Highlights
What are the main findings?
- The daily consumption of 40 ml of organic extra-virgin olive oil with a high minor polar compound content, composed mainly of hydroxytyrosol and oleocanthal, for 9 weeks significantly improved lipid and purine metabolism, atherogenic indices and body composition in patients with chronic kidney disease.
- The minor polar compounds of extra-virgin olive oil counteracted low-grade inflammation and oxidative stress in patients with chronic kidney disease.
What is the implication of the main finding?
- This study highlighted the novel cardioprotective action of extra-virgin olive oil in nephropathic patients.
Abstract
The high mortality related to chronic kidney disease (CKD) is not only due to the disease itself; in fact, CKD also represents an important risk factor for cardiovascular (CV) morbidity and mortality. Among the functional foods that seems to have cardioprotective action, extra virgin olive oil (EVOO) plays a pivotal health-promoting role. The aim of this study was to evaluate the possible cardioprotective effects of an EVOO containing a very high content (>900 ppm) of minor phenolic compounds (MPCs). The selected EVOO was analyzed by HPLC-DAD-MS to establish the MPC content. The Olea extract obtained from the selected EVOO was tested against the RAW 264.7 cell line in order to investigate its anti-inflammatory activity. We enrolled 40 CKD patients under conservative therapy for in vivo clinical testing. All CKD patients consumed 40 mL/day of raw EVOO for 9 weeks (T1). At baseline (T0) and at T1, we monitored the patients’ blood and urinary parameters. The patients’ body composition was assessed using bioelectrical impedance analysis and the carotid intima-media thickness (CIMT) using ultrasound imaging. At T1, we observed a decrease in inflammatory parameters, CIMT, and oxidative stress biomarkers. We also noticed improvements in lipid and purine metabolism, atherogenic indices, and body composition. Thus, this study highlighted the cardioprotective action of EVOO in nephropathic patients.
1. Introduction
The incidence of chronic kidney disease (CKD) has increased significantly in the last three decades due to the explosive spread of related risk factors such as arterial hypertension, diabetes mellitus, metabolic syndrome, etc. In 2016, CKD reached 13th place in the leading causes of death in the world, and it has been hypothesized that by 2040, it will be the 5th leading cause of death in the world [1]. The high mortality related to CKD cannot be explained only by the disease itself; rather, CKD also represents an important risk factor for cardiovascular (CV) mortality and morbidity [2,3]. In fact, the presence of CKD has been observed to be associate with the onset of a series of comorbidities that are risk factors for CV diseases. Among these comorbidities, special mention is due to alterations of calcium–phosphorus metabolism [4,5], hyperhomocysteinemia [6,7], the increase of asymmetric dimethylarginine (ADMA), malnutrition, uremic sarcopenia [8,9], the accumulation of toxic substances, including those derived from the gut microbiota [10,11], a low-grade chronic inflammatory state, and dyslipidaemia and metabolic acidosis [12,13,14,15]. All these factors contribute to the development of accelerated atherosclerosis in which the primum movens is represented by endothelial dysfunction (ED) [16,17]. Physiologically, the endothelium can be considered a “pervasive” organ that performs numerous functions, among which is the maintenance of vascular tone. In fact, it regulates vascular permeability, the immune response, and angiogenesis [17]. During CKD, ED is amplified and all the previously mentioned CV risk factors contribute to its genesis. As a matter of fact, an increase in ED prevalence was previously observed in relation to CKD stage [18]. Moreover, several studies have demonstrated a worsening of ED directly related to the reduction of the glomerular filtration rate (GFR) in CKD patients [17]. Low-grade chronic inflammatory states are due to an increased production of pro-inflammatory cytokines (interleukin-IL-6, tumor necrosis factor-TNF-α, IL-1β) in association with an enhanced formation of reactive oxygen species (ROS), with consequent induction of oxidative stress (OS). Both inflammatory states and OS cause a reduction in the bioavailability of nitric oxide (NO), a compound with antithrombotic, anti-inflammatory, and vasorelaxation action [18,19]. In particular, a NO reduction induced by the accumulation of NO synthetase endogenous inhibitors through the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB) pathway was observed in CKD patients [20]. Furthermore, increased OS provokes the glycation of proteins, carbohydrates, and lipids and leads to the formation of advanced glycation end products (AGEs), which appear to have a direct toxic effect on the myocardium [21]. AGEs also increase the ED phenomenon by inhibiting the enzyme dimethylarginine dimethylaminohydrolase (DDAH), which degrades the ADMA, an endogenous inhibitor of NO production via reduction of the activity of endothelial NO synthetase [22,23,24].
Extra virgin olive oil (EVOO) plays a pivotal role among the functional foods that seem to reduce the risk of CV morbidity and mortality. In fact, the European Prospective Investigation (EPIC)-Spain study [25], the PREvención con DIeta MEDditerránea (PREDIMED) study [26], and the EUROLIVE study [27] have all highlighted the important cardioprotective action of EVOO. This beneficial effect seems to be related to the presence of minor phenolic compounds (MPCs), such as oleocanthal, oleacin, tyrosol and hydroxytyrosol (HT), and monounsaturated fatty acids (MUFAs), in which EVOO is rich [28,29,30]. In fact, these compounds exert an anti-inflammatory, antioxidant, and antiatherogenic effect, reducing the global CV risk.
To date, we have tested two EVOOs other than the one selected for this clinical study. In particular, we previously administered two EVOOs to CKD patients—the first was characterized by a medium (>400 ppm) MPC content and the second one was characterized by a high (>700 ppm) MPC content—in order to assess EVOO’s potential beneficial effects on quality of life and on CKD-related comorbidities [31,32]. Moreover, in the study conducted by Noce et al. [32], we assessed whether the beneficial effects were maintained over time, namely after a two-month washout period. At this regard, we demonstrated that only the consumption of EVOO with a high MPC content resulted in the positive effects being maintained over time.
In the light of our previous results, in this study, we evaluated the possible cardioprotective action of an EVOO with a very high (>900 ppm) MPC content in CKD patients under conservative therapy. This EVOO has never been tested before in in vivo study. Furthermore, in this study, we examined several parameters that were not investigated in our previous studies, namely atherogenic indices, carotid intima-media thickness (CIMT), and some inflammatory biomarkers (namely TNF-α, platelet-to-lymphocyte ratio (PLR), neutrophil-to-lymphocyte ratio (NLR), and lymphocyte-to-monocyte ratio (LMR)). In addition, in this study, we conducted a preliminary in vitro test on an extract, rich in oleocanthal, obtained from the selected EVOO in order to evaluate the effective anti-inflammatory activity of our EVOO.
2. Materials and Methods
2.1. Selection and Characterization of the Organic EVOO
For this study, we selected an organic EVOO from Ophena (Abruzzo), a town in central Italy, with a very high content of active MPCs [31]. This EVOO is produced from a mix of two cultivars of Olea europaea L., namely Leccino and Intosso, which are widespread in the Abruzzo region.
This EVOO was produced using a biphasic and controlled pressing technology in order to preserve and maintain the very high content of active MPCs. The selected EVOO was analyzed using high-performance liquid chromatography coupled with diode array detector mass spectrometry (HPLC-DAD-MS) (Agilent Technologies, Palo Alto, CA, USA) to evaluate the contents of single active molecules such as HT, tyrosol, elenolic acid and its derivatives, oleacin, oleocanthal, oleuropein aglycone, secoiridoid derivatives, and lignans. The analyses were performed according to the protocol described by Romani et al. [31].
EVOO product analysis was carried out using the Oxitester system (CDR srl Florence, Italy) for parameters such as acidity (expressed as % of oleic acid), peroxides (expressed as meq O2/kg), and total polyphenols (expressed in mg of tyrosol) [31].
2.1.1. Olea Extract
The used EVOO for the extraction had a total MPC content of 1145.95 mg/kg. The extract tested in this study was obtained using a liquid/liquid multiple extraction method starting with 10 L of EVOO. The selected EVOO blend was extracted with a 70% EtOH-H2O solution adjusted to pH 3.2 with formic acid in the dark for 30 min at room temperature in a mechanical orbital shaker. The solution was transferred to a separating funnel to remove the lipid portion with hexane. The liquid extract was dried under vacuum (Laborota 4000, Heidolph, Schwabach, Germany) and dissolved in 50 mL of 70% EtOH-H2O solution adjusted to pH 3.2 with formic acid. The obtained extract was mixed and dried again and then dissolved in 50 mL of H2O. The final redissolved extract was then degreased twice with hexane directly in the test tube and subjected to HPLC-DAD-MS analysis for qualitative and quantitative characterization.
