The Effect of Dietary Supplementations on Delaying the Progression of Age-Related Macular Degeneration: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy and Data Sources
2.2. Eligibility Criteria: Inclusion and Exclusion
2.3. Screening, Data Extraction, and Quality Assessment
2.4. Data Preparation
2.5. Statistical Analyses
3. Results
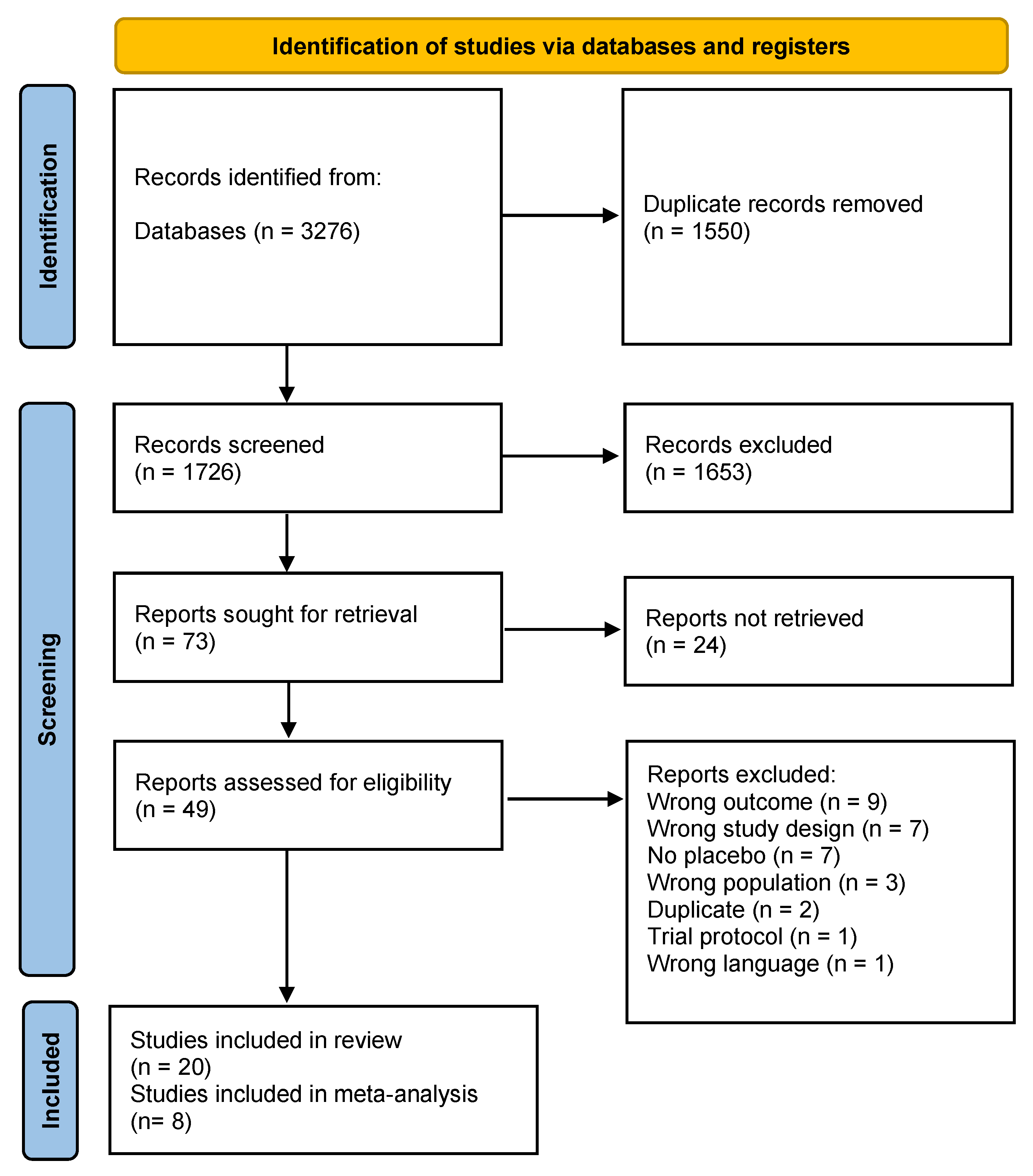
3.1. Search Results and Study Characteristics
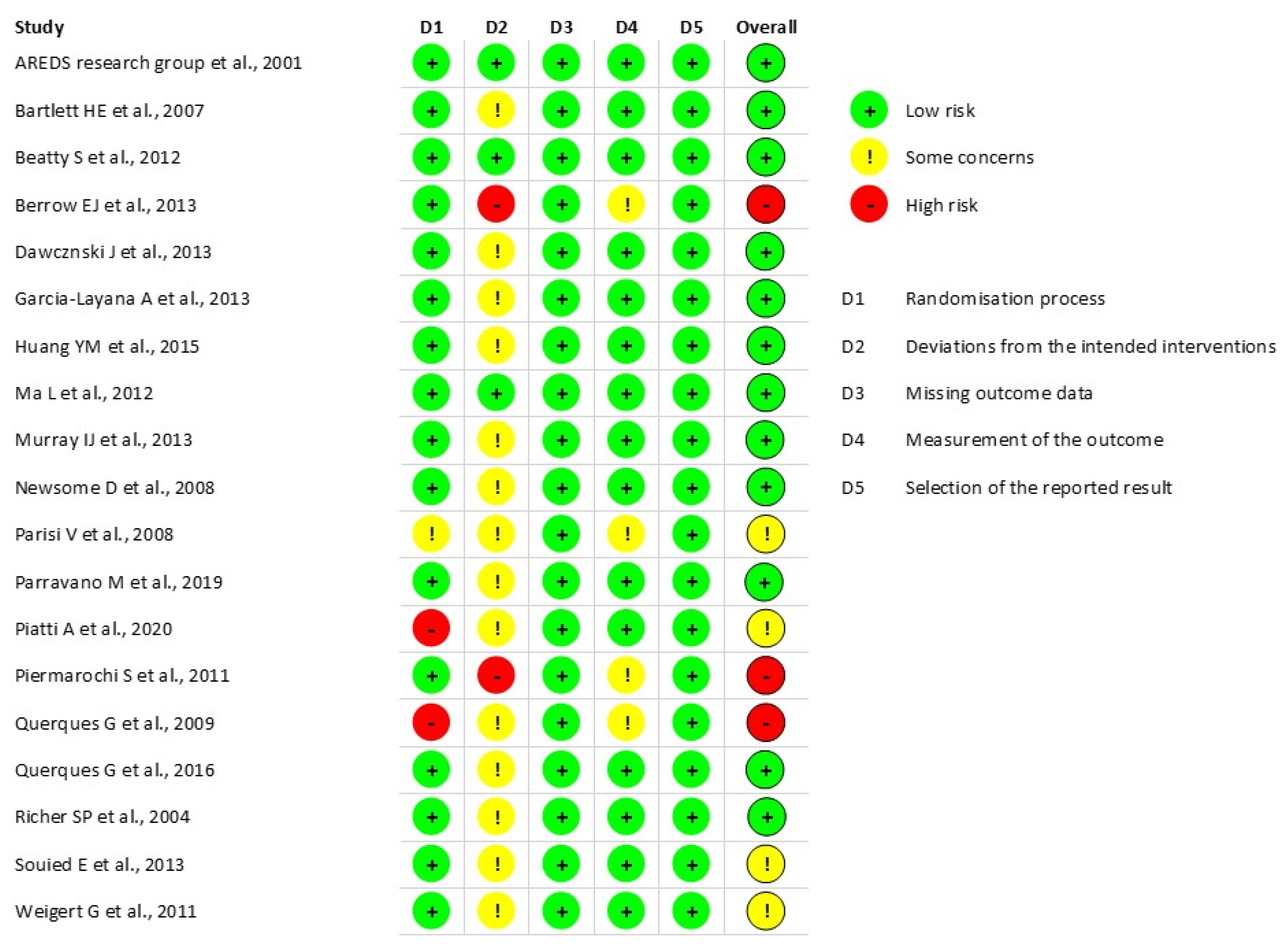
3.2. Quality Assessment
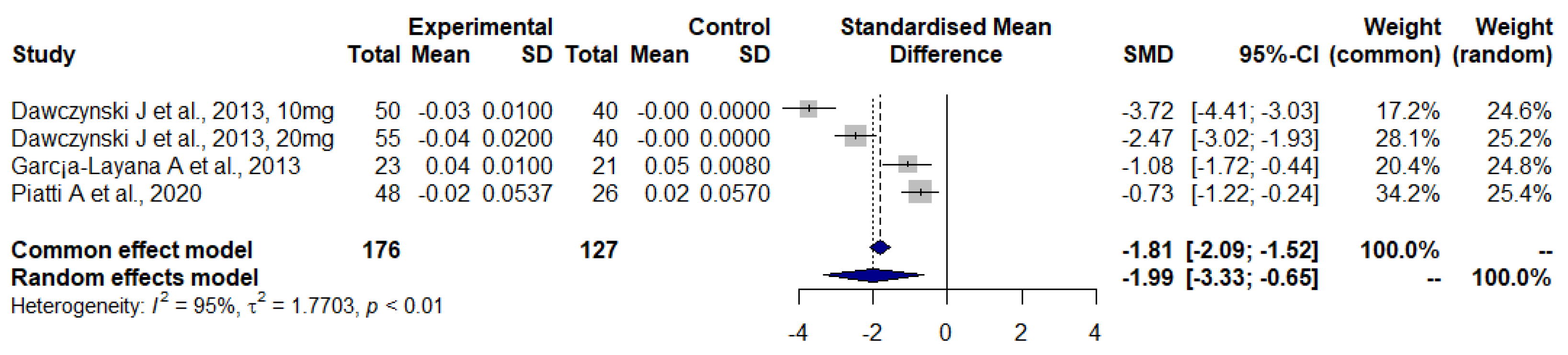
3.3. Dietary Supplements Reports and Meta-Analysis
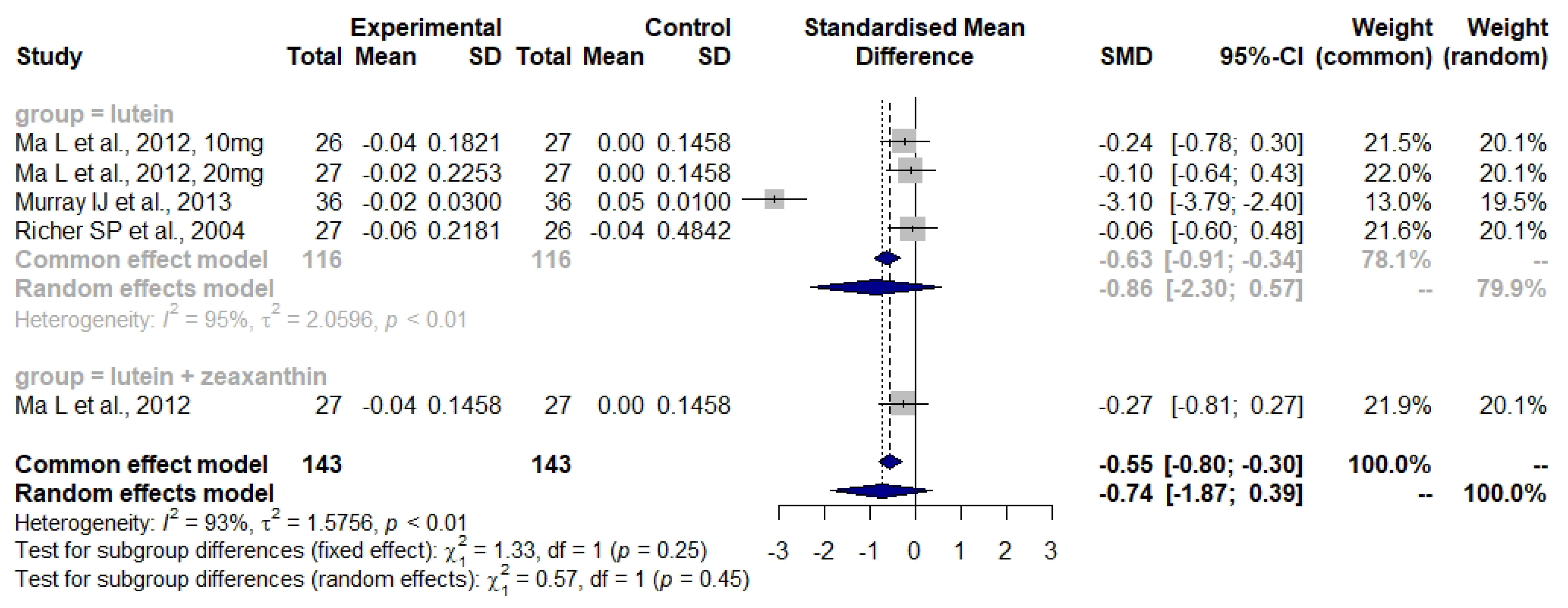
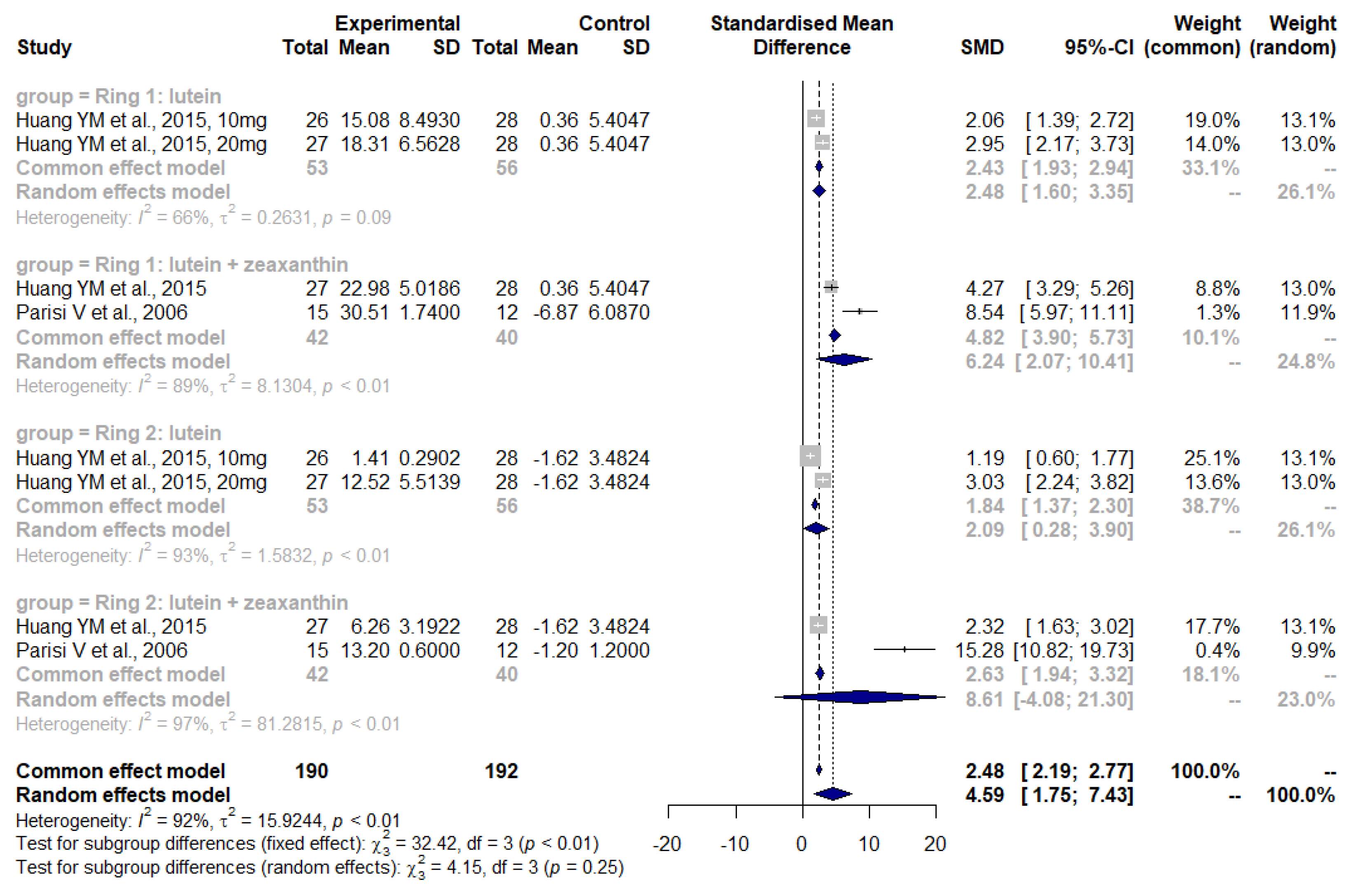
3.4. Lutein and Zeaxanthin
3.5. Lutein and Zeaxanthin Plus n-3 LC-PUFA
3.6. Zinc
3.7. Curcumin
4. Discussion
4.1. Clinical Relevance
4.2. Strength and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fleckenstein, M.; Keenan, T.D.L.; Guymer, R.H.; Chakravarthy, U.; Schmitz-Valckenberg, S.; Klaver, C.C.; Wong, W.T.; Chew, E.Y. Age-Related Macular Degeneration. Nat. Rev. Dis. Primers 2021, 7, 31. [Google Scholar] [CrossRef] [PubMed]
- Stahl, A. The Diagnosis and Treatment of Age-Related Macular Degeneration. Dtsch. Arztebl. Int. 2020, 117, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Wong, W.L.; Su, X.; Li, X.; Cheung, C.M.G.; Klein, R.; Cheng, C.-Y.; Wong, T.Y. Global Prevalence of Age-Related Macular Degeneration and Disease Burden Projection for 2020 and 2040: A Systematic Review and Meta-Analysis. Lancet Glob. Health 2014, 2, e106–e116. [Google Scholar] [CrossRef]
- Bowes Rickman, C.; Farsiu, S.; Toth, C.A.; Klingeborn, M. Dry Age-Related Macular Degeneration: Mechanisms, Therapeutic Targets, and Imaging. Investig. Ophthalmol. Vis. Sci. 2013, 54, ORSF68. [Google Scholar] [CrossRef]
- Curcio, C.A.; Johnson, M.; Huang, J.-D.; Rudolf, M. Aging, Age-Related Macular Degeneration, and the Response-to-Retention of Apolipoprotein B-Containing Lipoproteins. Prog. Retin. Eye Res. 2009, 28, 393–422. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, P.; Liew, G.; Gopinath, B.; Wong, T.Y. Age-Related Macular Degeneration. Lancet 2018, 392, 1147–1159. [Google Scholar] [CrossRef]
- Awh, C.C.; Lane, A.-M.; Hawken, S.; Zanke, B.; Kim, I.K. CFH and ARMS2 Genetic Polymorphisms Predict Response to Antioxidants and Zinc in Patients with Age-Related Macular Degeneration. Ophthalmology 2013, 120, 2317–2323. [Google Scholar] [CrossRef]
- García-Layana, A.; Cabrera-López, F.; García-Arumí, J.; Arias-Barquet, L.; Ruiz-Moreno, J.M. Early and Intermediate Age-Related Macular Degeneration: Update and Clinical Review. Clin. Interv. Aging 2017, 12, 1579–1587. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.J.; Mirza, R.G.; Gill, M.K. Age-Related Macular Degeneration. Med. Clin. N. Am. 2021, 105, 473–491. [Google Scholar] [CrossRef]
- Hernández-Zimbrón, L.F.; Zamora-Alvarado, R.; Ochoa-De la Paz, L.; Velez-Montoya, R.; Zenteno, E.; Gulias-Cañizo, R.; Quiroz-Mercado, H.; Gonzalez-Salinas, R. Age-Related Macular Degeneration: New Paradigms for Treatment and Management of AMD. Oxid. Med. Cell Longev. 2018, 2018, 8374647. [Google Scholar] [CrossRef]
