Addressing Anxiety and Stress for Healthier Eating in Teens (ASSET): A Pilot Randomized Controlled Trial Protocol for Reducing Anxiety, Disinhibited Eating, Excess Weight Gain, and Cardiometabolic Risk in Adolescent Girls
Abstract
1. Introduction
1.1. Disinhibited Eating
1.2. Anxiety Symptoms and Disinhibited Eating
1.3. Targeting Anxiety to Reduce Disinhibited Eating/Excess Weight Gain
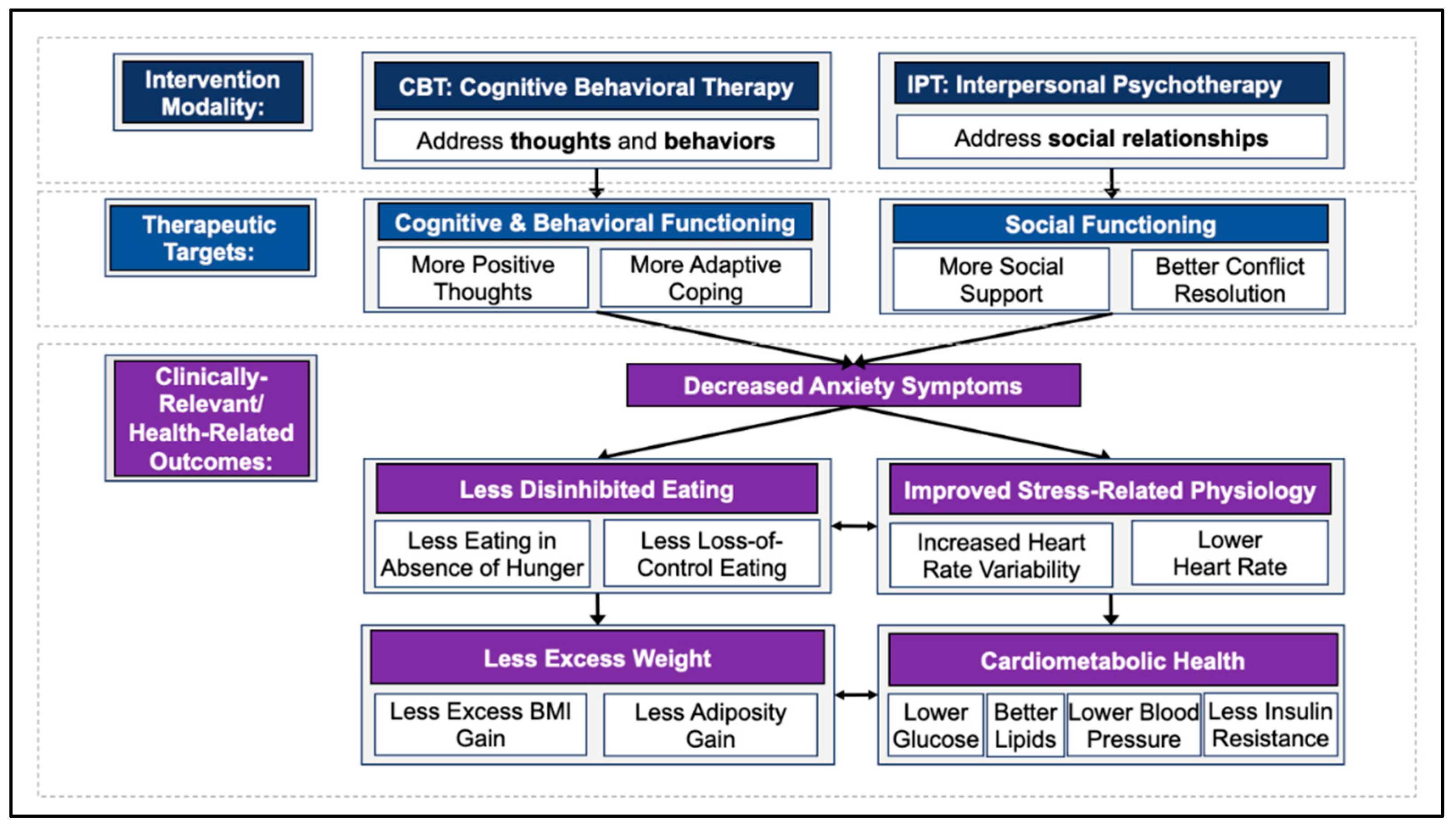
1.3.1. Cognitive Behavioral Therapy (CBT)
1.3.2. Interpersonal Psychotherapy (IPT)
1.4. Study Objectives
2. Materials and Methods
2.1. Clinically Relevant and Health-Related Measures
2.1.1. Weight and Body Composition
2.1.2. Cardiometabolic Health
2.1.3. Stress-Related Physiology
2.1.4. Daily Physical Activity
2.1.5. Disinhibited Eating
2.1.6. Anxiety and Depression Symptoms
2.1.7. Ecological Momentary Assessments
2.2. Therapeutic Targets
2.2.1. Cognitive and Behavioral Functioning
2.2.2. Social Functioning
2.2.3. Affect and Emotion Regulation
2.2.4. Self-Efficacy
2.3. Procedures and Participant Timeline
2.3.1. Pre-Screening Assessment
2.3.2. Screening/Baseline Assessment
2.3.3. Baseline Real-Time, Real-World Data Collection
2.3.4. The 12-Week Group Telehealth Intervention
2.3.5. Post-Treatment Assessment
2.3.6. The 1-Year Follow-Up
2.3.7. The 2- and 3-Year Follow-Ups
2.4. Description of the Interventions
2.4.1. General Intervention Structure and Format
2.4.2. Cognitive Behavioral Therapy (CBT)
2.4.3. Interpersonal Psychotherapy (IPT)
3. Results
3.1. Feasibility Indicators
3.2. Acceptability Indicators
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Disclaimer
References
- About Child & Teen Bmi. Available online: https://www.cdc.gov/healthyweight/assessing/bmi/childrens_bmi/about_childrens_bmi.html (accessed on 12 December 2021).
