Adherence to the EAT–Lancet Diet: Unintended Consequences for the Brain?
Abstract
1. Introduction
2. Materials and Methods
2.1. Procedure
2.2. Participants
2.3. Habitual Diet
2.3.1. Alternate Healthy Eating Index 2010 (AHEI-2010) Score
2.3.2. World Index for Sustainability and Health (WISH)
2.4. Heart Rate Variability
Pre-Processing
2.5. Cognition and Mood
2.5.1. Episodic Memory: Word-List Recall
2.5.2. Working Memory
2.5.3. Focused Attention
2.5.4. Depressed Mood
2.6. Additional Covariates
2.6.1. Body Mass Index (BMI)
2.6.2. Physical Activity
2.7. Statistical Analysis
2.8. Cluster Analysis
3. Results
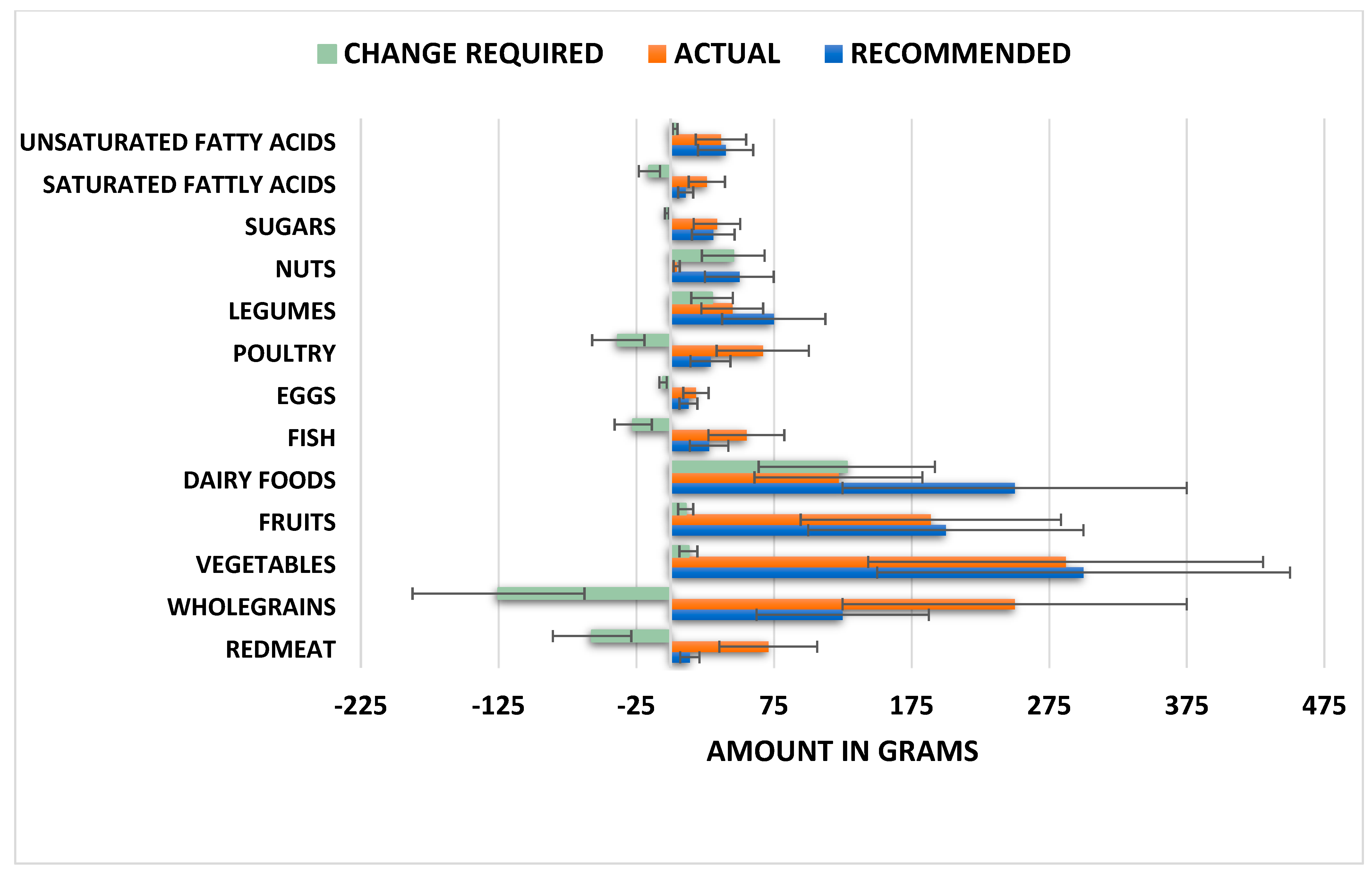
3.1. Sample Adherence to the Planetary Health Diet (WISH)
3.2. Associations between the EAT-Lancet Diet (WISH) and Health Outcomes
3.2.1. Heart Rate Variability
EAT-Lancet Diet (WISH)
Alternative Healthy Eating Index (AHEI)
3.2.2. Mood
EAT-Lancet Diet (WISH)
Alternative Healthy Eating Index (AHEI)
3.2.3. Immediate and Delayed Episodic Memory
EAT-Lancet Diet (WISH)
Alternative Healthy Eating Index (AHEI)
3.2.4. Working Memory Reaction Times
EAT-Lancet Diet (WISH)
Alternative Healthy Eating Index (AHEI)
3.2.5. Working Memory Accuracy
EAT-Lancet Diet (WISH)
Alternative Healthy Eating Index (AHEI)
3.2.6. Focused Attention Reaction Times
EAT-Lancet Diet (WISH)
Alternative Healthy Eating Index (AHEI)
3.2.7. Focused Attention Accuracy
EAT-Lancet Diet (WISH)
Alternative Healthy Eating Index (AHEI)
3.3. Identification of Healthy and Unhealthy Sustainable Dietary Patterns
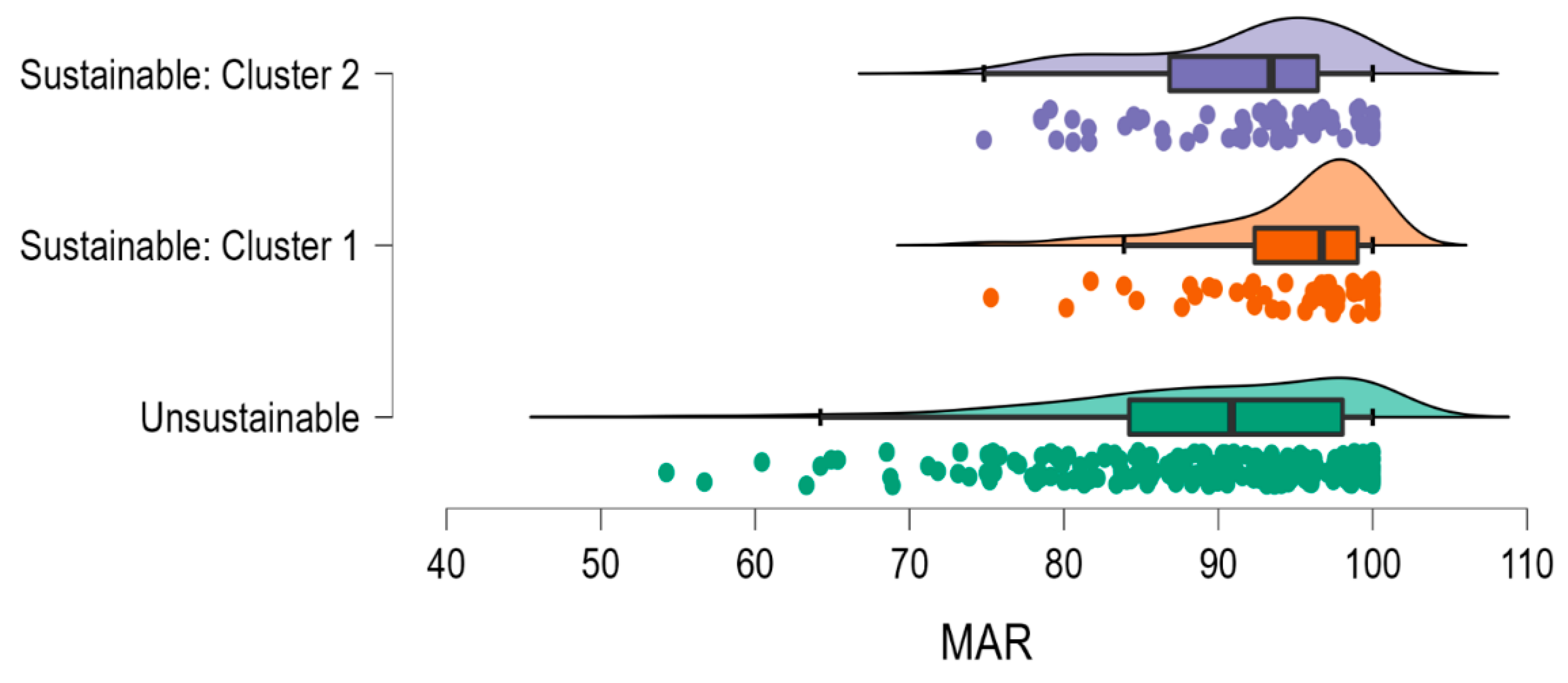
3.4. Association between Healthy and Unhealthy Sustainable Dietary Patterns and Nutrient Adequacy
3.5. Association between Healthy and Unhealthy Sustainable Dietary Patterns and Health Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Willett, W.J.; Rockström, B.; Loken, M.; Springmann, T.; Lang, S.; Vermeulen, T.; Garnett, D.; Tilman, F.; DeClerck, A.; Wood, M.; et al. Food in the Anthropocene: The EAT–Lancet Commission on healthy diets from sustainable food systems. Lancet 2019, 393, 447–492. [Google Scholar] [CrossRef]
- World Health Organization. Sustainable Healthy Diets: Guiding Principles; Food & Agriculture Org: Wageningen, The Netherlands, 2019. [Google Scholar]
- Zagmutt, F.J.; Pouzou, J.G.; Costard, S. The EAT-Lancet Commission’s dietary composition may not prevent noncommunicable disease mortality. J. Nutr. 2020, 150, 985–988. [Google Scholar] [CrossRef] [PubMed]
- Zagmutt, F.J.; Pouzou, J.G.; Costard, S. The EAT–Lancet Commission: A flawed approach? Lancet 2019, 394, 1140–1141. [Google Scholar] [CrossRef]
- Harcombe, Z. This is not the EAT–Lancet Diet. Lancet 2020, 395, 271–272. [Google Scholar] [CrossRef]
- Kaiser, M. What is wrong with the EAT Lancet report? In Justice and Food Security in a Changing Climate; Wageningen Academic Publishers: Wageningen, The Netherlands, 2021; pp. 1140–1141. [Google Scholar]