- Nashine, S. Potential Therapeutic Candidates for Age-Related Macular Degeneration (AMD). Cells 2021, 10, 2483. [Google Scholar] [CrossRef] [PubMed]
- Grzybowski, A.; Told, R.; Sacu, S.; Bandello, F.; Moisseiev, E.; Loewenstein, A.; Schmidt-Erfurth, U.; Euretina Board. 2018 Update on Intravitreal Injections: Euretina Expert Consensus Recommendations. Ophthalmologica 2018, 239, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Age-Related Eye Disease Study Research Group. A Randomized, Placebo-Controlled, Clinical Trial of High-Dose Supplementation with Vitamins C and E, Beta Carotene, and Zinc for Age-Related Macular Degeneration and Vision Loss: AREDS Report No. 8. Arch. Ophthalmol. 2001, 119, 1417–1436. [Google Scholar] [CrossRef] [PubMed]
- The Age-Related Eye Disease Study 2 (AREDS2) Research Group. Lutein + Zeaxanthin and Omega-3 Fatty Acids for Age-Related Macular Degeneration: The Age-Related Eye Disease Study 2 (AREDS2) Randomized Clinical Trial. JAMA 2013, 309, 2005. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. PLOS Med. 2021, 18, e1003583. [Google Scholar] [CrossRef]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 Explanation and Elaboration: Updated Guidance and Exemplars for Reporting Systematic Reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A Web and Mobile App for Systematic Reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A Revised Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A Tool for Assessing Risk of Bias in Non-Randomised Studies of Interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef]
- Beck, R.W.; Moke, P.S.; Turpin, A.H.; Ferris, F.L.; SanGiovanni, J.P.; Johnson, C.A.; Birch, E.E.; Chandler, D.L.; Cox, T.A.; Blair, R.C.; et al. A Computerized Method of Visual Acuity Testing: Adaptation of the Early Treatment of Diabetic Retinopathy Study Testing Protocol. Am. J. Ophthalmol. 2003, 135, 194–205. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (Updated February 2022) Cochrane. 2022. Available online: https://training.cochrane.org/handbook (accessed on 14 March 2022).
- Richer, S.; Stiles, W.; Statkute, L.; Pulido, J.; Frankowski, J.; Rudy, D.; Pei, K.; Tsipursky, M.; Nyland, J. Double-Masked, Placebo-Controlled, Randomized Trial of Lutein and Antioxidant Supplementation in the Intervention of Atrophic Age-Related Macular Degeneration: The Veterans LAST Study (Lutein Antioxidant Supplementation Trial). Optometry 2004, 75, 216–230. [Google Scholar] [CrossRef]
- Huang, Y.-M.; Dou, H.-L.; Huang, F.-F.; Xu, X.-R.; Zou, Z.-Y.; Lu, X.-R.; Lin, X.-M. Changes Following Supplementation with Lutein and Zeaxanthin in Retinal Function in Eyes with Early Age-Related Macular Degeneration: A Randomised, Double-Blind, Placebo-Controlled Trial. Br. J. Ophthalmol. 2015, 99, 371–375. [Google Scholar] [CrossRef] [PubMed]
- Parisi, V.; Tedeschi, M.; Gallinaro, G.; Varano, M.; Saviano, S.; Piermarocchi, S.; CARMIS Study Group. Carotenoids and Antioxidants in Age-Related Maculopathy Italian Study: Multifocal Electroretinogram Modifications after 1 Year. Ophthalmology 2008, 115, 324–333.e2. [Google Scholar] [CrossRef] [PubMed]