- Hales, C.M.; Carrol, M.D.; Fryar, C.D.; Ogden, C.L. Prevalence of obesity among adults and youth: United States, 2015–2016. NCHS Data Briefs 2017, 288, 1–8. [Google Scholar]
- Umer, A.; Kelley, G.A.; Cottrell, L.E.; Giacobbi, P., Jr.; Innes, K.E.; Lilly, C.L. Childhood obesity and adult cardiovascular disease risk factors: A systematic review with meta-analysis. BMC Public Health 2017, 17, 683. [Google Scholar] [CrossRef]
- Ward, Z.J.; Long, M.W.; Resch, S.C.; Giles, C.M.; Cradock, A.L.; Gortmaker, S.L. Simulation of Growth Trajectories of Childhood Obesity into Adulthood. N. Engl. J. Med. 2017, 377, 2145–2153. [Google Scholar] [CrossRef]
- Field, A.E.; Cook, N.R.; Gillman, M.W. Weight Status in Childhood as a Predictor of Becoming Overweight or Hypertensive in Early Adulthood. Obes. Res. 2005, 13, 163–169. [Google Scholar] [CrossRef]
- Nader, P.R.; O’Brien, M.; Houts, R.; Bradley, R.; Belsky, J.; Crosnoe, R.; Friedman, S.; Mei, Z.; Susman, E.J. Identifying risk for obesity in early childhood. Pediatrics 2006, 118, e594–e601. [Google Scholar] [CrossRef]
- Geng, T.; Smith, C.E.; Li, C.; Huang, T. Childhood BMI and Adult Type 2 Diabetes, Coronary Artery Diseases, Chronic Kidney Disease, and Cardiometabolic Traits: A Mendelian Randomization Analysis. Diabetes Care 2018, 41, 1089–1096. [Google Scholar] [CrossRef] [PubMed]
- Freedman, D.S.; Mei, Z.; Srinivasan, S.R.; Berenson, G.S.; Dietz, W.H. Cardiovascular Risk Factors and Excess Adiposity among Overweight Children and Adolescents: The Bogalusa Heart Study. J. Pediatr. 2007, 150, 12–17.e2. [Google Scholar] [CrossRef] [PubMed]
- Meigs, J.B.; Wilson, P.W.F.; Fox, C.S.; Vasan, R.S.; Nathan, D.M.; Sullivan, L.; D’Agostino, R.B. Body Mass Index, Metabolic Syndrome, and Risk of Type 2 Diabetes or Cardiovascular Disease. J. Clin. Endocrinol. Metab. 2006, 91, 2906–2912. [Google Scholar] [CrossRef]
- Morrison, J.A.; Friedman, L.A.; Gray-McGuire, C. Metabolic Syndrome in Childhood Predicts Adult Cardiovascular Disease 25 Years Later: The Princeton Lipid Research Clinics Follow-up Study. Pediatrics 2007, 120, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Morrison, J.A.; Friedman, L.A.; Wang, P.; Glueck, C.J. Metabolic Syndrome in Childhood Predicts Adult Metabolic Syndrome and Type 2 Diabetes Mellitus 25 to 30 Years Later. J. Pediatr. 2008, 152, 201–206. [Google Scholar] [CrossRef]
- Morrison, J.A.; Glueck, C.J.; Woo, J.G.; Wang, P. Risk factors for cardiovascular disease and type 2 diabetes retained from childhood to adulthood predict adult outcomes: The Princeton LRC Follow-up Study. Int. J. Pediatr. Endocrinol. 2012, 2012, 6. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, D.R., Jr.; Woo, J.G.; Sinaiko, A.R.; Daniels, S.R.; Ikonen, J.; Juonala, M.; Kartiosuo, N.; Lehtimäki, T.; Magnussen, C.G.; Viikari, J.S.; et al. Childhood Cardiovascular Risk Factors and Adult Cardiovascular Events. N. Engl. J. Med. 2022, 386, 1877–1888. [Google Scholar] [CrossRef]
- Alberga, A.S.; Sigal, R.J.; Goldfield, G.; Homme, D.P.; Kenny, G.P. Overweight and obese teenagers: Why is adolescence a critical period? Pediatr. Obes. 2012, 7, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Yanovski, S.Z.; Yanovski, J.A. Toward Precision Approaches for the Prevention and Treatment of Obesity. JAMA 2018, 319, 223–224. [Google Scholar] [CrossRef] [PubMed]
- Kelly, A.S.; Marcus, M.D.; Yanovski, J.A.; Yanovski, S.Z.; Osganian, S.K. Working toward precision medicine approaches to treat severe obesity in adolescents: Report of an NIH workshop. Int. J. Obes. 2018, 42, 1834–1844. [Google Scholar] [CrossRef] [PubMed]
- Wilfley, D.E.; Tibbs, T.L.; Van Buren, D.; Reach, K.P.; Walker, M.S.; Epstein, L.H. Lifestyle interventions in the treatment of childhood overweight: A meta-analytic review of randomized controlled trials. Health Psychol. 2007, 26, 521–532. [Google Scholar] [CrossRef]
- Field, A.E.; Camargo, C.A.; Ogino, S. The merits of subtyping obesity: One size does not fit all. JAMA 2013, 310, 2147–2148. [Google Scholar] [CrossRef]
- Shomaker, L.B.; Tanofsky-Kraff, M.; Yanovski, J.A. Disinhibited Eating and Body Weight in Youth. In Handbook of Behavior, Food and Nutrition; Springer: New York, NY, USA, 2011; pp. 2183–2200. [Google Scholar] [CrossRef]
- Goossens, L.; Braet, C.; Van Vlierberghe, L.; Mels, S. Loss of control over eating in overweight youngsters: The role of anxiety, depression and emotional eating. Eur. Eat. Disord. Rev. 2009, 17, 68–78. [Google Scholar] [CrossRef]
- He, J.; Cai, Z.; Fan, X. Prevalence of binge and loss of control eating among children and adolescents with overweight and obesity: An exploratory meta-analysis. Int. J. Eat. Disord. 2017, 50, 91–103. [Google Scholar] [CrossRef]
