Impact of Transferrin Saturation and Anemia on Radial Artery Calcification in Patients with End-Stage Kidney Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Overview
2.2. Procedures
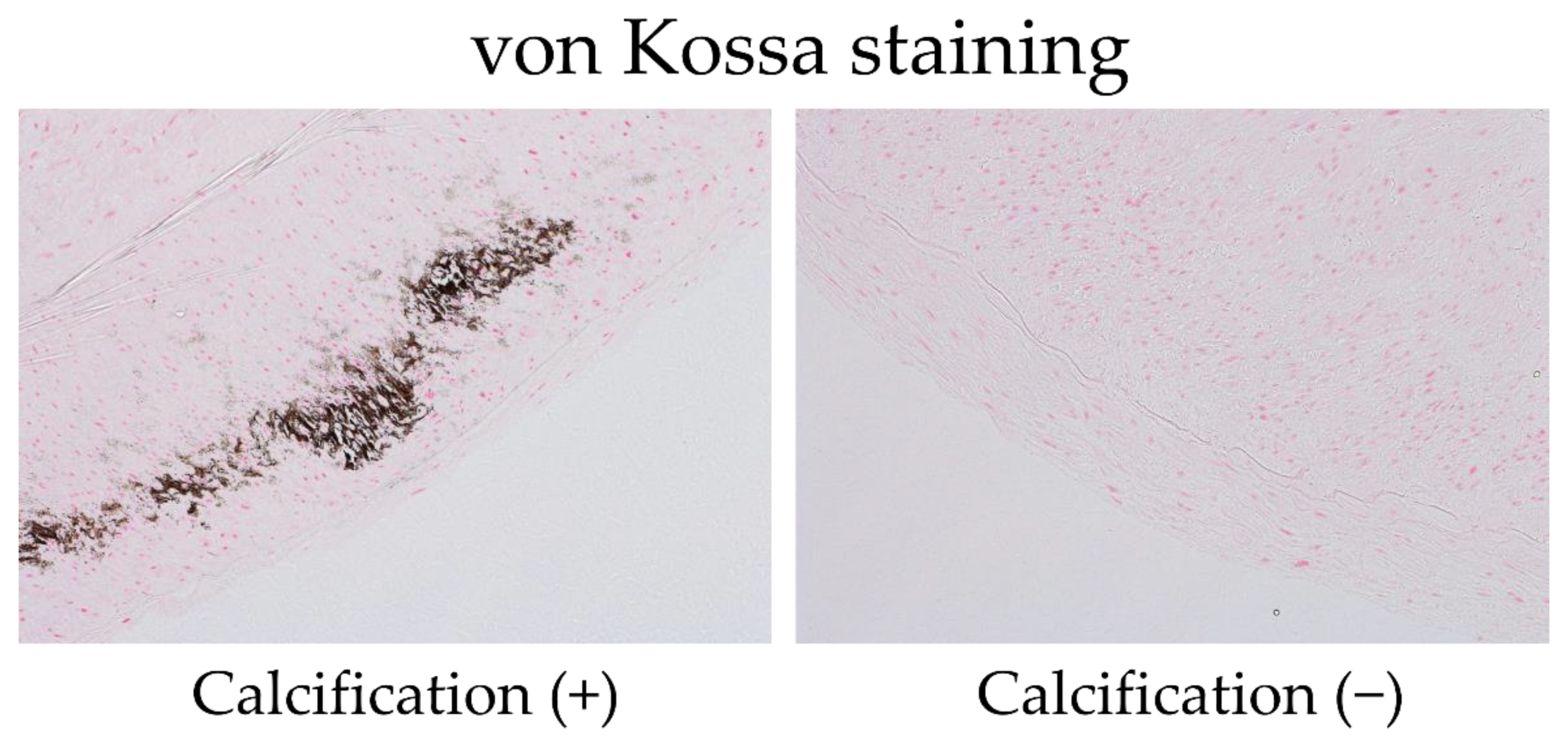
2.3. Pathological Studies of Arterial Specimens
2.4. Surgical Technique
2.5. Follow-Up
2.6. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Factors Contributing to Radial Artery Calcification
3.3. Clinical CVE/AVF Outcomes after the AVF Operation
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lanzer, P.; Boehm, M.; Sorribas, V.; Thiriet, M.; Janzen, J.; Zeller, T.; St Hilaire, C.; Shanahan, C. Medial Vascular Calcification Revisited: Review and Perspectives. Eur. Heart J. 2014, 35, 1515–1525. [Google Scholar] [CrossRef]
- Shigematsu, T.; Sonou, T.; Ohya, M.; Yokoyama, K.; Yoshida, H.; Yokoo, T.; Okuda, K.; Masumoto, A.R.; Iwashita, Y.; Iseki, K.; et al. Preventive Strategies for Vascular Calcification in Patients with Chronic Kidney Disease. Contrib. Nephrol. 2017, 189, 169–177. [Google Scholar] [CrossRef]
- Ohya, M.; Otani, H.; Kimura, K.; Saika, Y.; Fujii, R.; Yukawa, S.; Shigematsu, T. Vascular Calcification Estimated by Aortic Calcification Area Index Is a Significant Predictive Parameter of Cardiovascular Mortality in Hemodialysis Patients. Clin. Exp. Nephrol. 2011, 15, 877–883. [Google Scholar] [CrossRef]
- Blacher, J.; Guerin, A.P.; Pannier, B.; Marchais, S.J.; London, G.M. Arterial Calcifications, Arterial Stiffness, and Cardiovascular Risk in End-Stage Renal Disease. Hypertension 2001, 38, 938–942. [Google Scholar] [CrossRef]
- Block, G.A.; Hulbert-Shearon, T.E.; Levin, N.W.; Port, F.K. Association of Serum Phosphorus and Calcium × Phosphate Product with Mortality Risk in Chronic Hemodialysis Patients: A National Study. Am. J. Kidney Dis. 1998, 31, 607–617. [Google Scholar] [CrossRef]
- Yamada, S.; Tokumoto, M.; Tatsumoto, N.; Taniguchi, M.; Noguchi, H.; Nakano, T.; Masutani, K.; Ooboshi, H.; Tsuruya, K.; Kitazono, T. Phosphate Overload Directly Induces Systemic Inflammation and Malnutrition as Well as Vascular Calcification in Uremia. Am. J. Physiol. Ren. Physiol. 2014, 306, F1418–F1428. [Google Scholar] [CrossRef]