- Borenstein, M.; Hedges, L.V.; Higgins, J.P.T.; Rothstein, H.R. A Basic Introduction to Fixed-Effect and Random-Effects Models for Meta-Analysis. Res. Synth. Methods 2010, 1, 97–111. [Google Scholar] [CrossRef]
- Dawczynski, J.; Jentsch, S.; Schweitzer, D.; Hammer, M.; Lang, G.E.; Strobel, J. Long Term Effects of Lutein, Zeaxanthin and Omega-3-LCPUFAs Supplementation on Optical Density of Macular Pigment in AMD Patients: The LUTEGA Study. Graefes Arch. Clin. Exp. Ophthalmol. 2013, 251, 2711–2723. [Google Scholar] [CrossRef]
- Ma, L.; Yan, S.-F.; Huang, Y.-M.; Lu, X.-R.; Qian, F.; Pang, H.-L.; Xu, X.-R.; Zou, Z.-Y.; Dong, P.-C.; Xiao, X.; et al. Effect of Lutein and Zeaxanthin on Macular Pigment and Visual Function in Patients with Early Age-Related Macular Degeneration. Ophthalmology 2012, 119, 2290–2297. [Google Scholar] [CrossRef]
- Berrow, E.J.; Bartlett, H.E.; Eperjesi, F.; Gibson, J.M. The Effects of a Lutein-Based Supplement on Objective and Subjective Measures of Retinal and Visual Function in Eyes with Age-Related Maculopathy—A Randomised Controlled Trial. Br. J. Nutr. 2013, 109, 2008–2014. [Google Scholar] [CrossRef]
- García-Layana, A.; Recalde, S.; Alamán, A.S.; Robredo, P.F. Effects of Lutein and Docosahexaenoic Acid Supplementation on Macular Pigment Optical Density in a Randomized Controlled Trial. Nutrients 2013, 5, 543–551. [Google Scholar] [CrossRef]
- Parravano, M.; Tedeschi, M.; Manca, D.; Costanzo, E.; Di Renzo, A.; Giorno, P.; Barbano, L.; Ziccardi, L.; Varano, M.; Parisi, V. Effects of Macuprev® Supplementation in Age-Related Macular Degeneration: A Double-Blind Randomized Morpho-Functional Study Along 6 Months of Follow-Up. Adv. Ther. 2019, 36, 2493–2505. [Google Scholar] [CrossRef]
- Weigert, G.; Kaya, S.; Pemp, B.; Sacu, S.; Lasta, M.; Werkmeister, R.M.; Dragostinoff, N.; Simader, C.; Garhöfer, G.; Schmidt-Erfurth, U.; et al. Effects of Lutein Supplementation on Macular Pigment Optical Density and Visual Acuity in Patients with Age-Related Macular Degeneration. Invest. Ophthalmol. Vis. Sci. 2011, 52, 8174–8178. [Google Scholar] [CrossRef]
- Querques, G.; Merle, B.M.J.; Pumariega, N.M.; Benlian, P.; Delcourt, C.; Zourdani, A.; Leisy, H.B.; Lee, M.D.; Smith, R.T.; Souied, E.H. Dynamic Drusen Remodelling in Participants of the Nutritional AMD Treatment-2 (NAT-2) Randomized Trial. PLoS ONE 2016, 11, e0149219. [Google Scholar] [CrossRef] [PubMed]
- Allegrini, D.; Raimondi, R.; Angi, M.; Ricciardelli, G.; Montericcio, A.; Borgia, A.; Romano, M.R. Curcuma-Based Nutritional Supplement in Patients with Neovascular Age-Related Macular Degeneration. J. Med. Food 2021, 24, 1191–1196. [Google Scholar] [CrossRef] [PubMed]
- Piermarocchi, S.; Saviano, S.; Parisi, V.; Tedeschi, M.; Panozzo, G.; Scarpa, G.; Boschi, G.; Lo Giudice, G.; Carmis Study Group. Carotenoids in Age-Related Maculopathy Italian Study (CARMIS): Two-Year Results of a Randomized Study. Eur. J. Ophthalmol. 2011, 22, 216–225. [Google Scholar] [CrossRef]
