Calf Circumference, a Valuable Tool to Predict Sarcopenia in Older People Hospitalized with Hip Fracture
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Demographic and Clinical Data
2.3. Anthropometric Measurements
2.4. Functional Status
2.5. Nutritional Status
2.6. Adherence to the Mediterranean Diet (MedDiet)
2.7. Diagnosis of Sarcopenia
3. Statistical Analysis
4. Results
4.1. General Characteristics of the Participants
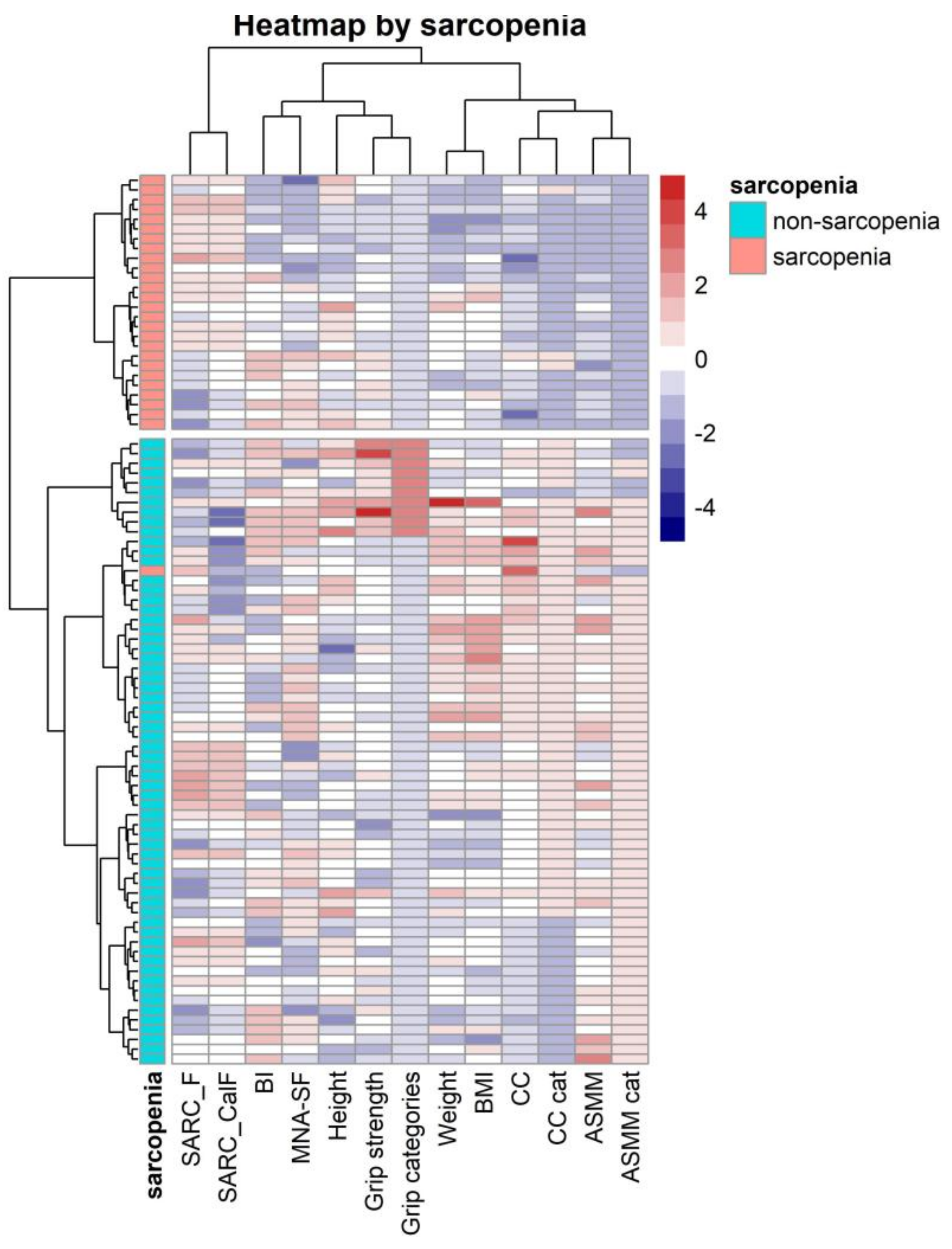
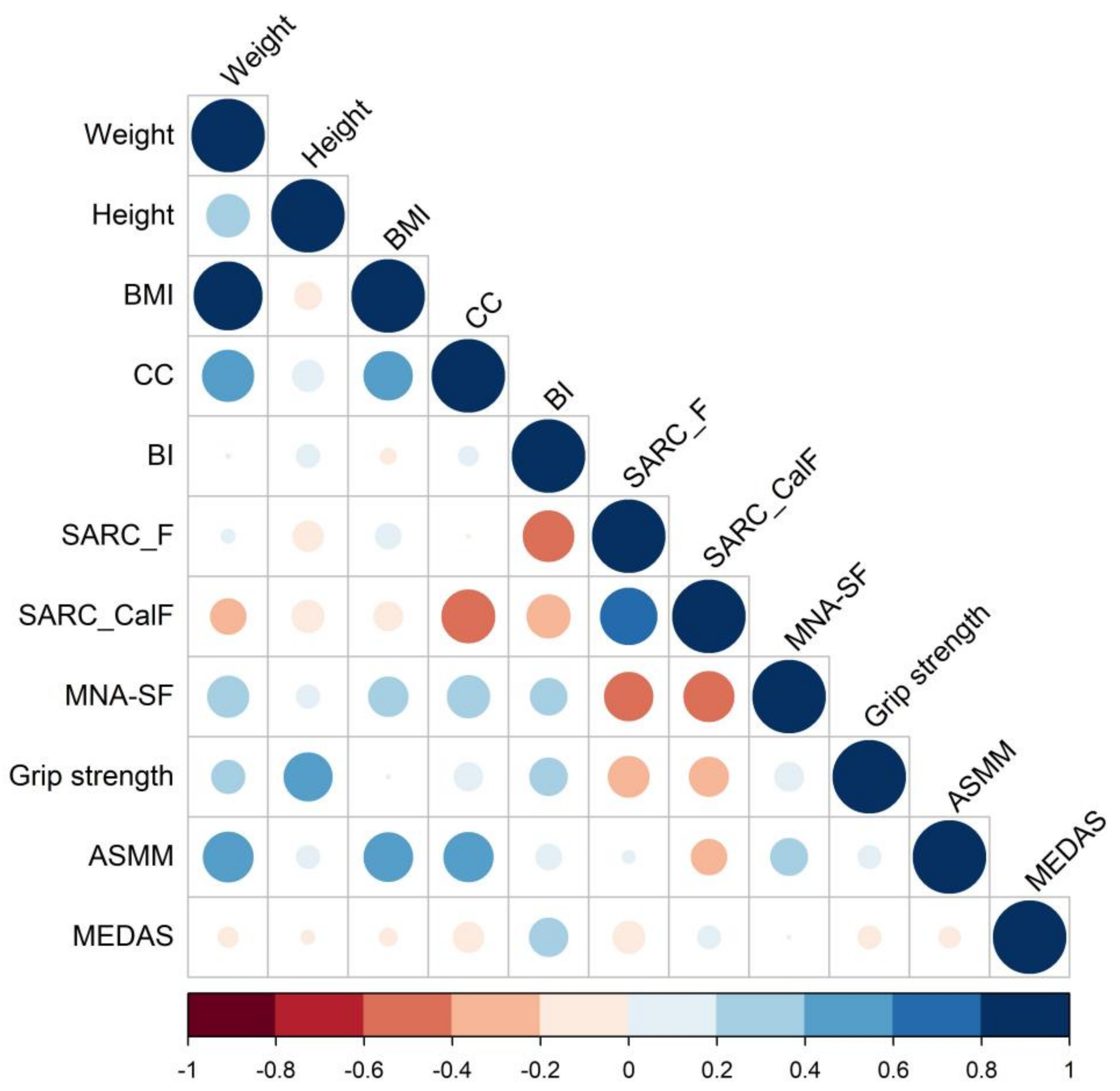
4.2. Correlation and Clustering Heatmaps Analysis
4.3. Factors Associated with Sarcopenia: Multivariable Regression Models and AIC Criteria
4.4. Prediction Models for Sarcopenia
4.5. Cut-off Value of CC to Predict Sarcopenia and ASMM
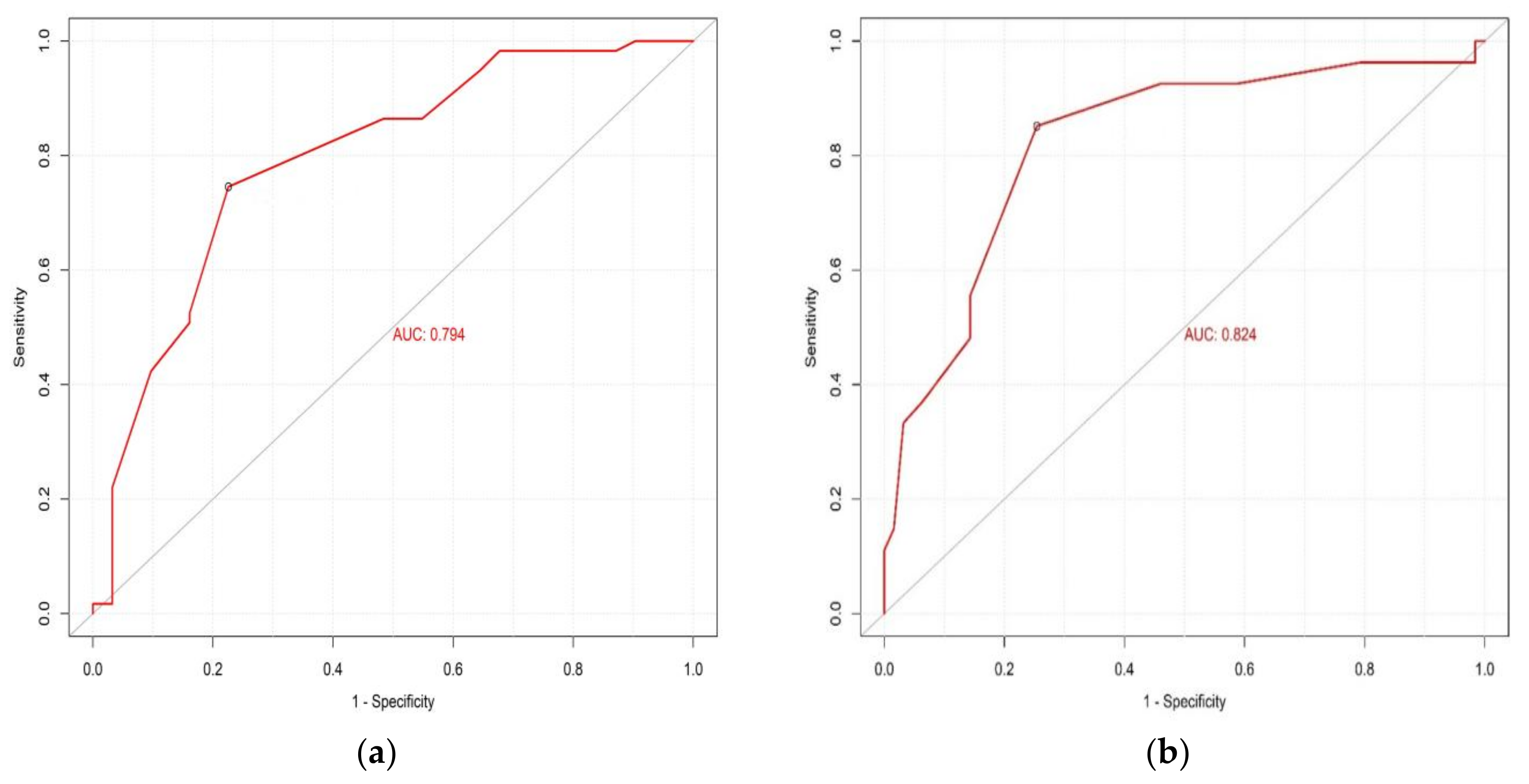
4.6. Performance of Screening Tools (SARC-F and SARC-CalF) and CC in the Detection of Sarcopenia Risk
5. Discussion
6. Strengths and Limitations
