Changes Induced by Aging and Long-Term Exercise and/or DHA Supplementation in Muscle of Obese Female Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Treatments
2.2. Training Protocol
2.3. mRNA Expression (Real-Time PCR)
2.4. Protein Extraction and Western Blot Analysis
2.5. Statistical Analysis
3. Results
3.1. Effects of Aging on Skeletal Muscle
3.1.1. Effects on Whole Body Lean Mass and Muscle Relative Weights
3.1.2. Effects on mRNA Expression of Genes Related to Inflammation, Muscle Damage and Muscle Regeneration
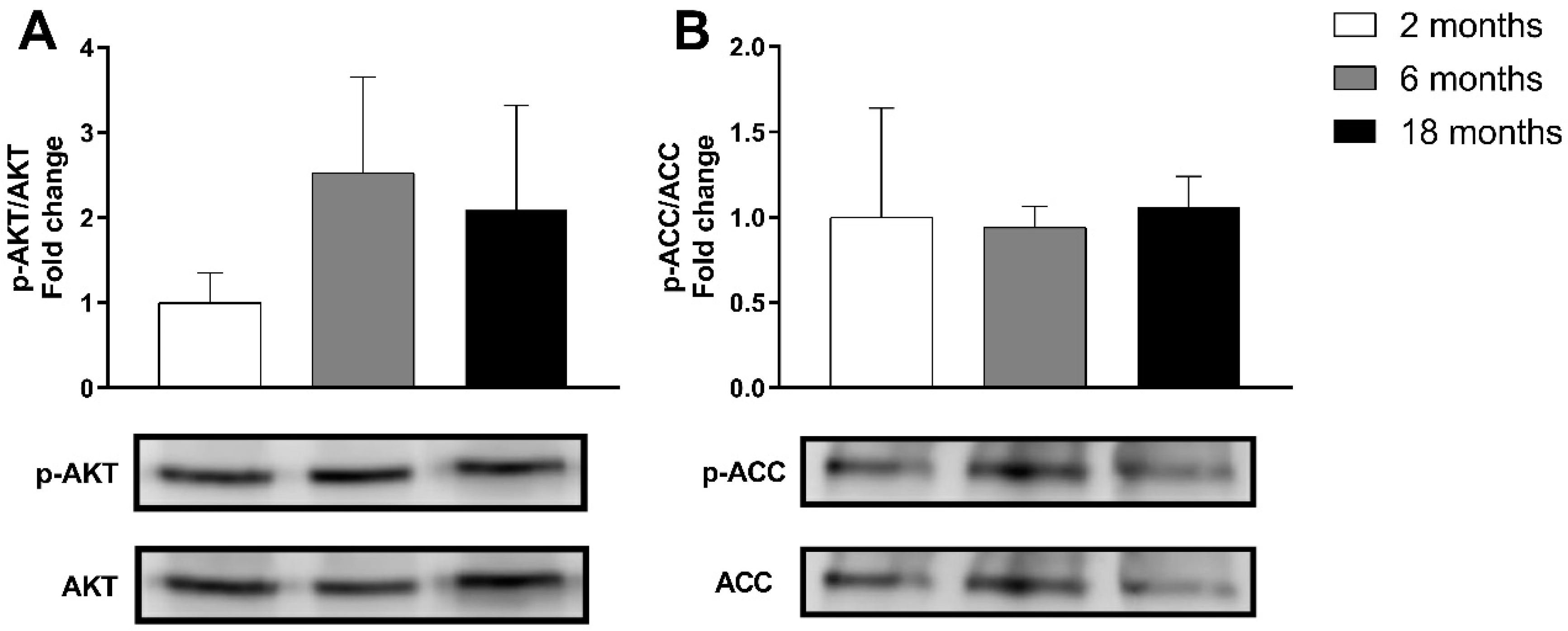
3.1.3. Effects on Genes and Proteins Related to Muscle Metabolism
3.1.4. Effects on mRNA Expression of Genes Related to Myokines
3.2. Effects of Long-Term DHA Supplementation and/or Treadmill Training on Muscle in DIO Old Mice
3.2.1. Effects on Whole Body Lean Mass and Muscle Relative Weights
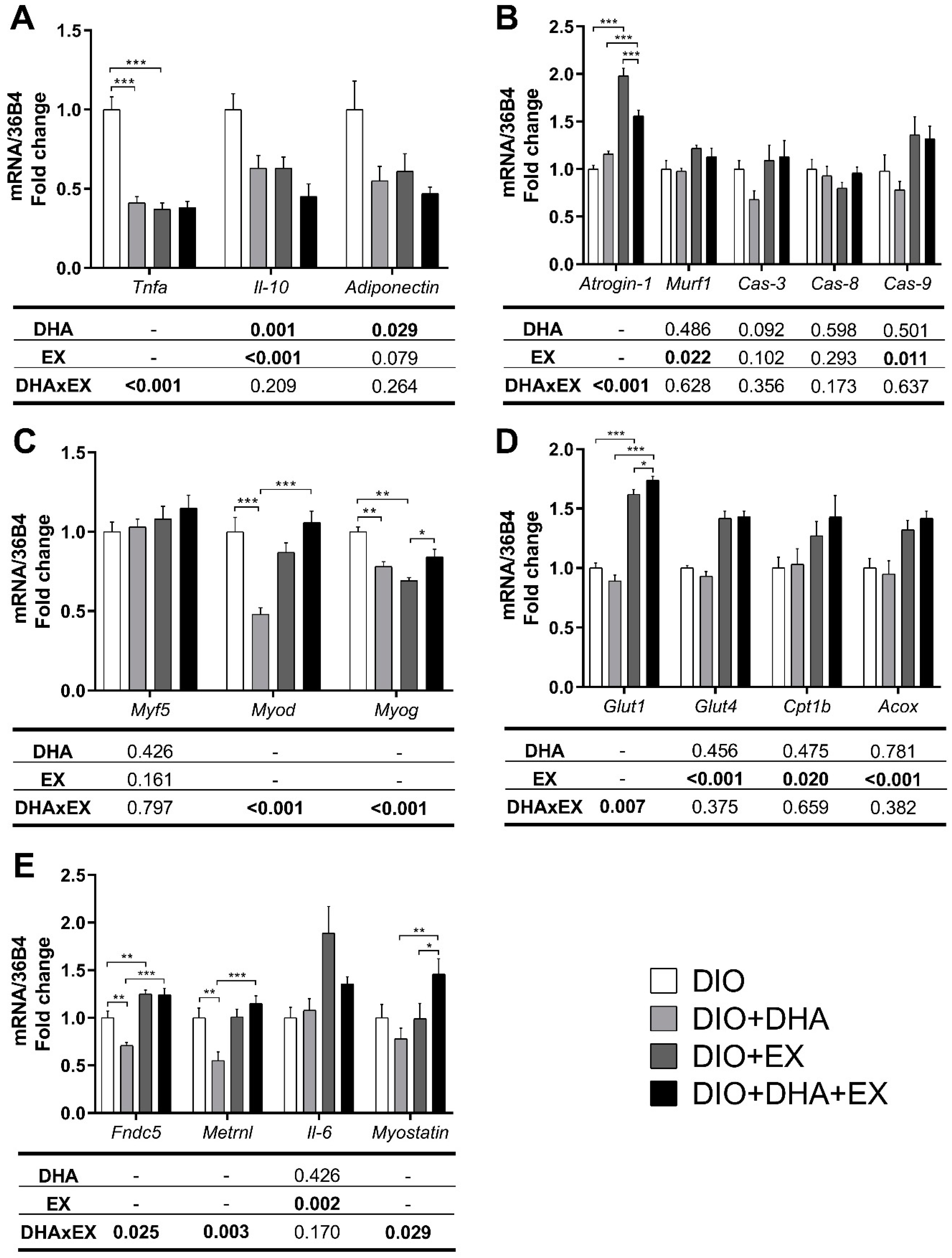
3.2.2. Effects on mRNA Expression of Pro-Inflammatory, Muscle Damage- and Muscle Regeneration-Related Genes
3.2.3. Effects on Genes and Proteins Related to Muscle Metabolism
3.2.4. Effects on mRNA Expression of Genes Related to Myokines
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO—World Health Organization. Noncommunicable Diseases. Available online: https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases (accessed on 26 May 2020).