2.1.2. HPLC-DAD-MS Analysis of Olea Extract
The analyses for the qualitative and quantitative characterization of bioactive compounds of the Olea extract were achieved using an HP-1260 liquid chromatograph equipped with a DAD (Agilent-Technologies, Palo Alto, CA, USA). The HPLC system was interfaced with an Agilent MS system equipped with an ESI source (Agilent Corp, Santa Clara, CA, USA). The analyses were acquired in full-scan mode and the mass range was set to m/z 100–1500 in negative mode. The analytical columns and chromatographic methods used were described in a previous study of ours [31]. Polyphenols found in the extract were identified by comparing retention times and UV/Vis spectra with those of the authentic standards. Each compound was quantified at the selected wavelength (240, 280, 330, 350 nm) using a five-point regression curve and applying the correction of the molecular weights [31].
2.2. In Vitro Study
Murine macrophage RAW 264.7 cell line was purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA). Cells were cultured in Dulbecco’s modified Eagle’s medium/high glucose (DMEM 4500, EuroClone, Milan, Italy) supplemented with 10% fetal bovine serum (FBS, EuroClone) and maintained at 37 °C in a humidified atmosphere containing 90% air and 10% CO2. Cells were harvested from subconfluent cultures via incubation with a trypsin-EDTA solution (EuroClone) and propagated every three days. Viability of the cells was determined by trypan blue exclusion test. Cultures were periodically monitored for Mycoplasma contamination using Chen’s fluorochrome test, as described in a previous study of ours [33].
2.2.1. MTT Assay
Cell viability was assessed using a 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay (Sigma Aldrich, Milan, Italy). Cells were placed into 96-well plates in complete medium in the absence and in the presence of different concentrations of oleocanthal standard (oleo std) or Olea extract that was rich in oleocanthal fraction (oleo fr) for 72 h. Next, MTT reagent was added to the medium and incubated at 37 °C. After 2 h, the blue MTT–formazan product was solubilized with dimethyl sulfoxide (DMSO, Sigma Aldrich) and its absorbance was read at 595 nm using a microplate reader (Bio-Rad, Milan, Italy), as previously described [34].
2.2.2. Nitrite Assay
NO metabolite concentration was measured in the culture medium of RAW cells, using the Griess reaction, which is specific for nitrite dosage. First, 100 µL of cell culture medium from RAW264.7 cells was mixed with an equal volume of Griess reagent (1% sulfanilamide, 0.1% N-1-naphthalenediamine dihydrochloride, and 2.5% H3PO4) and transferred to 96-well plates. Plates were incubated at room temperature for 10 min. The absorbance was then measured at 540 nm in a microplate reader (BioTek, Winooski, VT, USA). The amount of nitrite in the media was calculated from the sodium nitrite (NaNO2) standard curve. Results were normalized to protein concentration. For RAW264.7 cells, nitrite production was expressed with reference to lipopolysaccharide (LPS; Sigma) as 100% [33].
2.2.3. Western Blotting Analysis
Cells were lysed and separated using electrophoresis as previously described in the literature [35]. The primary antibodies were as follows: rabbit anti-inducible NO syntethase (iNOS) (1:1000, Cell Signaling Technology, Danvers, MA, USA) and rabbit anti-cyclooxigenase (COX)-2 (1:1000, Cell Signaling Technology, Danvers, MA, USA). The membrane was washed in T-PBS buffer, incubated for 1 h at room temperature with goat anti-rabbit IgG Alexa Fluor 750 antibody or with goat anti-mouse IgG Alexa Fluor 680 antibody (Invitrogen, Monza, Italy), and then visualized using an Odyssey Infrared Imaging System (LI-COR® Bioscience, Lincoln, NE, USA). Mouse anti-alpha tubulin monoclonal antibody (1:1000, Cell Signaling Technology) was used to confirm that equal amounts of protein were loaded into each lane.
2.3. In Vivo Study
The aim of in vivo study was to evaluate the potential beneficial effects of an organic EVOO with a very high content of MPCs (>900 ppm) in CKD patients under conservative therapy.
2.3.1. Sample Size
In this study, we enrolled 40 CKD patients under conservative therapy distributed according to a Gauss curve of known mean and unknown variance. Therefore, the aforementioned sample size of 40 patients derived from the following probabilistic considerations:
- The null hypothesis was rejected if the mean of the population from which the sample was extracted differed from the sample mean by an amount, expressed in absolute value, equal to or greater than 46.2% of the standard deviation;
- For the hypotheses’ (null and alternative) verification, the one-tailed Gauss z test with α = 0.05 (first type error) and β = 0.10 (second type error) was adopted in order to give a test power equal to 90%.
2.3.2. Enrolled Patients
Consequently, 40 CKD patients under conservative therapy, stage I-IV (according to kidney disease: improving global outcomes—KDIGO guidelines [36]) were enrolled at the Hypertension and Nephrology Unit of Policlinico Tor Vergata (PTV), Rome (Italy). The inclusion criteria of study population were as follows: acceptance and signature of informed consent, age between 18 and 80 years, both sexes, and CKD stage I-IV. The exclusion criteria were as follows: the presence of either solid or hematological malignancies in the active phase, or of inflammatory and/or infectious pathologies.
At the time of enrolment, all patients were instructed to consume 40 mL/day of the EVOO selected for clinical experimentation. The EVOO was to be consumed raw in the patients’ culinary preparations [37]. The study lasted 9 weeks. At baseline (T0) and after 9 weeks (T1), all the enrolled patients underwent blood and urinary analysis, body composition assessment via bioelectrical impedance analysis (BIA), Doppler ultrasound imaging of epiaortic vessels, monitoring of systolic and diastolic blood pressure, and evaluation of style and quality of life through specific questionnaires described below. The study protocol was declared to be compliant with the Helsinki Declaration by the PTV Independent Ethics Committee (protocol number 36/20). The flowchart of the study is represented in Figure 1.
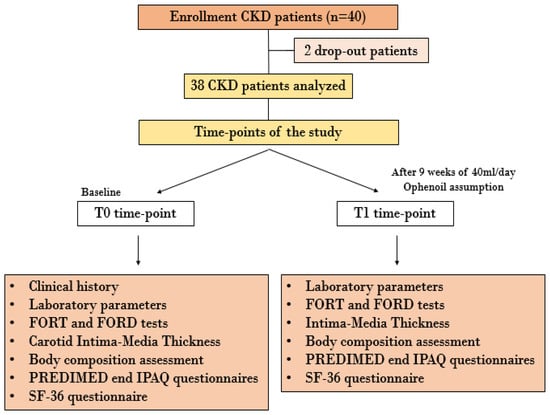
Figure 1.
Flowchart of in vivo study. Abbreviations: CKD, chronic kidney disease; FORD, Free Oxygen Radical Defense; FORT, Free Oxygen Radical Test; IPAQ, International Physical Activity Questionnaires; PREDIMED, Preventiòn con dieta Mediterrànea; SF-36, Short Form Health Survey-36.
2.3.3. Blood and Urinary Analysis
At baseline (T0) and after 9 weeks (T1), all patients were assessed via blood and urinary analysis. In particular, glycemia, uricemia, intact parathyroid hormone, serum albumin, renal function parameters (like creatininemia, azotemia, albuminuria), electrolytes (like potassium, calcium, phosphorus, sodium), and lipid profile (like total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), and triglycerides) were detected. Moreover, the patients underwent evaluation of inflammatory status via C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), IL-6, TNF-α, PLR, NLR, and LMR and evaluation of OS biomarkers (like free oxygen radical defense (FORD) and free oxygen radical test (FORT)). In particular, the last ones allowed us to evaluate the antioxidant defenses and OS of the patients by means of capillary sampling, with a CR4000 instrument used for analysis [38].
2.3.4. Atherogenic Indices
At both time-points of the study, we calculated the atherogenic indices. In particular, we calculated TC/HDL-C, LDL-C/HDL-C, and log(triglycerides/HDL-C) indices. These indices can provide additional information regarding CV risk, as they are closely related to the patient lipid profile [39].