- Querques, G.; Benlian, P.; Chanu, B.; Portal, C.; Coscas, G.; Soubrane, G.; Souied, E.H. Nutritional AMD Treatment Phase I (NAT-1): Feasibility of Oral DHA Supplementation in Age-Related Macular Degeneration. Eur. J. Ophthalmol. 2009, 19, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, H.E.; Eperjesi, F. Effect of Lutein and Antioxidant Dietary Supplementation on Contrast Sensitivity in Age-Related Macular Disease: A Randomized Controlled Trial. Eur. J. Clin. Nutr. 2007, 61, 1121–1127. [Google Scholar] [CrossRef]
- Murray, I.J.; Makridaki, M.; van der Veen, R.L.P.; Carden, D.; Parry, N.R.A.; Berendschot, T.T.J.M. Lutein Supplementation over a One-Year Period in Early AMD Might Have a Mild Beneficial Effect on Visual Acuity: The CLEAR Study. Invest. Ophthalmol. Vis. Sci. 2013, 54, 1781–1788. [Google Scholar] [CrossRef] [PubMed]
- Beatty, S.; Chakravarthy, U.; Nolan, J.M.; Muldrew, K.A.; Woodside, J.V.; Denny, F.; Stevenson, M.R. Secondary Outcomes in a Clinical Trial of Carotenoids with Coantioxidants versus Placebo in Early Age-Related Macular Degeneration. Ophthalmology 2013, 120, 600–606. [Google Scholar] [CrossRef]
- Piatti, A.; Croce, A.; Mazzacane, D.; Traina, G.; Ambrosino, L.; Boni, L.; Lisi, L.; Cascella, M.C.; Grunberger, A. Effect of 2-Year Nutritional Supplementation on Progression of Age-Related Macular Degeneration. Eur. J. Ophthalmol. 2020, 30, 376–381. [Google Scholar] [CrossRef]
- Souied, E.H.; Delcourt, C.; Querques, G.; Bassols, A.; Merle, B.; Zourdani, A.; Smith, T.; Benlian, P.; Nutritional AMD Treatment 2 Study Group. Oral Docosahexaenoic Acid in the Prevention of Exudative Age-Related Macular Degeneration: The Nutritional AMD Treatment 2 Study. Ophthalmology 2013, 120, 1619–1631. [Google Scholar] [CrossRef]
- Newsome, D.A. A Randomized, Prospective, Placebo-Controlled Clinical Trial of a Novel Zinc-Monocysteine Compound in Age-Related Macular Degeneration. Curr. Eye Res. 2008, 33, 591–598. [Google Scholar] [CrossRef]
- Lai, T.Y.Y.; Chan, W.-M.; Lai, R.Y.K.; Ngai, J.W.S.; Li, H.; Lam, D.S.C. The Clinical Applications of Multifocal Electroretinography: A Systematic Review. Surv. Ophthalmol. 2007, 52, 61–96. [Google Scholar] [CrossRef]
- Berg, H. Carotenoid Interactions. Nutr. Rev. 1999, 57, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Castenmiller, J.J.M.; West, C.E. Bioavailability and Bioconversion of Carotenoids. Annu. Rev. Nutr. 1998, 18, 19–38. [Google Scholar] [CrossRef] [PubMed]
- Moran, N.E.; Mohn, E.S.; Hason, N.; Erdman, J.W.; Johnson, E.J. Intrinsic and Extrinsic Factors Impacting Absorption, Metabolism, and Health Effects of Dietary Carotenoids. Adv. Nutr. 2018, 9, 465–492. [Google Scholar] [CrossRef] [PubMed]
- Bone, R.A.; Landrum, J.T.; Mayne, S.T.; Gomez, C.M.; Tibor, S.E.; Twaroska, E.E. Macular Pigment in Donor Eyes with and without AMD: A Case-Control Study. Investig. Ophthalmol. Vis. Sci. 2001, 42, 235–240. [Google Scholar]