- WHO—World Health Organization. Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 26 May 2020).
- World Obesity Prevalence of Obesity. Available online: https://www.worldobesity.org/about/about-obesity/prevalence-of-obesity (accessed on 3 June 2020).
- Crimmins, E.M. Lifespan and Healthspan: Past, Present, and Promise. Gerontologist 2015, 55, 901–911. [Google Scholar] [CrossRef] [PubMed]
- UN—United Nations. 2019 Revision of World Population Prospects. Available online: https://population.un.org/wpp/ (accessed on 5 June 2020).
- Bollheimer, L.C.; Buettner, R.; Pongratz, G.; Brunner-Ploss, R.; Hechtl, C.; Banas, M.; Singler, K.; Hamer, O.W.; Stroszczynski, C.; Sieber, C.C.; et al. Sarcopenia in the Aging High-Fat Fed Rat: A Pilot Study for Modeling Sarcopenic Obesity in Rodents. Biogerontology 2012, 13, 609–620. [Google Scholar] [CrossRef] [PubMed]
- Vasto, S.; Candore, G.; Balistreri, C.R.; Caruso, M.; Colonna-Romano, G.; Grimaldi, M.P.; Listi, F.; Nuzzo, D.; Lio, D.; Caruso, C. Inflammatory Networks in Ageing, Age-Related Diseases and Longevity. Mech. Ageing Dev. 2007, 128, 83–91. [Google Scholar] [CrossRef]
- Garg, S.K.; Delaney, C.; Shi, H.; Yung, R. Changes in Adipose Tissue Macrophages and T Cells during Aging. Crit. Rev. Immunol. 2014, 34, 1. [Google Scholar] [CrossRef]
- Ahima, R.S. Connecting Obesity, Aging and Diabetes. Nat. Med. 2009, 15, 996–997. [Google Scholar] [CrossRef] [PubMed]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic Inflammation in the Etiology of Disease across the Life Span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef]
- Steensberg, A.; Van Hall, G.; Osada, T.; Sacchetti, M.; Saltin, B.; Pedersen, B.K. Production of Interleukin-6 in Contracting Human Skeletal Muscles Can Account for the Exercise-Induced Increase in Plasma Interleukin-6. J. Physiol. 2000, 529, 237–242. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Febbraio, M.A. Muscles, Exercise and Obesity: Skeletal Muscle as a Secretory Organ. Nat. Rev. Endocrinol. 2012, 8, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Whitham, M.; Febbraio, M.A. The Ever-Expanding Myokinome: Discovery Challenges and Therapeutic Implications. Nat. Rev. Drug Discov. 2016, 15, 719–729. [Google Scholar] [CrossRef]
- Stanford, K.I.; Middelbeek, R.J.W.; Goodyear, L.J. Exercise Effects on White Adipose Tissue: Beiging and Metabolic Adaptations. Diabetes 2015, 64, 2361–2368. [Google Scholar] [CrossRef] [PubMed]
- Boström, P.; Wu, J.; Jedrychowski, M.P.; Korde, A.; Ye, L.; Lo, J.C.; Rasbach, K.A.; Boström, E.A.; Choi, J.H.; Long, J.Z.; et al. A PGC1-α-Dependent Myokine that Drives Brown-Fat-like Development of White Fat and Thermogenesis. Nature 2012, 481, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Rao, R.R.; Long, J.Z.; White, J.P.; Svensson, K.J.; Lou, J.; Lokurkar, I.; Jedrychowski, M.P.; Ruas, J.L.; Wrann, C.D.; Lo, J.C.; et al. Meteorin-like Is a Hormone That Regulates Immune-Adipose Interactions to Increase Beige Fat Thermogenesis. Cell 2014, 157, 1279–1291. [Google Scholar] [CrossRef] [PubMed]
- Kirkendall, D.T.; Garrett, W.E. The Effects of Aging and Training on Skeletal Muscle. Am. J. Sports Med. 1998, 26, 598–602. [Google Scholar] [CrossRef] [PubMed]
- Tallis, J.; Shelley, S.; Degens, H.; Hill, C. Age-Related Skeletal Muscle Dysfunction Is Aggravated by Obesity: An Investigation of Contractile Function, Implications and Treatment. Biomolecules 2021, 11, 372. [Google Scholar] [CrossRef] [PubMed]
- Khadilkar, S.S. Musculoskeletal Disorders and Menopause. J. Obstet. Gynaecol. India 2019, 69, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Morgan, P.T.; Smeuninx, B.; Breen, L. Exploring the Impact of Obesity on Skeletal Muscle Function in Older Age. Front. Nutr. 2020, 7, 569904. [Google Scholar] [CrossRef] [PubMed]
- Goisser, S.; Kemmler, W.; Porzel, S.; Volkert, D.; Sieber, C.C.; Bollheimer, L.C.; Freiberger, E. Sarcopenic Obesity and Complex Interventions with Nutrition and Exercise in Community-Dwelling Older Persons—A Narrative Review. Clin. Interv. Aging 2015, 10, 1267–1282. [Google Scholar] [CrossRef]
- Meng, P.; Hu, Y.-X.; Fan, L.; Zhang, Y.; Zhang, M.-X.; Sun, J.; Liu, Y.; Li, M.; Yang, Y.; Wang, L.-H.; et al. Sarcopenia and Sarcopenic Obesity among Men Aged 80 Years and Older in Beijing: Prevalence and Its Association with Functional Performance. Geriatr. Gerontol. Int. 2014, 14 (Suppl. S1), 29–35. [Google Scholar] [CrossRef] [PubMed]
- Kalinkovich, A.; Livshits, G. Sarcopenic Obesity or Obese Sarcopenia: A Cross Talk between Age-Associated Adipose Tissue and Skeletal Muscle Inflammation as a Main Mechanism of the Pathogenesis. Ageing Res. Rev. 2017, 35, 200–221. [Google Scholar] [CrossRef] [PubMed]
- Ji, T.; Li, Y.; Ma, L. Sarcopenic Obesity: An Emerging Public Health Problem. Aging Dis. 2022, 13, 379–388. [Google Scholar] [CrossRef]
- Moreno-Aliaga, M.J.; Lorente-Cebrián, S.; Martínez, J.A. Regulation of Adipokine Secretion by N-3 Fatty Acids. Proc. Nutr. Soc. 2010, 69, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Krause, M.; Milne, K.; Hawke, T. Adiponectin—Consideration for Its Role in Skeletal Muscle Health. Int. J. Mol. Sci. 2019, 20, 1528. [Google Scholar] [CrossRef] [PubMed]