- American Psychiatric, A. Diagnostic and statistical manual of mental disorders. Am. Psychiatric. Assoc. 2013, 21, 591–643. [Google Scholar]
- Tanofsky-Kraff, M.; Ba, K.R.T.; Yanovski, J.; Bassett, A.M.; Burns, N.P.; Bs, L.M.R.; Ma, D.R.G.; Yanovski, J.A. Validation of the emotional eating scale adapted for use in children and adolescents (EES-C). Int. J. Eat. Disord. 2007, 40, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Byrne, M.E.; Tanofsky-Kraff, M.; Kelly, N.R.; Grammer, A.C.; Jaramillo, M.; Mi, S.J.; Stojek, M.; Shank, L.; Burke, N.; Cassidy, O.; et al. Pediatric Loss-of-Control Eating and Anxiety in Relation to Components of Metabolic Syndrome. J. Pediatr. Psychol. 2019, 44, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Sonneville, K.R.; Horton, N.; Micali, N.; Crosby, R.; Swanson, S.A.; Solmi, F.; Field, A.E. Longitudinal Associations between Binge Eating and Overeating and Adverse Outcomes among Adolescents and Young Adults: Does loss of control matter? JAMA Pediatr. 2013, 167, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Tanofsky-Kraff, M.; Cohen, M.L.; Yanovski, S.Z.; Cox, C.; Theim, K.R.; Keil, M.; Reynolds, J.C.; Yanovski, J.A. A Prospective Study of Psychological Predictors of Body Fat Gain among Children at High Risk for Adult Obesity. Pediatrics 2006, 117, 1203–1209. [Google Scholar] [CrossRef] [PubMed]
- Tanofsky-Kraff, M.; Yanovski, J.; Ba, N.A.S.; Olsen, C.H.; Bs, J.G.; Yanovski, J.A. A prospective study of loss of control eating for body weight gain in children at high risk for adult obesity. Int. J. Eat. Disord. 2009, 42, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Stojek, M.; Tanofsky-Kraff, M.; Shomaker, L.B.; Kelly, N.R.; Ba, K.A.T.; Mehari, R.D.; Bs, S.E.M.; Demidowich, A.P.; Galescu, O.A.; Brady, S.M.; et al. Associations of adolescent emotional and loss of control eating with 1-year changes in disordered eating, weight, and adiposity. Int. J. Eat. Disord. 2017, 50, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Radin, R.; Tanofsky-Kraff, M.; Shomaker, L.B.; Kelly, N.R.; Pickworth, C.K.; Shank, L.; Altschul, A.M.; Brady, S.M.; Demidowich, A.P.; Yanovski, S.Z.; et al. Metabolic characteristics of youth with loss of control eating. Eat. Behav. 2015, 19, 86–89. [Google Scholar] [CrossRef] [PubMed]
- Ranzenhofer, L.M.; Engel, S.G.; Crosby, R.D.; Haigney, M.; Anderson, M.; McCaffery, J.M.; Tanofsky-Kraff, M. Real-time assessment of heart rate variability and loss of control eating in adolescent girls: A pilot study. Int. J. Eat. Disord. 2016, 49, 197–201. [Google Scholar] [CrossRef]
- Shank, L.; Tanofsky-Kraff, M.; Kelly, N.R.; Schvey, N.A.; Marwitz, S.E.; Mehari, R.D.; Brady, S.M.; Demidowich, A.P.; Broadney, M.M.; Galescu, O.A.; et al. Pediatric Loss of Control Eating and High-Sensitivity C-Reactive Protein Concentrations. Child. Obes. 2017, 13, 1–8. [Google Scholar] [CrossRef]
- Koren, R.; Duncan, A.E.; Munn-Chernoff, M.; Bucholz, K.K.; Lynskey, M.T.; Heath, A.C.; Agrawal, A. Preliminary evidence for the role of HTR2A variants in binge eating in young women. Psychiatr. Genet. 2014, 24, 28–33. [Google Scholar] [CrossRef]
- Tomkins, S.S. Affect theory. Approaches Emot. 1984, 163, 31–65. [Google Scholar]
- Ranzenhofer, L.M.; Hannallah, L.; Field, S.E.; Shomaker, L.B.; Stephens, M.; Sbrocco, T.; Kozlosky, M.; Reynolds, J.; Yanovski, J.A.; Tanofsky-Kraff, M. Pre-meal affective state and laboratory test meal intake in adolescent girls with loss of control eating. Appetite 2013, 68, 30–37. [Google Scholar] [CrossRef]
- Shank, L.M.; Crosby, R.D.; Grammer, A.C.; Shomaker, L.B.; Vannucci, A.; Burke, N.L.; Stojek, M.; Brady, S.M.; Kozlosky, M.; Reynolds, J.C.; et al. Examination of the interpersonal model of loss of control eating in the laboratory. Compr. Psychiatry 2017, 76, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Merikangas, K.R.; He, J.-P.; Burstein, M.; Swanson, S.A.; Avenevoli, S.; Cui, L.; Benjet, C.; Georgiades, K.; Swendsen, J. Lifetime Prevalence of Mental Disorders in U.S. Adolescents: Results from the National Comorbidity Survey Replication–Adolescent Supplement (NCS-A). J. Am. Acad. Child Adolesc. Psychiatry 2010, 49, 980–989. [Google Scholar] [CrossRef] [PubMed]
- Aune, T.; Stiles, T.C. The Effects of Depression and Stressful Life Events on the Development and Maintenance of Syndromal Social Anxiety: Sex and Age Differences. J. Clin. Child Adolesc. Psychol. 2009, 38, 501–512. [Google Scholar] [CrossRef] [PubMed]
