutrients 14, no. 20: 4271. https://doi.org/10.3390/nu14204271
APA StyleRiseberg, E., Chui, K., James, K. A., Melamed, R., Alderete, T. L., & Corlin, L. (2022). A Longitudinal Study of Exposure to Manganese and Incidence of Metabolic Syndrome. Nutrients, 14(20), 4271. https://doi.org/10.3390/nu14204271