- Ibañez, J.; Izquierdo, M.; Argüelles, I.; Forga, L.; Larrión, J.L.; García-Unciti, M.; Idoate, F.; Gorostiaga, E.M. Twice-Weekly Progressive Resistance Training Decreases Abdominal Fat and Improves Insulin Sensitivity in Older Men with Type 2 Diabetes. Diabetes Care 2005, 28, 662–667. [Google Scholar] [CrossRef] [PubMed]
- Hurley, B.F.; Roth, S.M. Strength Training in the Elderly. Sport Med. 2000, 30, 249–268. [Google Scholar] [CrossRef]
- Spite, M.; Clària, J.; Serhan, C.N. Resolvins, Specialized Proresolving Lipid Mediators, and Their Potential Roles in Metabolic Diseases. Cell Metab. 2014, 19, 21–36. [Google Scholar] [CrossRef] [PubMed]
- Lorente-Cebrián, S.; Costa, A.G.V.; Navas-Carretero, S.; Zabala, M.; Laiglesia, L.M.; Martínez, J.A.; Moreno-Aliaga, M.J. An Update on the Role of Omega-3 Fatty Acids on Inflammatory and Degenerative Diseases. J. Physiol. Biochem. 2015, 71, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Rodacki, C.L.; Rodacki, A.L.; Pereira, G.; Naliwaiko, K.; Coelho, I.; Pequito, D.; Fernandes, L.C. Fish-Oil Supplementation Enhances the Effects of Strength Training in Elderly Women. Am. J. Clin. Nutr. 2012, 95, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Strandberg, E.; Ponsot, E.; Piehl-Aulin, K.; Falk, G.; Kadi, F. Resistance Training Alone or Combined with N-3 PUFA-Rich Diet in Older Women: Effects on Muscle Fiber Hypertrophy. J. Gerontol. Ser. A 2019, 74, 489–494. [Google Scholar] [CrossRef] [PubMed]
- Da Boit, M.; Sibson, R.; Sivasubramaniam, S.; Meakin, J.R.; Greig, C.A.; Aspden, R.M.; Thies, F.; Jeromson, S.; Hamilton, D.L.; Speakman, J.R.; et al. Sex Differences in the Effect of Fish-Oil Supplementation on the Adaptive Response to Resistance Exercise Training in Older People: A Randomized Controlled Trial. Am. J. Clin. Nutr. 2017, 105, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Cornish, S.M.; Myrie, S.B.; Bugera, E.M.; Chase, J.E.; Turczyn, D.; Pinder, M. Omega-3 Supplementation with Resistance Training Does not Improve Body Composition or Lower Biomarkers of Inflammation More so than Resistance Training Alone in Older Men. Nutr. Res. 2018, 60, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, H.; Nishimura, M.; Uehara, M.; Kuribara-Souta, A.; Yamamoto, M.; Yoshikawa, N.; Morohashi, K.-I.; Tanaka, H. Eicosapentaenoic Acid Changes Muscle Transcriptome and Intervenes in Aging-Related Fiber Type Transition in Male Mice. Am. J. Physiol. Metab. 2021, 320, E346–E358. [Google Scholar] [CrossRef] [PubMed]
- Aoki, K.; Konno, M.; Honda, K.; Abe, T.; Nagata, T.; Takehara, M.; Sugasawa, T.; Takekoshi, K.; Ohmori, H. Habitual Aerobic Exercise Diminishes the Effects of Sarcopenia in Senescence-Accelerated Mice Prone8 Model. Geriatrics 2020, 5, 48. [Google Scholar] [CrossRef] [PubMed]
- Takigawa, K.; Matsuda, R.; Uchitomi, R.; Onishi, T.; Hatazawa, Y.; Kamei, Y. Effects of Long-Term Physical Exercise on Skeletal Muscles in Senescence-Accelerated Mice (SAMP8). Biosci. Biotechnol. Biochem. 2019, 83, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Félix-Soriano, E.; Martínez-Gayo, A.; Cobo, M.J.; Pérez-Chávez, A.; Ibáñez-Santos, J.; Palacios Samper, N.; Goikoetxea Galarza, I.; Cuervo, M.; García-Unciti, M.; González-Muniesa, P.; et al. Effects of DHA-Rich n-3 Fatty Acid Supplementation and/or Resistance Training on Body Composition and Cardiometabolic Biomarkers in Overweight and Obese Post-Menopausal Women. Nutrients 2021, 13, 2465. [Google Scholar] [CrossRef]
- Yang, J.; Sáinz, N.; Félix-Soriano, E.; Gil-Iturbe, E.; Castilla-Madrigal, R.; Fernández-Galilea, M.; Martínez, J.A.; Moreno-Aliaga, M.J. Effects of Long-Term DHA Supplementation and Physical Exercise on Non-Alcoholic Fatty Liver Development in Obese Aged Female Mice. Nutrients 2021, 13, 501. [Google Scholar] [CrossRef]
- Félix-Soriano, E.; Sáinz, N.; Gil-Iturbe, E.; Collantes, M.; Fernández-Galilea, M.; Castilla-Madrigal, R.; Ly, L.; Dalli, J.; Moreno-Aliaga, M.J. Changes in Brown Adipose Tissue Lipid Mediator Signatures with Aging, Obesity, and DHA Supplementation in Female Mice. FASEB J. 2021, 35, e21592. [Google Scholar] [CrossRef]
- Moreno-Aliaga, M.J.; Pérez-Echarri, N.; Marcos-Gómez, B.; Larequi, E.; Gil-Bea, F.J.; Viollet, B.; Gimenez, I.; Martínez, J.A.; Prieto, J.; Bustos, M. Cardiotrophin-1 Is a Key Regulator of Glucose and Lipid Metabolism. Cell Metab. 2011, 14, 242–253. [Google Scholar] [CrossRef]
- López-Yoldi, M.; Fernández-Galilea, M.; Laiglesia, L.M.; Larequi, E.; Prieto, J.; Martínez, J.A.; Bustos, M.; Moreno-Aliaga, M.J. Cardiotrophin-1 Stimulates Lipolysis through the Regulation of Main Adipose Tissue Lipases. J. Lipid Res. 2014, 55, 2634–2643. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2-ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Curran-Everett, D. Explorations in Statistics: Permutation Methods. Adv. Physiol. Educ. 2012, 36, 181–187. [Google Scholar] [CrossRef]
- Gil-Iturbe, E.; Félix-Soriano, E.; Sáinz, N.; Idoate-Bayón, A.; Castilla-Madrigal, R.; Moreno-Aliaga, M.J.; Lostao, M.P. Effect of Aging and Obesity on GLUT12 Expression in Small Intestine, Adipose Tissue, Muscle, and Kidney and Its Regulation by Docosahexaenoic Acid and Exercise in Mice. Appl. Physiol. Nutr. Metab. 2020, 45, 957–967. [Google Scholar] [CrossRef] [PubMed]