- Chavkin, N.W.; Chia, J.J.; Crouthamel, M.H.; Giachelli, C.M. Phosphate Uptake-Independent Signaling Functions of the Type III Sodium-Dependent Phosphate Transporter, PiT-1, in Vascular Smooth Muscle Cells. Exp. Cell Res. 2015, 333, 39–48. [Google Scholar] [CrossRef]
- Steitz, S.A.; Speer, M.Y.; Curinga, G.; Yang, H.Y.; Haynes, P.; Aebersold, R.; Schinke, T.; Karsenty, G.; Giachelli, C.M. Smooth Muscle Cell Phenotypic Transition Associated with Calcification: Upregulation of Cbfa1 and Downregulation of Smooth Muscle Lineage Markers. Circ. Res. 2001, 89, 1147–1154. [Google Scholar] [CrossRef]
- Seto, T.; Hamada, C.; Tomino, Y. Suppressive Effects of Iron Overloading on Vascular Calcification in Uremic Rats. J. Nephrol. 2014, 27, 135–142. [Google Scholar] [CrossRef]
- Schaeffer, C.; Thomassin, L.; Rochette, L.; Connat, J.L. Apoptosis Induced in Vascular Smooth Muscle Cells by Oxidative Stress Is Partly Prevented by Pretreatment with CGRP. Ann. N. Y. Acad. Sci. 2003, 1010, 733–737. [Google Scholar] [CrossRef]
- Sandberg, E.M.; Sayeski, P.P. Jak2 Tyrosine Kinase Mediates Oxidative Stress-Induced Apoptosis in Vascular Smooth Muscle Cells. J. Biol. Chem. 2004, 279, 34547–34552. [Google Scholar] [CrossRef]
- Kempe, D.S.; Lang, P.A.; Duranton, C.; Akel, A.; Lang, K.S.; Huber, S.M.; Wieder, T.; Lang, F. Enhanced programmed cell death of iron-deficient erythrocytes. FASEB J. 2006, 20, 368–370. [Google Scholar] [CrossRef]
- Watanabe, Y.; Yamagata, K.; Nishi, S.; Hirakata, H.; Hanafusa, N.; Saito, C.; Hattori, M.; Itami, N.; Komatsu, Y.; Kawaguchi, Y.; et al. Japanese society for dialysis therapy clinical guideline for “hemodialysis initiation for maintenance hemodialysis”. Ther. Apher. Dial. 2015, 19, 93–107. [Google Scholar] [CrossRef]
- Mune, S.; Shibata, M.; Hatamura, I.; Saji, F.; Okada, T.; Maeda, Y.; Sakaguchi, T.; Negi, S.; Shigematsu, T. Mechanism of Phosphate-Induced Calcification in Rat Aortic Tissue Culture: Possible Involvement of Pit-1 and Apoptosis. Clin. Exp. Nephrol. 2009, 13, 571–577. [Google Scholar] [CrossRef]
- Zarjou, A.; Jeney, V.; Arosio, P.; Poli, M.; Zavaczki, E.; Balla, G.; Balla, J. Ferritin Ferroxidase Activity: A Potent Inhibitor of Osteogenesis. J. Bone Miner. Res. 2010, 25, 164–172. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Regidor, D.L.; McAllister, C.J.; Michael, B.; Warnock, D.G. Time-Dependent Associations between Iron and Mortality in Hemodialysis Patients. J. Am. Soc. Nephrol. 2005, 16, 3070–3080. [Google Scholar] [CrossRef]
