The Impact of Volume Overload on the Longitudinal Change of Adipose and Lean Tissue Mass in Incident Chinese Peritoneal Dialysis Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Data Collection
2.3. Echocardiography
2.4. Body Composition Measurement
2.5. Statistical Analyses
3. Results
3.1. Baseline Characteristics of Study Participants
3.2. Relationship between Body Composition and Cardiac Markers
3.3. Change in Body Composition over Time
3.4. Predictors of Change in Lean Tissue and Fat Tissue Mass
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, P.K.-T.; Lu, W.; Mak, S.-K.; Boudville, N.; Yu, X.; Wu, M.J.; Cheng, Y.-L.; Chan, C.T.; Goh, B.L.; Tian, N.; et al. Peritoneal dialysis first policy in Hong Kong for 35 years: Global impact. Nephrology 2022, 27, 787–794. [Google Scholar] [CrossRef] [PubMed]
- Li, P.K.; Chow, K.M. Peritoneal dialysis-first policy made successful: Perspectives and actions. Am. J. Kidney Dis. 2013, 62, 993–1005. [Google Scholar] [CrossRef] [PubMed]
- Brown, E.A.; Blake, P.G.; Boudville, N.; Davies, S.; de Arteaga, J.; Dong, J.; Finkelstein, F.; Foo, M.; Hurst, H.; Johnson, D.W.; et al. International Society for Peritoneal Dialysis practice recommendations: Prescribing high-quality goal-directed peritoneal dialysis. Perit. Dial. Int. 2020, 40, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Kramer, H.J.; Saranathan, A.; Luke, A.; Durazo-Arvizu, R.A.; Guichan, C.; Hou, S.; Cooper, R. Increasing body mass index and obesity in the incident ESRD population. J. Am. Soc. Nephrol. 2006, 17, 1453–1459. [Google Scholar] [CrossRef]
- Than, W.H.; Ng, J.K.; Chan, G.C.; Fung, W.W.; Chow, K.M.; Szeto, C.C. The change in the prevalence of obesity and new-onset diabetes in Chinese peritoneal dialysis patients over 25 years. Clin. Kidney J. 2022, 15, 70–78. [Google Scholar] [CrossRef]
- Ng, J.K.-C.; Than, W.H.; Szeto, C.C. Obesity, Weight Gain, and Fluid Overload in Peritoneal Dialysis. Front. Nephrol. 2022, 2, 880097. [Google Scholar] [CrossRef]
- Adequacy of dialysis and nutrition in continuous peritoneal dialysis: Association with clinical outcomes. Canada-USA (CANUSA) Peritoneal Dialysis Study Group. J. Am. Soc. Nephrol. 1996, 7, 198–207. [CrossRef]
- Szeto, C.C.; Wong, T.Y.; Leung, C.B.; Wang, A.Y.; Law, M.C.; Lui, S.F.; Li, P.K. Importance of dialysis adequacy in mortality and morbidity of chinese CAPD patients. Kidney Int. 2000, 58, 400–407. [Google Scholar] [CrossRef]
- Ikizler, T.A.; Burrowes, J.D.; Byham-Gray, L.D.; Campbell, K.L.; Carrero, J.-J.; Chan, W.; Fouque, D.; Friedman, A.N.; Ghaddar, S.; Goldstein-Fuchs, D.J.; et al. KDOQI Clinical Practice Guideline for Nutrition in CKD: 2020 Update. Am. J. Kidney Dis. 2020, 76, S1–S107. [Google Scholar] [CrossRef]