- Zelová, H.; Hošek, J. TNF-α Signalling and Inflammation: Interactions between Old Acquaintances. Inflamm. Res. 2013, 62, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Saraiva, M.; Vieira, P.; O’Garra, A. Biology and Therapeutic Potential of Interleukin-10. J. Exp. Med. 2020, 217, e20190418. [Google Scholar] [CrossRef]
- Krause, M.P.; Liu, Y.; Vu, V.; Chan, L.; Xu, A.; Riddell, M.C.; Sweeney, G.; Hawke, T.J. Adiponectin Is Expressed by Skeletal Muscle Fibers and Influences Muscle Phenotype and Function. Am. J. Physiol.-Cell Physiol. 2008, 295, C203–C212. [Google Scholar] [CrossRef] [PubMed]
- Qiao, L.; Kinney, B.; Yoo, H.S.; Lee, B.; Schaack, J.; Shao, J. Adiponectin Increases Skeletal Muscle Mitochondrial Biogenesis by Suppressing Mitogen-Activated Protein Kinase Phosphatase-1. Diabetes 2012, 61, 1463–1470. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bodine, S.C.; Latres, E.; Baumhueter, S.; Lai, V.K.M.; Nunez, L.; Clarke, B.A.; Poueymirou, W.T.; Panaro, F.J.; Erqian, N.; Dharmarajan, K.; et al. Identification of Ubiquitin Ligases Required for Skeletal Muscle Atrophy. Science 2001, 294, 1704–1708. [Google Scholar] [CrossRef]
- Edström, E.; Altun, M.; Hägglund, M.; Ulfhake, B. Atrogin-1/MAFbx and MuRF1 Are Downregulated in Aging-Related Loss of Skeletal Muscle. J. Gerontol. Ser. A 2006, 61, 663–674. [Google Scholar] [CrossRef]
- McIlwain, D.R.; Berger, T.; Mak, T.W. Caspase Functions in Cell Death and Disease. Cold Spring Harb. Perspect. Biol. 2013, 5, a008656. [Google Scholar] [CrossRef]
- Bharathy, N.; Ling, B.M.T.; Taneja, R. Epigenetic Regulation of Skeletal Muscle Development and Differentiation. Subcell. Biochem. 2013, 61, 139–150. [Google Scholar] [CrossRef]
- Jaiswal, N.; Gavin, M.G.; Quinn, W.J.; Luongo, T.S.; Gelfer, R.G.; Baur, J.A.; Titchenell, P.M. The Role of Skeletal Muscle Akt in the Regulation of Muscle Mass and Glucose Homeostasis. Mol. Metab. 2019, 28, 1–13. [Google Scholar] [CrossRef]
- Houten, S.M.; Wanders, R.J.A. A General Introduction to the Biochemistry of Mitochondrial Fatty Acid β-Oxidation. J. Inherit. Metab. Dis. 2010, 33, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, N.; Shinohara, Y.; Shima, A.; Terada, H. High Expression of a Novel Carnitine Palmitoyltransferase I like Protein in Rat Brown Adipose Tissue and Heart: Isolation and Characterization of Its CDNA Clone. FEBS Lett. 1995, 363, 41–45. [Google Scholar] [CrossRef]
- Reubsaet, F.A.G.; Veerkamp, J.H.; Bukkens, S.G.F.; Trijbels, J.M.F.; Monnens, L.A.H. Acyl-CoA Oxidase Activity and Peroxisomal Fatty Acid Oxidation in Rat Tissues. Biochim. Biophys. Acta (BBA)/Lipids Lipid Metab. 1988, 958, 434–442. [Google Scholar] [CrossRef]
- Brownsey, R.W.; Boone, A.N.; Elliott, J.E.; Kulpa, J.E.; Lee, W.M. Regulation of Acetyl-CoA Carboxylase. Biochem. Soc. Trans. 2006, 34, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Roca-Rivada, A.; Castelao, C.; Senin, L.L.; Landrove, M.O.; Baltar, J.; Crujeiras, A.B.; Seoane, L.M.; Casanueva, F.F.; Pardo, M. FNDC5/Irisin Is Not Only a Myokine but Also an Adipokine. PLoS ONE 2013, 8, e60563. [Google Scholar] [CrossRef]
- Rana, K.S.; Arif, M.; Hill, E.J.; Aldred, S.; Nagel, D.A.; Nevill, A.; Randeva, H.S.; Bailey, C.J.; Bellary, S.; Brown, J.E. Plasma Irisin Levels Predict Telomere Length in Healthy Adults. Age 2014, 36, 995–1001. [Google Scholar] [CrossRef]
- Jørgensen, J.R.; Fransson, A.; Fjord-Larsen, L.; Thompson, L.H.; Houchins, J.P.; Andrade, N.; Torp, M.; Kalkkinen, N.; Andersson, E.; Lindvall, O.; et al. Cometin Is a Novel Neurotrophic Factor that Promotes Neurite Outgrowth and Neuroblast Migration in Vitro and Supports Survival of Spiral Ganglion Neurons in Vivo. Exp. Neurol. 2012, 233, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.L.; Li, Z.Y.; Song, J.; Liu, J.M.; Miao, C.Y. Metrnl: A Secreted Protein with New Emerging Functions. Acta Pharmacol. Sin. 2016, 37, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.Y.; Song, J.; Zheng, S.L.; Fan, M.B.; Guan, Y.F.; Qu, Y.; Xu, J.; Wang, P.; Miao, C.Y. Adipocyte Metrnl Antagonizes Insulin Resistance through Pparg Signaling. Diabetes 2015, 64, 4011–4022. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Cánoves, P.; Scheele, C.; Pedersen, B.K.; Serrano, A.L. Interleukin-6 Myokine Signaling in Skeletal Muscle: A Double-Edged Sword? FEBS J. 2013, 280, 4131–4148. [Google Scholar] [CrossRef]
- Lee, S.-J.; McPherron, A.C. Myostatin and the Control of Skeletal Muscle Mass. Curr. Opin. Genet. Dev. 1999, 9, 604–607. [Google Scholar] [CrossRef]
- Sharma, M.; Langley, B.; Bass, J.; Kambadur, R. Myostatin in Muscle Growth and Repair. Exerc. Sport Sci. Rev. 2001, 29, 155–158. [Google Scholar] [CrossRef]
- Baylis, D.; Bartlett, D.B.; Patel, H.P.; Roberts, H.C. Understanding How We Age: Insights into Inflammaging. Longev. Health 2013, 2, 8. [Google Scholar] [CrossRef] [PubMed]
- Dayan, M.; Segal, R.; Globerson, A.; Habut, B.; Shearer, G.M.; Mozes, E. Effect of Aging on Cytokine Production in Normal and Experimental Systemic Lupus Erythematosus-Afflicted Mice. Exp. Gerontol. 2000, 35, 225–236. [Google Scholar] [CrossRef]
- Clavel, S.; Coldefy, A.S.; Kurkdjian, E.; Salles, J.; Margaritis, I.; Derijard, B. Atrophy-Related Ubiquitin Ligases, Atrogin-1 and MuRF1 Are up-Regulated in Aged Rat Tibialis Anterior Muscle. Mech. Ageing Dev. 2006, 127, 794–801. [Google Scholar] [CrossRef]