- Young, H.A.; Benton, D. Heart-rate variability: A biomarker to study the influence of nutrition on physiological and psychological health? Behav. Pharmacol. 2018, 29, 140–151. [Google Scholar] [CrossRef] [PubMed]
- Katz, D.; Meller, S. Can We Say What Diet Is Best for Health? Annu. Rev. Public Health 2014, 35, 83–103. [Google Scholar] [CrossRef] [PubMed]
- Qian, F.; Liu, G.; Hu, F.B.; Bhupathiraju, S.N.; Sun, Q. Association Between Plant-Based Dietary Patterns and Risk of Type 2 Diabetes: A Systematic Review and Meta-analysis. JAMA Intern. Med. 2019, 179, 1335–1344. [Google Scholar] [CrossRef] [PubMed]
- Patel, H.; Chandra, S.; Alexander, S.; Soble, J.; Williams, K.A. Plant-Based Nutrition: An Essential Component of Cardiovascular Disease Prevention and Management. Curr. Cardiol. Rep. 2017, 19, 104. [Google Scholar] [CrossRef] [PubMed]
- Kahleova, H.; Levin, S.; Barnard, N. Cardio-Metabolic Benefits of Plant-Based Diets. Nutrients 2017, 9, 848. [Google Scholar] [CrossRef]
- Walker, M.E.; O’Donnell, A.A.; Himali, J.J.; Rajendran, I.; van Lent, D.M.; Ataklte, F.; Jacques, P.F.; Beiser, A.S.; Seshadri, S.; Vasan, R.S.; et al. Associations of the Mediterranean-Dietary Approaches to Stop Hypertension Intervention for Neurodegenerative Delay diet with cardiac remodelling in the community: The Framingham Heart Study. Br. J. Nutr. 2021, 126, 1888–1896. [Google Scholar] [CrossRef]
- Knuppel, A.; Papier, K.; Key, T.J.; Travis, R.C. EAT-Lancet score and major health outcomes: The EPIC-Oxford study. Lancet 2019, 394, 213–214. [Google Scholar] [CrossRef]
- Ibsen, D.B.; Christiansen, A.H.; Olsen, A.; Tjønneland, A.; Overvad, K.; Wolk, A.; Mortensen, J.K.; Dahm, C.C. Adherence to the EAT-Lancet Diet and Risk of Stroke and Stroke Subtypes: A Cohort Study. Stroke 2022, 53, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Stubbendorff, A.; Sonestedt, E.; Ramne, S.; Drake, I.; Hallström, E.; Ericson, U. Development of an EAT-Lancet index and its relation to mortality in a Swedish population. Am. J. Clin. Nutr. 2021, 115, 705–716. [Google Scholar] [CrossRef] [PubMed]
- Gijsbers, L.; Ding, E.L.; Malik, V.S.; de Goede, J.; Geleijnse, J.M.; Soedamah-Muthu, S.S. Consumption of dairy foods and diabetes incidence: A dose-response meta-analysis of observational studies. Am. J. Clin. Nutr. 2016, 103, 1111–1124. [Google Scholar] [CrossRef] [PubMed]
- Petti, A.; Palmieri, B.; Vadalà, M.; Laurino, C. Vegetarianism and veganism: Not only benefits but also gaps. A review. Prog. Nutr. 2017, 19, 229–242. [Google Scholar] [CrossRef]