- Vishwanathan, R.; Chung, M.; Johnson, E.J. A Systematic Review on Zinc for the Prevention and Treatment of Age-Related Macular Degeneration. Investig. Ophthalmol. Vis. Sci. 2013, 54, 3985–3998. [Google Scholar] [CrossRef] [PubMed]
- Blasiak, J.; Pawlowska, E.; Chojnacki, J.; Szczepanska, J.; Chojnacki, C.; Kaarniranta, K. Zinc and Autophagy in Age-Related Macular Degeneration. Int. J. Mol. Sci. 2020, 21, 4994. [Google Scholar] [CrossRef] [PubMed]
- Peddada, K.V.; Brown, A.; Verma, V.; Nebbioso, M. Therapeutic Potential of Curcumin in Major Retinal Pathologies. Int. Ophthalmol. 2019, 39, 725–734. [Google Scholar] [CrossRef]
- Metzler, M.; Pfeiffer, E.; Schulz, S.I.; Dempe, J.S. Curcumin Uptake and Metabolism. BioFactors 2013, 39, 14–20. [Google Scholar] [CrossRef]
- Salehi, B.; Mishra, A.; Nigam, M.; Sener, B.; Kilic, M.; Sharifi-Rad, M.; Fokou, P.; Martins, N.; Sharifi-Rad, J. Resveratrol: A Double-Edged Sword in Health Benefits. Biomedicines 2018, 6, 91. [Google Scholar] [CrossRef]
- Gliemann, L.; Nyberg, M.; Hellsten, Y. Effects of Exercise Training and Resveratrol on Vascular Health in Aging. Free. Radic. Biol. Med. 2016, 98, 165–176. [Google Scholar] [CrossRef]
- Klein, R.; Klein, B.E.K.; Knudtson, M.D.; Wong, T.Y.; Cotch, M.F.; Liu, K.; Burke, G.; Saad, M.F.; Jacobs, D.R. Prevalence of Age-Related Macular Degeneration in 4 Racial/Ethnic Groups in the Multi-Ethnic Study of Atherosclerosis. Ophthalmology 2006, 113, 373–380. [Google Scholar] [CrossRef]
- Fenwick, E.K.; Man, R.E.K.; Cheung, C.M.G.; Sabanayagam, C.; Cheng, C.-Y.; Neelam, K.; Chua, J.; Gan, A.T.L.; Mitchell, P.; Wong, T.Y.; et al. Ethnic Differences in the Association Between Age-Related Macular Degeneration and Vision-Specific Functioning. JAMA Ophthalmol. 2017, 135, 469–476. [Google Scholar] [CrossRef][Green Version]
- DeAngelis, M.M.; Owen, L.A.; Morrison, M.A.; Morgan, D.J.; Li, M.; Shakoor, A.; Vitale, A.; Iyengar, S.; Stambolian, D.; Kim, I.K.; et al. Genetics of Age-Related Macular Degeneration (AMD). Hum. Mol. Genet. 2017, 26, R45–R50. [Google Scholar] [CrossRef] [PubMed]
- Warwick, A.; Lotery, A. Genetics and Genetic Testing for Age-Related Macular Degeneration. Eye 2018, 32, 849–857. [Google Scholar] [CrossRef]
- Zhang, Q.-Y.; Tie, L.-J.; Wu, S.-S.; Lv, P.-L.; Huang, H.-W.; Wang, W.-Q.; Wang, H.; Ma, L. Overweight, Obesity, and Risk of Age-Related Macular Degeneration. Invest. Ophthalmol. Vis. Sci. 2016, 57, 1276–1283. [Google Scholar] [CrossRef]
- Jaisankar, D.; Swaminathan, G.; Roy, R.; Kulothungan, V.; Sharma, T.; Raman, R. Association of Obesity and Age-Related Macular Degeneration in Indian Population. Indian J. Ophthalmol. 2018, 66, 976–983. [Google Scholar] [CrossRef] [PubMed]
- Ng Yin Ling, C.; Lim, S.C.; Jonas, J.B.; Sabanayagam, C. Obesity and Risk of Age-Related Eye Diseases: A Systematic Review of Prospective Population-Based Studies. Int. J. Obes. 2021, 45, 1863–1885. [Google Scholar] [CrossRef]