- Baek, K.; Hwang, H.R.; Park, H.-J.; Kwon, A.; Qadir, A.S.; Ko, S.-H.; Woo, K.M.; Ryoo, H.-M.; Kim, G.-S.; Baek, J.-H. TNF-α Upregulates Sclerostin Expression in Obese Mice Fed a High-Fat Diet. J. Cell. Physiol. 2014, 229, 640–650. [Google Scholar] [CrossRef]
- Hotamisligil, G.S.; Shargill, N.S.; Spiegelman, B.M. Adipose Expression of Tumor Necrosis Factor-Alpha: Direct Role in Obesity-Linked Insulin Resistance. Science 1993, 259, 87–91. [Google Scholar] [CrossRef]
- Fang, H.; Judd, R.L. Adiponectin Regulation and Function. Compr. Physiol. 2018, 8, 1031–1063. [Google Scholar] [CrossRef]
- Fasshauer, M.; Neumann, S.; Eszlinger, M.; Paschke, R.; Klein, J. Hormonal Regulation of Adiponectin Gene Expression in 3T3-L1 Adipocytes. Biochem. Biophys. Res. Commun. 2002, 290, 1084–1089. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, J.M.; Benite-Ribeiro, S.A.; Queiroz, G.; Duarte, J.A. The Effect of Age on Glucose Uptake and GLUT1 and GLUT4 Expression in Rat Skeletal Muscle. Cell Biochem. Funct. 2012, 30, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Larkin, L.M.; Reynolds, T.H.; Supiano, M.A.; Kahn, B.B.; Halter, J.B. Effect of Aging and Obesity on Insulin Responsiveness and Glut-4 Glucose Transporter Content in Skeletal Muscle of Fischer 344 X Brown Norway Rats. J. Gerontol. Ser. A 2001, 56, B486–B492. [Google Scholar] [CrossRef] [PubMed]
- Qiang, W.; Weiqiang, K.; Qing, Z.; Pengju, Z.; Yi, L. Aging Impairs Insulin-Stimulated Glucose Uptake in Rat Skeletal Muscle via Suppressing AMPKalpha. Exp. Mol. Med. 2007, 39, 535–543. [Google Scholar] [CrossRef]
- Garvey, W.T.; Maianu, L.; Hancock, J.A.; Golichowski, A.M.; Baron, A. Gene Expression of GLUT4 in Skeletal Muscle from Insulin-Resistant Patients with Obesity, IGT, GDM, and NIDDM. Diabetes 1992, 41, 465–475. [Google Scholar] [CrossRef]
- Kahn, B.B.; Pedersen, O. Suppression of GLUT4 Expression in Skeletal Muscle of Rats That Are Obese from High Fat Feeding but not from High Carbohydrate Feeding or Genetic Obesity. Endocrinology 1993, 132, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Reznick, R.M.; Zong, H.; Li, J.; Morino, K.; Moore, I.K.; Yu, H.J.; Liu, Z.-X.; Dong, J.; Mustard, K.J.; Hawley, S.A.; et al. Aging-Associated Reductions in AMP-Activated Protein Kinase Activity and Mitochondrial Biogenesis. Cell Metab. 2007, 5, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Turdi, S.; Fan, X.; Li, J.; Zhao, J.; Huff, A.F.; Du, M.; Ren, J. AMP-Activated Protein Kinase Deficiency Exacerbates Aging-Induced Myocardial Contractile Dysfunction. Aging Cell 2010, 9, 592–606. [Google Scholar] [CrossRef] [PubMed]
- Hadem, I.K.H.; Sharma, R. Age- and Tissue-Dependent Modulation of IGF-1/PI3K/Akt Protein Expression by Dietary Restriction in Mice. Horm. Metab. Res. 2016, 48, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Yamashita, H.; Qiao, L.; Friedman, J. Decreased Akt Kinase Activity and Insulin Resistance in C57BL/KsJ-Leprdb/Db Mice. J. Endocrinol. 2000, 167, 107–115. [Google Scholar] [CrossRef]
- He, M.-Q.; Wang, J.-Y.; Wang, Y.; Sui, J.; Zhang, M.; Ding, X.; Zhao, Y.; Chen, Z.-Y.; Ren, X.-X.; Shi, B.-Y. High-Fat Diet-Induced Adipose Tissue Expansion Occurs Prior to Insulin Resistance in C57BL/6J Mice. Chronic Dis. Transl. Med. 2020, 6, 198–207. [Google Scholar] [CrossRef]
- Li, M.; Verdijk, L.B.; Sakamoto, K.; Ely, B.; van Loon, L.J.C.; Musi, N. Reduced AMPK-ACC and MTOR Signaling in Muscle from Older Men, and Effect of Resistance Exercise. Mech. Ageing Dev. 2012, 133, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Nilwik, R.; Snijders, T.; Leenders, M.; Groen, B.B.L.; van Kranenburg, J.; Verdijk, L.B.; Van Loon, L.J.C. The Decline in Skeletal Muscle Mass with Aging Is Mainly Attributed to a Reduction in Type II Muscle Fiber Size. Exp. Gerontol. 2013, 48, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Saghizadeh, M.; Ong, J.M.; Garvey, W.T.; Henry, R.R.; Kern, P.A. The Expression of TNFα by Human Muscle: Relationship to Insulin Resistance. J. Clin. Investig. 1996, 97, 1111–1116. [Google Scholar] [CrossRef]
- Hamann, A.; Benecke, H.; Le Marchand-Brustel, Y.; Susulic, V.S.; Lowell, B.B.; Flier, J.S. Characterization of Insulin Resistance and NIDDM in Transgenic Mice with Reduced Brown Fat. Diabetes 1995, 44, 1266–1273. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Q.; Huang, Y.; Yang, L.; Ruan, J.; Gu, W.; Zhang, X.; Zhang, Y.; Zhang, W.; Yu, Z. The Effects of Both Age and Sex on Irisin Levels in Paired Plasma and Cerebrospinal Fluid in Healthy Humans. Peptides 2019, 113, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Huh, J.Y.; Mougios, V.; Kabasakalis, A.; Fatouros, I.; Siopi, A.; Douroudos, I.I.; Filippaios, A.; Panagiotou, G.; Park, K.H.; Mantzoros, C.S. Exercise-Induced Irisin Secretion Is Independent of Age or Fitness Level and Increased Irisin May Directly Modulate Muscle Metabolism through AMPK Activation. J. Clin. Endocrinol. Metab. 2014, 99, E2154–E2161. [Google Scholar] [CrossRef] [PubMed]
- Kurdiova, T.; Balaz, M.; Vician, M.; Maderova, D.; Vlcek, M.; Valkovic, L.; Srbecky, M.; Imrich, R.; Kyselovicova, O.; Belan, V.; et al. Effects of Obesity, Diabetes and Exercise on Fndc5 Gene Expression and Irisin Release in Human Skeletal Muscle and Adipose Tissue: In Vivo and in Vitro Studies. J. Physiol. 2014, 592, 1091–1107. [Google Scholar] [CrossRef]