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Yeung, S.S.Y.; Reijnierse, E.M.; Pham, V.K.; Trappenburg, M.C.; Lim, W.K.; Meskers, C.G.M.; Maier, A.B. Sarcopenia and its association with falls and fractures in older adults: A systematic review and meta-analysis. J. Cachexia Sarcopenia Muscle 2019, 10, 485–500. [Google Scholar] [CrossRef] [PubMed]
- Landi, F.; Calvani, R.; Ortolani, E.; Salini, S.; Martone, A.M.; Santoro, L.; Santoliquido, A.; Sisto, A.; Picca, A.; Marzetti, E. The association between sarcopenia and functional outcomes among older patients with hip fracture undergoing in-hospital rehabilitation. Osteoporos. Int. 2017, 28, 1569–1576. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.I.; Ha, Y.C.; Choi, H.; Kim, K.H.; Lee, Y.K.; Koo, K.H.; Park, K.S. Malnutrition and chronic inflammation as risk factors for sarcopenia in elderly patients with hip fracture. Asia Pac. J. Clin. Nutr. 2018, 27, 527–532. [Google Scholar] [CrossRef]
- Malafarina, V.; Malafarina, C.; Ugarte, A.B.; Martinez, J.A.; Goñi, I.A.; Zulet, M.A. Factors Associated with Sarcopenia and 7-Year Mortality in Very Old Patients with Hip Fracture Admitted to Rehabilitation Units: A Pragmatic Study. Nutrients 2019, 11, 2243. [Google Scholar] [CrossRef]
- Kim, H.S.; Jang, G.; Park, J.W.; Lee, Y.K.; Koo, K.H. Vitamin D Deficiency and Sarcopenia in Hip Fracture Patients. J. Bone Metab. 2021, 28, 79–83. [Google Scholar] [CrossRef]
- Kim, S.H.; Park, S. A Meta-Analysis of the Correlates of Successful Aging in Older Adults. Res. Aging 2017, 39, 657–677. [Google Scholar] [CrossRef]
- Bartra, A.; Caeiro, J.R.; Mesa-Ramos, M.; Etxebarría-Foronda, I.; Montejo, J.; Carpintero, P.; Sorio-Vilela, F.; Gatell, S.; Canals, L. Cost of osteoporotic hip fracture in Spain per Autonomous Region. Rev. Esp. Cir. Ortop. Traumatol. 2019, 63, 56–68. [Google Scholar] [CrossRef]
- INE. Instituto Nacional de Estadística. INEbase/Clasificaciones Estadísticas. Available online: https://www.ine.es/prensa/pp_2020_2070.pdf (accessed on 20 September 2022).