- Choi, S.J.; Kim, N.R.; Hong, S.A.; Lee, W.B.; Park, M.Y.; Kim, J.K.; Hwang, S.D.; Lee, H.K. Changes in body fat mass in patients after starting peritoneal dialysis. Perit. Dial. Int. 2011, 31, 67–73. [Google Scholar] [CrossRef]
- Jager, K.J.; Merkus, M.P.; Huisman, R.M.; Boeschoten, E.W.; Dekker, F.W.; Korevaar, J.C.; Tijssen, J.G.P.; Krediet, R.T. Nutritional Status over Time in Hemodialysis and Peritoneal Dialysis. J. Am. Soc. Nephrol. 2001, 12, 1272–1279. [Google Scholar] [CrossRef] [PubMed]
- Van Biesen, W.; Verger, C.; Heaf, J.; Vrtovsnik, F.; Britto, Z.M.L.; Do, J.Y.; Prieto-Velasco, M.; Martínez, J.P.; Crepaldi, C.; De Los Ríos, T.; et al. Evolution Over Time of Volume Status and PD-Related Practice Patterns in an Incident Peritoneal Dialysis Cohort. Clin. J. Am. Soc. Nephrol. 2019, 14, 882–893. [Google Scholar] [CrossRef] [PubMed]
- Ng, J.K.; Kwan, B.C.; Chow, K.M.; Pang, W.F.; Cheng, P.M.; Leung, C.B.; Li, P.K.; Szeto, C.C. Asymptomatic fluid overload predicts survival and cardiovascular event in incident Chinese peritoneal dialysis patients. PLoS ONE 2018, 13, e0202203. [Google Scholar] [CrossRef] [PubMed]
- Henriques, V.T.; Martinez, E.Z.; Divino-Filho, J.C.; Pecoits-Filho, R.; da Costa, J.A. Increase in BMI over time is associated with fluid overload and signs of wasting in incident peritoneal dialysis patients. J. Ren. Nutr. 2013, 23, e51–e57. [Google Scholar] [CrossRef]
- Ng, J.K.; Li, P.K. Fluid management and bioimpedance study in peritoneal dialysis. Curr. Opin. Nephrol. Hypertens 2019, 28, 58–64. [Google Scholar] [CrossRef]
- O’Lone, E.L.; Visser, A.; Finney, H.; Fan, S.L. Clinical significance of multi-frequency bioimpedance spectroscopy in peritoneal dialysis patients: Independent predictor of patient survival. Nephrol. Dial. Transpl. 2014, 29, 1430–1437. [Google Scholar] [CrossRef]
- Parthasarathy, R.; Oei, E.; Fan, S.L. Clinical value of body composition monitor to evaluate lean and fat tissue mass in peritoneal dialysis. Eur. J. Clin. Nutr. 2019, 73, 1520–1528. [Google Scholar] [CrossRef]
- Konings, C.J.; Kooman, J.P.; Schonck, M.; van Kreel, B.; Heidendal, G.A.; Cheriex, E.C.; van der Sande, F.M.; Leunissen, K.M. Influence of fluid status on techniques used to assess body composition in peritoneal dialysis patients. Perit. Dial. Int. 2003, 23, 184–190. [Google Scholar] [CrossRef]
- Andersen, O.S.; Smiseth, O.A.; Dokainish, H.; Abudiab, M.M.; Schutt, R.C.; Kumar, A.; Sato, K.; Harb, S.; Gude, E.; Remme, E.W.; et al. Estimating Left Ventricular Filling Pressure by Echocardiography. J. Am. Coll. Cardiol. 2017, 69, 1937–1948. [Google Scholar] [CrossRef]