2.3.5. Body Composition Assessment
At T0 and T1, all patients underwent an anthropometric parameter assessment. Body weight was measured to the nearest 0.01 Kg using a balance (Seca 711, Hamburg, Germany), and height was measured using a stadiometer to the nearest 0.1 cm (Seca 220, Hamburg, Germany). Subsequently, the body mass index (BMI) was calculated as weight divided by the height squared.
Moreover, the study population underwent BIA to evaluate the body composition at T0 and at T1. The BIA parameters were determined at 50 KHz frequency, using a BIA 101 S instrument (Akern/RIL System, Florence, Italy). In particular, resistance, reactance, phase angle, fat mass (FM), fat-free mass (FFM), intracell water (ICW), extracell water (ECW), total body water (TBW), body cellular mass (BCM), body cellular mass index (BCMI), and basal metabolic rate (BMR) were evaluated [40].
2.3.6. Doppler Ultrasound of Epiaortic Vessels
All nephropathic patients underwent Doppler ultrasound to estimate CIMT. This examination was done using a MyLab70 VXG ultrasound device (Esaote, Genova, Italy) with a linear LA523 probe, at a 2–9 MHz frequency range. All examinations were conducted by the same operator (A.N.) to rule out operator-dependent bias. The CIMT was measured through a B-mode ultrasound examination at the level of the right common carotid artery. A longitudinal section of the right common carotid artery was obtained; three different CIMT measurements were performed, about 1 cm below the bifurcation, in the plaque-free area on the distal wall of the right common carotid artery, using a semiautomatic application. The CIMT was calculated as the average value of the three different measurements. This procedure was executed in accordance with the Mannheim protocol [41].
2.3.7. Questionnaires
Three questionnaires were administered to all enrolled patients, evaluating both their lifestyle (PREDIMED and International Physical Activity Questionnaires-IPAQ) and their quality of life (Short Form 36 Health Survey-SF-36), at T0 and at T1.
The lifestyle questionnaires were administered in order to exclude any bias due to lifestyle changes during the study period. In particular, the PREDIMED questionnaire monitors eating habits and adherence to the Mediterranean diet, while the IPAQ monitors the degree of physical activity of the patient [26,42].
The SF-36 questionnaire evaluates the quality of life as perceived by the patient through 36 questions regarding nine areas: general health, health change, physical functioning, pain, social functioning, emotional well-being, energy/fatigue, role limitation due to physical health, and role limitation due to emotional problems.
2.3.8. Statistical Analysis
All data were entered into an Excel spreadsheet (Microsoft, Redmond, Washington, DC, USA) and the analyses were done using the Windows Social Science Statistics Package, version 25.0 (IBM_SPSS, Chicago, IL, USA).
The descriptive analysis results are represented as mean ± standard deviation (SD) for the parameters with normal distributions (after confirmation with the histograms and the Kolgomorov–Smirnov test), while for the non-normal variables, the median and the interval (minimum:maximum) are used.
The comparisons between the normal variables were done with one-way ANOVA, while the comparisons between the two time-points of the study (T0 versus T1) were performed using paired T-tests for normal variables, while Mann–Whitney tests were used for non-normal variables. Regarding occurrences (percentages), the chi-square test, possibly corrected by Fisher’s exact test, was applied. A p value < 0.05 was considered statistically significant.
3. Results
3.1. Qualitative and Quantitative Characterization of the Organic EVOO
The HPLC-DAD-MS characterization of the EVOO selected for this study showed a very high content of MPCs, equal to 1145.98 mg/L. By evaluating the single molecules, we confirmed that the EVOO met the criteria required in order to claim CV protection according to the European Food Safety Authority (EFSA), as reported in Commission Regulation n. 432/2012 [43,44]. This regulation establishes that a daily intake of 20 g EVOO containing 5 mg of HT and its derivatives protects against blood lipid oxidation. In particular, in the EVOO selected for this study, the content of HT and its derivatives reached 778.43 mg/L. Moreover, in the EVOO administered in this study, we detected the contents of oleacin (315.46 mg/L) and oleocanthal (197.84 mg/L), molecules characterized by important anti-inflammatory activities [45,46].
Table 1 shows the chemical characterization of the single compounds identified in the EVOO sample.

Table 1.
HPLC-DA-MS analysis of Ophena EVOO.
Analyses relating to qualitative parameters of EVOO were carried out using the Oxitester system. We obtained low peroxide values (4.98 meqO2/kg) and acidity equal to 0.17%, indicating a high quality of the selected product (Table 2).

Table 2.
Acidity, peroxides, and polyphenols of EVOO.
All results are expressed as average of three determinations. The standard error was <3% for acidity, <5% for peroxides, and <10% for polyphenols.
Oleocanthal Fraction Characterization
The HPLC-DAD-MS characterization analyses of the Olea extract showed high contents of oleacin (411.73 mg/g) and oleocanthal (331.73 mg/g) (Table 3). As we previously reported, for the analyzed EVOO sample, the high presence of these molecules permitted us to hypothesize a possible anti-inflammatory action of this extract. In fact, this action is added to the massive antioxidant properties already ascribed to these molecules and to HT, tyrosol, elenolic acid, oleuropein aglycone, ligstriside, and secoiridoidic derivatives [28,47,48].

Table 3.
HPLC-DAD-MS characterization of Olea extract.
3.2. In Vitro Study
To evaluate whether the oleo fr extracted from EVOO was more effective in terms of anti-inflammatory properties than the oleocanthal standard (oleo std), we compared their potential toxicities. In this way, equimolar doses of both oleo std and fr were administered to RAW 264.7 cells for 72 h and cell viability was determined via MTT assay.
Figure 2A shows that oleo fr was nontoxic up to a 15 μM dose, whereas oleo std did not affect cell viability even at high doses. To assess the anti-inflammatory property of oleo fr, we measured NO metabolite (specifically nitrite) production induced by 1 μg/mL of LPS (a marker of inflammation) in the same RAW 264.7culture medium, in either the presence or the absence of oleo fr. Oleo fr showed a higher capability to reduce NO production (Figure 2C) compared to oleo std (Figure 2B) and also compared to LPS, which was used as the positive control for nitrite production. In fact, it was already effective at a dose of 7.5 μM, compared to 30 μM of the oleo std. To study the anti-inflammatory capability of oleo fr in more depth, we also analyzed the expression levels of two enzymes directly implicated in the inflammatory response: iNOS and COX-2. Western blotting and densitometric analysis showed significant decreases of both iNOS and COX-2 expression, starting from a concentration of 15 μM (Figure 3).

Figure 2.
Cell viability assessed by MTT assay. (a) Cell viability assessed by MTT assay after 72 h of exposure to different doses of oleocanthal fraction (oleo fr) or oleocanthal standard (oleo std). Significance is indicated with * and relates to the untreated control (UT). Statistics were determined using two-way ANOVA and Sidak’s multiple-comparisons test; (b) Nitrite production by RAW 264.7 treated for 24 h with 1 μg/mL LPS and oleo std at different doses. Significance is indicated with * and relates to LPS as 100% of nitrite production. Statistics were determined using ordinary one-way ANOVA and Dunnett’s multiple-comparisons test; (c) Nitrite production by RAW 264.7 treated for 24 h with 1 μg/mL LPS and oleol fr at different doses. Significance is indicated with * and relates to LPS as 100% of nitrite production. Statistics were determined using ordinary one-way ANOVA and Dunnett’s multiple-comparisons test.
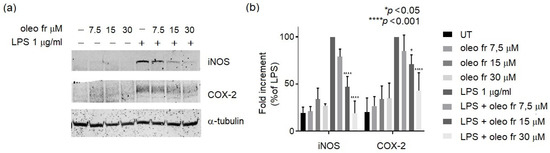
Figure 3.
(a) Representative western blot panels of iNOS and COX-2 protein levels. Each band in the western blot was quantified via densitometric analysis and (b) the corresponding histogram was constructed by normalizing the density of each band to that of α-tubulin. The significance is indicated with * and relates to LPS. Statistical analyses were conducted using two-way ANOVA and Dunnett’s multiple-comparisons test.
These results suggest a greater effectiveness of the fraction compared to the standard, probably due to the synergistic effect induced by all components present in the fraction.