- Hanley-Cook, G.T.; Argaw, A.A.; de Kok, B.P.; Vanslambrouck, K.W.; Toe, L.C.; Kolsteren, P.W.; Jones, A.D.; Lachat, C.K. EAT–Lancet diet score requires minimum intake values to predict higher micronutrient adequacy of diets in rural women of reproductive age from five low- and middle-income countries. Br. J. Nutr. 2021, 126, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Bakaloudi, D.R.; Halloran, A.; Rippin, H.L.; Oikonomidou, A.C.; Dardavesis, T.I.; Williams, J.; Wickramasinghe, K.; Breda, J.; Chourdakis, M. Intake and adequacy of the vegan diet. A systematic review of the evidence. Clin. Nutr. 2021, 40, 3503–3521. [Google Scholar] [CrossRef] [PubMed]
- Iguacel, I.; Huybrechts, I.; A Moreno, L.; Michels, N. Vegetarianism and veganism compared with mental health and cognitive outcomes: A systematic review and meta-analysis. Nutr. Rev. 2020, 79, 361–381. [Google Scholar] [CrossRef] [PubMed]
- Tardy, A.-L.; Pouteau, E.; Marquez, D.; Yilmaz, C.; Scholey, A. Vitamins and Minerals for Energy, Fatigue and Cognition: A Narrative Review of the Biochemical and Clinical Evidence. Nutrients 2020, 12, 228. [Google Scholar] [CrossRef]
- Grosso, G.; Pajak, A.; Marventano, S.; Castellano, S.; Galvano, F.; Bucolo, C.; Drago, F.; Caraci, F. Role of Omega-3 Fatty Acids in the Treatment of Depressive Disorders: A Comprehensive Meta-Analysis of Randomized Clinical Trials. PLoS ONE 2014, 9, e96905. [Google Scholar] [CrossRef]
- Sandstead, H.H. Causes of Iron and Zinc Deficiencies and Their Effects on Brain. J. Nutr. 2000, 130, 347S–349S. [Google Scholar] [CrossRef] [PubMed]
- Yurko-Mauro, K.; Alexander, D.D.; Van Elswyk, M.E. Docosahexaenoic Acid and Adult Memory: A Systematic Review and Meta-Analysis. PLoS ONE 2015, 10, e0120391. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, M.F.; da Silva, G.M.; Freiria, C.N.; Borim, F.S.A.; de Brito, T.R.P.; Milanski, M.; Corona, L.P. Association between zinc deficiency and cognitive decline in community-dwelling older adults. Ciência Saúde Coletiva 2022, 27, 2805–2816. [Google Scholar] [CrossRef] [PubMed]
- Vogel, T.; Dali-Youcef, N.; Kaltenbach, G.; Andrès, E. Homocysteine, vitamin B12, folate and cognitive functions: A systematic and critical review of the literature. Int. J. Clin. Pract. 2009, 63, 1061–1067. [Google Scholar] [CrossRef] [PubMed]
- Fairbairn, P.; Dyall, S.; Tsofliou, F. The Effects of Multi-Nutrient Formulas containing a Combination of Omega-3 Polyunsaturated Fatty Acids and B vitamins on Cognition in the older adult: A Systematic Review and Meta-analysis. Br. J. Nutr. Int. J. Nutr. Sci. 2022, in press. [Google Scholar]
- Georgieff, M.K. Nutrition and the developing brain: Nutrient priorities and measurement. Am. J. Clin. Nutr. 2007, 85, 614S–620S. [Google Scholar]
- Black, M.M. Effects of Vitamin B12 and Folate Deficiency on Brain Development in Children. Food Nutr. Bull. 2008, 29, S126–S131. [Google Scholar] [CrossRef]
- Dalile, B.; Kim, C.; Challinor, A.; Geurts, L.; Gibney, E.R.; Galdos, M.V.; La Fata, G.; Layé, S.; Mathers, J.C.; Vauzour, D.; et al. The EAT–Lancet reference diet and cognitive function across the life course. Lancet Planet. Health 2022, 6, e749–e759. [Google Scholar] [CrossRef]