- Lancellotti, P.; Galderisi, M.; Edvardsen, T.; Donal, E.; Goliasch, G.; Cardim, N.; Magne, J.; Laginha, S.; Hagendorff, A.; Haland, T.F.; et al. Echo-Doppler estimation of left ventricular filling pressure: Results of the multicentre EACVI Euro-Filling study. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 961–968. [Google Scholar] [CrossRef]
- Chamney, P.W.; Wabel, P.; Moissl, U.M.; Müller, M.J.; Bosy-Westphal, A.; Korth, O.; Fuller, N.J. A whole-body model to distinguish excess fluid from the hydration of major body tissues. Am. J. Clin. Nutr. 2007, 85, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Kam, K.; Ng, J.; Kwong, V.; Szeto, C.; Lee, A. The prognostic value of reduced global longitudinal strain (GLS) on cardiac outcomes in patients on peritoneal dialysis: A prospective study. Eur. Heart J. Cardiovasc. Imaging 2022, 23. [Google Scholar] [CrossRef]
- Beddhu, S.; Zeidel, M.L.; Saul, M.; Seddon, P.; Samore, M.H.; Stoddard, G.J.; Bruns, F.J. The effects of comorbid conditions on the outcomes of patients undergoing peritoneal dialysis. Am. J. Med. 2002, 112, 696–701. [Google Scholar] [CrossRef]
- Papakrivopoulou, E.; Lillywhite, S.; Davenport, A. Is N-terminal probrain-type natriuretic peptide a clinically useful biomarker of volume overload in peritoneal dialysis patients? Nephrol. Dial. Transpl. 2012, 27, 396–401. [Google Scholar] [CrossRef]
- Davenport, A. Changes in N-terminal pro-brain natriuretic peptide correlate with fluid volume changes assessed by bioimpedance in peritoneal dialysis patients. Am. J. Nephrol. 2012, 36, 371–376. [Google Scholar] [CrossRef]
- Bergström, J.; Heimbürger, O.; Lindholm, B. Calculation of the protein equivalent of total nitrogen appearance from urea appearance. Which formulas should be used? Perit. Dial. Int. 1998, 18, 467–473. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39.e14. [Google Scholar] [CrossRef]
- Xu, X.; Moissl, U.; Cai, H.; Zhang, W.; Lim, P.S.; Ha Phan, H.A.; Wabel, P.; Etter, M. Reference ranges for lean and fat tissue index (LTI, FTI) in a large Asian population (SUN-260). Kidney Int. Rep. 2019, 4, S267–S268. [Google Scholar] [CrossRef]
- Fiechter, M.; Fuchs, T.A.; Gebhard, C.; Stehli, J.; Klaeser, B.; Stahli, B.E.; Manka, R.; Manes, C.; Tanner, F.C.; Gaemperli, O.; et al. Age-related normal structural and functional ventricular values in cardiac function assessed by magnetic resonance. BMC Med. Imaging 2013, 13, 6. [Google Scholar] [CrossRef]
- Broers, N.J.H.; Canaud, B.; Dekker, M.J.E.; van der Sande, F.M.; Stuard, S.; Wabel, P.; Kooman, J.P. Three compartment bioimpedance spectroscopy in the nutritional assessment and the outcome of patients with advanced or end stage kidney disease: What have we learned so far? Hemodial. Int. 2020, 24, 148–161. [Google Scholar] [CrossRef]