- Korta, P.; Pocheć, E.; Mazur-Biały, A. Irisin as a Multifunctional Protein: Implications for Health and Certain Diseases. Medicina 2019, 55, 485. [Google Scholar] [CrossRef]
- Kawada, S.; Tachi, C.; Ishii, N. Content and Localization of Myostatin in Mouse Skeletal Muscles during Aging, Mechanical Unloading and Reloading. J. Muscle Res. Cell Motil. 2001, 22, 627–633. [Google Scholar] [CrossRef]
- Baumann, A.P.; Ibebunjo, C.; Grasser, W.A.; Paralkar, V.M. Myostatin Expression in Age and Denervation-Induced Skeletal Muscle Atrophy. J. Musculoskelet. Neuronal Interact. 2003, 3, 8–16. [Google Scholar]
- DeRuisseau, K.C.; Kavazis, A.N.; Powers, S.K. Selective Downregulation of Ubiquitin Conjugation Cascade MRNA Occurs in the Senescent Rat Soleus Muscle. Exp. Gerontol. 2005, 40, 526–531. [Google Scholar] [CrossRef]
- Sierra, H.; Cordova, M.; Chen, C.S.J.; Rajadhyaksha, M. Confocal Imaging-Guided Laser Ablation of Basal Cell Carcinomas: An Ex Vivo Study. J. Investig. Dermatol. 2015, 135, 612–615. [Google Scholar] [CrossRef] [PubMed]
- Siu, P.M.; Pistilli, E.E.; Alway, S.E. Apoptotic Responses to Hindlimb Suspension in Gastrocnemius Muscles from Young Adult and Aged Rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 289, R1015–R1026. [Google Scholar] [CrossRef] [PubMed]
- Musarò, A.; Cusella De Angelis, M.G.; Germani, A.; Ciccarelli, C.; Molinaro, M.; Zani, B.M. Enhanced Expression of Myogenic Regulatory Genes in Aging Skeletal Muscle. Exp. Cell Res. 1995, 221, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Day, K.; Shefer, G.; Shearer, A.; Yablonka-Reuveni, Z. The Depletion of Skeletal Muscle Satellite Cells with Age Is Concomitant with Reduced Capacity of Single Progenitors to Produce Reserve Progeny. Dev. Biol. 2010, 340, 330–343. [Google Scholar] [CrossRef] [PubMed]
- Keller, C.; Keller, P.; Giralt, M.; Hidalgo, J.; Pedersen, B.K. Exercise Normalises Overexpression of TNF-α in Knockout Mice. Biochem. Biophys. Res. Commun. 2004, 321, 179–182. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, F.; Odle, J.; Lin, X.; Jacobi, S.K.; Zhu, H.; Wu, Z.; Hou, Y. Inhibits TLR4 and NOD2 Signaling Pathways in Weaned Pigs after LPS Challenge1-3. J. Nutr. 2012, 142, 2017–2024. [Google Scholar] [CrossRef]
- Williams-Bey, Y.; Boularan, C.; Vural, A.; Huang, N.N.; Hwang, I.Y.; Shan-Shi, C.; Kehrl, J.H. Omega-3 Free Fatty Acids Suppress Macrophage Inflammasome Activation by Inhibiting NF-ΚB Activation and Enhancing Autophagy. PLoS ONE 2014, 9, e97957. [Google Scholar] [CrossRef]
- Castilla-Madrigal, R.; Gil-Iturbe, E.; López de Calle, M.; Moreno-Aliaga, M.J.; Lostao, M.P. DHA and Its Derived Lipid Mediators MaR1, RvD1 and RvD2 Block TNF-α Inhibition of Intestinal Sugar and Glutamine Uptake in Caco-2 Cells. J. Nutr. Biochem. 2020, 76, 108264. [Google Scholar] [CrossRef]
- Tartibian, B.; Maleki, B.H.; Abbasi, A. Omega-3 Fatty Acids Supplementation Attenuates Inflammatory Markers after Eccentric Exercise in Untrained Men. Clin. J. Sport Med. 2011, 21, 131–137. [Google Scholar] [CrossRef]
- Petersen, A.M.W.; Pedersen, B.K. The Anti-Inflammatory Effect of Exercise. J. Appl. Physiol. 2005, 98, 1154–1162. [Google Scholar] [CrossRef]
- Capel, F.; Acquaviva, C.; Pitois, E.; Laillet, B.; Rigaudière, J.P.; Jouve, C.; Pouyet, C.; Gladine, C.; Comte, B.; Vianey Saban, C.; et al. DHA at Nutritional Doses Restores Insulin Sensitivity in Skeletal Muscle by Preventing Lipotoxicity and Inflammation. J. Nutr. Biochem. 2015, 26, 949–959. [Google Scholar] [CrossRef] [PubMed]
- Hoffman-Goetz, L.; Spagnuolo, P.A.; Guan, J. Repeated Exercise in Mice Alters Expression of IL-10 and TNF-α in Intestinal Lymphocytes. Brain. Behav. Immun. 2008, 22, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Li, C.; Lv, Y.; Zhang, Y.; Amakye, W.K.; Mao, L. Dha Increases Adiponectin Expression More Effectively than Epa at Relative Low Concentrations by Regulating Pparγ and Its Phosphorylation at Ser273 in 3t3-L1 Adipocytes. Nutr. Metab. 2017, 14, 52. [Google Scholar] [CrossRef] [PubMed]
- Bueno, A.A.; Oyama, L.M.; De Oliveira, C.; Pisani, L.P.; Ribeiro, E.B.; Silveira, V.L.F.; Oller Do Nascimento, C.M. Effects of Different Fatty Acids and Dietary Lipids on Adiponectin Gene Expression in 3T3-L1 Cells and C57BL/6J Mice Adipose Tissue. Pflugers Arch. Eur. J. Physiol. 2008, 455, 701–709. [Google Scholar] [CrossRef]
- Lefils, J.; Géloën, A.; Vidal, H.; Lagarde, M.; Bernoud-Hubac, N. Dietary DHA: Time Course of Tissue Uptake and Effects on Cytokine Secretion in Mice. Br. J. Nutr. 2010, 104, 1304–1312. [Google Scholar] [CrossRef]
- Menzaghi, C.; Trischitta, V. The Adiponectin Paradox for All-Cause and Cardiovascular Mortality. Diabetes 2018, 67, 12–22. [Google Scholar] [CrossRef]
- Pinel, A.; Rigaudière, J.P.; Laillet, B.; Pouyet, C.; Malpuech-Brugère, C.; Prip-Buus, C.; Morio, B.; Capel, F. N—3PUFA Differentially Modulate Palmitate-Induced Lipotoxicity through Alterations of Its Metabolism in C2C12 Muscle Cells. Biochim. Biophys. Acta-Mol. Cell Biol. Lipids 2016, 1861, 12–20. [Google Scholar] [CrossRef]
- Slonim, A.E. Skeletal Muscle Metabolism in Exercise and Diabetes; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1999; Volume 70. [Google Scholar]