- Trijsburg, L.; Talsma, E.; Crispim, S.; Garrett, J.; Kennedy, G.; de Vries, J.; Brouwer, I. Method for the Development of WISH, a Globally Applicable Index for Healthy Diets from Sustainable Food Systems. Nutrients 2020, 13, 93. [Google Scholar] [CrossRef]
- Cacau, L.T.; De Carli, E.; de Carvalho, A.M.; Lotufo, P.A.; Moreno, L.A.; Bensenor, I.M.; Marchioni, D.M. Development and Validation of an Index Based on EAT-Lancet Recommendations: The Planetary Health Diet Index. Nutrients 2021, 13, 1698. [Google Scholar] [CrossRef]
- Gallagher, C.T.; Hanley, P.; Lane, K.E. Pattern analysis of vegan eating reveals healthy and unhealthy patterns within the vegan diet. Public Health Nutr. 2021, 25, 1310–1320. [Google Scholar] [CrossRef] [PubMed]
- Rajaram, S.; Jones, J.; Lee, G.J. Plant-based dietary patterns, plant foods, and age-related cognitive decline. Adv. Nutr. 2019, 10 (Suppl. 4), S422–S436. [Google Scholar] [CrossRef] [PubMed]
- Young, H.; Benton, D. We should be using nonlinear indices when relating heart-rate dynamics to cognition and mood. Sci. Rep. 2015, 5, 16619. [Google Scholar] [CrossRef] [PubMed]
- Young, H.; Benton, D.; Carter, N. The Effect of Chicken Extract on Mood, Cognition and Heart Rate Variability. Nutrients 2015, 7, 887–904. [Google Scholar] [CrossRef] [PubMed]
- Young, H.A.; Cousins, A.; Johnston, S.; Fletcher, J.M.; Benton, D. Autonomic adaptations mediate the effect of hydration on brain functioning and mood: Evidence from two randomized controlled trials. Sci. Rep. 2019, 9, 16412. [Google Scholar] [CrossRef]
- Young, H.A.; Cousins, A.L.; Watkins, H.T.; Benton, D. Is the link between depressed mood and heart rate variability explained by disinhibited eating and diet? Biol. Psychol. 2017, 123, 94–102. [Google Scholar] [CrossRef]
- Young, H.A.; Davies, J.; Freegard, G.; Benton, D. Nonsuicidal Self-Injury Is Associated with Attenuated Interoceptive Responses to Self-Critical Rumination. Behav. Ther. 2021, 52, 1123–1136. [Google Scholar] [CrossRef]
- Young, H.A.; Gaylor, C.M.; De Kerckhove, D.; Watkins, H.; Benton, D. Interoceptive accuracy moderates the response to a glucose load: A test of the predictive coding framework. Proc. R. Soc. B Boil. Sci. 2019, 286, 20190244. [Google Scholar] [CrossRef]
- Young, H.A.; Watkins, H. Eating disinhibition and vagal tone moderate the postprandial response to glycemic load: A randomized controlled trial. Sci. Rep. 2016, 6, 35740. [Google Scholar] [CrossRef]
- Young, H.A.; Williams, C.; Pink, A.E.; Freegard, G.; Owens, A.; Benton, D. Getting to the heart of the matter: Does aberrant interoceptive processing contribute towards emotional eating? PLoS ONE 2017, 12, e0186312. [Google Scholar] [CrossRef]