3.3. In Vivo Study
The main epidemiological features of the study population (40 CKD patients under conservative therapy) are shown in Table 4. In particular, the mean age of the study population was 70.7 ± 10.6 years (CI: 49.5–80). The values of systolic and diastolic blood pressure monitored in the different time-points did not demonstrate any statistical significance.

Table 4.
Epidemiological features of study population.
Only 38 patients completed the clinical trial. We recorded two drop-outs, for reasons not related to the experiment.
Table 5 shows the results of the laboratory and urinary parameters assessed at T0 and at T1. At the end of the study (T1), we observed an improvement of renal function parameters, with a significant reduction of serum level of creatinine (p = 0.013), azotemia (p = 0.017), and albuminuria (p = 0.027). Moreover, we noticed an amelioration of lipid and purine metabolism, with a significant increase of HDL-C and a significant decrease of triglycerides (p = 0.002) and uric acid serum levels (p = 0.039). The results also showed an improvement in the nutritional status of the patients after EVOO consumption, with a significant increase in serum albumin levels (p = 0.001).

Table 5.
Laboratory and urinary parameters assessed at baseline and after 9 weeks of study.
As for the OS and the inflammation parameters, after 9 weeks of EVOO consumption, we observed significant reductions of FORT level (p = 0.013) and of CRP (p = 0.046), ESR (0.0006), TNF-α (p = 0.0001), and IL-6 (p = 0.019) serum levels, as reported in Table 6. In contrast, the patients’ antioxidant defenses seemed to remain stable.

Table 6.
Oxidative stress and inflammatory parameters assessed at baseline and after 9 weeks of study.
Table 7 expresses the data regarding atherogenic indices. At the end of the study, we observed significant reductions of TC/HDL-C (p = 0.0171), LDL-C/HDL-C (p = 0.0098), and log(triglycerides/HDL-C) (p = 0.0067).

Table 7.
Atherogenic indices.
After 9 weeks of EVOO consumption, we also observed an amelioration in the other inflammatory parameters, namely the PLR (p = 0.0003) and LMR (p = 0.0406), and in the absolute number of lymphocytes (p = 0.0007), as reported in Table 8.

Table 8.
Other inflammatory parameters.
The Doppler ultrasound imaging showed a significant reduction of CIMT (p = 0.017), as summarized in Table 9.

Table 9.
CIMT values assessed at baseline and after 9 weeks of study.
Finally, the body composition assessment by BIA highlighted a significant increase of reactance (0.007) and of BCM % (p = 0.025), as reported in Table 10.

Table 10.
Body composition parameters assessed at baseline and after 9 weeks of study.
The lifestyle questionnaires PREDIMED and IPAQ did not show statistically significant differences between the two observation time-points, highlighting that the results obtained may be attributable to EVOO consumption. Moreover, at baseline (T0) and after 9 weeks (T1) of in vivo study, the SF-36 questionnaire was administered to assess patients’ perception of their quality of life. At T1, we observed an improvement in the general health and emotional well-being spheres (Figure 4).

Figure 4.
Visual scores, expressed in % for each sphere, of the SF-36 questionnaire administered at baseline and after 9 weeks of study.
4. Discussion
In our study population, we observed a significant improvement of lipid metabolism after 9 weeks of treatment with an EVOO with a very high MPC content. In particular, we observed a significant increase of HDL-C and a reduction of triglycerides. These results could be partly related to the high content of HT. In fact, in a previous animal study, Jemai et al. [49] demonstrated a lipid-lowering action of HT and its derivatives, related to their antioxidant effects which counteract the lipid peroxidation. Moreover, Cao and co-authors [50] showed that HT supplementation, at a dose of 50 mg/kg/day for 17 weeks in obese mice, induced a decrease of sterol regulatory element-binding protein 1 (SREBP-1c) levels, which is a cholesterol-sensitive transcription factor that stimulates the activity of hydroxy-methyl-glutaryl-coenzyme A (HMG-CoA) reductase. In subsequent human studies, conducted on metabolic syndrome patients treated with oral food supplements with a high HT content [51,52], the hypolipemic effect of HT was confirmed.
Interestingly, in our study population, we also observed an amelioration of atherogenic indices. In detail, we detected significant decreases of TC/HDL-C, LDL-C/HDL-C, and log (triglycerides/HDL-C). These data are in accordance with the results obtained in the EUROLIVE Study [27], which reported an amelioration of fatty acid profile in LDL-C, probably correlated to a decrease in lipid oxidative injury. The observed reduction of TC/HDL-C is very important as it highlights the possible cardioprotective action of EVOO with a very high MPC content in nephropathic patients. In fact, this ratio represents a milestone biomarker in lipid atherogenesis, because it is linked to the balance between the passage of cholesterol in and out of the arterial intima [53]. Moreover, the Framingham Offspring Study showed that TC/HDL-C and LDL-C/HDL-C are the most important biomarkers for CV events, among all lipid parameters [54].
In the light of the CV protection induced by the EVOO administered to our study population, it was of utmost importance to observe not only an amelioration of lipid profile, but also a reduction of the chronic inflammatory state typical of CKD patients. In fact, atherosclerotic CV disease cannot be considered only a pathological condition related to the cholesterol accumulation, but is characterized by a more complex pathogenic mechanism and is frequently triggered by a chronic inflammatory status [55]. Inflammatory cell infiltrates have been isolated in atherosclerotic plaque [56], corroborating this hypothesis. Moreover, in the Physicians’ Health Study [57] and Women’s Health Study [58], a direct association was shown between pro-inflammatory cytokine (such as IL-6 and IL-1β) levels and atherosclerotic CV disease. In our study, we highlighted significant reductions of CRP, ESR, TNF-α, and IL-6 after 9 weeks of EVOO administration, confirming the cardioprotective action of EVOO with a very high MPC content in CKD patients. This anti-inflammatory effect was related to the high content of oleocanthal in the EVOO selected. In fact, several studies have pointed out the anti-inflammatory action induced by oleocanthal, as it is an unselective inhibitor of COX-1 and -2 [46,59]. This anti-inflammatory action is also supported by our in vitro data, as discussed above.
In addition, the inflammatory status improvement seemed to positively influence the degree of ED related to CKD. In fact, in the initial stages of atherosclerotic disease, pro-inflammatory cytokines (like IL-6 and TNF-α, which are significantly reduced at T1 in our study) play a key role. These molecules, on one hand, induce the migration of leukocytes into the subendothelial space and, on the other hand, alter the gene expression of the adhesion molecules. The latter, in turn, favor the adhesion, chemotaxis, and subendothelial migration of the same leukocytes [60]. In detail, the physiopathological process that induces atherosclerotic plaque formation is mediated by the accumulation of monocytes in the subendothelial space [61]. We consider the reduction of CIMT observed at the end of our study to support this hypothesis. In detail, upon analyzing the data obtained from the ultrasound examination of the epiaortic vessels, we detected a CIMT improvement in patients presenting a slight increase of CIMT, but not in those who had already developed a carotid atherosclerotic plaque. In this regard, in the carotid atherosclerosis progression study (CAPS), the authors observed a direct correlation between serum CRP levels and an increase in CIMT [62]; at the T1 time-point, we registered a significant decrease of CRP levels, as previously described. In addition to traditional inflammatory biomarkers such as ESR and CRP, we also calculated, the following ratios in order to monitor the inflammatory state: NLR, PLR, and LMR. The latter parameters, which are easy and quite cheap to monitor, were significantly decreased at the end of the study. Furthermore, they are associated with the inflammatory state of the subject and they seem to be correlated to the CV risk. In particular, NLR appears to be a reliable predictor of cardiac arrhythmia and it represents a short- and long-term biomarker of CV disease [63]. On the other hand, PLR seems to be an integrated parameter able to monitor both thrombotic and inflammatory pathways [64]. Historically, it has been used as inflammatory biomarker in cancer patients [65,66], and a possible role has only recently identified as a predictor for CV events [67]. Finally, in a human study conducted on 199 patients that underwent coronary angiography, LMR was associated directly and independently with the severity of coronary atherosclerosis [68]. Therefore, the reduction of these three ratios observed in our study allows further speculation regarding the strong CV protection induced by EVOO with a very high MPC content. A further possible explanation of the CIMT improvement relies on the reduction of LDL-C/HDL-C observed. In fact, a meta-analysis demonstrated that this ratio is related to CIMT. Moreover, low HDL-C concentrations seem to be inversely related to CIMT [69].