- Burke, N.; Storch, E.A. A Meta-analysis of Weight Status and Anxiety in Children and Adolescents. J. Dev. Behav. Pediatr. 2015, 36, 133–145. [Google Scholar] [CrossRef]
- Friedman, J.M. The Function of Leptin in Nutrition, Weight, and Physiology. Nutr. Rev. 2002, 60, S1–S14; discussion S68–S84, S85–S87. [Google Scholar] [CrossRef]
- Byrne, M.E.; Tanofsky-Kraff, M.; Jaramillo, M.; Shank, L.M.; LeMay-Russell, S.; Rubin, S.G.; Ramirez, S.; Altman, D.R.; Schvey, N.A.; Brady, S.M.; et al. Relationships of Trait Anxiety and Loss of Control Eating with Serum Leptin Concentrations among Youth. Nutrients 2019, 11, 2198. [Google Scholar] [CrossRef]
- Grammer, A.C.; Tanofsky-Kraff, M.; Burke, N.L.; Byrne, M.E.; Mi, S.J.; Jaramillo, M.; Shank, L.M.; Kelly, N.R.; Stojek, M.M.; Schvey, N.A.; et al. An examination of the associations between pediatric loss of control eating, anxiety, and body composition in children and adolescents. Eat. Behav. 2018, 30, 109–114. [Google Scholar] [CrossRef]
- Thayer, J.F.; Lane, R.D. Claude Bernard and the heart–brain connection: Further elaboration of a model of neurovisceral integration. Neurosci. Biobehav. Rev. 2009, 33, 81–88. [Google Scholar] [CrossRef]
- Tully, P.J.; Harrison, N.; Cheung, P.; Cosh, S. Anxiety and Cardiovascular Disease Risk: A Review. Curr. Cardiol. Rep. 2016, 18, 120. [Google Scholar] [CrossRef] [PubMed]
- Al-Khudairy, L.; Loveman, E.; Colquitt, J.L.; Mead, E.; Johnson, R.E.; Fraser, H.; Olajide, J.; Murphy, M.; Velho, R.M.; O’Malley, C.; et al. Diet, physical activity and behavioural interventions for the treatment of overweight or obese adolescents aged 12 to 17 years. Cochrane Database Syst. Rev. 2017, 6, CD012651. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, S.; Wilson, C.; Austin, J.; Hooper, L. Effects of psychotherapy for anxiety in children and adolescents: A meta-analytic review. Clin. Psychol. Rev. 2012, 32, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Wergeland, G.J.H.; Fjermestad, K.W.; Marin, C.E.; Haugland, B.S.-M.; Bjaastad, J.F.; Oeding, K.; Bjelland, I.; Silverman, W.K.; Öst, L.-G.; Havik, O.E.; et al. An effectiveness study of individual vs. group cognitive behavioral therapy for anxiety disorders in youth. Behav. Res. Ther. 2014, 57, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Manassis, K.; Mendlowitz, S.L.; Scapillato, D.; Avery, D.; Fiksenbaum, L.; Freire, M.; Monga, S.; Owens, M. Group and Individual Cognitive-Behavioral Therapy for Childhood Anxiety Disorders: A Randomized Trial. J. Am. Acad. Child Adolesc. Psychiatry 2002, 41, 1423–1430. [Google Scholar] [CrossRef]
- Childs, A.W.; Bacon, S.M.; Klingensmith, K.; Li, L.; Unger, A.; Wing, A.M.; Fortunati, F. Showing Up Is Half the Battle: The Impact of Telehealth on Psychiatric Appointment Attendance for Hospital-Based Intensive Outpatient Services during COVID-19. Telemed. E-Health 2021, 27, 835–842. [Google Scholar] [CrossRef]
- Cunningham, N.R.; Ely, S.L.; Garcia, B.N.B.; Bowden, J. Addressing Pediatric Mental Health Using Telehealth during Coronavirus Disease-2019 and Beyond: A Narrative Review. Acad. Pediatr. 2021, 21, 1108–1117. [Google Scholar] [CrossRef]
- De France, K.; Hancock, G.R.; Stack, D.M.; Serbin, L.A.; Hollenstein, T. The mental health implications of COVID-19 for adolescents: Follow-up of a four-wave longitudinal study during the pandemic. Am. Psychol. 2022, 77, 85–99. [Google Scholar] [CrossRef]
- Woolford, S.J.; Sidell, M.; Li, X.; Else, V.; Young, D.R.; Resnicow, K.; Koebnick, C. Changes in Body Mass Index among Children and Adolescents during the COVID-19 Pandemic. JAMA 2021, 326, 1434–1436. [Google Scholar] [CrossRef]
- Schmitt, J.A.; Ashraf, A.P.; Becker, D.J.; Sen, B. Changes in Type 2 Diabetes Trends in Children and Adolescents during the COVID-19 Pandemic. J. Clin. Endocrinol. Metab. 2022, 107, e2777–e2782. [Google Scholar] [CrossRef]
- Hilbert, A.; Bishop, M.E.; Stein, R.I.; Tanofsky-Kraff, M.; Swenson, A.K.; Welch, R.R.; Wilfley, D.E. Long-term efficacy of psychological treatments for binge eating disorder. Br. J. Psychiatry 2012, 200, 232–237. [Google Scholar] [CrossRef]
- Kass, A.E.; Kolko, R.P.; Wilfley, D.E. Psychological treatments for eating disorders. Curr. Opin. Psychiatry 2013, 26, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Linardon, J.; Fairburn, C.G.; Fitzsimmons-Craft, E.E.; Wilfley, D.E.; Brennan, L. The empirical status of the third-wave behaviour therapies for the treatment of eating disorders: A systematic review. Clin. Psychol. Rev. 2017, 58, 125–140. [Google Scholar] [CrossRef] [PubMed]