- Mulligan, A.A.; Luben, R.N.; Bhaniani, A.; Parry-Smith, D.J.; O’Connor, L.; Khawaja, A.P.; Forouhi, N.G.; Khaw, K.-T. A new tool for converting food frequency questionnaire data into nutrient and food group values: FETA research methods and availability. BMJ Open 2014, 4, e004503. [Google Scholar] [CrossRef]
- Lorr, M.; McNair, D.M.; Fisher, S. Evidence for bipolar mood states. J. Personal. Assess. 1982, 46, 432–436. [Google Scholar] [CrossRef] [PubMed]
- Watson, D.; Clark, L.A.; Tellegen, A. Development and validation of brief measures of positive and negative affect: The PANAS scales. J. Personal. Soc. Psychol. 1988, 54, 1063. [Google Scholar] [CrossRef]
- Fortier, I.; Raina, P.; Heuvel, E.R.V.D.; Griffith, L.E.; Craig, C.; Saliba, M.; Doiron, D.; Stolk, R.P.; Knoppers, B.M.; Ferretti, V.; et al. Maelstrom Research guidelines for rigorous retrospective data harmonization. Int. J. Epidemiol. 2017, 46, 103–105. [Google Scholar] [CrossRef] [PubMed]
- Wey, T.W.; Doiron, D.; Wissa, R.; Fabre, G.; Motoc, I.; Noordzij, J.M.; Ruiz, M.; Timmermans, E.; van Lenthe, F.J.; Bobak, M.; et al. Overview of retrospective data harmonisation in the MINDMAP project: Process and results. J. Epidemiol. Community Health 2021, 75, 433–441. [Google Scholar] [CrossRef]
- Bingham, S.A.; Gill, C.; Welch, A.; Day, K.; Cassidy, A.; Khaw, K.; Sneyd, M.; Key, T.; Roe, L.; Day, N. Comparison of dietary assessment methods in nutritional epidemiology: Weighed records v. 24 h recalls, food-frequency questionnaires and estimated-diet records. Br. J. Nutr. 1994, 72, 619–643. [Google Scholar] [CrossRef]
- Bingham, S.A.; Welch, A.A.; McTaggart, A.; Mulligan, A.A.; Runswick, S.A.; Luben, R.; Oakes, S.; Khaw, K.T.; Wareham, N.; Day, N.E. Nutritional methods in the European Prospective Investigation of Cancer in Norfolk. Public Health Nutr. 2001, 4, 847–858. [Google Scholar] [CrossRef]
- Paul, A.; Southgate, D. McCance and Widdowson’s the Composition of Foods. In European Food Composition Tables in Translation; Springer: Berlin/Heidelberg, Germany, 1987; pp. 129–131. [Google Scholar]
- McCullough, M.L.; Feskanich, D.; Stampfer, M.J.; Giovannucci, E.L.; Rimm, E.B.; Hu, F.B.; Spiegelman, D.; Hunter, D.J.; A Colditz, G.; Willett, W.C. Diet quality and major chronic disease risk in men and women: Moving toward improved dietary guidance. Am. J. Clin. Nutr. 2002, 76, 1261–1271. [Google Scholar] [CrossRef]
- Wang, D.D.; Leung, C.W.; Li, Y.; Ding, E.; Chiuve, S.; Hu, F.B.; Willett, W.C. Trends in Dietary Quality Among Adults in the United States, 1999 Through 2010. JAMA Intern. Med. 2014, 174, 1587–1595. [Google Scholar] [CrossRef]
- Estrella, M.L.; A Durazo-Arvizu, R.; Mattei, J.; Mossavar-Rahmani, Y.; Perreira, K.M.; Siega-Riz, A.M.; Sotres-Alvarez, D.; González, H.M.; Gallo, L.C.; Daviglus, M.L.; et al. Alternate Healthy Eating Index is Positively Associated with Cognitive Function Among Middle-Aged and Older Hispanics/Latinos in the HCHS/SOL. J. Nutr. 2020, 150, 1478–1487. [Google Scholar] [CrossRef]