In this paper, we confirmed the positive impact of EVOO on slowing CKD progression, as already demonstrated in our previous studies [16,32]. In fact, we observed at T1 a significant reduction of creatinine and uric acid, and an increase of estimated-GFR. Moreover, at the end of the study, we noticed a reduction of microalbuminuria, which on one hand represents a biomarker of renal dysfunction and CKD progression [29,31,70], and on the other hand is related to the CV risk [71]. Therefore, its significant reduction confirms both the cardioprotective and nephroprotective action of EVOO.
We also obtained an additional result, namely a reduction of OS as monitored by FORT. This interesting observation supports the cardioprotective action induced by EVOO and it is probably related to its high content of oleocanthal. This molecule also exerts an antioxidant action, reducing the activity of nicotamide adenine dinucleotide phosphate oxidase and, at the same time, ROS production [72].
Finally, we showed an improvement of the nutritional status of patients, related to the significant enhancement of albuminemia observed at the end of the study. In fact, hypoalbuminemia is frequently present in CKD patients under conservative therapy, and it is correlated with both a low caloric intake and a chronic inflammatory status. In this regard, EVOO consumption at a dose of 40 mL/day is able to counteract the inflammation and, at the same time, provide the correct caloric intake [73].
Moreover, regarding body composition assessment, we observed a significant increase of BCM% after our intervention. This parameter is not only directly related to albumin, as previous demonstrated in the literature, but it is a useful tool to evaluate inflammation in CKD and elderly patients [74].
As for the SF-36 questionnaire, this test allowed us to underline an enhancement of quality of life as perceived by patients, likely related to their enhanced health status.
5. Conclusions
We would like to stress that this study highlighted the cardioprotective action of EVOO in nephropathic patients for the first time. This positive health effect could be due to the reduction of the chronic inflammatory state produced by the oleocanthal and to the improvement of lipid metabolism induced by HT and oleuropein.
Previous clinical trials (such as PREDIMED [26], EPIC [25], and EUROLIVE [27]) have shown this beneficial effect only in other CV high-risk populations, but not in CKD patients.
In order to reduce CV mortality in CKD, the traditional nutritional treatment, planned according to CKD stage, should be combined with functional foods. Among these, according also to EFSA’s health claim [43,44], EVOO with a high content of MPCs seems to be one of the most important functional foods exerting cardioprotective action.
Author Contributions
Conceptualization, A.N.; methodology, S.U., J.R., F.I., P.V. and C.N.; investigation, G.M., M.D.L. and C.G.; data curation, G.M.; writing—original draft preparation, G.M., M.D.L. and A.N.; writing—review and editing, N.D.D. and A.N.; visualization, M.D.L., J.R. and F.D.D.; supervision, N.D.D. and A.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Independent Ethics Committee of Policlinico Tor Vergata (protocol code 36, approved in 2020).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data supporting reported results can be found upon request to the corresponding author.
Acknowledgments
This article is dedicated to the memory of our beloved and esteemed full professor Annalisa Romani, who recently passed away. We would like to thank Gabriella Venafro for the English language revision. We would like to thank Lazio Region and ARSIAL (Regional Agency for Development and Innovation of Lazio Agriculture)—Research project “Food culture well-being and natural cuisine” and Tuscany Region (BIOSYNOL PSGO 52 2017). We would like to thank Goldmed Suisse for the extra-virgin olive oil supply for free.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviation
| ADMA | Asymmetric Dimethylarginine |
| AGEs | Advanced Glycation End Products |
| BCM | Body Cellular Mass |
| BCMI | Body Cellular Mass Index |
| BIA | Bioelectrical Impedance Analysis |
| BMI | Body Mass Index |
| BMR | Basal Metabolic Rate |
| CAPS | Carotid Atherosclerosis Progression Study |
| CIMT | Carotid Intima-Media Thickness |
| CKD | Chronic Kidney Disease |
| COX | Cyclooxygenase Enzymes |
| CRP | C-Reactive Protein |
| CV | Cardiovascular |
| DDAH | Enzyme Dimethylarginine Di-Methylaminohydrolase |
| ECW | Extra Cell Water |
| ED | Endothelial Dysfunction |
| EFSA | European Food Safety Authority |
| EPIC | European Prospective Investigation |
| ESR | Erythrocyte Sedimentation Rate |
| EVOO | Extra Virgin Olive Oil |
| FFM | Fat Free Mass |
| FM | Fat Mass |
| FORD | Free Oxygen Radical Defense |
| FORT | Free Oxygen Radical Test |
| SF-36 | Short Form 36 Health Survey |
| GFR | Glomerular Filtration Rate |
| HDL-C | High-Density Lipoprotein Cholesterol |
| HMG-CoA | Hydroxy-methyl-glutaryl-coenzyme A |
| HPLC-DAD-MS | High-Performance Liquid Chromatography Coupled with Diode Array Detector-Mass Spectrometry |
| HT | Hydroxytyrosol |
| ICW | Intra Cell Water |
| IL | Interleukin |
| IPAQ | International Physical Activity Questionnaires |
| KDIGO | Kidney Disease: Improving Global Outcomes |
| LDL-C | Low-Density Lipoprotein Cholesterol |
| LMR | Lymphocyte-To-Monocyte Ratio |
| LPS | Lipopolysaccharide |
| MPCs | Minor Phenolic Compounds |
| MTT | 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| MUFAs | Monounsaturated Fatty Acids |
| NF-kB | Nuclear Factor Kappa-Light-Chain-Enhancer of Activated B Cells |
| NLR | Neutrophil-To-Lymphocyte Ratio |
| NO | Nitric Oxide |
| Oleo fr | Oleocanthal fraction |
| Oleo std | Oleocanthal standard |
| OS | Oxidative Stress |
| PLR | Platelet-To-Lymphocyte Ratio |
| PREDIMED | Prevención Con Dieta Mediterránea |
| PTV | Policlinico Tor Vergata |
| ROS | Reactive Oxygen Species |
| SD | Standard Deviation |
| TBW | Total Body Water |
| TC | Total Cholesterol |
| TNF | Tumor Necrosis Factor |
References
- Foreman, K.J.; Marquez, N.; Dolgert, A.; Fukutaki, K.; Fullman, N.; McGaughey, M.; Pletcher, M.A.; Smith, A.E.; Tang, K.; Yuan, C.W.; et al. Forecasting life expectancy, years of life lost, and all-cause and cause-specific mortality for 250 causes of death: Reference and alternative scenarios for 2016-40 for 195 countries and territories. Lancet 2018, 392, 2052–2090. [Google Scholar] [CrossRef]