- McGuinness, M.B.; Le, J.; Mitchell, P.; Gopinath, B.; Cerin, E.; Saksens, N.T.M.; Schick, T.; Hoyng, C.B.; Guymer, R.H.; Finger, R.P. Physical Activity and Age-Related Macular Degeneration: A Systematic Literature Review and Meta-Analysis. Am. J. Ophthalmol. 2017, 180, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Yip, J.L.Y.; Muthy, Z.; Peto, T.; Lotery, A.; Foster, P.J.; Patel, P. Socioeconomic Risk Factors and Age-Related Macular Degeneration in the UK Biobank Study. BMJ Open Ophthalmol. 2021, 6, e000585. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Cotch, M.F.; Ryskulova, A.; Primo, S.A.; Nair, P.; Chou, C.-F.; Geiss, L.S.; Barker, L.E.; Elliott, A.F.; Crews, J.E.; et al. Vision Health Disparities in the United States by Race/Ethnicity, Education, and Economic Status: Findings from Two Nationally Representative Surveys. Am. J. Ophthalmol. 2012, 154, S53–S62.e1. [Google Scholar] [CrossRef] [PubMed]
- French, S.A.; Tangney, C.C.; Crane, M.M.; Wang, Y.; Appelhans, B.M. Nutrition Quality of Food Purchases Varies by Household Income: The SHoPPER Study. BMC Public Health 2019, 19, 231. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Erfurth, U.; Chong, V.; Loewenstein, A.; Larsen, M.; Souied, E.; Schlingemann, R.; Eldem, B.; Monés, J.; Richard, G.; Bandello, F. Guidelines for the Management of Neovascular Age-Related Macular Degeneration by the European Society of Retina Specialists (EURETINA). Br. J. Ophthalmol. 2014, 98, 1144–1167. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, E.B.; Farah, M.E.; Maia, M.; Penha, F.M.; Regatieri, C.; Melo, G.B.; Pinheiro, M.M.; Zanetti, C.R. Therapeutic Monoclonal Antibodies in Ophthalmology. Prog. Retin. Eye Res. 2009, 28, 117–144. [Google Scholar] [CrossRef]
- van Asten, F.; Michels, C.T.J.; Hoyng, C.B.; van der Wilt, G.J.; Klevering, B.J.; Rovers, M.M.; Grutters, J.P.C. The Cost-Effectiveness of Bevacizumab, Ranibizumab and Aflibercept for the Treatment of Age-Related Macular Degeneration—A Cost-Effectiveness Analysis from a Societal Perspective. PLoS ONE 2018, 13, e0197670. [Google Scholar] [CrossRef]
- Chew, E.Y.; Clemons, T.E.; Agrón, E.; Domalpally, A.; Keenan, T.D.L.; Vitale, S.; Weber, C.; Smith, D.C.; Christen, W.; AREDS2 Research Group. Long-Term Outcomes of Adding Lutein/Zeaxanthin and ω-3 Fatty Acids to the AREDS Supplements on Age-Related Macular Degeneration Progression: AREDS2 Report 28. JAMA Ophthalmol. 2022, 140, 692–698. [Google Scholar] [CrossRef]
- Alpha-Tocopherol, Beta Carotene Cancer Prevention Study Group. The Effect of Vitamin E and Beta Carotene on the Incidence of Lung Cancer and Other Cancers in Male Smokers. N. Engl. J. Med. 1994, 330, 1029–1035. [Google Scholar] [CrossRef]
| First Author, Year of Publication, Country | Study Design | Sample Size (F/M) | Mean Age (SD) (Years) | AMD Stage | Dietary Supplements (Total Daily Dose) | Control | Outcome Measurements | Intervention Duration (Months) |
|---|---|---|---|---|---|---|---|---|
| Bartlett HE and Epejersi F 2007, UK [36] | double-masked RCT | I: 13 (7/6) C: 7 (4/3) | I: 69.2 (7.8) C: 69.2 (7.8) | ARM, atrophic AMD | 6 mg L, retinol, vitamin C, vitamin E, Zn, Cu | placebo (cellulose) | CS (Pelli-Robson chart) | 9 |