- Tomova, G.D.; Arnold, K.F.; Gilthorpe, M.S.; Tennant, P.W.G. Adjustment for energy intake in nutritional research: A causal inference perspective. Am. J. Clin. Nutr. 2021, 115, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Tarvainen, M.P.; Niskanen, J.-P.; Lipponen, J.A.; Ranta-Aho, P.O.; Karjalainen, P.A. Kubios HRV–heart rate variability analysis software. Comput. Methods Programs Biomed. 2014, 113, 210–220. [Google Scholar] [CrossRef] [PubMed]
- Recommendations, G.D. Government Recommendations for Energy and Nutrients for Males and Females Aged 1–18 Years and 19+ Years. Public Health Engl. 2016, 1–12. [Google Scholar]
- Angelino, D.; Godos, J.; Ghelfi, F.; Tieri, M.; Titta, L.; Lafranconi, A.; Marventano, S.; Alonzo, E.; Gambera, A.; Sciacca, S.; et al. Fruit and vegetable consumption and health outcomes: An umbrella review of observational studies. Int. J. Food Sci. Nutr. 2019, 70, 652–667. [Google Scholar] [CrossRef] [PubMed]
- Godos, J.; Currenti, W.; Angelino, D.; Mena, P.; Castellano, S.; Caraci, F.; Galvano, F.; Del Rio, D.; Ferri, R.; Grosso, G. Diet and Mental Health: Review of the Recent Updates on Molecular Mechanisms. Antioxidants 2020, 9, 346. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, H.R. Amino acid and protein requirements: Cognitive performance, stress and brain function. In The Role of Protein and Amino Acids in Sustaining and Enhancing Performance; Committee of Military Nutrition Research and Institute of Medicine, Ed.; National Academy Press: Washington, DC, USA, 1999; pp. 289–307. [Google Scholar]
- Strasser, B.; Gostner, J.M.; Fuchs, D. Mood, food, and cognition: Role of tryptophan and serotonin. Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Dobersek, U.; Wy, G.; Adkins, J.; Altmeyer, S.; Krout, K.; Lavie, C.J.; Archer, E. Meat and mental health: A systematic review of meat abstention and depression, anxiety, and related phenomena. Crit. Rev. Food Sci. Nutr. 2021, 61, 622–635. [Google Scholar] [CrossRef]
- Ocklenburg, S.; Borawski, J. Vegetarian diet and depression scores: A meta-analysis. J. Affect. Disord. 2021, 294, 813–815. [Google Scholar] [CrossRef]
- Berkins, S.; Schiöth, H.; Rukh, G. Depression and Vegetarians: Association between Dietary Vitamin B6, B12 and Folate Intake and Global and Subcortical Brain Volumes. Nutrients 2021, 13, 1790. [Google Scholar] [CrossRef]
- Lassen, A.D.; Christensen, L.M.; Trolle, E. Development of a Danish Adapted Healthy Plant-Based Diet Based on the EAT-Lancet Reference Diet. Nutrients 2020, 12, 738. [Google Scholar] [CrossRef]