- Tanofsky-Kraff, M.; Shomaker, L.B.; Wilfley, D.E.; Young, J.F.; Sbrocco, T.; Stephens, M.; Brady, S.M.; Galescu, O.; Demidowich, A.; Olsen, C.H.; et al. Excess weight gain prevention in adolescents: Three-year outcome following a randomized controlled trial. J. Consult. Clin. Psychol. 2017, 85, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Wilfley, D.E.; Kolko, R.P.; Kass, A.E. Cognitive-Behavioral Therapy for Weight Management and Eating Disorders in Children and Adolescents. Child Adolesc. Psychiatr. Clin. N. Am. 2011, 20, 271–285. [Google Scholar] [CrossRef] [PubMed]
- Wilson, G.T.; Wilfley, D.E.; Agras, S.; Bryson, S.W. Psychological Treatments of Binge Eating Disorder. Arch. Gen. Psychiatry 2010, 67, 94–101. [Google Scholar] [CrossRef]
- Banneyer, K.N.; Bonin, L.; Price, K.; Goodman, W.K.; Storch, E.A. Cognitive Behavioral Therapy for Childhood Anxiety Disorders: A Review of Recent Advances. Curr. Psychiatry Rep. 2018, 20, 65. [Google Scholar] [CrossRef]
- Young, J.F.; Mufson, L.; Davies, M. Impact of Comorbid Anxiety in an Effectiveness Study of Interpersonal Psychotherapy for Depressed Adolescents. J. Am. Acad. Child Adolesc. Psychiatry 2006, 45, 904–912. [Google Scholar] [CrossRef]
- Gunlicks-Stoessel, M.; Mufson, L.; Jekal, A.; Turner, J.B. The impact of perceived interpersonal functioning on treatment for adolescent depression: IPT-A versus treatment as usual in school-based health clinics. J. Consult. Clin. Psychol. 2010, 78, 260–267. [Google Scholar] [CrossRef]
- Tanofsky-Kraff, M.; Shomaker, L.B.; E Wilfley, D.; Young, J.F.; Sbrocco, T.; Stephens, M.; Ranzenhofer, L.M.; Elliott, C.; Brady, S.; Radin, R.; et al. Targeted prevention of excess weight gain and eating disorders in high-risk adolescent girls: A randomized controlled trial. Am. J. Clin. Nutr. 2014, 100, 1010–1018. [Google Scholar] [CrossRef]
- Shank, L.; Tanofsky-Kraff, M.; Radin, R.M.; Shomaker, L.B.; Wilfley, D.E.; Young, J.F.; Brady, S.; Olsen, C.H.; Reynolds, J.C.; Yanovski, J.A. Remission of loss of control eating and changes in components of the metabolic syndrome. Int. J. Eat. Disord. 2018, 51, 565–573. [Google Scholar] [CrossRef]
- Kendall, P.C.; Southam-Gerow, M.A. Long-term follow-up of a cognitive–behavioral therapy for anxiety-disordered youth. J. Consult. Clin. Psychol. 1996, 64, 724–730. [Google Scholar] [CrossRef] [PubMed]
- James, A.C.; James, G.; A Cowdrey, F.; Soler, A.; Choke, A. Cognitive behavioural therapy for anxiety disorders in children and adolescents. Cochrane Database Syst. Rev. 2015, 2020, CD004690. [Google Scholar] [CrossRef]
- Sullivan, H.S. The Interpersonal Theory of Psychiatry; W W Norton & Co.: New York, NY, USA, 1953. [Google Scholar]
- Meyer, A. Psychobiology: A Science of Man; Charles C. Thomas: Springfield, IL, USA, 1957. [Google Scholar]
- Strauss, R.S.; Pollack, H.A. Social Marginalization of Overweight Children. Arch. Pediatr. Adolesc. Med. 2003, 157, 746–752. [Google Scholar] [CrossRef] [PubMed]
- Settipani, C.A.; Kendall, P.C. Social Functioning in Youth with Anxiety Disorders: Association with Anxiety Severity and Outcomes from Cognitive-Behavioral Therapy. Child Psychiatry Hum. Dev. 2013, 44, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Ranzenhofer, L.M.; Columbo, K.M.; Tanofsky-Kraff, M.; Shomaker, L.B.; Cassidy, O.; Matheson, B.E.; Kolotkin, R.L.; Checchi, J.M.; Keil, M.; McDuffie, J.R.; et al. Binge Eating and Weight-Related Quality of Life in Obese Adolescents. Nutrients 2012, 4, 167–180. [Google Scholar] [CrossRef]
- Wilfley, D.E.; Stein, R.; Saelens, B.; Mockus, D.S.; Matt, G.; Hayden-Wade, H.A.; Welch, R.R.; Schechtman, K.B.; Thompson, P.A.; Epstein, L.H. Efficacy of Maintenance Treatment Approaches for Childhood Overweight. JAMA 2007, 298, 1661–1673. [Google Scholar] [CrossRef]
- Tanofsky-Kraff, M.; Wilfley, D.E.; Young, J.F.; Mufson, L.; Yanovski, S.Z.; Glasofer, D.R.; Salaita, C.G. Preventing Excessive Weight Gain in Adolescents: Interpersonal Psychotherapy for Binge Eating. Obesity 2007, 15, 1345–1355. [Google Scholar] [CrossRef]
- Flynn, L. The Benefits and Challenges of Multisite Studies: Lessons learned. AACN Adv. Crit. Care 2009, 20, 388–391. [Google Scholar] [CrossRef]
- Spielberger, C.D.; Edwards, C.D.; Montouri, J.; Lushene, R. State-Trait Anxiety Inventory for Children; Consulting Psychologists Press: Palo Alto, CA, USA, 1973. [Google Scholar] [CrossRef]
- Cocks, K.; Torgerson, D.J. Sample size calculations for pilot randomized trials: A confidence interval approach. J. Clin. Epidemiol. 2013, 66, 197–201. [Google Scholar] [CrossRef]
- BMI Percentile Calculator for Child and Teen. Available online: https://www.cdc.gov/healthyweight/bmi/calculator.html (accessed on 8 October 2021).