- Sánchez-Castellano, C.; Martín-Aragón, S.; Vaquero-Pinto, N.; Bermejo-Bescós, P.; Merello de Miguel, A.; Cruz-Jentoft, A.J. Prevalencia de sarcopenia y características de los sarcopénicos en pacientes mayores de 80 años ingresados por fractura de cadera. Nutr. Hosp. 2019, 36, 813–818. [Google Scholar] [CrossRef]
- Antunes, A.C.; Araújo, D.A.; Veríssimo, M.T.; Amaral, T.F. Sarcopenia and hospitalisation costs in older adults: A cross-sectional study. Nutr. Diet. J. Dietit. Assoc. Aust. 2017, 74, 46–50. [Google Scholar] [CrossRef]
- Cuervo, M.; Ansorena, D.; García, A.; Martínez, M.A.G.; Astiasarán, I.; Martínez, J.A. Assessment of calf circumference as an indicator of the risk for hyponutrition in the elderly. Nutr. Hosp. 2009, 24, 63–67. [Google Scholar]
- Abdalla, P.P.; Venturini, A.C.R.; dos Santos, A.P.; Junior, M.F.T.; Marini, J.A.G.; Alves, T.C.; de Sousa Oliveira, A.; Mota, J.; Stratton, G.; Machado, D.R.L. Normalizing calf circumference to identify low skeletal muscle mass in older women: A cross-sectional study. Nutr. Hosp. 2021, 38, 729–735. [Google Scholar] [CrossRef]
- Pérez-Zepeda, M.U.; Gutiérrez-Robledo, L.M. Calf circumference predicts mobility disability: A secondary analysis of the Mexican health and ageing study. Eur. Geriatr. Med. 2016, 7, 262–266. [Google Scholar] [CrossRef]
- Wei, J.; Jiao, J.; Chen, C.L.; Tao, W.Y.; Ying, Y.J.; Zhang, W.W.; Wu, X.J.; Zhang, X.M. The association between low calf circumference and mortality: A systematic review and meta-analysis. Eur. Geriatr. Med. 2022, 13, 597–609. [Google Scholar] [CrossRef]
- Zhang, X.M.; Wu, X.; Ma, Y.; Zhu, C.; Cao, J.; Liu, G.; Li, F.F.; Cheng, A.S.K. Comparing the Performance of Calf Circumference, Albumin, and BMI for Predicting Mortality in Immobile Patients. Risk Manag. Healthc. Policy 2021, 14, 2289–2300. [Google Scholar] [CrossRef]
- Singh, R.; Gupta, S. Relationship of calf circumference with bone mineral density and hip geometry: A hospital-based cross-sectional study. Arch. Osteoporos. 2015, 10, 17. [Google Scholar] [CrossRef]
- Endo, K.; Sato, T.; Kakisaka, K.; Takikawa, Y. Calf and arm circumference as simple markers for screening sarcopenia in patients with chronic liver disease. Hepatol. Res. 2021, 51, 176–189. [Google Scholar] [CrossRef]
- González-Correa, C.H.; Pineda-Zuluaga, M.C.; Marulanda-Mejía, F. Skeletal Muscle Mass by Bioelectrical Impedance Analysis and Calf Circumference for Sarcopenia Diagnosis. J. Electr. Bioimpedance 2020, 11, 57–61. [Google Scholar] [CrossRef]
- Chen, H.; Ma, J.; Liu, A.; Cui, Y.; Ma, X. The association between sarcopenia and fracture in middle-aged and elderly people: A systematic review and meta-analysis of cohort studies. Injury 2020, 51, 804–811. [Google Scholar] [CrossRef]
- Nishikawa, H.; Yoh, K.; Enomoto, H.; Nishimura, T.; Nishiguchi, S.; Iijima, H. Combined grip strength and calf circumference as a useful prognostic system in patients with liver diseases: A large cohort study. Ann. Transl. Med. 2021, 9, 624. [Google Scholar] [CrossRef]
- Kim, S.; Kim, M.; Lee, Y.; Kim, B.; Yoon, T.Y.; Won, C.W. Calf Circumference as a Simple Screening Marker for Diagnosing Sarcopenia in Older Korean Adults: The Korean Frailty and Aging Cohort Study (KFACS). J. Korean Med. Sci. 2018, 33, e151. [Google Scholar] [CrossRef]
- Johnell, O.; Kanis, J.A. An estimate of the worldwide prevalence, mortality and disability associated with hip fracture. Osteoporos. Int. 2004, 15, 897–902. [Google Scholar] [CrossRef]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Jyrkkä, J.; Enlund, H.; Korhonen, M.; Sulkava, R.; Hartikainen, S. Polypharmacy Status as an Indicator of Mortality in an Elderly Population. Drugs Aging 2009, 26, 1039–1048. [Google Scholar] [CrossRef]
- Wanden-Berghe, C. Recomendaciones Nutricionales en el Anciano: Recomendaciones Practicas de los Expertos en Geriatria y Nutrición; SENPE Sociedad Española Nutrición Parenteral Enteral: São Geraldo, Brazil, 2007; ISBN 9788495364555. [Google Scholar]