- Weiner, D.E.; Tighiouart, H.; Elsayed, E.F.; Griffith, J.L.; Salem, D.N.; Levey, A.S.; Sarnak, M.J. The Framingham predictive instrument in chronic kidney disease. J. Am. Coll. Cardiol. 2007, 50, 217–224. [Google Scholar] [CrossRef]
- Reichel, H.; Zee, J.; Tu, C.; Young, E.; Pisoni, R.L.; Stengel, B.; Duttlinger, J.; Lonnemann, G.; Robinson, B.M.; Pecoits-Filho, R.; et al. Chronic kidney disease progression and mortality risk profiles in Germany: Results from the Chronic Kidney Disease Outcomes and Practice Patterns Study. Nephrol. Dial. Transplant. 2020, 35, 803–810. [Google Scholar] [CrossRef] [PubMed]
- Noce, A.; Canale, M.P.; Capria, A.; Rovella, V.; Tesauro, M.; Splendiani, G.; Annicchiarico-Petruzzelli, M.; Manzuoli, M.; Simonetti, G.; Di Daniele, N. Coronary artery calcifications predict long term cardiovascular events in non diabetic Caucasian hemodialysis patients. Aging 2015, 7, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Pires, A.; Sobrinho, L.; Ferreira, H.G. The Calcium/Phosphorus Homeostasis in Chronic Kidney Disease: From Clinical Epidemiology to Pathophysiology. Acta Med. Port. 2017, 30, 485–492. [Google Scholar] [CrossRef]
- Pastore, A.; Noce, A.; Di Giovamberardino, G.; De Stefano, A.; Calla, C.; Zenobi, R.; Dessi, M.; Di Daniele, N. Homocysteine, cysteine, folate and vitamin B(1)(2) status in type 2 diabetic patients with chronic kidney disease. J. Nephrol. 2015, 28, 571–576. [Google Scholar] [CrossRef]
- Cianciolo, G.; De Pascalis, A.; Di Lullo, L.; Ronco, C.; Zannini, C.; La Manna, G. Folic Acid and Homocysteine in Chronic Kidney Disease and Cardiovascular Disease Progression: Which Comes First? Cardiorenal. Med. 2017, 7, 255–266. [Google Scholar] [CrossRef]
- Pereira, R.A.; Cordeiro, A.C.; Avesani, C.M.; Carrero, J.J.; Lindholm, B.; Amparo, F.C.; Amodeo, C.; Cuppari, L.; Kamimura, M.A. Sarcopenia in chronic kidney disease on conservative therapy: Prevalence and association with mortality. Nephrol. Dial. Transplant. 2015, 30, 1718–1725. [Google Scholar] [CrossRef]
- Noce, A.; Marrone, G.; Ottaviani, E.; Guerriero, C.; Di Daniele, F.; Pietroboni Zaitseva, A.; Di Daniele, N. Uremic Sarcopenia and Its Possible Nutritional Approach. Nutrients 2021, 13, 147. [Google Scholar] [CrossRef]
- Noce, A.; Marchetti, M.; Marrone, G.; Di Renzo, L.; Di Lauro, M.; Di Daniele, F.; Albanese, M.; Di Daniele, N.; De Lorenzo, A. Link between gut microbiota dysbiosis and chronic kidney disease. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 2057–2074. [Google Scholar] [CrossRef] [PubMed]
- Noce, A.; Marrone, G.; Di Daniele, F.; Ottaviani, E.; Wilson Jones, G.; Bernini, R.; Romani, A.; Rovella, V. Impact of Gut Microbiota Composition on Onset and Progression of Chronic Non-Communicable Diseases. Nutrients 2019, 11, 1073. [Google Scholar] [CrossRef]
- Noce, A.; Marrone, G.; Di Lauro, M.; Urciuoli, S.; Pietroboni Zaitseva, A.; Wilson Jones, G.; Di Daniele, N.; Romani, A. Cardiovascular Protection of Nephropathic Male Patients by Oral Food Supplements. Cardiovasc. Ther. 2020, 2020, 1807941. [Google Scholar] [CrossRef]
- Noce, A.; Marrone, G.; Wilson Jones, G.; Di Lauro, M.; Pietroboni Zaitseva, A.; Ramadori, L.; Celotto, R.; Mitterhofer, A.P.; Di Daniele, N. Nutritional Approaches for the Management of Metabolic Acidosis in Chronic Kidney Disease. Nutrients 2021, 13, 2534. [Google Scholar] [CrossRef]
- Mihai, S.; Codrici, E.; Popescu, I.D.; Enciu, A.M.; Albulescu, L.; Necula, L.G.; Mambet, C.; Anton, G.; Tanase, C. Inflammation-Related Mechanisms in Chronic Kidney Disease Prediction, Progression, and Outcome. J. Immunol. Res. 2018, 2018, 2180373. [Google Scholar] [CrossRef]
- Noce, A.; Di Lauro, M.; Di Daniele, F.; Pietroboni Zaitseva, A.; Marrone, G.; Borboni, P.; Di Daniele, N. Natural Bioactive Compounds Useful in Clinical Management of Metabolic Syndrome. Nutrients 2021, 13, 630. [Google Scholar] [CrossRef]
- Grazioli, E.; Romani, A.; Marrone, G.; Di Lauro, M.; Cerulli, C.; Urciuoli, S.; Murri, A.; Guerriero, C.; Tranchita, E.; Tesauro, M.; et al. Impact of Physical Activity and Natural Bioactive Compounds on Endothelial Dysfunction in Chronic Kidney Disease. Life 2021, 11, 841. [Google Scholar] [CrossRef]
- Roumeliotis, S.; Mallamaci, F.; Zoccali, C. Endothelial Dysfunction in Chronic Kidney Disease, from Biology to Clinical Outcomes: A 2020 Update. J. Clin. Med. 2020, 9, 2359. [Google Scholar] [CrossRef]
- Vanhoutte, P.M.; Zhao, Y.; Xu, A.; Leung, S.W. Thirty Years of Saying NO: Sources, Fate, Actions, and Misfortunes of the Endothelium-Derived Vasodilator Mediator. Circ. Res. 2016, 119, 375–396. [Google Scholar] [CrossRef]
- Bocedi, A.; Fabrini, R.; Lai, O.; Alfieri, L.; Roncoroni, C.; Noce, A.; Pedersen, J.Z.; Ricci, G. Erythrocyte glutathione transferase: A general probe for chemical contaminations in mammals. Cell Death Discov. 2016, 2, 16029. [Google Scholar] [CrossRef]
- Vila Cuenca, M.; van Bezu, J.; Beelen, R.H.J.; Vervloet, M.G.; Hordijk, P.L. Stabilization of cell-cell junctions by active vitamin D ameliorates uraemia-induced loss of human endothelial barrier function. Nephrol. Dial. Transplant. 2019, 34, 252–264. [Google Scholar] [CrossRef]
- Noce, A.; Rovella, V.; Marrone, G.; Cattani, G.; Zingaretti, V.; Limongi, D.; D’Agostini, C.; Sorge, R.; Casasco, M.; Di Daniele, N.; et al. Hemodialysis biomarkers: Total advanced glycation end products (AGEs) against oxidized human serum albumin (HSAox). Acta Diabetol. 2019, 56, 1323–1331. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, M.I.; Saglam, M.; Caglar, K.; Cakir, E.; Sonmez, A.; Ozgurtas, T.; Aydin, A.; Eyileten, T.; Ozcan, O.; Acikel, C.; et al. The determinants of endothelial dysfunction in CKD: Oxidative stress and asymmetric dimethylarginine. Am. J. Kidney Dis. 2006, 47, 42–50. [Google Scholar] [CrossRef]
- Ando, R.; Ueda, S.; Yamagishi, S.; Miyazaki, H.; Kaida, Y.; Kaifu, K.; Yokoro, M.; Nakayama, Y.; Obara, N.; Fukami, K.; et al. Involvement of advanced glycation end product-induced asymmetric dimethylarginine generation in endothelial dysfunction. Diab. Vasc. Dis. Res. 2013, 10, 436–441. [Google Scholar] [CrossRef]
- Goligorsky, M.S. Pathogenesis of endothelial cell dysfunction in chronic kidney disease: A retrospective and what the future may hold. Kidney Res. Clin. Pract. 2015, 34, 76–82. [Google Scholar] [CrossRef]