- Mccrory, M.A.; Gomez, T.D.; Bernauer, E.M.; Mol, P.A. Evaluation of a new air displacement plethysmograph for measuring human body composition. Med. Sci. Sports Exerc. 1995, 27, 1686–1691. [Google Scholar] [CrossRef] [PubMed]
- Siri, W.E. Body composition from fluid spaces and density: Analysis of methods. Tech. Meas. Body Compos. 1961, 61, 223–244. [Google Scholar]
- Katz, A.; Nambi, S.S.; Mather, K.; Baron, A.D.; Follmann, D.A.; Sullivan, G.; Quon, M.J. Quantitative Insulin Sensitivity Check Index: A Simple, Accurate Method for Assessing Insulin Sensitivity in Humans. J. Clin. Endocrinol. Metab. 2000, 85, 2402–2410. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Monnier, L.; Colette, C. Glycemic Variability: Should we and can we prevent it? Diabetes Care 2008, 31, S150–S154. [Google Scholar] [CrossRef]
- Fairburn, C.G.; Cooper, Z.; O’Connor, M. The eating disorder examination. Int. J. Eat. Disord. 1993, 6, 1–8. [Google Scholar]
- Glasofer, D.R.; Tanofsky-Kraff, M.; Eddy, K.T.; Yanovski, S.Z.; Theim, K.R.; Mirch, M.C.; Ghorbani, S.; Ranzenhofer, L.M.; Haaga, D.; Yanovski, J.A. Binge Eating in Overweight Treatment-Seeking Adolescents. J. Pediatr. Psychol. 2007, 32, 95–105. [Google Scholar] [CrossRef]
- Sysko, R.; Glasofer, D.R.; Hildebrandt, T.; Klimek, P.; Mitchell, J.E.; Berg, K.C.; Peterson, C.B.; Wonderlich, S.A.; Walsh, B.T. The eating disorder assessment for DSM-5 (EDA-5): Development and validation of a structured interview for feeding and eating disorders. Int. J. Eat. Disord. 2015, 48, 452–463. [Google Scholar] [CrossRef]
- Vannucci, A.; Tanofsky-Kraff, M.; Shomaker, L.B.; Ranzenhofer, L.M.; E Matheson, B.; Cassidy, O.L.; Zocca, J.M.; Kozlosky, M.; Yanovski, S.Z.; Yanovski, J. Construct validity of the Emotional Eating Scale Adapted for Children and Adolescents. Int. J. Obes. 2012, 36, 938–943. [Google Scholar] [CrossRef]
- Shomaker, L.B.; Tanofsky-Kraff, M.; Mooreville, M.; Reina, S.A.; Courville, A.B.; Field, S.E.; Matheson, B.E.; Brady, S.M.; Yanovski, S.Z.; Yanovski, J.A. Links of adolescent- and parent-reported eating in the absence of hunger with observed eating in the absence of hunger. Obesity 2013, 21, 1243–1250. [Google Scholar] [CrossRef]
- Beidel, D.C. Assessment of childhood social phobia: Construct, convergent, and discriminative validity of the Social Phobia and Anxiety inventory for Children (SPA-C). Psychol. Assess. 1996, 8, 235–240. [Google Scholar] [CrossRef]
- Beidel, D.C.; Turner, S.M.; Hamlin, K.; Morris, T.L. The social phobia and anxiety inventory for children (SPAI-C): External and discriminative validity. Behav. Ther. 2000, 31, 75–87. [Google Scholar] [CrossRef]
- Radloff, L.S. The CES-D Scale. Appl. Psychol. Meas. 2016, 1, 385–401. [Google Scholar] [CrossRef]
- Phillips, G.; Shadish, W.; Murray, D.; Kubik, M.; Lytle, L.; Birnbaum, A. The Center for Epidemiologic Studies Depression Scale with a Young Adolescent Population: A Confirmatory Factor Analysis. Multivar. Behav. Res. 2006, 41, 147–163. [Google Scholar] [CrossRef] [PubMed]
- Hogendoorn, S.M.; Wolters, L.H.; Vervoort, L.; Prins, P.J.M.; Boer, F.; Kooij, E.; de Haan, E. Measuring Negative and Positive Thoughts in Children: An Adaptation of the Children’s Automatic Thoughts Scale (CATS). Cogn. Ther. Res. 2010, 34, 467–478. [Google Scholar] [CrossRef]
- Ayers, T.S.; Sandler, I.N. Manual for the Children’s Coping Strategies Checklist & The How I Coped under Pressure Scale. 1999. Available online: http://asuprc.asu.edu (accessed on 8 October 2021).