- Pollak, V.E.; Lorch, J.A.; Shukla, R.; Satwah, S. The Importance of Iron in Long-Term Survival of Maintenance Hemodialysis Patients Treated with Epoetin-Alfa and Intravenous Iron: Analysis of 9.5 Years of Prospectively Collected Data. BMC Nephrol. 2009, 10, 6. [Google Scholar] [CrossRef]
- Ueda, N.; Takasawa, K. Impact of Inflammation on Ferritin, Hepcidin and the Management of Iron Deficiency Anemia in Chronic Kidney Disease. Nutrients 2018, 10, 1173. [Google Scholar] [CrossRef]
- Ketteler, M.; Bongartz, P.; Westenfeld, R.; Wildberger, J.E.; Mahnken, A.H.; Böhm, R.; Metzger, T.; Wanner, C.; Jahnen-Dechent, W.; Floege, J. Association of Low Fetuin-A (AHSG) Concentrations in Serum with Cardiovascular Mortality in Patients on Dialysis: A Cross-Sectional Study. Lancet 2003, 361, 827–833. [Google Scholar] [CrossRef]
- Moriguchi, R.; Otaki, Y.; Hazeki, S.; Shimada, T.; Matsumoto, A.; Kakita, N.; Kaibe, S.; Kuragano, T.; Nonoguchi, H.; Masayoshi, N.; et al. High levels of tumor necrosis factor-alpha downregulate antimicrobial iron transport protein, Nramp1, in chronic hemodialysis patients: A key factor for infection risk. Am. J. Nephrol. 2012, 35, 372–378. [Google Scholar] [CrossRef]
- Alayoud, A.; El Amrani, M.; Belarbi, M.; El Kharras, A.; Chtioui, M.; Elfilali, K. Risk Factors for Progression of Coronary Artery Calcification Over 5 Years in Hemodialysis Patients. Ann. Cardiol. Angeiol. 2020, 69, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Mokas, S.; Larivière, R.; Lamalice, L.; Gobeil, S.; Cornfield, D.N.; Agharazii, M.; Richard, D.E. Hypoxia-Inducible Factor-1 Plays a Role in Phosphate-Induced Vascular Smooth Muscle Cell Calcification. Kidney Int. 2016, 90, 598–609. [Google Scholar] [CrossRef]
- Okazaki, M.; Komatsu, M.; Kawaguchi, H.; Tsuchiya, K.; Nitta, K. Erythropoietin Resistance Index and the All-Cause Mortality of Chronic Hemodialysis Patients. Blood Purif. 2014, 37, 106–112. [Google Scholar] [CrossRef]