- Popovic, V.; Zerahn, B.; Heaf, J.G. Comparison of Dual Energy X-ray Absorptiometry and Bioimpedance in Assessing Body Composition and Nutrition in Peritoneal Dialysis Patients. J. Ren. Nutr. 2017, 27, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Reis, N.; Vaninni, F.C.D.; Silva, M.Z.C.; de Oliveira, R.C.; Reis, F.M.; Costa, F.L.; Martin, L.C.; Barretti, P. Agreement of Single-Frequency Electrical Bioimpedance in the Evaluation of Fat Free Mass and Fat Mass in Peritoneal Dialysis Patients. Front. Nutr. 2021, 8, 686513. [Google Scholar] [CrossRef] [PubMed]
- Bhatla, B.; Moore, H.; Emerson, P.; Keshaviah, P.; Prowant, B.; Nolph, K.D.; Singh, A. Lean body mass estimation by creatinine kinetics, bioimpedance, and dual energy x-ray absorptiometry in patients on continuous ambulatory peritoneal dialysis. ASAIO J. 1995, 41, M442–M446. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.H.; Do, J.Y. Effects of volume status on body composition in incident peritoneal dialysis patients. Eur. J. Clin. Nutr. 2020, 74, 633–641. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Kim, J.K.; Lee, H.S.; Kim, S.G.; Song, Y.R. Longitudinal changes in body composition are associated with all-cause mortality in patients on peritoneal dialysis. Clin. Nutr. 2021, 40, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Law, S.; Davenport, A. Glucose absorption from peritoneal dialysate is associated with a gain in fat mass and a reduction in lean body mass in prevalent peritoneal dialysis patients. Br. J. Nutr. 2020, 123, 1269–1276. [Google Scholar] [CrossRef]
- Verger, C.; Ronco, C.; Van Biesen, W.; Heaf, J.; Vrtovsnik, F.; Vera Rivera, M.; Puide, I.; Azar, R.; Gauly, A.; Atiye, S.; et al. Association of Prescription With Body Composition and Patient Outcomes in Incident Peritoneal Dialysis Patients. Front. Med. 2021, 8, 737165. [Google Scholar] [CrossRef]
- Karava, V.; Stabouli, S.; Dotis, J.; Liakopoulos, V.; Papachristou, F.; Printza, N. Tracking hydration status changes by bioimpedance spectroscopy in children on peritoneal dialysis. Perit. Dial. Int. 2021, 41, 217–225. [Google Scholar] [CrossRef]
- Edefonti, A.; Carcano, A.; Damiani, B.; Ghio, L.; Consalvo, G.; Picca, M. Changes in body composition assessed by bioimpedance analysis in the first 6 months of chronic peritoneal dialysis. Adv. Perit. Dial. 1997, 13, 267–270. [Google Scholar]
- Ng, J.K.; Kwan, B.C.; Chan, G.C.; Chow, K.M.; Pang, W.F.; Cheng, P.M.; Leung, C.B.; Li, P.K.; Szeto, C.C. Predictors and prognostic significance of persistent fluid overload: A longitudinal study in Chinese peritoneal dialysis patients. Perit. Dial. Int. 2022, 08968608221110491. [Google Scholar] [CrossRef]