- Camisasca, E.; Caravita, S.; Milani, L.; Di Blasio, P. The Children’s Coping Strategies Checklist-Revision1: A validation study in the Italian population. TPM Test. Psychom. Methodol. Appl. Psychol. 2012, 19, 197–218. [Google Scholar] [CrossRef]
- Weems, C.F.; Silverman, W.K.; Rapee, R.M.; Pina, A.A. The Role of Control in Childhood Anxiety Disorders. Cogn. Ther. Res. 2003, 27, 557–568. [Google Scholar] [CrossRef]
- Hogendoorn, S.M.; Wolters, L.H.; de Haan, E.; Lindauer, R.J.L.; Tillema, A.; Vervoort, L.; Weems, C.F.; Boer, F.; Prins, P.J.M. Advancing an Understanding of the Anxiety Control Questionnaire for Children (ACQ-C) in Clinically Anxious and Non-Anxious Youth: Psychometric Properties, Incremental Prediction and Developmental Differences. J. Psychopathol. Behav. Assess. 2013, 36, 288–299. [Google Scholar] [CrossRef]
- Weissman, M.M.; Bothwell, S. Assessment of Social Adjustment by Patient Self-Report. Arch. Gen. Psychiatry 1976, 33, 1111–1115. [Google Scholar] [CrossRef]
- Rzepa, S.; Weissman, M. Social adjustment scale self-report (SAS-SR). In Encyclopedia of Quality of Life and Well-Being Research; Springer: Dordrecht, The Netherlands, 2014; pp. 6017–6021. [Google Scholar]
- Gameroff, M.J.; Wickramaratne, P.; Weissman, M.M. Testing the Short and Screener versions of the Social Adjustment Scale–Self-report (SAS-SR). Int. J. Methods Psychiatr. Res. 2012, 21, 52–65. [Google Scholar] [CrossRef]
- Furman, W.; Buhrmester, D. Children’s perceptions of the personal relationships in their social networks. Dev. Psychol. 1985, 21, 1016–1024. [Google Scholar] [CrossRef]
- Robin, A.L.; Foster, S.L. Negotiating Parent-Adolescent Conflict: A Behavioral-Family Systems Approach; Guilford Press: New York, NY, USA, 1989. [Google Scholar]
- Robin, A.L.; Weiss, J.G. Criterion-related validity of behavioral and self-report measures of problem-solving communication-skills in distressed and non-distressed parent-adolescent dyads. Behav. Assess. 1980, 2, 339–352. [Google Scholar]
- Watson, D.; Clark, L.A. The PANAS-X: Manual for the Positive and Negative Affect Schedule—Expanded Form [Data Set]; University of Iowa: Iowa City, IA, USA, 1994. [Google Scholar] [CrossRef]
- Sanmartín, R.; Inglés, C.J.; Vicent, M.; Gonzálvez, C.; Díaz-Herrero, A.; García-Fernández, J.M. Positive and negative affect as predictors of social functioning in Spanish children. PLoS ONE 2018, 13, e0201698. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, E.A.; Xia, M.; Fosco, G.; Yaptangco, M.; Skidmore, C.R.; Crowell, S.E. The Difficulties in Emotion Regulation Scale Short Form (DERS-SF): Validation and Replication in Adolescent and Adult Samples. J. Psychopathol. Behav. Assess. 2015, 38, 443–455. [Google Scholar] [CrossRef]
- Gratz, K.L.; Roemer, L. Multidimensional Assessment of Emotion Regulation and Dysregulation: Development, Factor Structure, and Initial Validation of the Difficulties in Emotion Regulation Scale. J. Psychopathol. Behav. Assess. 2004, 26, 41–54. [Google Scholar] [CrossRef]
- Muris, P. A Brief Questionnaire for Measuring Self-Efficacy in Youths. J. Psychopathol. Behav. Assess. 2001, 23, 145–149. [Google Scholar] [CrossRef]
- Kendall, P.C.; Choudhury, M. The CAT Project Manual for the Cognitive-Behavioral Treatment of Anxious Adolescents; Workbook Publishing: Ardmore, PA, USA, 2002. [Google Scholar]
- Young, J.F.; Mufson, L.; Schueler, C.M. Preventing Adolescent Depression: Interpersonal Psychotherapy-Adolescent Skills Training; Oxford University Press: Oxford, UK, 2016. [Google Scholar]
- Welch, R.R.; Mills, M.S.; Wilfley, D.E. Interpersonal Psychotherapy for Group (IPT-G); Basic Books: New York, NY, USA, 2012; pp. 365–392. [Google Scholar] [CrossRef]
- Mufson, L. Interpersonal Psychotherapy for Depressed Adolescents; Guilford Press: New York, NY, USA, 2004. [Google Scholar]
- Eldridge, S.M.; Chan, C.L.; Campbell, M.J.; Bond, C.M.; Hopewell, S.; Thabane, L.; Lancaster, G.A. CONSORT 2010 statement: Extension to randomised pilot and feasibility trials. BMJ 2016, 355, i5239. [Google Scholar] [CrossRef]
- Hunsley, J. Development of the treatment acceptability questionnaire. J. Psychopathol. Behav. Assess. 1992, 14, 55–64. [Google Scholar] [CrossRef]
- American Psychological Association. Clinical Practice Guideline for the Treatment of Depression across Three Age Cohorts. 2019. Available online: https://www.apa.org/depression-guideline (accessed on 15 April 2022).
- DeBar, L.L.; Wilson, G.T.; Yarborough, B.J.; Burns, B.; Oyler, B.; Hildebrandt, T.; Clarke, G.N.; Dickerson, J.; Striegel, R.H. Cognitive Behavioral Treatment for Recurrent Binge Eating in Adolescent Girls: A Pilot Trial. Cogn. Behav. Pract. 2013, 20, 147–161. [Google Scholar] [CrossRef]
- Shiffman, S.; Stone, A.A.; Hufford, M.R. Ecological momentary assessment. Annu. Rev. Clin. Psychol. 2008, 4, 32. [Google Scholar] [CrossRef]
- Gloff, N.E.; LeNoue, S.R.; Novins, D.K.; Myers, K. Telemental health for children and adolescents. Int. Rev. Psychiatry 2015, 27, 513–524. [Google Scholar] [CrossRef] [PubMed]
- Comer, J.S.; Barlow, D.H. The occasional case against broad dissemination and implementation: Retaining a role for specialty care in the delivery of psychological treatments. Am. Psychol. 2014, 69, 1. [Google Scholar] [CrossRef] [PubMed]
| Construct: Assessment | Baseline | Post-Treatment | 1-Year | 2- and 3-Year | |
|---|---|---|---|---|---|
| Informed consent/assent | X | Randomization: CBT or IPT 12-week Intervention Group | |||
| Demographics questionnaire | X | ||||
| At-home device acceptability questionnaire | X | X | X | ||
| Treatment expectation questionnaire | X | ||||
| Intervention acceptability questionnaire | X | ||||
| Weight and body composition | |||||
| BMI indices: Height and weight | X | X | X | X | |