- Mahoney, F.I.; Barthel, D.W. Functional Evaluation: The Barthel Index. Md. State Med. J. 1965, 14, 61–65. [Google Scholar]
- Helminen, H.; Luukkaala, T.; Saarnio, J.; Nuotio, M. Comparison of the Mini-Nutritional Assessment short and long form and serum albumin as prognostic indicators of hip fracture outcomes. Injury 2017, 48, 903–908. [Google Scholar] [CrossRef]
- Kaiser, M.J.; Bauer, J.M.; Ramsch, C.; Uter, W.; Guigoz, Y.; Cederholm, T.; Thomas, D.R.; Anthony, P.; Charlton, K.E.; Maggio, M.; et al. Validation of the Mini Nutritional Assessment short-form (MNA-SF): A practical tool for identification of nutritional status. J. Nutr. Health Aging 2009, 13, 782–788. [Google Scholar] [CrossRef]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N. Engl. J. Med. 2013, 368, 1279–1290. [Google Scholar] [CrossRef]
- Rodríguez-Rejón, A.I.; Artacho, R.; Ruiz-López, M.D. Anthropometric Measurements and Cognitive Impairment Rather Than Nutrition Status Are Associated with Sarcopenia in Long-Term Care Residents. Nutr. Clin. Pract. 2020, 35, 642–648. [Google Scholar] [CrossRef]
- Sergi, G.; De Rui, M.; Veronese, N.; Bolzetta, F.; Berton, L.; Carraro, S.; Bano, G.; Coin, A.; Manzato, E.; Perissinotto, E. Assessing appendicular skeletal muscle mass with bioelectrical impedance analysis in free-living Caucasian older adults. Clin. Nutr. 2015, 34, 667–673. [Google Scholar] [CrossRef]
- Gould, H.; Brennan, S.L.; Kotowicz, M.A.; Nicholson, G.C.; Pasco, J.A. Total and appendicular lean mass reference ranges for Australian men and women: The Geelong osteoporosis study. Calcif. Tissue Int. 2014, 94, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Malmstrom, T.K.; Morley, J.E. SARC-F: A simple questionnaire to rapidly diagnose sarcopenia. J. Am. Med. Dir. Assoc. 2013, 14, 531–532. [Google Scholar] [CrossRef] [PubMed]
- Parra-Rodríguez, L.; Szlejf, C.; García-González, A.I.; Malmstrom, T.K.; Cruz-Arenas, E.; Rosas-Carrasco, O. Cross-Cultural Adaptation and Validation of the Spanish-Language Version of the SARC-F to Assess Sarcopenia in Mexican Community-Dwelling Older Adults. J. Am. Med. Dir. Assoc. 2016, 17, 1142–1146. [Google Scholar] [CrossRef] [PubMed]
- Barbosa-Silva, T.G.; Menezes, A.M.; Bielemann, R.M.; Malmstrom, T.K.; Gonzalez, M.C. Enhancing SARC-F: Improving Sarcopenia Screening in the Clinical Practice. J. Am. Med. Dir. Assoc. 2016, 17, 1136–1141. [Google Scholar] [CrossRef]
- Cervera-Díaz, M.d.C.; López-Gómez, J.J.; García-Virto, V.; Aguado-Hernández, H.J.; De Luis-Román, D.A. Prevalencia de sarcopenia en pacientes mayores de 75 años ingresados por fractura de cadera. Endocrinol. Diabetes Nutr. 2022, in press. [Google Scholar] [CrossRef]
- Sousa-Santos, A.R.; Afonso, C.; Borges, N.; Santos, A.; Padrão, P.; Moreira, P.; Amaral, T.F. Factors associated with sarcopenia and undernutrition in older adults. Nutr. Diet. 2019, 76, 604–612. [Google Scholar] [CrossRef]
- Inoue, T.; Maeda, K.; Nagano, A.; Shimizu, A.; Ueshima, J.; Murotani, K.; Sato, K.; Tsubaki, A. Undernutrition, Sarcopenia, and Frailty in Fragility Hip Fracture: Advanced Strategies for Improving Clinical Outcomes. Nutrients 2020, 12, 3743. [Google Scholar] [CrossRef]
- Santos, L.P.; Gonzalez, M.C.; Orlandi, S.P.; Bielemann, R.M.; Barbosa-Silva, T.G.; Heymsfield, S.B. New Prediction Equations to Estimate Appendicular Skeletal Muscle Mass Using Calf Circumference: Results from NHANES 1999–2006. J. Parenter. Enter. Nutr. 2019, 43, 998–1007. [Google Scholar] [CrossRef]
- Kawakami, R.; Murakami, H.; Sanada, K.; Tanaka, N.; Sawada, S.S.; Tabata, I.; Higuchi, M.; Miyachi, M. Calf circumference as a surrogate marker of muscle mass for diagnosing sarcopenia in Japanese men and women. Geriatr. Gerontol. Int. 2015, 15, 969–976. [Google Scholar] [CrossRef]