- Buckland, G.; Mayen, A.L.; Agudo, A.; Travier, N.; Navarro, C.; Huerta, J.M.; Chirlaque, M.D.; Barricarte, A.; Ardanaz, E.; Moreno-Iribas, C.; et al. Olive oil intake and mortality within the Spanish population (EPIC-Spain). Am. J. Clin. Nutr. 2012, 96, 142–149. [Google Scholar] [CrossRef]
- Estruch, R.; Ros, E.; Salas-Salvado, J.; Covas, M.I.; Corella, D.; Aros, F.; Gomez-Gracia, E.; Ruiz-Gutierrez, V.; Fiol, M.; Lapetra, J.; et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N. Engl. J. Med. 2018, 378, e34. [Google Scholar] [CrossRef]
- Cicero, A.F.; Nascetti, S.; Lopez-Sabater, M.C.; Elosua, R.; Salonen, J.T.; Nyyssonen, K.; Poulsen, H.E.; Zunft, H.J.; Kiesewetter, H.; de la Torre, K.; et al. Changes in LDL fatty acid composition as a response to olive oil treatment are inversely related to lipid oxidative damage: The EUROLIVE study. J. Am. Coll. Nutr. 2008, 27, 314–320. [Google Scholar] [CrossRef]
- Romani, A.; Ieri, F.; Urciuoli, S.; Noce, A.; Marrone, G.; Nediani, C.; Bernini, R. Health Effects of Phenolic Compounds Found in Extra-Virgin Olive Oil, By-Products, and Leaf of Olea europaea L. Nutrients 2019, 11, 1776. [Google Scholar] [CrossRef]
- Romani, A.; Campo, M.; Urciuoli, S.; Marrone, G.; Noce, A.; Bernini, R. An Industrial and Sustainable Platform for the Production of Bioactive Micronized Powders and Extracts Enriched in Polyphenols from Olea europaea L. and Vitis vinifera L. Wastes. Front. Nutr. 2020, 7, 120. [Google Scholar] [CrossRef]
- Senesi, R.; Andreani, C.; Baglioni, P.; Batista de Carvalho, L.A.E.; Licoccia, S.; Marques, M.P.M.; Moretti, G.; Noce, A.; Paolesse, R.; Parker, S.F.; et al. Looking for Minor Phenolic Compounds in Extra Virgin Olive Oils Using Neutron and Raman Spectroscopies. Antioxidants 2021, 10, 643. [Google Scholar] [CrossRef]
- Romani, A.; Bernini, R.; Noce, A.; Urciuoli, S.; Di Lauro, M.; Pietroboni Zaitseva, A.; Marrone, G.; Di Daniele, N. Potential Beneficial Effects of Extra Virgin Olive Oils Characterized by High Content in Minor Polar Compounds in Nephropathic Patients: A Pilot Study. Molecules 2020, 25, 4757. [Google Scholar] [CrossRef] [PubMed]
- Noce, A.; Marrone, G.; Urciuoli, S.; Di Daniele, F.; Di Lauro, M.; Pietroboni Zaitseva, A.; Di Daniele, N.; Romani, A. Usefulness of Extra Virgin Olive Oil Minor Polar Compounds in the Management of Chronic Kidney Disease Patients. Nutrients 2021, 13, 581. [Google Scholar] [CrossRef] [PubMed]
- Ruzzolini, J.; Chioccioli, S.; Monaco, N.; Peppicelli, S.; Andreucci, E.; Urciuoli, S.; Romani, A.; Luceri, C.; Tortora, K.; Calorini, L.; et al. Oleuropein-Rich Leaf Extract as a Broad Inhibitor of Tumour and Macrophage iNOS in an Apc Mutant Rat Model. Antioxidants 2021, 10, 1577. [Google Scholar] [CrossRef] [PubMed]
- Ruzzolini, J.; Peppicelli, S.; Bianchini, F.; Andreucci, E.; Urciuoli, S.; Romani, A.; Tortora, K.; Caderni, G.; Nediani, C.; Calorini, L. Cancer Glycolytic Dependence as a New Target of Olive Leaf Extract. Cancers 2020, 12, 317. [Google Scholar] [CrossRef]
- Ruzzolini, J.; Peppicelli, S.; Andreucci, E.; Bianchini, F.; Scardigli, A.; Romani, A.; la Marca, G.; Nediani, C.; Calorini, L. Oleuropein, the Main Polyphenol of Olea. europaea Leaf Extract, Has an Anti-Cancer Effect on Human BRAF Melanoma Cells and Potentiates the Cytotoxicity of Current Chemotherapies. Nutrients 2018, 10, 1950. [Google Scholar] [CrossRef]
- Holden, R.M.; Mustafa, R.A.; Alexander, R.T.; Battistella, M.; Bevilacqua, M.U.; Knoll, G.; Mac-Way, F.; Reslerova, M.; Wald, R.; Acott, P.D.; et al. KDIGO 2017 Clinical Practice Guideline Update for the Diagnosis, Evaluation, Prevention, and Treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int. Suppl. 2017, 7, 1–59. [Google Scholar] [CrossRef]
- Ramirez-Anaya, J.D.P.; Castaneda-Saucedo, M.C.; Olalla-Herrera, M.; Villalon-Mir, M.; Serrana, H.L.; Samaniego-Sanchez, C. Changes in the Antioxidant Properties of Extra Virgin Olive Oil after Cooking Typical Mediterranean Vegetables. Antioxidants 2019, 8, 246. [Google Scholar] [CrossRef]
- Gaman, M.A.; Epingeac, M.E.; Diaconu, C.C.; Gaman, A.M. Evaluation of oxidative stress levels in obesity and diabetes by the free oxygen radical test and free oxygen radical defence assays and correlations with anthropometric and laboratory parameters. World. J. Diabetes 2020, 11, 193–201. [Google Scholar] [CrossRef]
- Millan, J.; Pinto, X.; Munoz, A.; Zuniga, M.; Rubies-Prat, J.; Pallardo, L.F.; Masana, L.; Mangas, A.; Hernandez-Mijares, A.; Gonzalez-Santos, P.; et al. Lipoprotein ratios: Physiological significance and clinical usefulness in cardiovascular prevention. Vasc. Health Risk Manag. 2009, 5, 757–765. [Google Scholar]
- Bellizzi, V.; Scalfi, L.; Terracciano, V.; De Nicola, L.; Minutolo, R.; Marra, M.; Guida, B.; Cianciaruso, B.; Conte, G.; Di Iorio, B.R. Early changes in bioelectrical estimates of body composition in chronic kidney disease. J. Am. Soc. Nephrol. 2006, 17, 1481–1487. [Google Scholar] [CrossRef]
- Touboul, P.J.; Hennerici, M.G.; Meairs, S.; Adams, H.; Amarenco, P.; Bornstein, N.; Csiba, L.; Desvarieux, M.; Ebrahim, S.; Hernandez Hernandez, R.; et al. Mannheim carotid intima-media thickness and plaque consensus (2004-2006-2011). An update on behalf of the advisory board of the 3rd, 4th and 5th watching the risk symposia, at the 13th, 15th and 20th European Stroke Conferences, Mannheim, Germany, 2004, Brussels, Belgium, 2006, and Hamburg, Germany, 2011. Cerebrovasc. Dis. 2012, 34, 290–296. [Google Scholar] [CrossRef]
- Wanner, M.; Probst-Hensch, N.; Kriemler, S.; Meier, F.; Autenrieth, C.; Martin, B.W. Validation of the long international physical activity questionnaire: Influence of age and language region. Prev. Med. Rep. 2016, 3, 250–256. [Google Scholar] [CrossRef]
- EFSA. Scientific Opinion on the Substantiation of Health Claims Related to Polyphenols in Olive and Protection of LDL Particles from Oxidative Damage (ID 1333, 1638, 1639, 1696, 2865), Maintenance of Normal Blood HDL Cholesterol Concentrations (ID 1639), Maintenance of Normal Blood Pressure (ID 3781), “Anti-Inflammatory Properties” (ID 1882), “Contributes to the Upper Respiratory Tract Health” (ID 3468), “Can Help to Maintain a Normal Function of Gastrointestinal Tract” (3779), and “Contributes to Body Defences against External Agents” (ID 3467) Pursuant to Article 13(1) of Regulation (EC) No 1924/2006. Available online: https://www.efsa.europa.eu/en/efsajournal/pub/2033 (accessed on 5 August 2020).