- Ren, J.M.; Semenkovich, C.F.; Gulve, E.A.; Gao, J.; Holloszy, J.O. Exercise Induces Rapid Increases in GLUT4 Expression, Glucose Transport Capacity, and Insulin-Stimulated Glycogen Storage in Muscle. J. Biol. Chem. 1994, 269, 14396–14401. [Google Scholar] [CrossRef]
- Bunprajun, T.; Henriksen, T.I.; Scheele, C.; Pedersen, B.K.; Green, C.J. Lifelong Physical Activity Prevents Aging-Associated Insulin Resistance in Human Skeletal Muscle Myotubes via Increased Glucose Transporter Expression. PLoS ONE 2013, 8, e66628. [Google Scholar] [CrossRef]
- Łukaszuk, B.; Bialuk, I.; Górski, J.; Zajączkiewicz, M.; Winnicka, M.M.; Chabowski, A. A Single Bout of Exercise Increases the Expression of Glucose but not Fatty Acid Transporters in Skeletal Muscle of IL-6 KO Mice. Lipids 2012, 47, 763–772. [Google Scholar] [CrossRef]
- Jiang, H.; Jia, D.; Zhang, B.; Yang, W.; Dong, Z.; Sun, X.; Cui, X.; Ma, L.; Wu, J.; Hu, K.; et al. Exercise Improves Cardiac Function and Glucose Metabolism in Mice with Experimental Myocardial Infarction through Inhibiting HDAC4 and Upregulating GLUT1 Expression. Basic Res. Cardiol. 2020, 115, 28. [Google Scholar] [CrossRef] [PubMed]
- Holloszy, J.O. The Regulation of Carbohydrate and Fat Metabolism during and after Exercise. Front. Biosci. 1998, 3, A342. [Google Scholar] [CrossRef] [PubMed]
- Hultman, E. Fuel Selection, Muscle Fibre. Proc. Nutr. Soc. 1995, 54, 107–121. [Google Scholar] [CrossRef] [PubMed]
- Coggan, A.R.; Raguso, C.A.; Gastaldelli, A.; Sidossis, L.S.; Yeckel, C.W. Fat Metabolism during High-Intensity Exercise in Endurance-Trained and Untrained Men. Metabolism 2000, 49, 122–128. [Google Scholar] [CrossRef]
- Bae, J.Y. Aerobic Exercise Increases Meteorin-Like Protein in Muscle and Adipose Tissue of Chronic High-Fat Diet-Induced Obese Mice. Biomed Res. Int. 2018, 2018, 6283932. [Google Scholar] [CrossRef]
- Shen, Y.; Xu, X.; Yue, K.; Xu, G. Effect of Different Exercise Protocols on Metabolic Profiles and Fatty Acid Metabolism in Skeletal Muscle in High-Fat Diet-Fed Rats. Obesity 2015, 23, 1000–1006. [Google Scholar] [CrossRef]
- Kurauti, M.A.; Costa-Júnior, J.M.; Ferreira, S.M.; Dos Santos, G.J.; Protzek, A.O.P.; Nardelli, T.R.; de Rezende, L.F.; Boschero, A.C. Acute Exercise Restores Insulin Clearance in Diet-Induced Obese Mice. J. Endocrinol. 2016, 229, 221–232. [Google Scholar] [CrossRef]
- Xiao, Y.; Sharma, N.; Arias, E.B.; Castorena, C.M.; Cartee, G.D. A Persistent Increase in Insulin-Stimulated Glucose Uptake by Both Fast-Twitch and Slow-Twitch Skeletal Muscles after a Single Exercise Session by Old Rats. Age 2013, 35, 573–582. [Google Scholar] [CrossRef]
- Arias, E.B.; Kim, J.; Funai, K.; Cartee, G.D. Prior Exercise Increases Phosphorylation of Akt Substrate of 160 KDa (AS160) in Rat Skeletal Muscle. Am. J. Physiol. Endocrinol. Metab. 2007, 292, E1191–E1200. [Google Scholar] [CrossRef]
- Wang, T.; Maltez, M.T.; Lee, H.W.; Ahmad, M.; Wang, H.W.; Leenen, F.H.H. Effect of Exercise Training on the FNDC5/BDNF Pathway in Spontaneously Hypertensive Rats. Physiol. Rep. 2019, 7, e14323. [Google Scholar] [CrossRef]
- Bae, J.Y.; Woo, J.; Kang, S.; Shin, K.O. Effects of Detraining and Retraining on Muscle Energy-Sensing Network and Meteorin-like Levels in Obese Mice. Lipids Health Dis. 2018, 17, 97. [Google Scholar] [CrossRef] [PubMed]
- Amano, Y.; Nonaka, Y.; Takeda, R.; Kano, Y.; Hoshino, D. Effects of Electrical Stimulation-Induced Resistance Exercise Training on White and Brown Adipose Tissues and Plasma Meteorin-like Concentration in Rats. Physiol. Rep. 2020, 8, e14540. [Google Scholar] [CrossRef] [PubMed]
- Ostrowski, K.; Rohde, T.; Zacho, M.; Asp, S.; Pedersen, B.K. Evidence that Interleukin-6 Is Produced in Human Skeletal Muscle during Prolonged Running. J. Physiol. 1998, 508, 949–953. [Google Scholar] [CrossRef] [PubMed]
- Keller, C.; Steensberg, A.; Pilegaard, H.; Osada, T.; Saltin, B.; Pedersen, B.K.; Neufer, P.D. Transcriptional Activation of the IL-6 Gene in Human Contracting Skeletal Muscle: Influence of Muscle Glycogen Content. FASEB J. 2001, 15, 14. [Google Scholar] [CrossRef] [PubMed]
- Jonsdottir, I.H.; Schjerling, P.; Ostrowski, K.; Asp, S.; Richter, E.A.; Pedersen, B.K. Muscle Contractions Induce Interleukin-6 MRNA Production in Rat Skeletal Muscles. J. Physiol. 2000, 528, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Moradi, Y.; Zehsaz, F.; Nourazar, M.A. Concurrent Exercise Training and Murf-l and Atrogin-1 Gene Expression in the Vastus Lateralis Muscle of Male Wistar Rats. Apunt. Sport. Med. 2020, 55, 21–27. [Google Scholar] [CrossRef]
- Primeau, A.J.; Adhihetty, P.J.; Hood, D.A. Apoptosis in Heart and Skeletal Muscle. Can. J. Appl. Physiol. 2002, 27, 349–395. [Google Scholar] [CrossRef] [PubMed]
- Sandri, M.; Podhorska-Okolow, M.; Geromel, V.; Rizzi, C.; Arslan, P.; Franceschi, C.; Carraro, U. Exercise Induces Myonuclear Ubiquitination and Apoptosis in Dystrophin-Deficient Muscle of Mice. J. Neuropathol. Exp. Neurol. 1997, 56, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Koçtürk, S.; Kayatekin, B.M.; Resmi, H.; Açıkgöz, O.; Kaynak, C.; Özer, E. The Apoptotic Response to Strenuous Exercise of the Gastrocnemius and Solues Muscle Fibers in Rats. Eur. J. Appl. Physiol. 2008, 102, 515–524. [Google Scholar] [CrossRef] [PubMed]