| Weigert G et al. 2011, Austria [31] | RCT | 116 (66/50) | 71.6 (8.6) | AREDS stages 2, 3 and 4 | month 1–3: 20 mg L month 4–6: 10 mg L | placebo | MPOD, VA, MDLT | 6 |
| * Ma L et al. 2012, China [27] | RCT | I1: 26 (16/10) I2: 27 (15/12) I3: 27 (15/12) C: 27 (16/11) | I1: 69.9 (8.4) I2: 69.0 (6.8) I3: 68.6 (7.0) C: 68.9 (7.6) | early AMD | I1: 10 mg L I2: 20 mg L I3: 10 mg L + 10 mg Z | placebo | MPOD, BCVA, CS, photo recovery time, Amsler grid | 10.5 |
| * Murray IJ et al. 2013, UK [37] | double-masked RCT | I: 36 (20/16) C: 37 (24/12) | I: 71.9 (8.7) C: 69.2 (8.6) | early AMD | 10 mg L | placebo soya bean oil capsula | MPOD, BCVA | 12 |
| * Richer SP et al. 2004, USA [22] | RCT | I1: 29(2/27) I2: 30(1/29) C: 31(1/30) | I1: 74.4 (6.4) I2: 73.5 (8.5) C: 76.1 (6.4) | atrophic AMD | I1: 10 mg L (FloraGlo) I2: 10 mg L, antioxidants, vitamins, minerals (OcuPower) | placebo (maltodextrin) | MPOD, near + distance VA, glare recovery, CS, AMD retinopathy differences | 12 |
| * Huang YM et al. 2015, China [23] | double-masked RCT | I1: 26 (17/9) I2: 27 (14/13) I3: 27 (15/12) C: 28 (17/11) | I1: 69.7 (8.3) I2: 69.3 (6.9) I3: 68.5 (6.9) C: 69 (7.5) | early AMD | I1: 10 mg L I2: 20 mg L I3: 10 mg L + 10 mg Z | placebo | MPOD, mfERG, Microperimetry | 24 |
| * Parisi V et al. 2008, Italy [24] | RCT | I: 15 (9/6) C1: 12 (6/6) C2: 15 (9/6) | I: 69.4 (4.3) C1: 69.7 (6.2) C2: 69.6 (5.1) | AREDS stage 3 | 10 mg L, 1 mg Z, 4 mg AX, vitamin C, vitamin E, Zn, Cu | C1: no supplements C2: healthy age-matched subjects | mfERG | 12 |
| Beatty S et al. 2012, Ireland [38] | double-masked RCT | I: 216 (124/92) C: 217 (124/93) | ≥55 (NA) | early AMD | 12 mg L, 0.6 mg Z, vitamin E, vitamin C, Zn, Cu gluconate | placebo | BCVA, CS (Pelli-Robson chart), AMD grade using fundus photographs, Raman spectroscopy counts | 12–36 |
| Piermarocchi S et al. 2011, Italy [34] | open-labeled RCT | I: 103 (62/41) C: 42 (25/17) | I: 72.5 (6.8) C: 72.6 (7.5) | dry AMD | 10 mg L, 1 mg Z, 4 mg AX, vitamin C, vitamin E, ZN, Cu, | no supplement | BCVA, CS (Pelli-Robson chart), visual function via the Italian-validated version of the 25-item NEI VFQ test | 24 |
| Parravano M et al. 2019, Italy [30] | double-masked RCT | I: 15 (NA) C: 15 (NA) | I: 68.5 (8.8) C: 70.1 (9.9) | AREDS stage 3 | 20 mg L, 4 mg Z, N-acetylcysteine, vitamins, minerals, rutin (2 tablets Marcuprev/day) | placebo with cellulose | mfERG and SD-OCT | 6 |
| Berrow EJ et al. 2013, UK [28] | blinded RCT | I: 8 (NA) C: 6 (NA) | I: 70 (7.5) C: 65.5 (9.3) | ARM | 12 mg L, 0.6 mg Z, EPA 240 mg, DHA 840 mg, Vitamin C, Cu oxide, Vitamin E, Zn (Ocuvite Duo) | placebo | mfERG, VA, CS | 13 |
| * Dawczynski J et al. 2013, Germany [26] | double-masked RCT | I1: 50 I2:55 C:40 overall: (79/66) | 69 (10) | dry AMD | I1: 10 mg L + 1 mg Z, antioxidants, DHA (1 tablet FloraGLO®/day) I2: 2 tablets FloraGLO®/day | placebo | MPOD, BCVA | 12 |
| * García-Layana A et al. 2013, Spain [29] | RCT | I: 23 (12/10) C: 21 (13/8) | I: 69.2 (7.8 SEM) C: 67.8 (9.2 SEM) | early AMD | 12 mg L, 0.6 mg Z, 280 mg of DHA | placebo (sugar) | MPOD, BCVA, CS, OCT | 12 |