| Adiposity: Bod Pod measurement, waist circumference | X | X | X | X | |
| Cardiometabolic health | |||||
| Insulin resistance, glucose, lipids, and A1c: Fasting blood draw | X | X | X | X | |
| Glycemic variability: CGM * | X | X | X | ||
| Resting blood pressure: Automatic blood pressure monitor | X | X | X | X | |
| Stress-related physiology | |||||
| Heart rate and heart rate variability: CAM * | X | X | X | ||
| Disinhibited eating | |||||
| Eating in absence of hunger: EES-C, EAH-C-PR | X | X | X | X | |
| Loss-of-control eating: EDE-O, EDA-5, EMA * | X | X | X | X | |
| Internalizing symptoms | |||||
| Anxiety symptoms: SPAI-C, EMA * | X | X | X | X | |
| Depression symptoms: CES-D, EMA * | X | X | X | X | |
| Cognitive and behavioral functioning | |||||
| Negative and positive thoughts: CATS-N/P, EMA * | X | X | X | X | |
| Healthy coping and anxiety control: CCSC-R1, ACQ-C, SEQ-C | X | X | X | X | |
| Social functioning | |||||
| Social support and adjustment: NRI-Short Form, SAS | X | X | X | X | |
| Conflict resolution: CBQ-20 | X | X | X | X | |
| Other Outcomes | |||||
| Daily physical activity: activPAL * | X | X | X | ||
| Affect and emotion regulation: PANAS-C, DERS-SF, EMA * | X | X | X | X | |
| Session | Content |
|---|---|
| Pre- | Review feelings/thoughts/behaviors associated with participant’s own anxiety; set individual goals |
| 1 | Rules and purpose of group; psychoeducation about thoughts/behaviors, anxiety, disinhibited eating |
| 2 | Recognize emotions in self and others; learn somatic responses to anxiety in general |
| 3 | Identify individual’s own somatic responses to anxiety; introduce relaxation exercises |
| 4 | Recognize negative thoughts, challenge their utility and accuracy, and replace with coping thoughts |
| 5 | Learn problem-solving to actively cope with anxiety-related feelings, thoughts, and avoidance |
| 6 | Conduct self-evaluation and self-reinforcement for active coping with rewards |
| Mid- | Review progress towards individual goals and make a plan for individual work for rest of group |
| 7 | Discuss coping with hypothetical anxiety-provoking scenarios; introduce in-session exposures |
| 8–10 | Participants each plan and complete 10-min in-session exposure with facilitator/group support |
| 11 | Continue exposures; introduce participant presentations at Session 12 to review what they learned |
| 12 | Complete exposures; presentations; review, practice, maintain, and generalize skills; graduation |
| Post- | Review progress towards goals and make a plan for individual work now that group has ended |
| Session | Content |
|---|---|
| Pre- | Review relationship issues associated with participant’s own anxiety; set individual goals |
| 1 | Rules and purpose of group; psychoeducation about conflict/support, anxiety, disinhibited eating |
| 2 | Practice communication analysis to understand impact of verbal/non-verbal communication |
| 3 | Learn communication skills to decrease conflict and increase support |
| 4 | Introduce in-session role-play to practice using communication skills |
| 5–6 | Continue discussion, role play, and problem solving from participants’ current relationships |
| Mid- | Review progress towards individual goals and make a plan for individual work for rest of group |
| 7–9 | Continue discussion, role play, and problem solving from participants’ current relationships |
| 10, 11 | Identify useful communication strategies and discuss barriers and solutions to generalize skills |
| 12 | Recognize characteristics of positive/healthy relationships; maintaining skills; graduation |
| Post- | Review progress towards goals and make a plan for individual work now that group has ended |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Repke, H.E.; Gulley, L.D.; Rice, A.J.; Gallagher-Teske, J.H.; Markos, B.; Sanchez, N.; Bristol, M.; Haynes, H.; Lavender, J.M.; Higgins Neyland, M.K.; et al. Addressing Anxiety and Stress for Healthier Eating in Teens (ASSET): A Pilot Randomized Controlled Trial Protocol for Reducing Anxiety, Disinhibited Eating, Excess Weight Gain, and Cardiometabolic Risk in Adolescent Girls. Nutrients 2022, 14, 4246. https://doi.org/10.3390/nu14204246
Repke HE, Gulley LD, Rice AJ, Gallagher-Teske JH, Markos B, Sanchez N, Bristol M, Haynes H, Lavender JM, Higgins Neyland MK, et al. Addressing Anxiety and Stress for Healthier Eating in Teens (ASSET): A Pilot Randomized Controlled Trial Protocol for Reducing Anxiety, Disinhibited Eating, Excess Weight Gain, and Cardiometabolic Risk in Adolescent Girls. Nutrients. 2022; 14(20):4246. https://doi.org/10.3390/nu14204246
Chicago/Turabian StyleRepke, Hannah E., Lauren D. Gulley, Alexander J. Rice, Julia H. Gallagher-Teske, Bethelhem Markos, Natalia Sanchez, Madison Bristol, Hannah Haynes, Jason M. Lavender, Mary K. Higgins Neyland, and et al. 2022. "Addressing Anxiety and Stress for Healthier Eating in Teens (ASSET): A Pilot Randomized Controlled Trial Protocol for Reducing Anxiety, Disinhibited Eating, Excess Weight Gain, and Cardiometabolic Risk in Adolescent Girls" Nutrients 14, no. 20: 4246. https://doi.org/10.3390/nu14204246
APA StyleRepke, H. E., Gulley, L. D., Rice, A. J., Gallagher-Teske, J. H., Markos, B., Sanchez, N., Bristol, M., Haynes, H., Lavender, J. M., Higgins Neyland, M. K., Shank, L. M., Emerick, J. E., Gutierrez-Colina, A. M., Arnold, T., Thomas, V., Haigney, M. C., Shomaker, L. B., & Tanofsky-Kraff, M. (2022). Addressing Anxiety and Stress for Healthier Eating in Teens (ASSET): A Pilot Randomized Controlled Trial Protocol for Reducing Anxiety, Disinhibited Eating, Excess Weight Gain, and Cardiometabolic Risk in Adolescent Girls. Nutrients, 14(20), 4246. https://doi.org/10.3390/nu14204246