- Barnard, R.J.; Leung, P.S.; Aronson, W.J.; Cohen, P.; Golding, L.A. A Mechanism to Explain How Regular Exercise Might Reduce the Risk for Clinical Prostate Cancer. Eur. J. Cancer Prev. 2007, 16, 415–421. [Google Scholar] [CrossRef]
- Füchtbauer, E.M.; Westphal, H. MyoD and Myogenin Are Coexpressed in Regenerating Skeletal Muscle of the Mouse. Dev. Dyn. 1992, 193, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Woodworth-Hobbs, M.E.; Hudson, M.B.; Rahnert, J.A.; Zheng, B.; Franch, H.A.; Price, S.R. Docosahexaenoic Acid Prevents Palmitate-Induced Activation of Proteolytic Systems in C2C12 Myotubes. J. Nutr. Biochem. 2014, 25, 868–874. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. An Increase in the Omega-6/Omega-3 Fatty Acid Ratio Increases the Risk for Obesity. Nutrients 2016, 8, 128. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.K.; Holloway, G.P.; Reza-Lopez, S.; Jeram, S.M.; Kang, J.X.; Ma, D.W.L. A Decreased N-6/n-3 Ratio in the Fat-1 Mouse Is Associated with Improved Glucose Tolerance. Appl. Physiol. Nutr. Metab. 2010, 35, 699–706. [Google Scholar] [CrossRef]
- Varghese, M.; Griffin, C.; McKernan, K.; Eter, L.; Lanzetta, N.; Agarwal, D.; Abrishami, S.; Singer, K. Sex Differences in Inflammatory Responses to Adipose Tissue Lipolysis in Diet-Induced Obesity. Endocrinology 2019, 160, 293–312. [Google Scholar] [CrossRef] [PubMed]
- Mobbs, C.V.; Gee, D.M.; Finch, C.E. Reproductive Senescence in Female C57BL/6J Mice: Ovarian Impairments and Neuroendocrine Impairments that are Partially Reversible and Delayable by Ovariectomy. Endocrinology 1984, 115, 1653–1662. [Google Scholar] [CrossRef] [PubMed]
- Félix-Soriano, E.; Sáinz, N.; Fernández-Galilea, M.; Gil-Iturbe, E.; Celay, J.; Martínez-Climent, J.A.; Moreno-Aliaga, M.J. Chronic Docosahexaenoic Acid Supplementation Improves Metabolic Plasticity in Subcutaneous Adipose Tissue of Aged Obese Female Mice. J. Nutr. Biochem. 2022, 109153. [Google Scholar] [CrossRef] [PubMed]


| Gene | Forward Primer Sequence | Reverse Primer Sequence |
|---|---|---|
| Acox | CTATGGGATCAGCCAGAAAG | AGTCAAAGGCATCCACCAA |
| Adiponectin | CGAGGATTCTCTGGAACTGC | GGTCGCTTCTTCAAGGTCTG |
| Atrogin-1 | CTTTCAACAGACTGGACTTCTCGA | CAGCTCCAACAGCCTTACTACGT |
| Caspase-3 | CCTCAGAGAGACATTCATGG | GCAGTAGTCGCCTCTGAAGA |
| Caspase-8 | ACCGAGATCCTGTGAATGGAACC | TAAGAATGTCATCTCCTTGAGGA |
| Caspase-9 | AGTTCCCGGGTGCTGTCTAT | GCCATGGTCTTTCTGCTCAC |
| Cpt1b | CGAGGATTCTCTGGAACTGC | GGTCGCTTCTTCAAGGTCTG |
| Fndc5 | GGTGCTGATCATTGTTGTGG | CGCTCTTGGTTTTCTCCTTG |
| Glut1 | TCAACACGGCCTTCACTG | CACGATGCTCAGATAGGAC |
| Glut4 | AAAAGTGCCTGAAACCAGAG | TCACCTCCTGCTCTAAAAGG |
| Il-6 | GAGGATACCACTCCCAACAGACC | AAGTGCATCATCGTTGTTCATACA |
| Il-10 | AAGGCAGTGGAGCAGGTGAA | CCAGCAGACTCAATACACAC |
| Metrnl | AAGCCTTTCAGGGACTCCTC | CCCTGGTCGTACTCCACACT |
| Myf5 | CACCAACCCTAACCAGAGACTCCC | GCTGTTACATTCAGGCATGCCG |
| Myod | CGCGCTCCAACTGCTCTGATGG | CTCGACACAGCCGCACTCTTCC |
| Myog | GACCCTACAGACGCCCACAATC | ACACCCAGCCTGACAGACAATC |
| Myostatin | TGCAAAATTGGCTCAAACAG | GCAGTCAAGCCCAAAGTCTC |
| Murf1 | GCTGGTGGAAAACATCATTGACAT | CATCGGGTGGCTGCCTTT |
| Tnf-α | CATCTTCTCAAAATTCGAGTGACAA | TGGGAGTAGACAAGGTACAACCC |
| 36b4 | CACTGGTCTAGGACCCGAGAAG | GGTGCCTCTGGAGATTTTCG |
| Parameter | 2 Months (n = 10) | 6 Months (n = 7) | 18 Months (n = 9) |
|---|---|---|---|
| Lean mass (g) | 13.85 (0.55) | 15.96 (0.50) *** | 16.74 (0.74) *** # |
| Lean mass (%) | 77.84 (2.63) | 76.49 (2.74) | 57.76 (9.78) *** ### |
| Gastrocnemius (%BW) | 0.50 (0.06) | 0.47 (0.06) | 0.44 (0.05) |
| Soleus (%BW) | 0.04 (0.02) | 0.03 (0.01) * | 0.03 (0.00) ** |
| Parameter | DIO (n = 10) | DIO + DHA (n = 6) | DIO + EX (n = 8) | DIO + DHA + EX (n = 9) | 2 × 2 ANOVA | ||
|---|---|---|---|---|---|---|---|
| DHA | EX | DHAxEX | |||||
| p | p | p | |||||
| Lean mass (g) | 20.30 (1.66) | 18.85 (0.67) | 19.84 (1.68) | 20.27 (0.95) | 0.293 | 0.324 | 0.052 |
| Lean mass (%) | 38.27 (4.44) | 37.48 (2.86) | 37.18 (1.65) | 39.05 (3.61) | 0.647 | 0.834 | 0.265 |
| Gas (%BW) | 0.25 (0.04) | 0.27 (0.05) | 0.27 (0.04) | 0.26 (0.04) | 0.687 | 0.687 | 0.232 |
| Soleus (%BW) | 0.02 (0.00) | 0.02 (0.00) | 0.02 (0.00) | 0.02 (0.00) | 0.971 | 0.754 | 0.71 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Gayo, A.; Félix-Soriano, E.; Sáinz, N.; González-Muniesa, P.; Moreno-Aliaga, M.J. Changes Induced by Aging and Long-Term Exercise and/or DHA Supplementation in Muscle of Obese Female Mice. Nutrients 2022, 14, 4240. https://doi.org/10.3390/nu14204240
Martínez-Gayo A, Félix-Soriano E, Sáinz N, González-Muniesa P, Moreno-Aliaga MJ. Changes Induced by Aging and Long-Term Exercise and/or DHA Supplementation in Muscle of Obese Female Mice. Nutrients. 2022; 14(20):4240. https://doi.org/10.3390/nu14204240
Chicago/Turabian StyleMartínez-Gayo, Alejandro, Elisa Félix-Soriano, Neira Sáinz, Pedro González-Muniesa, and María J. Moreno-Aliaga. 2022. "Changes Induced by Aging and Long-Term Exercise and/or DHA Supplementation in Muscle of Obese Female Mice" Nutrients 14, no. 20: 4240. https://doi.org/10.3390/nu14204240
APA StyleMartínez-Gayo, A., Félix-Soriano, E., Sáinz, N., González-Muniesa, P., & Moreno-Aliaga, M. J. (2022). Changes Induced by Aging and Long-Term Exercise and/or DHA Supplementation in Muscle of Obese Female Mice. Nutrients, 14(20), 4240. https://doi.org/10.3390/nu14204240