| * Piatti A et al. 2020, Italy [39] | double-masked RCT | I: 48 (31/17) C: 26 (20/6) | I: 71.4 (6.5) C: 72.7 (5.5) | intermediate AMD | 10 mg L, 4 mg AX, 2 mg Z, vitamin C, vitamin E, Zn, Cu, fish oil 500 mg (EPA 185 mg + DHA 140 mg) | placebo | retinography, BCVA | 24 |
| Querques G et al. 2009, France [35] | Comparative pilot study | 38 (28/10) | 72.74 (6.25) | wet AMD | 720 mg EPA and 480 DHA mg (fish oil capsule) | no supplement | BCVA, FA, OCT | 6 |
| Querques G et al. 2016, France [32] | RCT | I: 87 (59/28) C: 80 (44/36) | I: 74.4 (6.7) C: 72.8 (6.9) | wet AMD in one eye, ARM in the study eye | 840 mg DHA, 270 mg EPA, 6 mg vitamin E | placebo (602 mg olive oil) | drusen burden and disease progression by fundus photography | 36 |
| Souied E et al. 2013, France [40] | RCT | I: 134 (92/42) C: 129 (78/51) | I: 73.9 (6.6) C: 73.2 (6.8) | wet AMD | 840 mg DHA, 270 mg EPA, 6 mg vitamin E per day (3 Reti-Nat1-capsules/day) | placebo (602 mg olive oil) | CNV progression + drusen formation by fundus photography, BCVA | 36 |
| Newsome D et al. 2008, USA [41] | RCT | I: 37 (30/7) C: 37 (29/8) | I: 72.1 (11.7) C: 73.3 (9.5) | dry AMD | 50 mg Zn (as monocysteine) | placebo (cellulose) | BCVA, CS, photo recovery time | 6 |
| AREDS research group et al. 2001, USA [13] | RCT | I1: 936 (55/881) I2: 897 (57/840) I3: 882 (56/826) C: 894 (56/838) | I1: 69 (NA) I2: 70 (NA) I3: 69 (NA) C: 69 (NA) | all 4 AREDS stages | I1: antioxidants 500 mg Vitamin C, 400 IU vitamin E, 15 mg Beta carotene I2: 80 mg Zn, 2 mg Cu oxide I3: antioxidants + Zn | placebo | fundus photographs | follow-up for 6.3 years |
| Allegrini D et al. 2021, Italy [33] | Controlled retrospective study | I: 18 (6/12) C: 24 (10/14) | I: 80 (75–87 IQR) C: 80 (78–86 IQR) | wet AMD | 50 mg of curcumin, AREDS2 components, 4 mg AX, 20 mg resveratrol | no supplement, intravitreal injections of anti-VEGF (aflibercept) | BCVA, CMT | 6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Csader, S.; Korhonen, S.; Kaarniranta, K.; Schwab, U. The Effect of Dietary Supplementations on Delaying the Progression of Age-Related Macular Degeneration: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 4273. https://doi.org/10.3390/nu14204273
Csader S, Korhonen S, Kaarniranta K, Schwab U. The Effect of Dietary Supplementations on Delaying the Progression of Age-Related Macular Degeneration: A Systematic Review and Meta-Analysis. Nutrients. 2022; 14(20):4273. https://doi.org/10.3390/nu14204273
Chicago/Turabian StyleCsader, Susanne, Sonja Korhonen, Kai Kaarniranta, and Ursula Schwab. 2022. "The Effect of Dietary Supplementations on Delaying the Progression of Age-Related Macular Degeneration: A Systematic Review and Meta-Analysis" Nutrients 14, no. 20: 4273. https://doi.org/10.3390/nu14204273
APA StyleCsader, S., Korhonen, S., Kaarniranta, K., & Schwab, U. (2022). The Effect of Dietary Supplementations on Delaying the Progression of Age-Related Macular Degeneration: A Systematic Review and Meta-Analysis. Nutrients, 14(20), 4273. https://doi.org/10.3390/nu14204273