- Schweizer, U.; Bohleber, S.; Zhao, W.; Fradejas-Villar, N. The Neurobiology of Selenium: Looking Back and to the Future. Front. Neurosci. 2021, 15, 652099. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Lapiscina, E.H.; Clavero, P.; Toledo, E.; Estruch, R.; Salas-Salvadó, J.; San Julián, B.; Sanchez-Tainta, A.; Ros, E.; Valls-Pedret, C.; Martinez-Gonzalez, M.Á. Mediterranean diet improves cognition: The PREDIMED-NAVARRA randomised trial. J. Neurol. Neurosurg. Psychiatry 2013, 84, 1318–1325. [Google Scholar] [CrossRef] [PubMed]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.-I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N. Engl. J. Med. 2018, 378, e34. [Google Scholar] [CrossRef] [PubMed]
- Onwezen, M.C.; Bouwman, E.P.; Reinders, M.J.; Dagevos, H. A systematic review on consumer acceptance of alternative proteins: Pulses, algae, insects, plant-based meat alternatives, and cultured meat. Appetite 2021, 159, 105058. [Google Scholar] [CrossRef] [PubMed]
- Gazan, R.; Brouzes, C.M.C.; Vieux, F.; Maillot, M.; Lluch, A.; Darmon, N. Mathematical Optimization to Explore Tomorrow’s Sustainable Diets: A Narrative Review. Adv. Nutr. 2018, 9, 602–616. [Google Scholar] [CrossRef] [PubMed]
- Yeh, T.-S.; Yuan, C.; Ascherio, A.; A Rosner, B.; Blacker, D.; Willett, W.C. Long-term dietary protein intake and subjective cognitive decline in US men and women. Am. J. Clin. Nutr. 2021, 115, 199–210. [Google Scholar] [CrossRef]
- Mazza, E.; Fava, A.; Ferro, Y.; Moraca, M.; Rotundo, S.; Colica, C.; Provenzano, F.; Terracciano, R.; Greco, M.; Foti, D.; et al. Impact of legumes and plant proteins consumption on cognitive performances in the elderly. J. Transl. Med. 2017, 15, 109. [Google Scholar] [CrossRef]
- Van Vliet, S.; Bain, J.R.; Muehlbauer, M.J.; Provenza, F.D.; Kronberg, S.L.; Pieper, C.F.; Huffman, K.M. A metabolomics comparison of plant-based meat and grass-fed meat indicates large nutritional differences despite comparable Nutrition Facts panels. Sci. Rep. 2021, 11, 13828. [Google Scholar] [CrossRef]
- Kamiński, M.; Skonieczna-Żydecka, K.; Nowak, J.K.; Stachowska, E. Global and local diet popularity rankings, their secular trends, and seasonal variation in Google Trends data. Nutrition 2020, 79–80, 110759. [Google Scholar] [CrossRef]
- Shekhar, M.; Rahnev, D. Sources of Metacognitive Inefficiency. Trends Cogn. Sci. 2021, 25, 12–23. [Google Scholar] [CrossRef]
- Briefel, R.R.; Flegal, K.M.; Winn, D.M.; Loria, C.M.; Johnson, C.L.; Sempos, C.T. Assessing the nation’s diet: Limitations of the food frequency questionnaire. J. Am. Diet. Assoc. 1992, 92, 959–963. [Google Scholar] [CrossRef]
- Chiuve, S.E.; Fung, T.T.; Rimm, E.B.; Hu, F.B.; McCullough, M.L.; Wang, M.; Stampfer, M.J.; Willett, W.C. Alternative Dietary Indices Both Strongly Predict Risk of Chronic Disease. J. Nutr. 2012, 142, 1009–1018. [Google Scholar] [CrossRef] [PubMed]
- Clark, M.A.; Springmann, M.; Hill, J.; Tilman, D. Multiple health and environmental impacts of foods. Proc. Natl. Acad. Sci. USA 2019, 116, 23357–23362. [Google Scholar] [CrossRef] [PubMed]