- Wang, X.H.; Mitch, W.E. Mechanisms of muscle wasting in chronic kidney disease. Nat. Rev. Nephrol. 2014, 10, 504–516. [Google Scholar] [CrossRef] [PubMed]
- Szeto, C.C.; McIntyre, C.W.; Li, P.K. Circulating Bacterial Fragments as Cardiovascular Risk Factors in CKD. J. Am. Soc. Nephrol. 2018, 29, 1601–1608. [Google Scholar] [CrossRef] [PubMed]
- Savica, V.; Calò, L.A.; Monardo, P.; Santoro, D.; Mallamace, A.; Muraca, U.; Bellinghieri, G. Salivary phosphorus and phosphate content of beverages: Implications for the treatment of uremic hyperphosphatemia. J. Ren. Nutr. 2009, 19, 69–72. [Google Scholar] [CrossRef][Green Version]
- Savica, V.; Santoro, D.; Monardo, P.; Mallamace, A.; Bellinghieri, G. Sevelamer carbonate in the treatment of hyperphosphatemia in patients with chronic kidney disease on hemodialysis. Ther. Clin. Risk Manag. 2008, 4, 821–826. [Google Scholar] [CrossRef] [PubMed]
- Stenvinkel, P.; Lindholm, B.; Lönnqvist, F.; Katzarski, K.; Heimbürger, O. Increases in serum leptin levels during peritoneal dialysis are associated with inflammation and a decrease in lean body mass. J. Am. Soc. Nephrol. 2000, 11, 1303–1309. [Google Scholar] [CrossRef] [PubMed]
- Chan, G.C.; Than, W.H.; Kwan, B.C.; Lai, K.B.; Chan, R.C.; Teoh, J.Y.; Ng, J.K.; Chow, K.M.; Fung, W.W.; Cheng, P.M.; et al. Adipose and serum zinc alpha-2-glycoprotein (ZAG) expressions predict longitudinal change of adiposity, wasting and predict survival in dialysis patients. Sci. Rep. 2022, 12, 9087. [Google Scholar] [CrossRef]
- Luce, M.; Barba, C.; Yi, D.; Mey, A.; Roussel, D.; Bres, E.; Benoit, B.; Pastural, M.; Granjon, S.; Szelag, J.C.; et al. Accumulation of natriuretic peptides is associated with protein energy wasting and activation of browning in white adipose tissue in chronic kidney disease. Kidney Int. 2020, 98, 663–672. [Google Scholar] [CrossRef]
- Paudel, K.; Visser, A.; Burke, S.; Samad, N.; Fan, S.L. Can Bioimpedance Measurements of Lean and Fat Tissue Mass Replace Subjective Global Assessments in Peritoneal Dialysis Patients? J. Ren. Nutr. 2015, 25, 480–487. [Google Scholar] [CrossRef]
- Kamimura, M.A.; Carrero, J.J.; Canziani, M.E.; Watanabe, R.; Lemos, M.M.; Cuppari, L. Visceral obesity assessed by computed tomography predicts cardiovascular events in chronic kidney disease patients. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 891–897. [Google Scholar] [CrossRef]
- Tian, N.; Yang, X.; Guo, Q.; Zhou, Q.; Yi, C.; Lin, J.; Cao, P.; Ye, H.; Chen, M.; Yu, X. Bioimpedance Guided Fluid Management in Peritoneal Dialysis: A Randomized Controlled Trial. Clin. J. Am. Soc. Nephrol. 2020, 15, 685–694. [Google Scholar] [CrossRef]
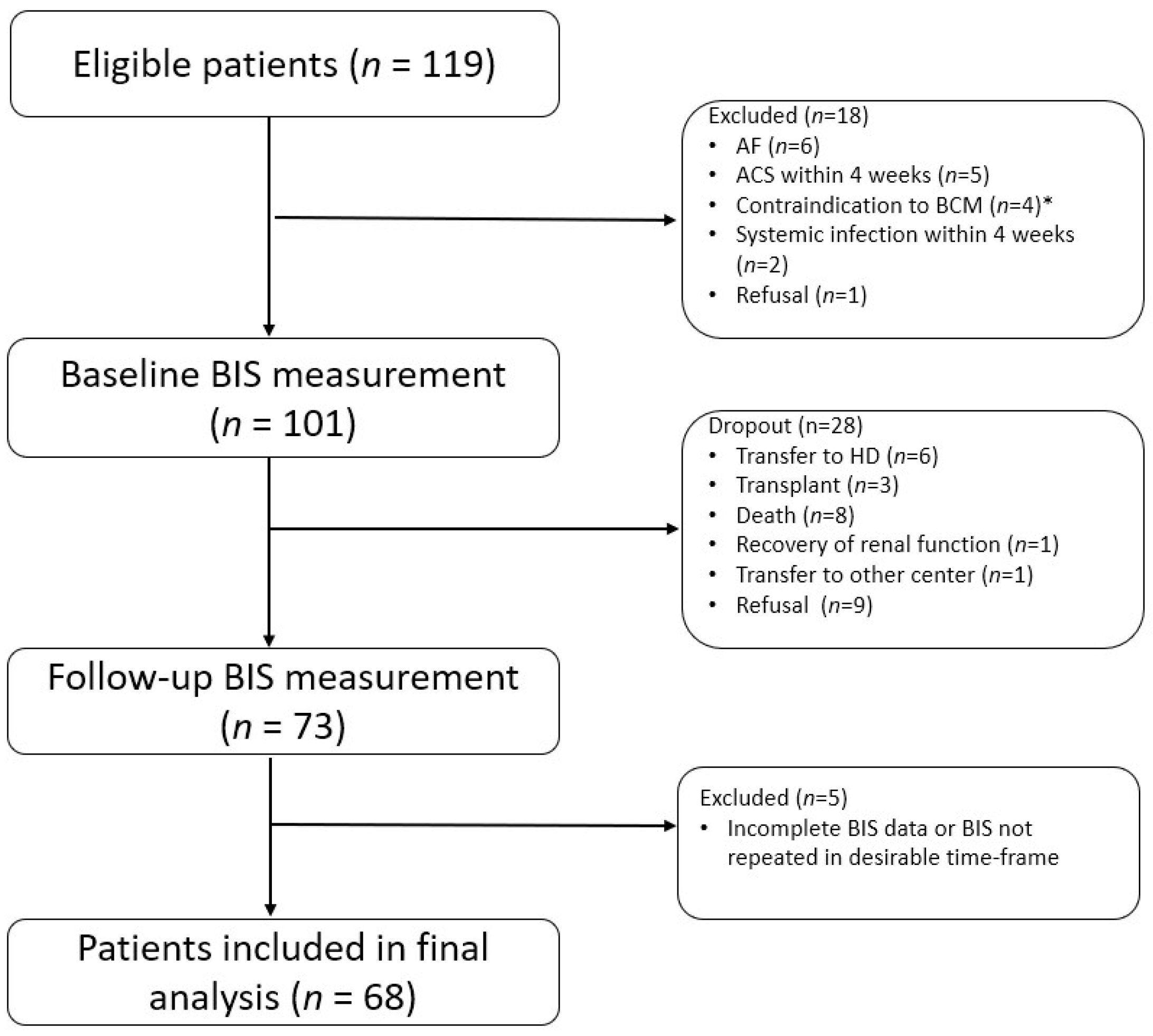