- Bae, M.N.; Kim, S.H.; Kim, Y.O.; Jin, D.C.; Song, H.C.; Choi, E.J.; Kim, Y.L.; Kim, Y.S.; Kang, S.W.; Kim, N.H.; et al. Association of Erythropoietin-Stimulating Agent Responsiveness with Mortality in Hemodialysis and Peritoneal Dialysis Patients. PLoS ONE 2015, 10, e0143348. [Google Scholar] [CrossRef]
- Mizuiri, S.; Nishizawa, Y.; Doi, T.; Yamashita, K.; Shigemoto, K.; Usui, K.; Arita, M.; Naito, T.; Doi, S.; Masaki, T. Iron, Coronary Artery Calcification, and Mortality in Patients Undergoing Hemodialysis. Ren. Fail. 2021, 43, 371–380. [Google Scholar] [CrossRef]
- Shroff, R.C.; Shanahan, C.M. The Vascular Biology of Calcification. Semin. Dial. 2007, 20, 103–109. [Google Scholar] [CrossRef]
- Kalra, S.S.; Shanahan, C.M. Vascular Calcification and Hypertension: Cause and Effect. Ann. Med. 2012, 44 (Suppl. S1), S85–S92. [Google Scholar] [CrossRef]
- Hénaut, L.; Chillon, J.M.; Kamel, S.; Massy, Z.A. Updates on the Mechanisms and the Care of Cardiovascular Calcification in Chronic Kidney Disease. Semin. Nephrol. 2018, 38, 233–250. [Google Scholar] [CrossRef]
- van Popele, N.M.; Grobbee, D.E.; Bots, M.L.; Asmar, R.; Topouchian, J.; Reneman, R.S.; Hoeks, A.P.G.; van der Kuip, D.A.M.; Hofman, A.; Witteman, J.C.M. Association between Arterial Stiffness and Atherosclerosis: The Rotterdam Study. Stroke 2001, 32, 454–460. [Google Scholar] [CrossRef]
- Allon, M.; Robbin, M.L.; Umphrey, H.R.; Young, C.J.; Deierhoi, M.H.; Goodman, J.; Hanaway, M.; Lockhart, M.E.; Barker-Finkel, J.; Litovsky, S. Preoperative Arterial Microcalcification and Clinical Outcomes of Arteriovenous Fistulas for Hemodialysis. Am. J. Kidney Dis. 2015, 66, 84–90. [Google Scholar] [CrossRef]
- Io, H.; Nakata, J.; Inoshita, H.; Ishizaka, M.; Tomino, Y.; Suzuki, Y. Relationship among Left Ventricular Hypertrophy, Cardiovascular Events, and Preferred Blood Pressure Measurement Timing in Hemodialysis Patients. J. Clin. Med. 2020, 9, 3512. [Google Scholar] [CrossRef] [PubMed]
- Chertow, G.M.; Burke, S.K.; Raggi, P.; Treat to Goal Working Group. Sevelamer attenuates the progression of coronary and aortic calcification in hemodialysis patients. Kidney Int. 2002, 62, 245–252. [Google Scholar] [CrossRef] [PubMed]


| Characteristics | Patients with RAC | Patients without RAC | p-Values | All Patients |
|---|---|---|---|---|
| (at the initiation of HD) | n = 29 | n = 35 | ||