- EFSA. Panel on Dietetic Products. EFSA J. 2011, 9, 2033–2058. [Google Scholar]
- Scotece, M.; Gomez, R.; Conde, J.; Lopez, V.; Gomez-Reino, J.J.; Lago, F.; Smith, A.B., 3rd; Gualillo, O. Further evidence for the anti-inflammatory activity of oleocanthal: Inhibition of MIP-1alpha and IL-6 in J774 macrophages and in ATDC5 chondrocytes. Life Sci. 2012, 91, 1229–1235. [Google Scholar] [CrossRef]
- Beauchamp, G.K.; Keast, R.S.; Morel, D.; Lin, J.; Pika, J.; Han, Q.; Lee, C.H.; Smith, A.B.; Breslin, P.A. Phytochemistry: Ibuprofen-like activity in extra-virgin olive oil. Nature 2005, 437, 45–46. [Google Scholar] [CrossRef]
- Lanza, B.; Ninfali, P. Antioxidants in Extra Virgin Olive Oil and Table Olives: Connections between Agriculture and Processing for Health Choices. Antioxidants 2020, 9, 41. [Google Scholar] [CrossRef]
- Carnevale, R.; Nocella, C.; Cammisotto, V.; Bartimoccia, S.; Monticolo, R.; D’Amico, A.; Stefanini, L.; Pagano, F.; Pastori, D.; Cangemi, R.; et al. Antioxidant activity from extra virgin olive oil via inhibition of hydrogen peroxide-mediated NADPH-oxidase 2 activation. Nutrition 2018, 55–56, 36–40. [Google Scholar] [CrossRef]
- Jemai, H.; Fki, I.; Bouaziz, M.; Bouallagui, Z.; El Feki, A.; Isoda, H.; Sayadi, S. Lipid-lowering and antioxidant effects of hydroxytyrosol and its triacetylated derivative recovered from olive tree leaves in cholesterol-fed rats. J. Agric. Food Chem. 2008, 56, 2630–2636. [Google Scholar] [CrossRef]
- Cao, K.; Xu, J.; Zou, X.; Li, Y.; Chen, C.; Zheng, A.; Li, H.; Li, H.; Szeto, I.M.; Shi, Y.; et al. Hydroxytyrosol prevents diet-induced metabolic syndrome and attenuates mitochondrial abnormalities in obese mice. Free Radic. Biol. Med. 2014, 67, 396–407. [Google Scholar] [CrossRef]
- Verhoeven, V.; Van der Auwera, A.; Van Gaal, L.; Remmen, R.; Apers, S.; Stalpaert, M.; Wens, J.; Hermans, N. Can red yeast rice and olive extract improve lipid profile and cardiovascular risk in metabolic syndrome?: A double blind, placebo controlled randomized trial. BMC Complement. Altern. Med. 2015, 15, 52. [Google Scholar] [CrossRef]
- Hermans, M.P.; Lempereur, P.; Salembier, J.P.; Maes, N.; Albert, A.; Jansen, O.; Pincemail, J. Supplementation Effect of a Combination of Olive (Olea europea L.) Leaf and Fruit Extracts in the Clinical Management of Hypertension and Metabolic Syndrome. Antioxidants 2020, 9, 872. [Google Scholar] [CrossRef]
- Kannel, W.B.; Wilson, P.W. Efficacy of lipid profiles in prediction of coronary disease. Am. Heart J. 1992, 124, 768–774. [Google Scholar] [CrossRef]
- Nam, B.H.; Kannel, W.B.; D’Agostino, R.B. Search for an optimal atherogenic lipid risk profile: From the Framingham Study. Am. J. Cardiol. 2006, 97, 372–375. [Google Scholar] [CrossRef]
- Alfaddagh, A.; Martin, S.S.; Leucker, T.M.; Michos, E.D.; Blaha, M.J.; Lowenstein, C.J.; Jones, S.R.; Toth, P.P. Inflammation and cardiovascular disease: From mechanisms to therapeutics. Am. J. Prev. Cardiol. 2020, 4, 100130. [Google Scholar] [CrossRef]
- Libby, P. Inflammation in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 2045–2051. [Google Scholar] [CrossRef]
- Ridker, P.M.; Rifai, N.; Stampfer, M.J.; Hennekens, C.H. Plasma concentration of interleukin-6 and the risk of future myocardial infarction among apparently healthy men. Circulation 2000, 101, 1767–1772. [Google Scholar] [CrossRef]
- Bermudez, E.A.; Rifai, N.; Buring, J.; Manson, J.E.; Ridker, P.M. Interrelationships among circulating interleukin-6, C-reactive protein, and traditional cardiovascular risk factors in women. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 1668–1673. [Google Scholar] [CrossRef]
- Lucas, L.; Russell, A.; Keast, R. Molecular mechanisms of inflammation. Anti-inflammatory benefits of virgin olive oil and the phenolic compound oleocanthal. Curr. Pharm. Des. 2011, 17, 754–768. [Google Scholar] [CrossRef]
- Tousoulis, D.; Antoniades, C.; Stefanadis, C. Assessing inflammatory status in cardiovascular disease. Heart 2007, 93, 1001–1007. [Google Scholar] [CrossRef]
- Pearson, T.A.; Mensah, G.A.; Alexander, R.W.; Anderson, J.L.; Cannon, R.O., 3rd; Criqui, M.; Fadl, Y.Y.; Fortmann, S.P.; Hong, Y.; Myers, G.L.; et al. Markers of inflammation and cardiovascular disease: Application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation 2003, 107, 499–511. [Google Scholar] [CrossRef]
- Nezu, T.; Hosomi, N.; Aoki, S.; Matsumoto, M. Carotid Intima-Media Thickness for Atherosclerosis. J. Atheroscler. Thromb. 2016, 23, 18–31. [Google Scholar] [CrossRef]
- Afari, M.E.; Bhat, T. Neutrophil to lymphocyte ratio (NLR) and cardiovascular diseases: An update. Expert Rev. Cardiovasc. Ther. 2016, 14, 573–577. [Google Scholar] [CrossRef]
- Kurtul, A.; Ornek, E. Platelet to Lymphocyte Ratio in Cardiovascular Diseases: A Systematic Review. Angiology 2019, 70, 802–818. [Google Scholar] [CrossRef]
- Malm, C.J.; Hansson, E.C.; Akesson, J.; Andersson, M.; Hesse, C.; Shams Hakimi, C.; Jeppsson, A. Preoperative platelet function predicts perioperative bleeding complications in ticagrelor-treated cardiac surgery patients: A prospective observational study. Br. J. Anaesth. 2016, 117, 309–315. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Li, D.; Xu, Y.; Wang, H.; Gao, J.; Zhang, Z.; Chen, K. Prognostic significance of metastatic lymph node ratio in patients with gastric cancer after curative gastrectomy: A single-center retrospective study. Scand. J. Gastroenterol. 2022, 57, 832–841. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhou, Y.; Ma, Y.; Han, S.; Zhou, L. The prognostic value of the platelet-to-lymphocyte ratio in acute coronary syndrome: A systematic review and meta-analysis. Kardiol. Pol. 2017, 75, 666–673. [Google Scholar] [CrossRef]
- Gong, S.; Gao, X.; Xu, F.; Shang, Z.; Li, S.; Chen, W.; Yang, J.; Li, J. Association of lymphocyte to monocyte ratio with severity of coronary artery disease. Medicine 2018, 97, e12813. [Google Scholar] [CrossRef]
- Touboul, P.J.; Labreuche, J.; Bruckert, E.; Schargrodsky, H.; Prati, P.; Tosetto, A.; Hernandez-Hernandez, R.; Woo, K.S.; Silva, H.; Vicaut, E.; et al. HDL-C, triglycerides and carotid IMT: A meta-analysis of 21,000 patients with automated edge detection IMT measurement. Atherosclerosis 2014, 232, 65–71. [Google Scholar] [CrossRef]
- Zhang, W.R.; Parikh, C.R. Biomarkers of Acute and Chronic Kidney Disease. Annu. Rev. Physiol. 2019, 81, 309–333. [Google Scholar] [CrossRef]
- Piepoli, M.F.; Hoes, A.W.; Agewall, S.; Albus, C.; Brotons, C.; Catapano, A.L.; Cooney, M.T.; Corra, U.; Cosyns, B.; Deaton, C.; et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts) Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur. Heart. J. 2016, 37, 2315–2381. [Google Scholar] [CrossRef]
- Pang, K.L.; Chin, K.Y. The Biological Activities of Oleocanthal from a Molecular Perspective. Nutrients 2018, 10, 570. [Google Scholar] [CrossRef]
- Perez Banasco, V.; Gil-Cunquero, J.M.; Borrego, F.J.; Grasso, M.; Segura, P.; Warletta, F.; Lozano, J.L.; Costa, L.A.; Torres, J.; Gaforio, J.J.; et al. Preliminary study on efficacy and tolerance of a “coupage” of olive oil in patients with chronic kidney disease. Nutritonal evaluation. Nefrologia 2007, 27, 472–481. [Google Scholar]
- Rondanelli, M.; Talluri, J.; Peroni, G.; Donelli, C.; Guerriero, F.; Ferrini, K.; Riggi, E.; Sauta, E.; Perna, S.; Guido, D. Beyond Body Mass Index. Is the Body Cell Mass Index (BCMI) a useful prognostic factor to describe nutritional, inflammation and muscle mass status in hospitalized elderly?: Body Cell Mass Index links in elderly. Clin. Nutr. 2018, 37, 934–939. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).