| Patients with Baseline BIS (n = 101) | Male (n = 54) | Female (n = 47) | p-Value * | |
|---|---|---|---|---|
| Age (year) | 59.4 ± 12.3 | 57.8 ± 12.7 | 61.2 ± 11.7 | 0.16 |
| Systolic blood pressure (mmHg) | 148.6 ± 18.4 | 146.8 ± 18.9 | 150.7 ± 17.7 | 0.29 |
| Diastolic blood pressure (mmHg) | 79.6 ± 11.3 | 80.1 ± 11.3 | 79.0 ± 11.5 | 0.66 |
| BW (kg) | 61.8 (53.9 to 75.6) | 67.3 (61.8 to 83.2) | 55.2 (50.9 to 58.9) | <0.0001 |
| BMI (kg/m2) | 23.9 (21.6 to 28.1) | 25.0 (22.4 to 30.2) | 22.7 (21.0 to 25.8) | 0.02 |
| Causes of renal failure, no. of cases (%) | 0.21 | |||
| Diabetic nephropathy | 55 (54.5%) | 33 (61.1%) | 22 (46.8%) | |
| Glomerulonephritis | 21 (20.8%) | 9 (16.7%) | 12 (25.5%) | |
| Hypertensive nephrosclerosis | 11 (10.9%) | 5 (9.3%) | 6 (12.8%) | |
| Polycystic kidney | 5 (5.0%) | 1 (1.9%) | 4 (8.5%) | |
| Comorbidities, no. of cases (%) | ||||
| Diabetes | 60 (59.4%) | 35 (64.8%) | 25 (53.2%) | 0.24 |
| Ischemic heart disease | 11 (10.9%) | 6 (11.1%) | 5 (10.6%) | 0.94 |
| Congestive heart failure | 8 (7.9%) | 5 (9.3%) | 3 (6.4%) | 0.72 |
| Cerebrovascular disease | 7 (6.9%) | 6 (11.1%) | 1 (2.1%) | 0.12 |
| Charlson’s Comorbidity Index | 5.2 ± 1.9 | 5.2 ± 1.8 | 5.1 ± 2.1 | 0.81 |
| Laboratory parameters | ||||
| Hemoglobin (g/dL) | 9.8 ± 1.4 | 10.0 ± 1.4 | 9.5 ± 1.5 | 0.10 |
| Albumin (g/L) | 31.7 ± 4.5 | 32.4 ± 4.3 | 30.8 ± 4.6 | 0.07 |
| Creatinine (µmol/L) | 792 (640 to 936) | 766 (615 to 950) | 824 (654 to 936) | 0.43 |
| Fasting glucose | 5.9 ± 3.0 | 5.7 ± 1.6 | 6.2 ± 4.0 | 0.42 |
| Total cholesterol (mmol/L) | 4.2 ± 1.2 | 3.7 ± 1.0 | 4.8 ± 1.2 | <0.0001 |
| C-reactive protein (mg/L) | 2.0 (0.7 to 5.8) | 1.7 (0.7 to 5.8) | 2.2 (0.8 to 6.0) | 0.80 |
| NT-proBNP (pg/mL) | 409.8 (184.8 to 857.4) | 382.3 (161.8 to 765.4) | 423.5 (206.7 to 1031.8) | 0.29 |
| Dialysis Characteristics | ||||
| Peritoneal glucose exposure (g/day) | 93.5 ± 21.6 | 92.9 ± 21.9 | 94.2 ± 21.4 | 0.75 |
| APD, no. of cases (%) | 22 (21.8%) | 13 (24.1%) | 9 (19.2%) | 0.55 |
| D/P creatinine at 4 h | 0.65 ± 0.12 | 0.64 ± 0.13 | 0.66 ± 0.11 | 0.34 |
| Dialysis adequacy | ||||
| Weekly total Kt/V | 2.04 (1.63 to 2.40) | 2.00 (1.65 to 2.23) | 2.04 (1.56 to 2.54) | 0.47 |
| Residual GFR (ml/min/1.73 m2) | 3.74 (1.71 to 6.55) | 5.68 (3.40 to 8.41) | 2.30 (1.48 to 3.84) | <0.0001 |
| Residual urine volume (L/day) | 1.15 ± 0.73 | 1.36 ± 0.78 | 0.92 ± 0.58 | 0.004 |
| NPNA (g/kg/day) | 1.05 ± 0.27 | 1.06 ± 0.25 | 1.04 ± 0.30 | 0.68 |
| Echocardiographic measurements | ||||
| EF (%) | 59.5 ± 6.7 | 58.8 ± 7.4 | 60.4 ± 5.8 | 0.26 |
| E/e’ | 13.8 (10.9 to 18.0) | 13.1 (9.9 to 17.3) | 15.4 (11.2 to 20.4) | 0.07 |
| LAVi (ml/m2) | 34.8 (27.9 to 43.4) | 34.9 (28.3 to 39.7) | 34.6 (27.9 to 43.7) | 0.92 |