| Age (year) | 64.2 ± 16.9 | 69.1 ± 11.2 | 0.17 | 66.8 ± 14.1 |
| Male sex (%) | 75.8 | 65.7 | 0.37 | 70.3 |
| DM (%) | 51.7 | 34.4 | 0.18 | 42.6 |
| Past CVD event (%) | 34.5 | 42.9 | 0.5 | 39.1 |
| sBP (mmHg) | 142.0 ± 18.4 | 131.8 ± 17.1 | <0.05 | 136.6 ± 17.8 |
| dBP (mmHg) | 67.4 ± 11.4 | 66.1 ± 7.6 | 0.59 | 66.7 ± 9.6 |
| Hb (g/dL) | 8.8 ± 1.2 | 9.5 ± 1.2 | <0.05 | 9.1 ± 1.2 |
| Fe (μg/dL) | 43.3 ± 16.7 | 54.5 ± 34.3 | 0.11 | 49.4 ± 28.1 |
| TSAT (%) | 20.1 ± 8.1 | 26.0 ± 13.0 | <0.05 | 23.1 ± 11.0 |
| Ferritin (ng/mL) | 174.5 ± 144.1 | 186.9 ± 221.2 | 0.79 | 181.3 ± 190.2 |
| Albumin (g/dL) | 3.07 ± 0.53 | 3.16 ± 0.48 | 0.5 | 3.12 ± 0.50 |
| Creatinine (mg/dL) | 7.88 ± 2.16 | 7.16 ± 1.91 | 0.17 | 7.49 ± 2.05 |
| eGFR (mL/min/1.73 m2) | 6.25 ± 1.67 | 6.61 ± 2.09 | 0.46 | 6.50 ± 1.94 |
| HsCRP (mg/dL) | 0.47 ± 0.86 | 0.65 ± 1.10 | 0.52 | 0.57 ± 0.99 |
| Int PTH (pg/dL) | 251.8 ± 197.1 | 180.6 ± 141.7 | 0.09 | 212.8 ± 168 |
| Alkaline phosphatase (U/L) | 233.9 ± 78.1 | 330.0 ± 419.2.4 | 0.23 | 286.5 ± 314.8 |
| Correct calcium (mg/dL) | 9.06 ± 0.43 | 9.19 ± 0.67 | 0.37 | 9.12 ± 0.57 |
| Phosphorous (mg/dL) | 5.42 ± 1.43 | 4.80 ± 1.05 | <0.05 | 5.09 ± 1.25 |
| Total cholesterol (mg/dL) | 160.6 ± 37.6 | 154.1 ± 35.1 | 0.5 | 157.2 ± 36.4 |
| LDL cholesterol (mg/dL) | 84.7 ± 5.14 | 78.8 ± 4.97 | 0.41 | 81.7 ± 27.7 |
| HDL cholesterol (mg/dL) | 42.7 ± 13.9 | 43.9 ± 14.4 | 0.73 | 43.3 ± 14.2 |
| Triglyceride (mg/dL) | 117.9 ± 56.3 | 106.3 ± 33.8 | 0.33 | 111.9 ± 46.1 |
| Oral iron use (%) | 20.7 | 17.1 | 0.64 | 18.6 |
| Phosphate binder use (%) | 44.8 | 25.7 | 0.11 | 34.4 |
| ESA use (%) | 86.2 | 77.1 | 0.68 | 81.3 |
| RAS-I use (%) | 72.4 | 71.4 | 0.70 | 71.9 |
| Duration of ESKD (mouth) | 21.6 ± 3.36 | 22.6 ± 2.91 | 0.83 | 22.1 ± 2.19 |
| Univariate | Multivariate | |||
|---|---|---|---|---|
| Parameters | HR (95% CI) | p-Value | HR (95% CI) | p-Value |
| Age (per 1 year) | 0.975 (0.941–1.011) | 0.16 | ||
| Male versus female | 0.61 (0.203–1.833) | 0.37 | ||
| DM | 2.05 (0.729–5.734) | 0.17 | ||
| Past CVD event | 0.702 (0.253–1.940) | 0.49 | ||
| sBP (per 1 mmHg) | 1.033 (1.0025–1.0654) | 0.0328 | 1.037 (1.001–1.073) | 0.0328 |
| dBP (per 1 mmHg) | 1.015 (0.962–1.071) | 0.581 | ||