- Afshin, A.; Sur, P.J.; Fay, K.A.; Cornaby, L.; Ferrara, G.; Salama, J.S.; Mullany, E.C.; Abate, K.H.; Cristiana, A.; Abebe, Z.; et al. Health effects of dietary risks in 195 countries, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2019, 393, 1958–1972. [Google Scholar] [CrossRef]
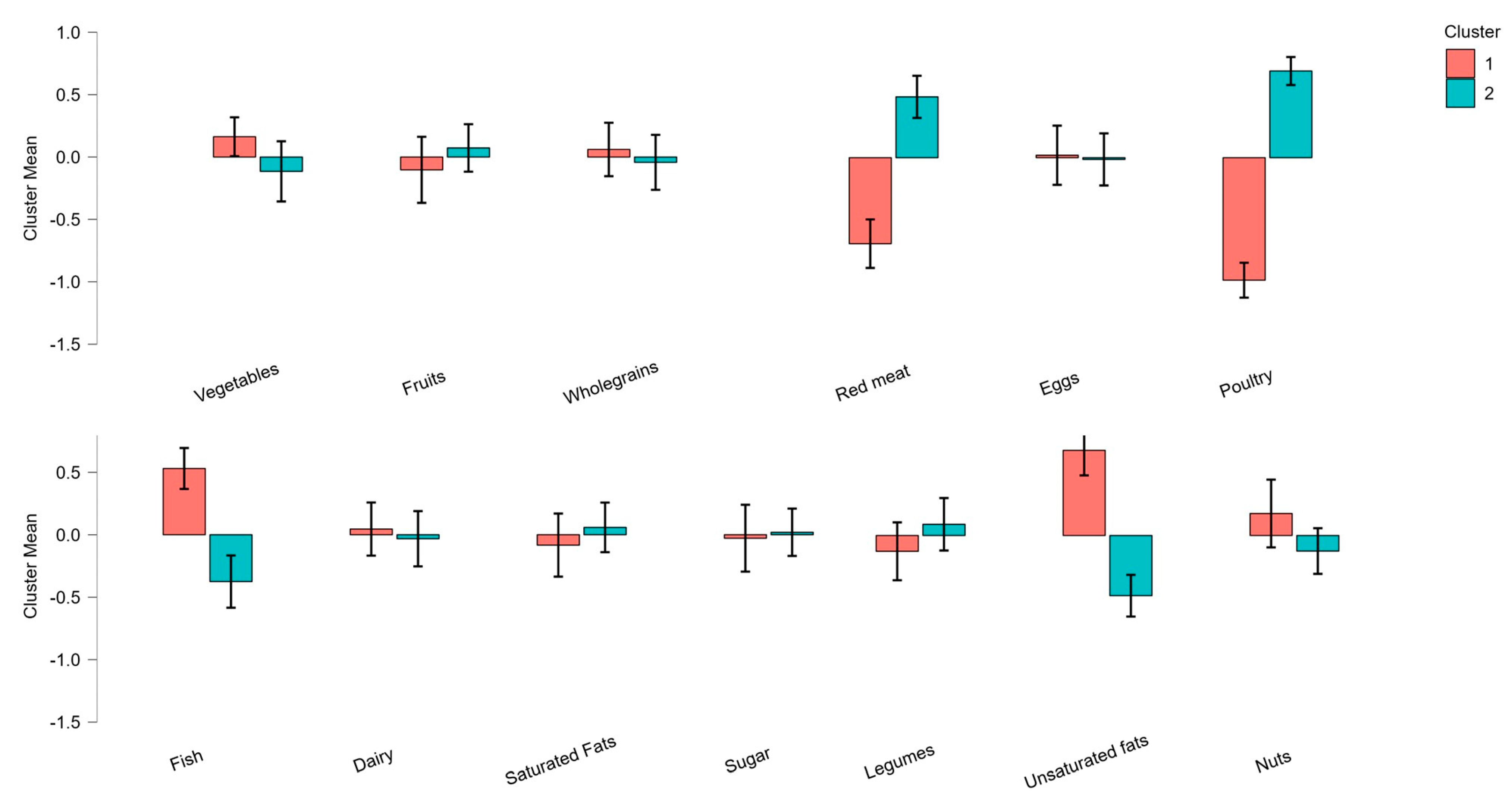
| Food Group | Cluster 1: Balanced (N = 54) | Cluster 2: Restricted (N = 57) | F | p |
|---|---|---|---|---|
| Vegetables | 9.38 (0.35) | 5.52 (0.34) | 4.477 | 0.037 |
| Fruit | 7.35 (0.53) | 7.76 (0.51) | 0.243 | 0.623 |
| Whole grains | 9.54 (0.29) | 9.92 (0.28) | 0.322 | 0.572 |
| Legumes | 5.74 (0.45) | 6.63 (0.43) | 0.069 | 0.793 |
| Unsaturated fats | 6.45 (0.52) | 2.25 (0.50) | 30.487 | <0.001 |
| Nuts | 1.33 (0.18) | 1.17 (0.17) | 0.035 | 0.853 |
| Fish | 8.72 (0.50) | 4.04 (0.48) | 51.476 | <0.001 |
| Dairy | 5.61 (0.35) | 5.58 (0.33) | 0.795 | 0.375 |
| Saturated fats | 6.41 (0.62) | 7.75 (0.59) | 10.448 | 0.002 |
| Sugar | 9.81 (0.18) | 9.82 (0.17) | 0.001 | 0.970 |
| Red meat | 2.26 (0.55) | 8.27 (0.38) | 155.136 | <0.001 |
| Eggs | 8.79 (0.38) | 9.18 (0.36) | 2.056 | 0.155 |
| Poultry | 1.73 (0.33) | 9.82 (0.32) | 82.225 | <0.001 |
| BMI (kg/m2) | 25.83 (0.76) | 25.54 (0.72) | 0.074 | 0.786 |
| Alcohol (g) | 6.91 (1.04) | 4.33 (1.00) | 3.187 | 0.077 |
| Exercise (min/week) | 130.90 (5.80) | 118.69 (5.54) | 2.316 | 0.131 |
| Kcal | 1850.57 (69.57) | 1671.54 (66.5) | 3.460 | 0.066 |
| Mood | 2.05 (0.18) | 2.63 (0.17) | 5.594 | 0.020 |
| HF HRV | 55.41 (3.44) | 50.24 (3.25) | 1.126 | 0.291 |
| Nutrient | Unsustainable (n = 215) | Cluster 1: Balanced (n = 54) | Cluster 2: Restricted (n = 57) | X2 Cluster 1 vs. Cluster 2 | p Cluster 1 vs. Cluster 2 |
|---|---|---|---|---|---|
| Calcium | 35.5% | 26.4% | 19.0% | 0.881 | 0.238 |
| Copper | 44.2% | 30.0% | 34.5% | 0.003 | 0.557 |
| Iron | 77.0% | 69.8% | 84.2% | 3.242 | 0.072 |
| Folate | 91.2% | 77.4% | 91.4% | 4.198 | 0.037 |
| Iodine | 61.1% | 34.0% | 44.8% | 1.366 | 0.165 |
| Magnesium | 60.8% | 28.3% | 32.8% | 0.259 | 0.382 |
| Selenium | 50.7% | 22.6% | 77.6% | 33.466 | 0.001 |
| Zinc | 65.8% | 41.5% | 82.8% | 20.229 | 0.001 |
| B12 | 8.3% | 3.8% | 10.3% | 1.788 | 0.167 |
| Protein | 7.2% | 0.0% | 15.5% | 8.950 | 0.002 |
| MAR | 89.43(0.58) | 95.01(1.19) | 91.66(1.12) | 9.254 | 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Young, H.A. Adherence to the EAT–Lancet Diet: Unintended Consequences for the Brain? Nutrients 2022, 14, 4254. https://doi.org/10.3390/nu14204254
Young HA. Adherence to the EAT–Lancet Diet: Unintended Consequences for the Brain? Nutrients. 2022; 14(20):4254. https://doi.org/10.3390/nu14204254
Chicago/Turabian StyleYoung, Hayley Anne. 2022. "Adherence to the EAT–Lancet Diet: Unintended Consequences for the Brain?" Nutrients 14, no. 20: 4254. https://doi.org/10.3390/nu14204254
APA StyleYoung, H. A. (2022). Adherence to the EAT–Lancet Diet: Unintended Consequences for the Brain? Nutrients, 14(20), 4254. https://doi.org/10.3390/nu14204254