- Inoue, T.; Maeda, K.; Shimizu, A.; Nagano, A.; Ueshima, J.; Sato, K.; Murotani, K. Calf circumference value for sarcopenia screening among older adults with stroke. Arch. Gerontol. Geriatr. 2021, 93, 104290. [Google Scholar] [CrossRef]
- Chen, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Chou, M.Y.; Iijima, K.; Jang, H.C.; Kang, L.; Kim, M.; Kim, S.; et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J. Am. Med. Dir. Assoc. 2020, 21, 300–307.e302. [Google Scholar] [CrossRef]
- Chen, C.Y.; Tseng, W.C.; Yang, Y.H.; Chen, C.L.; Lin, L.L.; Chen, F.P.; Wong, A.M.K. Calf Circumference as an Optimal Choice of Four Screening Tools for Sarcopenia Among Ethnic Chinese Older Adults in Assisted Living. Clin. Interv. Aging 2020, 15, 2415–2422. [Google Scholar] [CrossRef]
- Silva, R.; Pizato, N.; da Mata, F.; Figueiredo, A.; Ito, M.; Pereira, M.G. Mediterranean Diet and Musculoskeletal-Functional Outcomes in Community-Dwelling Older People: A Systematic Review and Meta-Analysis. J. Nutr. Health Aging 2018, 22, 655–663. [Google Scholar] [CrossRef]
- Kamijo, Y.; Kanda, E.; Ishibashi, Y.; Yoshida, M. Sarcopenia and Frailty in PD: Impact on Mortality, Malnutrition, and Inflammation. Perit. Dial. Int. 2018, 38, 447–454. [Google Scholar] [CrossRef]
- Hashemi, R.; Motlagh, A.D.; Heshmat, R.; Esmaillzadeh, A.; Payab, M.; Yousefinia, M.; Siassi, F.; Pasalar, P.; Baygi, F. Diet and its relationship to sarcopenia in community dwelling Iranian elderly: A cross sectional study. Nutrition 2015, 31, 97–104. [Google Scholar] [CrossRef]
- Tan, V.M.H.; Pang, B.W.J.; Lau, L.K.; Jabbar, K.A.; Seah, W.T.; Chen, K.K.; Ng, T.P.; Wee, S.L. Malnutrition and Sarcopenia in Community-Dwelling Adults in Singapore: Yishun Health Study. J. Nutr. Health Aging 2021, 25, 374–381. [Google Scholar] [CrossRef]
- Yazawa, A.; Inoue, Y.; Kondo, N.; Miyaguni, Y.; Ojima, T.; Kondo, K.; Kawachi, I. Accuracy of self-reported weight, height and body mass index among older people in Japan. Geriatr. Gerontol. Int. 2020, 20, 803–810. [Google Scholar] [CrossRef]
| n = 90 | |
|---|---|
| Age (years) | 83.4 (±7.2) |
| Sex | |
| Female | 80 (88.9%) |
| Male | 10 (11.1%) |
| Living arrangement | |
| Other family members | 62 (68.9%) |
| Nursing home residents | 10 (11.1%) |
| Alone | 9 (10.0%) |
| Partner | 9 (10.0%) |
| CCI | |
| High comorbidity | 89 (98.9%) |
| Low comorbidity | 1 (1.11%) |
| No comorbidity | 0 (0.0%) |
| Mdications/day | 6.7 (±3.56) |
| Length of hospital stay (days) | 10.9 (±7.38) |
| Weight (kg) | 68.2 (±11.5) |
| Height (m) | 1.59 (±0.06) |
| BMI (kg/m2) | 26.9 (±4.31) |
| Normal weight | 38 (42.2%) |
| Underweight | 11 (12.2%) |
| Overweight | 24 (26.7%) |
| Obese | 17 (18.9%) |
| Normal weight | 38 (42.2%) |
| CC (cm) | 31.1 (±3.33) |
| CC categories | |
| Low | 39 (43.3%) |
| Normal | 51 (56.7%) |
| BI | 58.8 (±27.4) |
| BI categories | |
| Independent | 3 (3.33%) |
| Mild dependent | 17 (18.9%) |
| Moderate dependent | 16 (17.8%) |
| Severe dependent | 47 (52.2%) |
| Total dependent | 7 (7.78%) |
| MNA-SF | 9.94 (±2.63) |
| MNA-SF categories | |
| Normal | 26 (28.9%) |
| At risk of nutrition | 45 (50.0%) |
| Malnourished | 19 (21.1%) |
| MEDAS | 0.70 (±0.46) |
| MEDAS categories | |
| Low | 18 (20.0%) |
| High | 72 (80.0%) |
| Grip Strength (kg) | 12.1 (±6.80) |
| Grip Strength categories | |
| Low | 81 (90.0%) |
| Normal | 9 (10.0%) |
| ASMM/height2 (kg/m2) | 6.80 (±1.35) |
| ASMM/height2categories | |
| Low | 31 (34.4%) |
| Normal | 59 (65.6%) |
| Probable sarcopenia | 81 (90.0%) |
| Sarcopenia | 27 (30.0%) |
| Non-Sarcopenic Group n = 63 | Sarcopenic Group n = 27 | OR [95% CI] | p | |
|---|---|---|---|---|
| Age (years) | 83.0 (±7.27) | 84.1 (±6.97) | 1.02 [0.96–1.09] | 0.503 |
| Sex | ||||
| Female | 55 (87.3%) | 25 (92.6%) | Ref. | |
| Male | 8 (12.7%) | 2 (7.41%) | 0.58 [0.08–2.60] | 0.506 |
| Living arrangement | ||||
| Other family members | 42 (66.7%) | 20 (74.1%) | Ref. | |