| Hb (per 1 g/dL) | 0.597 (0.371–0.960) | 0.023 | 0.516 (0.278–0.959) | 0.0217 |
| Fe (per 1 μg/dL) | 0.984 (0.965–1.004) | 0.11 | ||
| TSAT (per 1%) | 0.0043 (0.000018–1.00687) | 0.03 | 0.0012 (0.0000248–0.597) | 0.0173 |
| Ferritin (per 1 ng/mL) | 0.999 (0.997–1.002) | 0.199 | ||
| Albumin (per 1 g/dL) | 0.702 (0.253–1.946) | 0.494 | ||
| HsCRP (per 1 mg/dL) | 0.831 (0.474–1.456) | 0.508 | ||
| Int PTH (per 1 pg/dL) | 1.0026 (0.999–1.0057) | 0.091 | ||
| Alkaline phosphatase (per 1 U/L) | 0.998 (0.996–1.0011) | 0.191 | ||
| Correct Calcium (per 1 mg/dL) | 0.654 (0.260–1.643) | 0.358 | ||
| Phosphorous (per 1 mg/dL) | 1.53 (0.972–2.417) | 0.051 | 1.51 (0.818–2.775) | 0.1638 |
| Total cholesterol (per 1 mg/dL) | 1.005 (0.991–1.019) | 0.491 | ||
| LDL cholesterol (per 1 mg/dL) | 1.008 (0.989–1.027) | 0.401 | ||
| HDL cholesterol (per 1 mg/dL) | 0.993 (0.958–1.030) | 0.722 | ||
| Triglyceride (per 1 mg/dL) | 1.006 (0.994–1.017) | 0.324 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kano, T.; Io, H.; Nakata, J.; Sasaki, Y.; Muto, M.; Shimizu, Y.; Fukao, Y.; Fukuzaki, H.; Maeda, T.; Hosoya, R.; et al. Impact of Transferrin Saturation and Anemia on Radial Artery Calcification in Patients with End-Stage Kidney Disease. Nutrients 2022, 14, 4269. https://doi.org/10.3390/nu14204269
Kano T, Io H, Nakata J, Sasaki Y, Muto M, Shimizu Y, Fukao Y, Fukuzaki H, Maeda T, Hosoya R, et al. Impact of Transferrin Saturation and Anemia on Radial Artery Calcification in Patients with End-Stage Kidney Disease. Nutrients. 2022; 14(20):4269. https://doi.org/10.3390/nu14204269
Chicago/Turabian StyleKano, Toshiki, Hiroaki Io, Junichiro Nakata, Yu Sasaki, Masahiro Muto, Yuki Shimizu, Yusuke Fukao, Haruna Fukuzaki, Takuya Maeda, Reina Hosoya, and et al. 2022. "Impact of Transferrin Saturation and Anemia on Radial Artery Calcification in Patients with End-Stage Kidney Disease" Nutrients 14, no. 20: 4269. https://doi.org/10.3390/nu14204269
APA StyleKano, T., Io, H., Nakata, J., Sasaki, Y., Muto, M., Shimizu, Y., Fukao, Y., Fukuzaki, H., Maeda, T., Hosoya, R., & Suzuki, Y. (2022). Impact of Transferrin Saturation and Anemia on Radial Artery Calcification in Patients with End-Stage Kidney Disease. Nutrients, 14(20), 4269. https://doi.org/10.3390/nu14204269