| LVEDD (mm) | 46.4 ± 6.5 | 47.6 ± 6.9 | 44.9 ± 5.7 | 0.03 |
| Body composition | ||||
| OH (liter) | 3.3 (1.9 to 5.4) | 3.6 (2.4 to 5.5) | 3.2 (1.8 to 4.1) | 0.14 |
| RHI (%) | 20.2 ± 11.1 | 19.4 ± 10.8 | 21.1 ± 11.6 | 0.46 |
| LTI (kg/m2) | 14.3 ± 2.8 | 16.0 ± 2.4 | 12.4 ± 1.8 | <0.001 |
| FTI (kg/m2) | 9.1 ± 4.8 | 8.5 ± 4.6 | 9.7 ± 5.0 | 0.20 |
| Baseline Lean Tissue Index (kg/m2) | Baseline Fat Tissue Index (kg/m2) | Baseline Relative Hydration Index (%) | ||||
|---|---|---|---|---|---|---|
| β | p-Value | β | p-Value | β | p-Value | |
| E/e’ | 0.12 a | 0.11 | 0.05 a | 0.29 | 0.41 a | <0.0001 |
| LAVi | 0.07 a | 0.38 | −0.06 a | 0.38 | 0.35 a | <0.0001 |
| LVEDD | 0.07 a | 0.39 | −0.03 a | 0.72 | 0.25 a | 0.02 |
| EF (%) | 0.01 a | 0.90 | −0.03 a | 0.66 | −0.10 a | 0.32 |
| ln(NT-proBNP) | 0.01 b | 0.90 | −0.22 b | 0.03 | 0.52 b | <0.0001 |
| Change in LTI (kg/m2) | Change in FTI (kg/m2) | |||||||
|---|---|---|---|---|---|---|---|---|
| Univariable Analysis | Multivariable Analysis a | Univariable Analysis | Multivariable Analysis b | |||||
| β | p-Value | β (95%CI) | p-Value | β | p-Value | β (95% CI) | p-Value | |
| Demographics | ||||||||
| Age | 0.04 | 0.76 | - | - | −0.07 | 0.60 | - | - |
| Male gender | 0.08 | 0.52 | - | - | −0.05 | 0.69 | - | - |
| CCI | −0.14 | 0.27 | - | - | 0.12 | 0.35 | - | - |
| Laboratory parameters | ||||||||
| Albumin | 0.31 | 0.01 | 0.20 (−0.05–0.44) | 0.12 | −0.21 | 0.08 | - | - |
| ln(CRP) | −0.24 | 0.046 | −0.20 (−0.44–0.03) | 0.09 | 0.14 | 0.24 | - | - |
| FBG | −0.02 | 0.88 | - | - | 0.03 | 0.83 | - | - |
| Total cholesterol | −0.27 | 0.03 | −0.19 (−0.43–0.04) | 0.11 | 0.12 | 0.33 | - | - |
| Dialysis factors | ||||||||
| APD (vs. CAPD) | −0.02 | 0.91 | - | - | 0.04 | 0.74 | - | - |
| Peritoneal glucose exposure | −0.22 | 0.07 | - | - | 0.27 | 0.02 | - | - |
| D/PCr | −0.12 | 0.33 | - | - | 0.10 | 0.45 | - | - |
| Weekly total Kt/V | −0.09 | 0.49 | - | - | 0.14 | 0.26 | - | - |
| Residual urine volume | 0.10 | 0.41 | - | - | −0.16 | 0.19 | - | - |
| Body composition | ||||||||
| Baseline LTI | −0.11 | 0.39 | - | - | 0.06 | 0.63 | - | - |
| Baseline FTI | 0.22 | 0.07 | 0.26 (0.04–0.48) | 0.02 | −0.34 | 0.005 | −0.29 (−0.52–−0.06) | 0.01 |
| Baseline RHI | −0.30 | 0.01 | - | - | 0.33 | 0.01 | 0.28 (0.05–0.51) | 0.02 |
| Baseline BMI | 0.08 | 0.51 | - | - | −0.22 | 0.07 | - | - |
| Change in LTI (kg/m2) | Change in FTI (kg/m2) | |||||||
|---|---|---|---|---|---|---|---|---|
| Univariable Analysis | Multivariable Analysis a | Univariable Analysis | Multivariable Analysis b | |||||
| β | p-Value | β (95%CI) | p-Value | β | p-Value | β (95% CI) | p-Value | |
| Demographics | ||||||||
| Age | 0.04 | 0.76 | - | - | −0.07 | 0.60 | - | - |