| Nursing home residents | 7 (11.1%) | 3 (11.1%) | 0.92 [0.17–3.82] | 0.916 |
| Alone | 8 (12.7%) | 1 (3.70%) | 0.30 [0.01–1.83] | 0.219 |
| Partner | 6 (9.52%) | 3 (11.1%) | 1.07 [0.20–4.67] | 0.930 |
| CCI | ||||
| High comorbidity | 62 (98.4%) | 27 (100%) | Ref. | |
| Low comorbidity | 1 (1.59%) | 0 (0.00%) | ||
| No comorbidity | 0 (0.0%) | 0 (0.00%) | ND | |
| Nº of medications/day | 6.78 (±3.62) | 6.52 (±3.49) | 0.98 [0.86–1.11] | 0.751 |
| Length hospital stay (days) | 10.9 (±7.00) | 10.9 (±8.33) | 1.00 [0.94–1.06] | 0.990 |
| Weight (kg) | 70.9 (±11.5) | 62.0 (±8.87) | 0.92 [0.87–0.97] | 0.001 |
| Height (m) | 1.59 (±0.06) | 1.59 (±0.06) | 0.23 [0.00–366] | 0.694 |
| BMI (kg/m2) | 27.9 (±4.38) | 24.5 (±3.13) | 0.79 [0.68–0.91] | 0.001 |
| BMI categories | ||||
| Normal weight | 24 (38.1%) | 14 (51.9%) | Ref. | |
| Underweight | 4 (6.35%) | 7 (25.9%) | 2.89 [0.72–13.3] | 0.136 |
| Overweight | 19 (30.2%) | 5 (18.5%) | 0.46 [0.13–1.47] | 0.198 |
| Obese | 16 (25.4%) | 1 (3.70%) | 0.12 [0.00–0.72] | 0.016 |
| CC (cm) | 32.0 (±2.79) | 28.9 (±3.49) | 0.64 [0.51–0.81] | <0.001 |
| CC categories | ||||
| Low | 16 (25.4%) | 23 (85.2%) | Ref. | |
| Normal | 47 (74.6%) | 4 (14.8%) | 0.06 [0.02–0.19] | <0.001 |
| BI | 61.2 (±27.8) | 53.3 (±26.2) | 0.99 [0.97–1.01] | 0.213 |
| BI categories | ||||
| Independent | 3 (4.76%) | 0 (0.00%) | Ref. | |
| Mild dependent | 12 (19.0%) | 5 (18.5%) | ND | |
| Moderate dependent | 13 (20.6%) | 3 (11.1%) | ND | |
| Severe dependent | 29 (46.0%) | 18 (66.7%) | ND | |
| Total dependent | 6 (9.52%) | 1 (3.70%) | ND | |
| MNA-SF | 10.4 (±2.51) | 8.85 (±2.63) | 0.79 [0.66–0.95] | 0.012 |
| MNA-SF categories | ||||
| Normal | 23 (36.5%) | 3 (11.1%) | Ref. | |
| At risk of nutrition | 31 (49.2%) | 14 (51.9%) | 3.30 [0.93–16.3] | 0.067 |
| Malnourished | 9 (14.3%) | 10 (37.0%) | 7.84 [1.87–43.9] | 0.004 |
| MEDAS | 0.71 (±0.46) | 0.67 (±0.48) | 0.80 [0.30–2.11] | 0.652 |
| MEDAS categories | ||||
| Low | 13 (20.6%) | 5 (18.5%) | Ref. | |
| High | 50 (79.4%) | 22 (81.5%) | 1.13 [0.37–3.96] | 0.841 |
| Low CC Group | Normal CC Group | OR [95% CI] | p | |
|---|---|---|---|---|
| n = 39 | n = 51 | |||
| Age (years) | 86.0 (±6.90) | 81.3 (±6.75) | 0.90 [0.84–0.97] | 0.004 |
| Sex | ||||
| Female | 36 (92.3%) | 44 (86.3%) | Ref. | |
| Male | 3 (7.69%) | 7 (13.7%) | 1.85 [0.46–9.59] | 0.396 |
| Living arrangement | ||||
| Other family members | 28 (71.8%) | 34 (66.7%) | Ref. | |
| Nursing home residents | 5 (12.8%) | 5 (9.80%) | 0.83 [0.20–3.36] | 0.785 |
| Alone | 2 (5.13%) | 7 (13.7%) | 2.71 [0.58–21.3] | 0.218 |
| Partner | 4 (10.3%) | 5 (9.80%) | 1.02 [0.24–4.68] | 0.976 |
| CCI | ||||
| High comorbidity | 39 (100%) | 50 (98.0%) | Ref. | |
| Low comorbidity | 0 (0.00%) | 1 (1.96%) | ND | |
| Nº of medications/day | 6.74 (±3.09) | 6.67 (±3.91) | 0.99 [0.88–1.12] | 0.919 |
| Length hospital stay (days) | 10.2 (±7.30) | 11.5 (±7.46) | 1.03 [0.97–1.09] | 0.412 |
| Weight (kg) | 62.5 (±8.36) | 72.6 (±11.8) | 1.11 [1.05–1.18] | <0.001 |
| Height (cm) | 158 (±6.75) | 160 (±6.57) | 1.07 [1.00-1.14] | 0.047 |
| BMI (kg/m2) | 25.0 (±3.04) | 28.3 (±4.63) | 1.25 [1.09–1.42] | 0.001 |
| BMI categories | ||||
| Normal weight | 23 (59.0%) | 15 (29.4%) | Ref. | |
| Underweight | 7 (17.9%) | 4 (7.84%) | 0.89 [0.19–3.59] | 0.871 |
| Overweight | 7 (17.9%) | 17 (33.3%) | 3.61 [1.23–11.5] | 0.019 |
| Obese | 2 (5.13%) | 15 (29.4%) | 10.4 [2.44–80.0] | 0.001 |
| BI | 55.4 (±27.3) | 61.5 (±27.4) | 1.01 [0.99–1.02] | 0.295 |
| BI categories | ||||
| Independent | 1 (2.56%) | 2 (3.92%) | Ref. | |
| Mild dependent | 7 (17.9%) | 10 (19.6%) | 0.76 [0.02–11.2] | 0.849 |
| Moderate dependent | 4 (10.3%) | 12 (23.5%) | 1.53 [0.04–24.2] | 0.779 |
| Severe dependent | 23 (59.0%) | 24 (47.1%) | 0.56 [0.02–7.35] | 0.663 |
| Total dependent | 4 (10.3%) | 3 (5.88%) | 0.43 [0.01–7.81] | 0.583 |
| MNA-SF | 8.85 (±2.38) | 10.8 (±2.52) | 1.37 [1.13–1.65] | 0.001 |