| Male gender | 0.08 | 0.52 | - | - | −0.05 | 0.69 | - | - |
| Baseline CCI | −0.14 | 0.27 | - | - | 0.12 | 0.35 | - | - |
| Laboratory parameters | ||||||||
| Mean albumin | 0.33 | 0.01 | 0.21 (0.06–0.37) | 0.01 | −0.15 | 0.22 | - | - |
| Mean ln(CRP) | −0.29 | 0.02 | −0.12 (−0.28–0.03) | 0.10 | 0.20 | 0.11 | - | - |
| Mean FBG | 0.09 | 0.47 | - | - | −0.04 | 0.74 | - | - |
| Mean total cholesterol | −0.23 | 0.06 | - | - | 0.15 | 0.22 | - | - |
| Dialysis factors | ||||||||
| New APD user | −0.03 | 0.78 | - | - | 0.11 | 0.39 | - | - |
| New icodextrin user | −0.16 | 0.19 | - | - | 0.06 | 0.64 | - | - |
| Time-averaged peritoneal glucose exposure | −0.08 | 0.54 | - | - | 0.10 | 0.40 | - | - |
| Time-averaged weekly total Kt/V | −0.10 | 0.47 | - | - | 0.12 | 0.37 | - | - |
| Time-averaged residual urine volume | 0.07 | 0.60 | - | - | −0.03 | 0.83 | - | - |
| Body composition | ||||||||
| Change in LTI | - | - | - | - | −0.76 | <0.0001 | −0.72 (−0.76–−0.67) | <0.0001 |
| Change in FTI | −0.76 | <0.0001 | −0.71 (−0.87–−0.55) | <0.0001 | - | - | - | - |
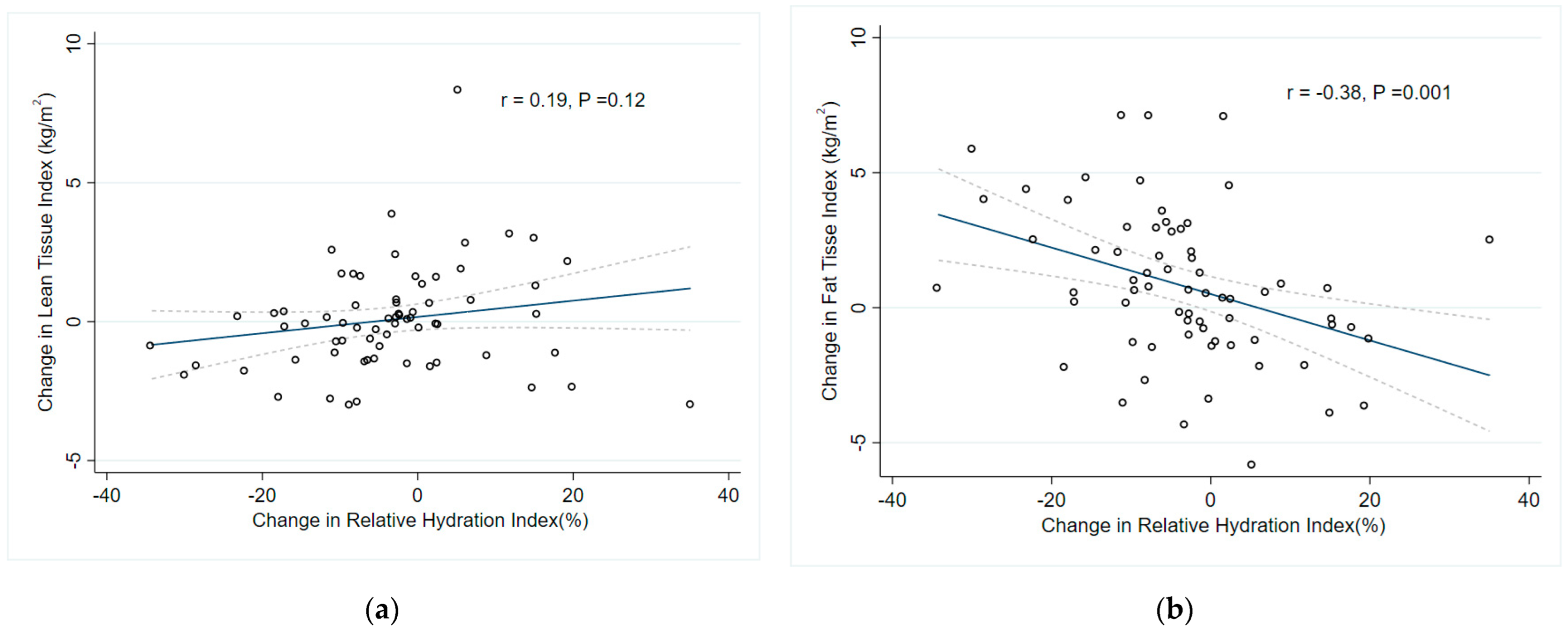
| Change in RHI | 0.19 | 0.12 | - | - | −0.38 | 0.001 | −0.35 (−0.37–−0.33) | <0.0001 |
| Change in BMI | 0.08 | 0.50 | - | - | 0.496 | <0.0001 | 0.59 (0.54–0.64) | <0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ng, J.K.-C.; Chan, G.C.-K.; Kam, K.K.-H.; Tian, N.; Than, W.H.; Cheng, P.M.-S.; Law, M.-C.; Pang, W.-F.; Szeto, C.-C.; Li, P.K.-T. The Impact of Volume Overload on the Longitudinal Change of Adipose and Lean Tissue Mass in Incident Chinese Peritoneal Dialysis Patients. Nutrients 2022, 14, 4076. https://doi.org/10.3390/nu14194076
Ng JK-C, Chan GC-K, Kam KK-H, Tian N, Than WH, Cheng PM-S, Law M-C, Pang W-F, Szeto C-C, Li PK-T. The Impact of Volume Overload on the Longitudinal Change of Adipose and Lean Tissue Mass in Incident Chinese Peritoneal Dialysis Patients. Nutrients. 2022; 14(19):4076. https://doi.org/10.3390/nu14194076
Chicago/Turabian StyleNg, Jack Kit-Chung, Gordon Chun-Kau Chan, Kevin Ka-Ho Kam, Na Tian, Win Hlaing Than, Phyllis Mei-Shan Cheng, Man-Ching Law, Wing-Fai Pang, Cheuk-Chun Szeto, and Philip Kam-Tao Li. 2022. "The Impact of Volume Overload on the Longitudinal Change of Adipose and Lean Tissue Mass in Incident Chinese Peritoneal Dialysis Patients" Nutrients 14, no. 19: 4076. https://doi.org/10.3390/nu14194076
APA StyleNg, J. K.-C., Chan, G. C.-K., Kam, K. K.-H., Tian, N., Than, W. H., Cheng, P. M.-S., Law, M.-C., Pang, W.-F., Szeto, C.-C., & Li, P. K.-T. (2022). The Impact of Volume Overload on the Longitudinal Change of Adipose and Lean Tissue Mass in Incident Chinese Peritoneal Dialysis Patients. Nutrients, 14(19), 4076. https://doi.org/10.3390/nu14194076