| MNA-SF categories | ||||
| Normal | 4 (10.3%) | 22 (43.1%) | Ref. | |
| At risk of nutrition | 22 (56.4%) | 23 (45.1%) | 0.20 [0.05–0.63] | 0.005 |
| Malnourished | 13 (33.3%) | 6 (11.8%) | 0.09 [0.02–0.36] | <0.001 |
| MEDAS | 0.74 (±0.44) | 0.67 (±0.48) | 0.69 [0.27–1.74] | 0.431 |
| MEDAS categories | ||||
| Low | 6 (15.4%) | 12 (23.5%) | Ref. | |
| High | 33 (84.6%) | 39 (76.5%) | 0.60 [0.19–1.75] | 0.356 |
| Grip Strength (kg) | 10.4 (±4.08) | 13.3 (±8.11) | 1.08 [1.00–1.18] | 0.054 |
| Grip categories | ||||
| Low | 38 (97.4%) | 43 (84.3%) | Ref. | |
| Normal | 1 (2.56%) | 8 (15.7%) | 6.22 [1.04–162] | 0.044 |
| ASMM/height2 (kg/m2) | 6.16 (±1.25) | 7.30 (±1.21) | 2.27 [1.46–3.53] | <0.001 |
| ASMM/height2 categories | ||||
| Low | 24 (61.5%) | 7 (13.7%) | Ref. | |
| Normal | 15 (38.5%) | 44 (86.3%) | 9.62 [3.58–29.0] | <0.001 |
| Sarcopenia diagnosis | ||||
| Non-sarcopenic | 16 (41.0%) | 47 (92.2%) | Ref. | |
| Sarcopenic | 23 (59.0%) | 4 (7.84%) | 0.06 [0.02–0.19] | <0.001 |
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | Lower CI | Upper CI | OR | Lower CI | Upper CI | OR | Lower CI | Upper CI | OR | Lower CI | Upper CI | OR | Lower CI | Upper CI | |
| BMI (per 1 unit) | 0.823 ** | 0.671 | 0.983 | 0.829 ** | 0.682 | 0.982 | 0.823 ** | 0.671 | 0.947 | 0.837 * | 0.688 | 0.995 | 0.845 * | 0.694 | 1.005 |
| CC < 31 cm (Ref.) | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | ||||||||||
| CC ≥ 31 cm | 0.090 *** | 0.022 | 0.295 | 0.085 *** | 0.022 | 0.272 | 0.086 *** | 0.021 | 0.279 | 0.086 *** | 0.021 | 0.282 | 0.091 *** | 0.022 | 0.304 |
| BI (per 1 unit) | 0.991 | 0.970 | 1.012 | ||||||||||||
| BI categories | |||||||||||||||
| BI Mild dependent Ref. | 1.00 | 1.00 | |||||||||||||
| BI Moderate dependent | 1.443 | 0.192 | 10.405 | ||||||||||||
| BI Severe dependent | 1.821 | 0.462 | 8.298 | ||||||||||||
| MNA-SF (per 1 unit) | 0.957 | 0.752 | 1.219 | ||||||||||||
| MNA-SF categories | |||||||||||||||
| MNA-SF normal (Ref.) | 1.00 | ||||||||||||||
| MNA-SF at risk | 1.349 | 0.278 | 7.492 | ||||||||||||
| MNA-malnourished | 1.976 | 0.323 | 13.232 | ||||||||||||
| Log likelihood | −38.075 | −37.716 | −37.691 | −38.009 | −37.782 | ||||||||||
| Akaike Inf. Criteria | 82.149 | 83.431 | 85.382 | 84.018 | 85.565 | ||||||||||
| Variable | Parameter Estimate | Standard Error | z | p |
|---|---|---|---|---|
| Intercept | 2.516 | 2.391 | 1.052 | 0.292 |
| BMI (kg/m2) | −0.185 | 0.091 | −2.037 | 0.04 |
| CC < 31 cm | 2.521 | 0.630 | 4.000 | <0.001 |
| Variables | Sensitivity (%) | Specificity (%) | NPV | PPV | Younden’s Index | AUC |
|---|---|---|---|---|---|---|
| SARC-F | 88.9 | 28.6 | 0.86 | 0.35 | 1.17 | 0.553 |
| SARC-CalF | 85.1 | 41.2 | 0.86 | 0.38 | 1.26 | 0.597 |
| CC | 85.2 | 74.6 | 0.92 | 0.58 | 1.59 | 0.824 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borges, K.; Artacho, R.; Jodar-Graus, R.; Molina-Montes, E.; Ruiz-López, M.D. Calf Circumference, a Valuable Tool to Predict Sarcopenia in Older People Hospitalized with Hip Fracture. Nutrients 2022, 14, 4255. https://doi.org/10.3390/nu14204255
Borges K, Artacho R, Jodar-Graus R, Molina-Montes E, Ruiz-López MD. Calf Circumference, a Valuable Tool to Predict Sarcopenia in Older People Hospitalized with Hip Fracture. Nutrients. 2022; 14(20):4255. https://doi.org/10.3390/nu14204255
Chicago/Turabian StyleBorges, Keith, Reyes Artacho, Rosa Jodar-Graus, Esther Molina-Montes, and María Dolores Ruiz-López. 2022. "Calf Circumference, a Valuable Tool to Predict Sarcopenia in Older People Hospitalized with Hip Fracture" Nutrients 14, no. 20: 4255. https://doi.org/10.3390/nu14204255
APA StyleBorges, K., Artacho, R., Jodar-Graus, R., Molina-Montes, E., & Ruiz-López, M. D. (2022). Calf Circumference, a Valuable Tool to Predict Sarcopenia in Older People Hospitalized with Hip Fracture. Nutrients, 14(20), 4255. https://doi.org/10.3390/nu14204255






